Abstract
Study Objectives:
Obstructive sleep apnea (OSA), common in Parkinson disease (PD), contributes to sleep disturbances and daytime sleepiness. We assessed the effect of continuous positive airway pressure (CPAP) on OSA, sleep, and daytime sleepiness in patients with PD.
Design:
This was a randomized placebo-controlled, crossover design. Patients with PD and OSA were randomized into 6 w of therapeutic treatment or 3 w of placebo followed by 3 w of therapeutic treatment. Patients were evaluated by polysomnography (PSG) and multiple sleep latency test (MSLT) pretreatment (baseline), after 3 w, and after 6 w of CPAP treatment. Analyses included mixed models, paired analysis, and within-group analyses comparing 3 w to 6 w of treatment.
Setting:
Sleep laboratory.
Participants:
Thirty-eight patients with PD (mean age = 67.2 ± 9.2 y; 12 females).
Intervention:
Continuous positive airway pressure.
Measurements:
PSG outcome measures: sleep efficiency, %sleep stages (N1, N2, N3, R), arousal index, apnea-hypopnea index (AHI), and % time oxygen saturation < 90% (%time SaO2 < 90%). MSLT outcome measures: mean sleep-onset latency (MSL).
Results:
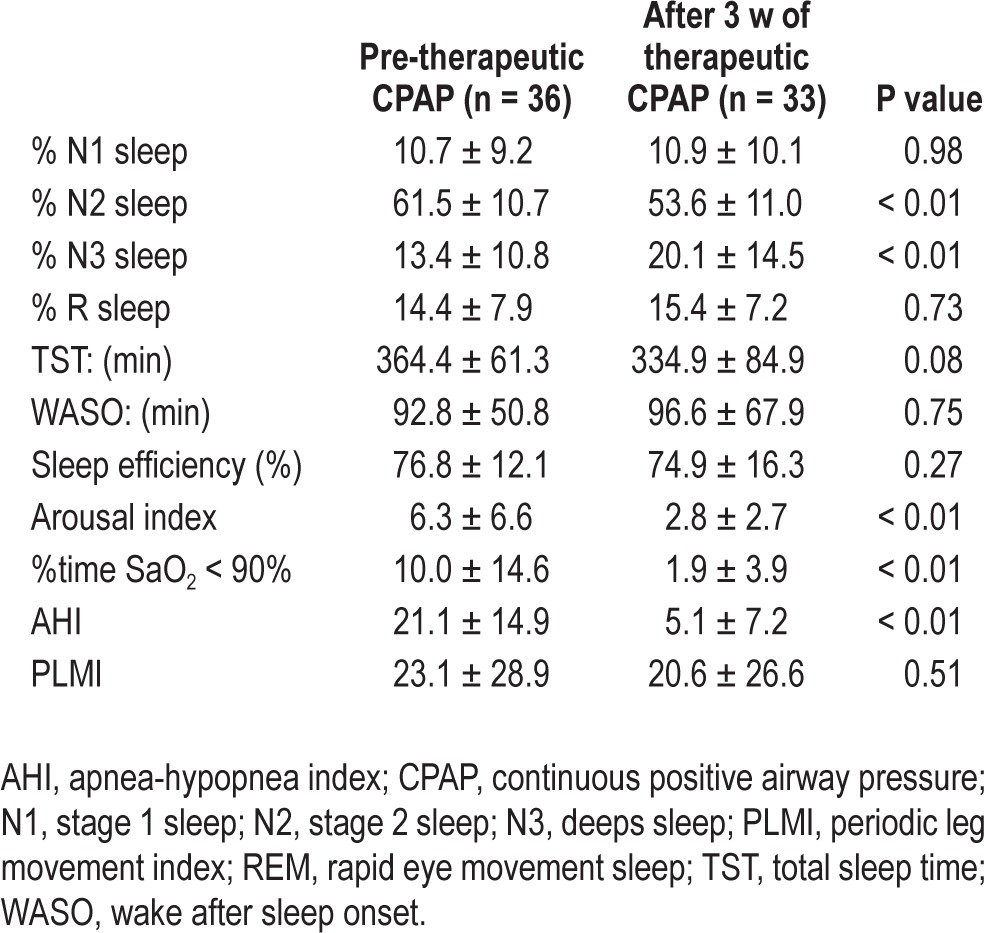
There were significant group-by-time interactions for AHI (P < 0.001), % time SaO2 < 90% (P = 0.02), %N2 (P = 0.015) and %N3 (P = 0.014). Subjects receiving therapeutic CPAP showed significant decrease in AHI, %time SaO2 < 90%, %N2, and significant increase in %N3 indicating effectiveness of CPAP in the treatment of OSA, improvement in nighttime oxygenation, and in deepening sleep. The paired sample analyses revealed that 3 w of therapeutic treatment resulted in significant decreases in arousal index (t = 3.4, P = 0.002). All improvements after 3 w were maintained at 6 w. Finally, 3 w of therapeutic CPAP also resulted in overall decreases in daytime sleepiness (P = 0.011).
