Abstract
Good sleep is essential to good health. Yet for most of its history, sleep medicine has focused on the definition, identification, and treatment of sleep problems. Sleep health is a term that is infrequently used and even less frequently defined. It is time for us to change this. Indeed, pressures in the research, clinical, and regulatory environments require that we do so. The health of populations is increasingly defined by positive attributes such as wellness, performance, and adaptation, and not merely by the absence of disease. Sleep health can be defined in such terms. Empirical data demonstrate several dimensions of sleep that are related to health outcomes, and that can be measured with self-report and objective methods. One suggested definition of sleep health and a description of self-report items for measuring it are provided as examples. The concept of sleep health synergizes with other health care agendas, such as empowering individuals and communities, improving population health, and reducing health care costs. Promoting sleep health also offers the field of sleep medicine new research and clinical opportunities. In this sense, defining sleep health is vital not only to the health of populations and individuals, but also to the health of sleep medicine itself.
Citation:
Buysse DJ. Sleep health: can we define it? Does it matter? SLEEP 2014;37(1):9-17.
Keywords: Sleep, health measurement, outcomes, public policy
Sleep Health: Is It Important?
Can we define sleep health? Can we measure it? Does it matter if we do? Isn't sleep health just the opposite of sleep problems or sleep deficiency?
For most of its brief history, sleep medicine has defined itself in terms of sleep disorders and, more recently, sleep deficiency.1,2 In this way, sleep medicine has followed the pattern established by other medical disciplines—focusing on disorders, diseases, and their treatment. But sleep medicine now finds itself embattled. Reductions in federal research funding threaten the pipeline of basic and clinical investigation. Simultaneously, reductions in reimbursement for laboratory-based polysomnography (PSG) threaten the financial viability of sleep medicine centers. The Patient Protection and Affordable Care Act (ACA) calls for large-scale re-engineering of medical care, and the role of sleep medicine in the patient-centered medical home and accountable care organizations remains uncertain.3
In this time of unprecedented challenge, how will sleep medicine demonstrate its relevance—indeed, its central position—in scientific and health care debates? Although we need multiple approaches, defining sleep health and promoting it as a crucial component of population health are important steps. Why?
Sleep health provides a positive frame of reference for sleep to patients, providers, and health care administrators. Although it is important to identify and treat disorders and deficits, sleep health is not simply their absence. Rather, sleep health indicates how well an individual or population is doing. By emphasizing the positive role of sleep in overall health, sleep health contrasts with the usual media and scientific attention on the negative role of sleep problems.
The concept of sleep health can serve an important educational function. It not only identifies what is “normal,” but quantifies degrees within the normal range.
It provides concrete targets for health promotion and prevention activities. This contrasts with treatments for disorders, which focus on the removal of symptoms or dysfunctions.
It ties in with broader agendas, such as improving population health.4,5 As accountable care organizations are required to improve outcomes and manage costs for all individuals under their care and within the larger communities in which they reside, greater emphasis will be placed on improving health.6 Sleep health provides a metric for health promotion efforts at the individual, group, and population level.
Examining the entire range of sleep health is useful for sleep research. Genetic, epigenetic, and proteomic studies have often focused on individuals with specific sleep disorders compared to unaffected controls, or the effects of sleep deprivation compared to undisturbed sleep. However, such studies can also be informed by examining individuals with varying degrees of a positive trait, or resilience to perturbation. As an example, studies of successful aging have helped to inform our understanding of pathological aging.7–9 Similarly, studying individuals with a wide range of sleep health may help to identify biomarkers more efficiently than simply studying affected and unaffected individuals.
Defining Sleep Health
Defining sleep health is a deceptively simple proposition, and one might assume that it has already been done. Principles and Practice of Sleep Medicine10 mentions “sleep health” twice, but does not define it. Neither do two other sleep medicine textbooks.11,12 A simple PubMed search for this exact term produced 150 results, and Google Scholar over 3,000, but the majority include a comma between the words “sleep” and “health,” indicating two items in a list of related concepts. Articles from countries including Japan, Australia, China, and the U.S. mention “sleep health,” but none of them explicitly defines the term, and each of them includes different constructs as part of sleep health, including sleep duration, sleep times, awakenings, sleepiness, and specific sleep disorder symptoms.13–18 The 2006 Institute of Medicine Report, Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem19 indicates that the first task of the ad hoc committee was to, “Review and quantify the public health significance of sleep health, sleep loss, and sleep disorders…” (italics added). Yet among the six occurrences of the term “sleep health” in the IOM report, none is a definition. The Centers for Disease Control and Prevention's mission statement for sleep and sleep disorders is, “To raise awareness about the problem of sleep insufficiency and sleep disorders and the importance of sleep health for the nation's overall health” (italics added). However, it also does not define sleep health. In short, “sleep health” is a term that is infrequently used in the literature, and when it is used, it is typically not defined.
Lessons from Definitions of General Health
The potential difficulties surrounding a definition of sleep health are illustrated by attempts to define health itself. Many have questioned whether it is even possible to define health.20 As summarized by Smith21 and Larson,22 paradigms for defining health have shifted from those that emphasize disease to those that focus on functioning, well-being, and interactions with the environment. Four major models of health can be summarized as follows: (1) The medical or clinical model, which defines health as an absence of disease or disability, and focuses on the causes, prevention, and care of illness; (2) The World Health Organization (WHO) model, which emphasizes health as well-being, and not merely the absence of disease (see below); (3) Wellness or role performance models, which emphasize function and the integration of body, mind, and spirit, and which treat health and illness as separable dimensions; and (4) Environmental or adaptive models, which emphasize health as resilience or potential and the individual's ability to adapt to challenges in the physical and social environment. These four models21,22 align well with how providers define health in clinical practice, emphasizing not only disease and disability, but also functioning and adaptation.23
The medical model has prevailed for the past several hundred years in Western medicine, and has led to dramatic advances in understanding the etiology pathophysiology, and treatment of diseases, including sleep disorders. A recent summary of US health in comparison with other countries focused on the presence/absence of specific diseases.24 However, medicine and public health have also begun to incorporate elements of the other three models, which place greater emphasis on health, wellness, and function. The WHO charter of 1948 adopted the following definition: “Health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.”25 Subsequent criticisms of this definition focused on its non-specificity, the absence of clear anchors or definitions of “well-being,” and the inclusion of “complete” as a qualifier, which renders most people unhealthy and health an unachievable goal.20 Nevertheless, the seemingly unrealistic WHO definition led to subsequent efforts at quantifying health in groundbreaking studies such as the RAND Health Insurance Experiment,26 the Medical Outcomes Survey,27,28 and the Alameda County Study.29,30 WHO and wellness models also paved the way for concepts such as health-related quality of life (HRQOL)31 which is now accepted as an important and measurable construct.22
The WHO definition of health offers several instructive points in considering a definition of sleep health. First, the WHO definition adopts a positive direction, describing health as a state of well-being and distinguishing it from the absence of disease. Second, it proposes notions of physical, mental, and social well-being that can, at least in theory, be quantified. Third, it places health in the context not only of the individual, but of society as well.
Dimensions of Sleep and Sleep Health
Just as health is a multidimensional concept, so too are sleep and sleep health. Carskadon and Dement offer the following definition of sleep: “Sleep is a recurring, reversible neuro-behavioral state of relative perceptual disengagement from and unresponsiveness to the environment. Sleep is typically accompanied (in humans) by postural recumbence, behavioral quiescence, and closed eyes.”32
The National Institute of Mental Health (NIMH), in its workshop on Arousal and Modulatory Systems, defined sleep and wakefulness in this way: “Sleep and wakefulness are endogenous, recurring, behavioral states that reflect coordinated changes in the dynamic functional organization of the brain and that optimize physiology, behavior, and health. Homeostatic and circadian processes regulate the propensity for wakefulness and sleep.”33
These definitions emphasize that human sleep can be measured across multiple levels of analysis and along multiple aspects or dimensions. For instance, sleep can be characterized across self-report, behavioral, physiological, circuit, cellular, and genetic levels of analysis. Within each level of analysis, sleep can be further characterized along multiple dimensions, such as quantity, continuity, and timing.34,35 Some dimensions are unique to a particular level of analysis: Satisfaction/quality in the self-report level, sleep stage architecture in the physiological level, and activation/deactivation of specific brain structures in the neural circuit level.
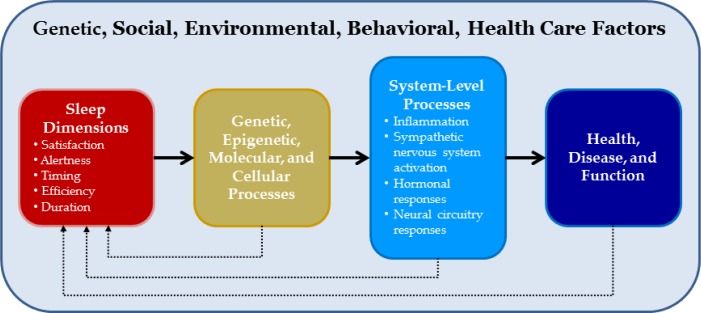
Definitions of sleep health should focus on those measurable characteristics of sleep that are most clearly associated with physical, mental, and neurobehavioral well-being. Multiple dimensions of sleep across multiple levels of analysis have been associated with such health outcomes, and should therefore be included in definitions of sleep health. A simple conceptual model of the relationship between sleep dimensions and health is presented in Figure 1.
Figure 1.
Conceptual model of sleep health. This model, similar to those proposed by many other authors, posits that various dimensions of sleep-wake function can affect distal outcomes of health and function. Intermediate processes may include epigenetic, molecular, and cellular processes that in turn affect systems-level processes. These processes, ranging from inflammation to altered function of neural circuits, are more proximally related to health outcomes. The model also recognizes that the relationships between sleep-wake function and molecular, cellular, systems and organism-level outcomes are reciprocal; just as sleep affects function and health, so too function and health influence sleep-wake function.
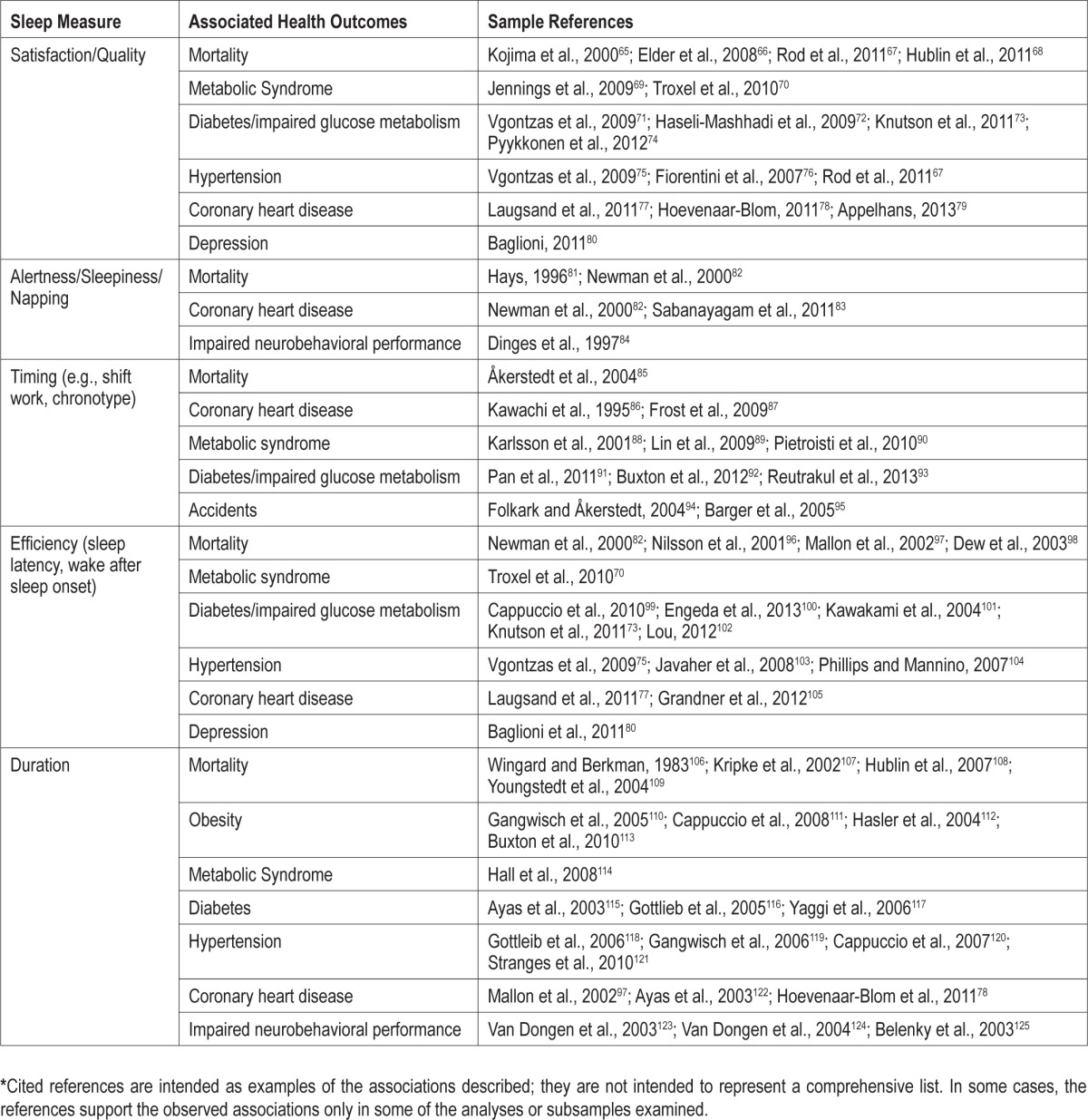
A comprehensive review of specific dimensions of sleep and their association with specific health outcomes is beyond the scope of this article, but numerous other articles have addressed such relationships. Table 1 provides a summary of key findings and references. Published studies have not always supported the relationships described in Table 1; rather, the table describes plausible correlates of sleep health that warrant further consideration. Based on these findings, the following five dimensions of sleep appear the most relevant to definitions and measurements of sleep health:
Sleep duration: The total amount of sleep obtained per 24 hours
Sleep continuity or efficiency: The ease of falling asleep and returning to sleep
Timing: The placement of sleep within the 24-hour day
Alertness/sleepiness: The ability to maintain attentive wakefulness
Satisfaction/Quality: The subjective assessment of “good” or “poor” sleep
Table 1.
Dimensions of sleep and potential health outcomes*
These five dimensions are appropriate indicators of sleep health for several reasons. First, each is associated with health outcomes, albeit with somewhat different outcomes for each dimension. Second, they can each be expressed in positive terms, i.e., we can characterize their “better” directions. This is not to say that these dimensions are all unidirectional. For instance, sleep duration and sleep timing are “good” if they fall within certain ranges, but “poor” if they deviate too far in either direction from these ranges. It is also important to acknowledge that, while these dimensions can be expressed in positive terms, the supporting studies in Table 1 largely focus on their negative directions and consequences; there have been few studies specifically examining the potential benefits of good sleep. Third, most of the dimensions can be measured across self-report, behavioral, and physiological levels of analysis. Self-report is readily obtained with retrospective questionnaires or sleep diaries. Behavioral data can be measured with actigraphy. Physiological data can be obtained with home or laboratory PSG. “Satisfaction/quality” is the potential exception to this rule. However, this dimension may have a physiological correlate in the amount of slow wave sleep (SWS) or EEG delta activity.36,37 Finally, each dimension has good face validity or ecological validity, i.e., can be readily understood by health professionals and members of the public.
There are, of course, many other potential dimensions of sleep. For example, sleep restoration or restfulness is a subjective dimension that has been assessed in several epidemiological and psychometric studies.38–40 However, it is often expressed in a negative sense, i.e., as “non-restorative sleep,” and its unique association with health outcomes is less well-established.
Another potential dimension is sleep “depth” or “soundness,” and its physiological correlates, SWS and EEG delta activity. SWS tracks the course of brain development in adolescence,41 decreases with aging42,43 and various neuropsychiatric conditions,44 and is associated with outcomes such as metabolic function45 and pain perception.46 One disadvantage of SWS as a fundamental dimension of sleep health is that it does not have a direct self-report analog. As noted above, SWS correlates with overall sleep quality36,37 in some studies, although it may dissociate from subjective sleep quality reports in studies comparing younger and older adults.47 Our psychometric studies suggest that subjective sleep depth falls along the same dimension as overall sleep quality/satisfaction.48 Therefore, it seems reasonable to propose SWS as a correlate, if not a direct analog, of sleep satisfaction in the physiological level of analysis.
Regularity vs. variability of sleep is important for understanding sleep disorders such as insomnia49 and circadian rhythm disorders.50 However, variability can be difficult to quantify in itself, and is typically measured with the proposed dimensions above. Variability is an important target for sleep disorder treatments, but it is less clearly related to adverse health outcomes. However, one recent report did link sleep regularity with performance outcomes in children.51 Similar arguments can be made regarding adaptability, i.e., the ability to sleep well under conditions of physical, psychosocial, or chronobiological stress. Adaptability is more difficult to measure and, while plausibly related to health outcomes, is less well-supported by current data.
A Proposed Definition of Sleep Health
Based on the concepts and data presented above, one possible definition of sleep health is the following:
Sleep health is a multidimensional pattern of sleep-wakefulness, adapted to individual, social, and environmental demands, that promotes physical and mental well-being. Good sleep health is characterized by subjective satisfaction, appropriate timing, adequate duration, high efficiency, and sustained alertness during waking hours.
This proposed definition does not include, nor is it specific to, any individual sleep disorder. Rather, it focuses on attributes of sleep-wakefulness per se that can be measured in any individual with or without sleep disorders. The definition is most appropriate for adults, but could be adapted to infants, children, and adolescents. It expresses sleep health as a positive attribute. It can be measured across self-report, behavioral, and physiological levels. Some elements of the definition are also measurable across circuit, cellular, and genetic levels. The definition recognizes that sleep health is best understood in the context of individual, social, and environmental demands, i.e., that good sleep health may not look the same in every situation or every individual. Finally, the definition provides definable anchors for the dimensions of sleep health.
Measuring Sleep Health: Are You SATED?
The proposed definition of sleep health incorporates a number of critical dimensions, but may still sound vague and difficult to quantify. Tools and criteria are needed to quantify sleep health. The existing literature provides guidance in devising such tools by identifying thresholds for health risk associated with different dimensions of sleep. One potential—but unvalidated—tool for measuring sleep health is a self-report scale referred to by the acronym SATED. The SATED scale assesses five key dimensions of sleep that have been consistently associated with health outcomes, and it incorporates specific quantitative criteria for four of the five. These dimensions are Satisfaction with sleep; Alertness during waking hours; Timing of sleep; Sleep Efficiency; and Sleep Duration. The SATED scale is brief and would take no more than a minute or two to complete. Consistent with the proposed definition of sleep health, the SATED scale addresses positive dimensions of sleep-wakefulness that are present to some degree in every person. This proposed scale is by no means the only, or perhaps even the best, tool to measure self-reported sleep health. Indeed, it would be premature to employ this or any other scale for measuring sleep health before developing broader consensus on its content and assessing its validity. The point is simply that it is possible to construct such scales. See the supplemental material and Figure S1 for a further description of the SATED scale.
Comparison of Sleep Health to Other Frameworks, and Potential Objections
As described above, “sleep health” is infrequently used and more rarely defined in the literature. The major competing construct is that of “sleep deficiency.”1,2 The 2011 National Institutes of Health Sleep Disorders Research Plan defines sleep deficiency as a “deficit in the quantity or quality of sleep obtained versus the amount needed for optimal health, performance, and well-being; sleep deficiency may result from prolonged wakefulness leading to sleep deprivation, insufficient sleep duration, sleep fragmentation, or a sleep disorder, such as in obstructive sleep apnea, that disrupts sleep and thereby renders sleep non-restorative.” The NHLBI includes additional specification in its public pages: “[Sleep deficiency] occurs if you have one or more of the following: You don't get enough sleep (sleep deprivation); You sleep at the wrong time of day (that is, you're out of sync with your body's natural clock); You don't sleep well or get all of the different types of sleep that your body needs; You have a sleep disorder that prevents you from getting enough sleep or causes poor quality sleep.”
The proposed definition of sleep health is in many ways the inverse of these definitions of sleep deficiency. Both sleep health and sleep deficiency emphasize the link to optimal health and well-being; both address multiple dimensions of sleep, including duration, efficiency, and timing. Sleep deficiency also incorporates sleep disorders. Dimensions incorporated in the proposed definition of sleep health, such as sleep duration, efficiency, and timing, clearly exist on a continuum with the constructs incorporated in sleep deficiency. In fact, we could define sleep deficiency and optimal sleep health as the anchors at either end of this continuum. Similarly, various domains of cardiovascular health such as atherosclerosis, blood pressure, and cardiac output each exist on a continuum from good to poor, or healthy to unhealthy. So is sleep health—or at least a definition of it—necessary? Several considerations suggest that sleep health is indeed distinct from sleep deficiency and important in its own right.
First, health is not merely the absence of disease. Cardiovascular health is not defined exclusively by the absence of a myocardial infarction, pulmonary health by the absence of emphysema, or mental health by the absence of schizophrenia. Similarly, sleep health should not be defined exclusively by the absence of sleep deprivation or a sleep disorder. In this regard sleep health is a broader and potentially more useful concept than sleep deficiency in some settings. It is also consistent with an increasing emphasis on promoting health—rather than simply focusing on disease—in other areas of medicine. For example, the CDC's Division for Heart Disease and Stroke Prevention “works to improve cardiovascular health through public health strategies and policies that promote healthy lifestyles and behaviors; healthy environments and communities; and access to early and affordable detection and treatment.” The American Heart Association defines “ideal cardiovascular health” by the absence of disease and the presence of seven quantifiable factors and behaviors.52 Ideal cardiovascular health predicts lower risk of cardiovascular disease and mortality.53
Second, deficiency states are framed in a negative rather than a positive light: as something to be avoided and replaced, rather than something to be sought and promoted. Framing sleep in a positive direction may help with education and health promotion initiatives.
Third, deficiencies usually refer to inadequate amounts of an exogenous or endogenous substance rather than to endogenous processes or states. Thus, we speak of vitamin or hormone deficiencies, but not of respiration or digestion deficiencies. We often refer to sleep in quantitative terms as though it were a substance, but it is fundamentally a process or state.
Fourth, deficiencies are usually defined in categorical terms: An individual is either deficient, and therefore in need of intervention, or not. Sleep characteristics exist on continua, like blood pressure or cholesterol, rather than as typical deficiency/ sufficiency states, like nutrient or hormone deficiencies. In a related way, deficiency states imply that a sufficiency state also exists, but this state remains undefined in the case of sleep deficiency. Gradations exist even within normal sleep, but these are more difficult to account for within the framework of sleep deficiency.
Finally, and perhaps most importantly, the continuum of sleep health can be measured and applied to every individual, whereas sleep deficiency by definition exists only in some individuals. For purposes of education, assessing populations, and health promotion, it is useful to include every individual, not only the minority with illness or disability.27
Aside from the comparison to sleep deficiency, several other potential objections to the concept of defining sleep health can also be raised:
Isn't it more important to focus on sleep disorders rather than the abstract concept of sleep health? Obviously, the identification and treatment of sleep disorders is important because of their morbidity, functional impairment, and mortality risk. However, sleep health and sleep disorders are not an either-or proposition. Diagnosing and treating sleep disorders is a proper function of sleep medicine centers. On the other hand, measuring sleep health may be important for characterizing populations and assessing risk, efficiently screening individuals, and potentially for measuring the outcome of interventions.
Isn't sleep health just a Platonic ideal that is unachievable in the real world? As proposed, sleep health can be measured and achieved. Everyone has some level of sleep health, from poor to good, just as everyone has some level of cardiovascular health or general health. Sleep health is not defined only as ideal or good sleep.
How does this definition of sleep health relate to sleep in other cultures? Human sleep is flexible enough to accommodate a variety of sleep schedules, ranging from the “segmented” nighttime sleep of pre-industrial society54 to the siesta pattern of Mediterranean and equatorial cultures, to the uniphasic sleep characteristic of most modern western cultures. While the proposed definition of sleep health allows for such variations, the proposed SATED scale was designed to measure the typical Western uniphasic nocturnal sleep pattern. However, it could be modified for other sleep cultures.
Next Steps and Future Directions
Standardization of the sleep health concept could lead to a number of new clinical, research, and public health applications. However, the first step will be to obtain broader consensus on a definition of sleep health and how to measure it. Stakeholders representing sleep research, clinical practice, public health, health care delivery agencies, and research funding agencies should participate in the development of such definitions.
A second step will be to refine and validate the actual components of sleep health. Existing data sets could be a useful and efficient means of conducting initial validation studies, since many of these studies have collected information on most of the suggested sleep dimensions, as well as information about physical and mental health. Retrospective studies could provide preliminary evidence regarding which potential dimensions of sleep health are associated with health; whether the various dimensions carry equal weight, and whether they act independently, additively, or synergistically; and the optimal thresholds to identify health risks for each dimension. Psychometric techniques such as item response theory (IRT) could help to address these questions. Retrospective studies could also be used to identify objective components of sleep health from actigraphy or PSG, and their respective threshold values.
Following preliminary validation of sleep health and its dimensions using archival data, prospective epidemiologic and treatment studies could further refine and validate the concept. Simple measures of sleep health could be readily incorporated into cohort and treatment studies that focus on other health outcomes. Understanding the determinants of sleep health, i.e., the factors that promote or constrain sleep health at the individual and population level, is another important area of investigation.
Perhaps most importantly, measures of sleep health should be incorporated into efforts to characterize and improve population health. A growing consensus recognizes that improving the U.S. health care system requires simultaneous pursuit of the so-called “Triple Aim”: Improving the experience of care, reducing per capita costs of health care, and improving the health of entire populations.5 While these are also the explicit goals for accountable care organizations,6 most attention thus far has focused on reducing costs and improving quality of care. Although “population” can be narrowly defined as the covered lives within an accountable care organization, as health care coverage expands it begins to include the entire population within a geographic area.6,55 Increasingly, then, health care is viewed not only as the treatment of disease in individuals within a particular health care delivery system, but as the production of health in the entire population.56,57
How do we produce health—and sleep health in particular— within a population? Multiple factors shape health in general and sleep health in particular, including genetic, social, environmental, behavioral, and medical care domains.4 Surprisingly, health-related behaviors and social and environmental factors account for a greater proportion of premature death (40% to 60%) than the proportion attributable to the availability or quality of health care (10%).4,58 In 1983, the Alameda County Study identified a number of health-related behaviors associated with premature mortality and disability: smoking, alcohol use, physical inactivity, obesity, poor nutrition—and short or long sleep duration.30 Analyses of health in the U.S. in 2010 show that poor diet, tobacco, obesity, inactivity, and alcohol use continue to be leading contributors to death and disability-adjusted life years24—although sleep was not examined in these analyses. Population-level interventions for these risk factors could plausibly reduce health care spending and increase longevity in the population.59 While a great deal of effort has been focused on individual and population-level interventions to address most of the health-related behaviors listed above, interventions aimed at sleep behaviors often have been notably absent. Indeed, high-profile recommendations for measuring population health have included each of the behavioral factors mentioned above—except sleep and sleep health.60 This oversight has been matched by sleep's exclusion in clinical practice and expert recommendations for routine preventive services in primary care settings.17 Recently, sleep has made some progress in entering the national health discussion: Sleep-related questions and measures have been included in the Center for Disease Control Behavioral Risk Factors Surveillance System (BRFSS) and the National Health and Nutritional Examination Survey (NHANES). Defining and measuring sleep health could provide another crucial step to developing population-level recognition and intervention efforts focused on sleep, similar to those for other important health behaviors.
Defining and measuring sleep health within the larger realm of population health also has important clinical implications for sleep medicine. Asch and Volpp provide a compelling argument that, “whereas the current health care delivery system focuses on health care, what people really want is health.”61 Thus, health care organizations may need to shift their focus from delivering health care to improving health. In a parallel way, sleep medicine has previously focused its efforts on the identification, understanding, and treatment of sleep disorders. As the reimbursement environment changes, greater emphasis is being placed on the chronic management of sleep disorder patients within the patient-centered medical home.3,62 In the future, our effort may need to broaden even further to include services such as sleep wellness or sleep health programs,62 which may be based in social service or public health organizations, rather than sleep medicine clinics or even health care organizations. To paraphrase Asch and Volpp: The current sleep medicine delivery system focuses on sleep testing and sleep disorders, but what people may really want is better sleep.
Considering sleep health from a population health perspective also has research implications. The Institute of Medicine has recommended better measurement of population health and increased funding for population-based prevention and health promotion activities, by reallocating clinical care funds under the ACA.63,64 Population health studies will require new partnerships between the health care delivery system, the public health sector, and the social services sector, as well as new methods, such as those proposed here, to assess sleep health status in the community and to provide targets for intervention.56,57
Finally, sleep health could become an important component of public education initiatives. A definition of sleep health would provide affirmative goals, as well as indicators of risk. Such initiatives would be most effective when coupled with education on behavioral and environmental strategies for achieving good sleep health. Previous educational campaigns related to other areas of behavioral health, such as those promoting healthy nutrition, physical activity, safe driving, and back-sleeping in infants, could provide important strategic and tactical lessons. Public education campaigns within sleep medicine, including those associated with National Sleep Week, would be further strengthened by having a clear definition of sleep health. Also, programs such as NHLBI's “Technologies to Assess Sleep Health Status in Populations” R43/R44 (RFAHL-14-013) could be used to develop objective biomarkers, and programs such as the “Education Research in Sleep Health and Sleep-Circadian Biology” R25 (PAR-11-098) could be used to provide education on sleep health.
Summary
Sleep is critical to health. As we enter new research and health care landscapes, the field of sleep medicine would benefit from having a clear definition not only of sleep disorders and sleep deficits, but also of sleep health. We know enough about sleep to construct such a definition. We know enough about measuring sleep to construct simple self-report and objective metrics. Defining sleep health will advance our science, and promote the health of our patients and the entire population.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Buysse has served as a paid consultant on scientific advisory boards for the following companies: Esai, Merck, Philips Respironics, Purdue Pharma, and General Sleep Corporation. Dr. Buysse has also spoken at single-sponsored educational meetings for Servier. He has also spoken at a single-sponsored lecture for Astellas. Dr. Buysse is supported by the following NIH grants: MH 024652, AG 020677, MH 078961, AR 052155. There is no off-label or investigational use of drugs.
ACKNOWLEDGMENTS
Several individuals provided valuable comments on the concepts and text in this manuscript. In particular, the author thanks Michael Twery, PhD; Martica Hall, PhD; Allison Harvey, PhD; and Christopher Kline, PhD. The author also thanks Dr. Kline for sharing the results of literature searches related to sleep and health.
SUPPLEMENTAL MATERIAL
Measuring Sleep Health: Are you SATED?
The proposed definition of sleep health in this paper incorporates a number of critical dimensions, but may still sound vague and difficult to quantify. Tools and criteria are needed to quantify sleep health. The existing literature provides guidance in devising such tools by identifying thresholds for health risk associated with different dimensions of sleep. Figure S1 presents one proposed tool for measuring sleep health, a self-report scale referred to by the acronym SATED. The SATED scale assesses five key dimensions of sleep that have been consistently associated with health outcomes, and it incorporates specific quantitative criteria for four of the five. These dimensions are Satisfaction with sleep; Alertness during waking hours; Timing of sleep; Sleep Efficiency; and Sleep Duration. The SATED scale is brief and takes no more than a minute or two to complete.
SATED, an example of a self-report sleep health questionnaire. This example of a self-report questionnaire could be used to measure dimensions of sleep health. Respondents indicate the frequency with which they experience or engage in each of 5 sleep-wake behaviors or characteristics. Sleep satisfaction is purely subjective. Each of the other questions is tied to measurable sleep-wake behavior, and includes a quantitative aspect. Individual items are score from 0-2, and item scores are totaled. A total score of “0” represents poor sleep health, and a score of “10” good sleep health. Psychometric techniques such as item response theory could be used to validate this or similar questionnaires, determining ideal threshold values item information for different dimensions. © 2013 University of Pittsburgh. All rights reserved. Used with permission.
Because it is based on empirical data rather than patient input regarding outcomes that that are important to them, SATED is not a patient-reported outcome (PRO) as typically defined.1 However, the dimensions of the SATED scale align well with previous research findings on the development of sleep-related PROs such as the Patient-Reported Outcomes Measurement Information System (PROMIS).2,3 The specific thresholds in the SATED scale are consistent with published observations in adults, but could be adjusted as additional information becomes available.
The SATED scale is a self-report measure of sleep health. Four of the five dimensions have parallel measures within the behavioral (actigraphy) and physiological (PSG) level of analysis. As noted above, sleep satisfaction is by definition subjective. Although there is no obvious actigraphy analog of sleep satisfaction, SWS is a potential physiological correlate.
The proposed definition of sleep health and the SATED scale address positive dimensions of sleep-wakefulness that are present to some degree in every person. As presented in Figure S1, the items on the SATED scale can be totaled to produce a single summary score, ranging from 0 (poor sleep health) to 10 (good sleep health). This is consistent with evidence from some studies that the number of sleep symptoms or problems have additive effects on health outcomes.4,5 Other response options, such as 5-point Likert scales, could also be used. It would also be possible to calculate scores based on specific numerical responses for most of the scales (e.g., actual sleep duration), although doing so would make it more complicated to score. The SATED scale is brief and simple enough to be used in a variety of community, health care, and research settings.
Comparison of SATED to Other Measurements, and Potential Objections
The SATED scale can also be compared to other instruments that assess constructs similar to sleep health. Hundreds of self-report sleep measures already exist,6–8 and a comprehensive comparison is beyond the scope of the current paper. However, a comparison to some of the more widely-used instruments is illustrates some key similarities and differences. The Epworth Sleepiness Scale9 is the most widely-used self-report sleep measure. It is brief, easy to complete and score, and has good face validity for adults in western societies. However, its assessment of a single dimension, the tendency to doze in specific situations, is not adequate for measuring the broad construct of sleep health. The Pittsburgh Sleep Quality Index (PSQI)10 is also widely-used and provides a single global score. It incorporates all the dimensions in the proposed definition of sleep health, and includes quantitative sleep information. However, the PSQI is lengthier (18 items), more complicated to score, and scaled to measure sleep problems rather than sleep health. The Medical Outcomes Survey (MOS) Sleep measure11 is a 12-item scale that assesses each of the constructs proposed for sleep health, as well as symptoms of sleep apnea and insomnia. The MOS sleep measure is summarized in seven subscales, ranging from one to nine items, but also has a complicated scoring algorithm that may limit widespread use. The PROMIS Sleep Disturbance and Sleep-Related Impairment scales2,3 cover the dimensions of sleep health, are calibrated to the general population, and have the precision associated with item response theory validation. The PROMIS scales were developed as PROs, with item selection following psychometric principles rather than research findings, and its items are not anchored to specific, quantifiable values. Scoring PROMIS measures requires scoring software or conversion tables. Thus, sleep health measures such as SATED may have utility and functionality beyond currently available scales.
Objections might also be raised to the particular format of the proposed SATED scale as a potential measure of sleep health:
The SATED scale has not been psychometrically validated. True. Rather, it is shown to indicate the type of measure, and of the type of items, that could be used to measure the general concept of sleep health. On the basis of data in Table 1, it seems likely that the items in SATED could be validated, but this remains an empirical question.
The SATED scale cannot be considered a PRO, since it was not developed with the formal methodology recommended for PROs, including input from patients themselves.1 However, the intent of this measure is somewhat different; it is intended to reflect what is known about sleep risk factors for adverse health outcomes, rather than outcomes important to patients themselves.
A more useful scale would assess the risk of actual sleep disorders. While it is clear that specific sleep disorders increase health risks, many screening, diagnostic, and severity tools already exist for these. Rather than assess the risk of any specific disorder, SATED evaluates aspects of sleep health that could be affected by multiple disorders. Importantly, all of the items in SATED refer to elements of sleep itself, and not to symptoms evident in association with sleep, such as snoring or restless legs.
Objective measures of sleep health would be more useful. There is a place for both self-report and objective measures of sleep health. Self-report measures can be made widely available, are simple and quick to complete, and have good face validity. Objective measures are best suited to smaller, well-characterized samples, and to funded cohort studies. Convincing arguments have been made previously for the need to identify and validate biomarkers for sleep processes and sleep deficiency.12,13 Self-reports such as SATED are not mutually exclusive with these measures. However, until such biomarkers are validated, self-report and objective sleep measures remain the most viable for the widest number of settings.
The scale does not measure adaptability of sleep. The proposed definition of sleep health incorporates adaptability, but as currently structured, the SATED scale does not. Some sleep circumstances, such as night shift work and sleep deprivation, clearly have an adverse effect on health and/or functioning. However, individuals vary widely in the degree of impairment they experience.14–17 The absence of significant impairment may be an indicator of sleep health. In practice, it is difficult to anchor sleep adaptability to observable behavioral dimensions outside of those already included in the SATED scale. Asking someone how they sleep at times of stress is possible,(e.g.,18) but still ultimately depends on variables such as sleep latency and sleep time. An alternative, simpler, and more direct way of measuring adaptability may be to assess a person's level of stress or “sleep challenge” at the time of completion of the SATED scale. It is also possible to objectively assess sleep under stressful circumstances,19 such assessments are difficult to conduct outside of research studies.
REFERENCES
- 1.Food and Drug Administration. U.S. Department of Health and Human Services; 2009. Guidance for Industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. [Google Scholar]
- 2.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–92. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS Sleep Disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2012;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: A population study. Circulation. 2011;124:2078–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moul DE, Hall M, Pilkonis PA, Buysse DJ. Self-report measures of insomnia adults: Rationales, choices, and needs. Sleep Med Rev. 2004;8:177–98. doi: 10.1016/S1087-0792(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;7:263–79. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6:41–4. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. National Institutes of Health Sleep Disorders Research Plan. Bethesda, MD: National Institutes of Health; 2011. [Google Scholar]
- 13.Czeisler CA. Impact of sleepiness and sleep deficiency on public health--utility of biomarkers. J Clin Sleep Med. 2011;7:S6–S8. doi: 10.5664/JCSM.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 15.Harma M. Individual differences in tolerance to shiftwork: a review. Ergonomics. 1993;36:101–9. doi: 10.1080/00140139308967860. [DOI] [PubMed] [Google Scholar]
- 16.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 17.Reinberg A, Ashkenazi I. Internal desynchronization of circadian rhythms and tolerance to shift work. Chronobiol Int. 2008;25:625–43. doi: 10.1080/07420520802256101. [DOI] [PubMed] [Google Scholar]
- 18.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 19.Hall M, Vasko R, Buysse DJ, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.National Institutes of Health. National Institutes of Health Sleep Disorders Research Plan. Bethesda, MD: National Institutes of Health; 2011. [Google Scholar]
- 2.Czeisler CA. Impact of sleepiness and sleep deficiency on public health--utility of biomarkers. J Clin Sleep Med. 2011;7:S6–S8. doi: 10.5664/JCSM.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strollo PJ, Jr., Badr MS, Coppola MP, Fleishman SA, Jacobowitz O, Kushida CA. The future of sleep medicine. Sleep. 2011;34:1613–9. doi: 10.5665/sleep.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood) 2002;21:78–93. doi: 10.1377/hlthaff.21.2.78. [DOI] [PubMed] [Google Scholar]
- 5.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27:759–69. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 6.Noble DJ, Casalino LP. Can accountable care organizations improve population health?: should they try? JAMA. 2013;309:1119–20. doi: 10.1001/jama.2013.592. [DOI] [PubMed] [Google Scholar]
- 7.Blazer DG. Successful aging. Am J Geriatr Psychiatry. 2006;14:2–5. doi: 10.1097/01.JGP.0000195222.93655.d1. [DOI] [PubMed] [Google Scholar]
- 8.Reichstadt J, Depp CA, Palinkas LA, Folsom DP, Jeste DV. Building blocks of successful aging: a focus group study of older adults' perceived contributors to successful aging. Am J Geriatr Psychiatry. 2007;15:194–201. doi: 10.1097/JGP.0b013e318030255f. [DOI] [PubMed] [Google Scholar]
- 9.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: A descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th ed. St. Louis: Saunders Elsevier Inc; 2011. [Google Scholar]
- 11.Chokroverty S. Sleep disorders medicine: basic science, technical considerations, and clinical aspects. 3rd ed. Philadelphia: Saunders Elsevier; 2009. [Google Scholar]
- 12.Carney PR, Berry RB, Geyer JD. Clinical sleep disorders. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 13.Uezu E, Taira K, Tanaka H, et al. Survey of sleep-health and lifestyle of the elderly in Okinawa. Psychiatry Clin Neurosci. 2000;54:311–3. doi: 10.1046/j.1440-1819.2000.00692.x. [DOI] [PubMed] [Google Scholar]
- 14.Taira K, Tanaka H, Arakawa M, Nagahama N, Uza M, Shirakawa S. Sleep health and lifestyle of elderly people in Ogimi, a village of longevity. Psychiatry Clin Neurosci. 2002;56:243–4. doi: 10.1046/j.1440-1819.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Shirakawa S. Sleep health, lifestyle and mental health in the Japanese elderly: ensuring sleep to promote a healthy brain and mind. J Psychosom Res. 2004;56:465–77. doi: 10.1016/j.jpsychores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett DJ, Marshall NS, Williams A, Grunstein RR. Sleep health New South Wales: chronic sleep restriction and daytime sleepiness. Intern Med J. 2008;38:24–31. doi: 10.1111/j.1445-5994.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 17.Sorscher AJ. How is your sleep: a neglected topic for health care screening. J Am Board Fam Med. 2008;21:141–8. doi: 10.3122/jabfm.2008.02.070167. [DOI] [PubMed] [Google Scholar]
- 18.Loredo JS, Soler X, Bardwell W, Ancoli-Israel S, Dimsdale JE, Palinkas LA. Sleep health in U.S. Hispanic population. Sleep. 2010;33:962–7. doi: 10.1093/sleep/33.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine. IOM report: Sleep disorders and sleep deprivation: An unmet public health problem. Washington, D.C.: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 20.Jadad AR, O'Grady L. How should health be defined? BMJ. 2008;337:a2900. doi: 10.1136/bmj.a2900. [DOI] [PubMed] [Google Scholar]
- 21.Smith JA. The idea of health: a philosophical inquiry. ANS Adv Nurs Sci. 1981;3:43–50. doi: 10.1097/00012272-198104000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Larson JS. The conceptualization of health. Med Care Res Rev. 1999;56:123–36. doi: 10.1177/107755879905600201. [DOI] [PubMed] [Google Scholar]
- 23.Julliard K, Klimenko E, Jacob MS. Definitions of health among healthcare providers. Nurs Sci Q. 2006;19:265–71. doi: 10.1177/0894318406289575. [DOI] [PubMed] [Google Scholar]
- 24.US Burden of Disease Collaborators. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference, New York, 19 June - 22 July 1946; signed on 22 July by the representatives of 61 States. Official Records of the World Health Organization. 1948;2:100. [Google Scholar]
- 26.Brook RH, Ware JE, Rogers WH, et al. The Effects of Coinsurance on the Health of Adults: Results from the RAND Health Insurance Experiment. Santa Monica, CA: RAND Corporation; 1984. [Google Scholar]
- 27.Ware JE, Jr., Brook RH, Davies AR, Lohr KN. Choosing measures of health status for individuals in general populations. Am J Public Health. 1981;71:620–5. doi: 10.2105/ajph.71.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–35. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Breslow L. A quantitative approach to the World Health Organization definition of health: physical, mental and social well-being. Int J Epidemiol. 1972;1:347–55. doi: 10.1093/ije/1.4.347. [DOI] [PubMed] [Google Scholar]
- 30.Berkman LF, Breslow L. Health and Ways of Living: the Alameda County Study. New York: Oxford University Press; 1983. [Google Scholar]
- 31.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 32.Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 13–23. [Google Scholar]
- 33.National Institute of Mental Health. Arousal and Regulatory Systems: Workshop Proceedings, 2013 [Google Scholar]
- 34.Hall MH, Okun ML, Atwood CW, Buysse DJ, Strollo PJ. Measurement of sleep by polysomnography. In: Luecken LL, Gallo LC, editors. Handbook of physiological research methods in health psychology. Sage Publications; 2008. pp. 341–67. [Google Scholar]
- 35.Hall M. Behavioral medicine and sleep: Concepts, measures and methods. In: Steptoe A, editor. Handbook of behavioral medicine: methods and applications. Springer; 2010. pp. 749–65. [Google Scholar]
- 36.Riedel BW, Lichstein KL. Objective sleep measures and subjective sleep satisfaction: how do older adults with insomnia define a good night's sleep? Psychol Aging. 1998;13:159–63. doi: 10.1037//0882-7974.13.1.159. [DOI] [PubMed] [Google Scholar]
- 37.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–S17. doi: 10.1016/S1389-9457(08)70011-X. [DOI] [PubMed] [Google Scholar]
- 38.Vernon MK, Dugar A, Revicki D, Treglia M, Buysse D. Measurement of non-restorative sleep in insomnia: A review of the literature. Sleep Med Rev. 2010;14:205–12. doi: 10.1016/j.smrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Roth T, Zammit G, Lankford A, et al. Nonrestorative sleep as a distinct component of insomnia. Sleep. 2010;33:449–58. doi: 10.1093/sleep/33.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165:35–41. doi: 10.1001/archinte.165.1.35. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg I, Thode HC, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 42.Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 43.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (aged 20-60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 44.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 45.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 47.Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 48.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–92. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly Y, Kelly J, Sacker A. Time for bed: associations with cognitive performance in 7-year-old children: a longitudinal population-based study. J Epidemiol Community Health. 2013;67:926–31. doi: 10.1136/jech-2012-202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 53.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–84. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ekirch AR. At Day's Close. New York: W.W. Norton & Company; 2005. [Google Scholar]
- 55.Gourevitch MN, Cannell T, Boufford JI, Summers C. The challenge of attribution: responsibility for population health in the context of accountable care. Am J Public Health. 2012;102(Suppl 3):S322–S324. doi: 10.2105/AJPH.2011.300642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shortell SM. Bridging the divide between health and health care. JAMA. 2013;309:1121–2. doi: 10.1001/jama.2013.887. [DOI] [PubMed] [Google Scholar]
- 57.Stine NW, Chokshi DA, Gourevitch MN. Improving population health in US cities. JAMA. 2013;309:449–50. doi: 10.1001/jama.2012.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–12. [PubMed] [Google Scholar]
- 59.Goldman DP, Zheng Y, Girosi F, et al. The benefits of risk factor prevention in Americans aged 51 years and older. Am J Public Health. 2009;99:2096–101. doi: 10.2105/AJPH.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stiefel M, Nolan K. Cambridge, MA: Institute for Healthcare Improvement; A guide to measuring the triple aim: population health, experience of care, and per capita cost. IHI Innovation Series white paper. 2012. [Google Scholar]
- 61.Asch DA, Volpp KG. What business are we in? The emergence of health as the business of health care. N Engl J Med. 2012;367:888–9. doi: 10.1056/NEJMp1206862. [DOI] [PubMed] [Google Scholar]
- 62.Quan SF, Epstein LJ. A warning shot across the bow: the changing face of sleep medicine. J Clin Sleep Med. 2013;9:301–2. doi: 10.5664/jcsm.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Institute of Medicine. For the Public's Health: Investing in a Healthier Future. Washington, DC: Institute of Medicine; 2012. [Google Scholar]
- 64.Institute of Medicine. For the Public's Health: The Role of Measurement in Action and Accountability. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 65.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10:87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 66.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 67.Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173:300–9. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. Sleep. 2011;34:957–64. doi: 10.5665/SLEEP.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 70.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haseli-Mashhadi N, Dadd T, Pan A, Yu Z, Lin X, Franco OH. Sleep quality in middle-aged and elderly Chinese: distribution, associated factors and associations with cardio-metabolic risk factors. BMC Public Health. 2009;9:130. doi: 10.1186/1471-2458-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–6. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pyykkonen AJ, Isomaa B, Pesonen AK, et al. Subjective sleep complaints are associated with insulin resistance in individuals without diabetes: the PPP-Botnia Study. Diabetes Care. 2012;35:2271–8. doi: 10.2337/dc12-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiorentini A, Valente R, Perciaccante A, Tubani L. Sleep's quality disorders in patients with hypertension and type 2 diabetes mellitus. Int J Cardiol. 2007;114:E50–E52. doi: 10.1016/j.ijcard.2006.07.213. [DOI] [PubMed] [Google Scholar]
- 77.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: A population study. Circulation. 2011;124:2078–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 78.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Appelhans BM, Janssen I, Cursio JF, et al. Sleep duration and weight change in midlife women: The SWAN Sleep Study. Obesity. 2013;21:77–84. doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 82.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 83.Sabanayagam C, Shankar A, Buchwald D, Goins RT. Insomnia symptoms and cardiovascular disease among older American Indians: the Native Elder Care Study. J Environ Public Health. 2011;2011:964617. doi: 10.1155/2011/964617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 85.Åkerstedt T, Kecklund G, Johansson SE. Shift work and mortality. Chronobiol Int. 2004;21:1055–61. doi: 10.1081/cbi-200038520. [DOI] [PubMed] [Google Scholar]
- 86.Kawachi I, Colditz GA, Stampfer MJ, et al. Prospective study of shift work and risk of coronary heart disease in women [see comments] Circulation. 1995;92:3178–82. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 87.Frost P, Kolstad HA, Bonde JP. Shift work and the risk of ischemic heart disease - a systematic review of the epidemiologic evidence. Scand J Work Environ Health. 2009;35:163–79. doi: 10.5271/sjweh.1319. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int. 2009;26:740–55. doi: 10.1080/07420520902929029. [DOI] [PubMed] [Google Scholar]
- 90.Pietroiusti A, Neri A, Somma G, et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–7. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 91.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reutrakul S, Hood MM, Crowley SJ, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36:2523–9. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Folkard S, Åkerstedt T. Trends in the risk of accidents and injuries and their implications for models of fatigue and performance. Aviat Space Environ Med. 2004;75:A161–A167. [PubMed] [Google Scholar]
- 95.Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–34. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 96.Nilsson PM, Nilsson JA, Hedblad B, Berglund G. Sleep disturbance in association with elevated pulse rate for prediction of mortality--consequences of mental strain? J Intern Med. 2001;250:521–9. doi: 10.1046/j.1365-2796.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 97.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 98.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 99.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engeda J, Mezuk B, Ratliff S, Ning Y. Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabetes Med. 2013;30:676–80. doi: 10.1111/dme.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–3. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 102.Lou P, Chen P, Zhang L, et al. Relation of sleep quality and sleep duration to type 2 diabetes: a population-based cross-sectional survey. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- 105.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21:427–33. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6:102–7. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- 107.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 108.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 111.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 113.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 114.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory J, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 116.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 117.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 118.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 119.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 120.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stranges S, Dorn JM, Cappuccio FP, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- 122.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 123.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 124.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 125.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SATED, an example of a self-report sleep health questionnaire. This example of a self-report questionnaire could be used to measure dimensions of sleep health. Respondents indicate the frequency with which they experience or engage in each of 5 sleep-wake behaviors or characteristics. Sleep satisfaction is purely subjective. Each of the other questions is tied to measurable sleep-wake behavior, and includes a quantitative aspect. Individual items are score from 0-2, and item scores are totaled. A total score of “0” represents poor sleep health, and a score of “10” good sleep health. Psychometric techniques such as item response theory could be used to validate this or similar questionnaires, determining ideal threshold values item information for different dimensions. © 2013 University of Pittsburgh. All rights reserved. Used with permission.