Conclusions:
Therapeutic continuous positive airway pressure versus placebo was effective in reducing apnea events, improving oxygen saturation, and deepening sleep in patients with Parkinson disease and obstructive sleep apnea. Additionally, arousal index was reduced and effects were maintained at 6 weeks. Finally, 3 weeks of continuous positive airway pressure treatment resulted in reduced daytime sleepiness measured by multiple sleep latency test. These results emphasize the importance of identifying and treating obstructive sleep apnea in patients with Parkinson disease.
Citation:
Neikrug AB; Liu L; Avanzino JA; Maglione JE; Natarajan L; Bradley L; Maugeri A; Corey-Bloom J; Palmer BW; Loredo JS; Ancoli-Israel S. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. SLEEP 2014;37(1):177-185.
Keywords: Continuous positive airway pressure, daytime sleepiness, obstructive sleep apnea, Parkinson disease, sleep disorders, sleep quality
INTRODUCTION
Sleep complaints are very common in patients with Parkinson disease (PD), with 60-98% complaining of sleep related difficulties.1–3 Nonmotor symptoms, including sleep dysfunction, have been shown to have significant effect on health-related quality of life in patients with PD.4,5 Polysomnography (PSG; overnight sleep recording) studies have confirmed substantial decreases in total sleep time (TST) and in the amount of time spent in deep sleep (i.e., decrease in %N3 sleep) relative to lighter sleep (increases in %N1 and %N2 sleep) in patients with PD.6–9 In addition to this disruption of sleep architecture, it is estimated that up to 60% of patients also have obstructive sleep apnea (OSA).10–14 OSA can cause hypoxemia and sleep fragmentation, which have been associated with cardiac arrhythmias, nocturnal hypertension, nighttime confusion, and neuropsychological impairment.15–17 Additionally, daytime sleepiness, a main symptom of OSA, is a common and debilitating nonmotor symptom of PD and has been shown to be related to OSA in this population.18,19
Continuous positive airway pressure (CPAP) is the gold standard treatment for OSA, and CPAP efficacy has been demonstrated in older adult populations, in chronic disease, and in Alzheimer disease.20–24 To our knowledge, there have been no studies demonstrating ability of patients with PD to tolerate CPAP and their ability to appropriately use the mask apparatus, which may be more complicated due to the motor disability. Additionally, because PD is associated with upper airway dysfunction due to the neurodegenerative processes and as sleep disturbances in PD are likely multifactorial, CPAP effectiveness in this population remains to be demonstrated and the effect of CPAP therapy on sleep and daytime sleepiness in patients with PD needs to be assessed.
As part of a larger study exploring the effect of CPAP on nonmotor symptoms in PD, we assessed the effect of CPAP on OSA, sleep architecture, and daytime sleepiness. We hypothesized that CPAP treatment would result in improvement in OSA (evident by decreased apnea-hypopnea index [AHI] and improved oxygenation), and in deeper sleep and fewer arousals during the night as well as improvement of daytime sleepiness.
METHODS
Participants
Patients with PD were referred by neurologists at the University of California, San Diego (UCSD), by community neurologists in San Diego County or volunteered after hearing a talk about the study at PD support group meetings. Inclusion criteria included having a diagnosis of PD confirmed by a neurologist, fluent English, and having overall stable health. Patients were excluded if they were receiving current treatment for OSA, had bronchospastic and symptomatic chronic obstructive pulmonary disease, coronary or cerebral vascular disease, active seizure disorder, any neurodegenerative disorder other than PD, deep brain stimulation treatment for PD or current alcohol or drug abuse, or any other physiological (e.g., incontinence) or psychological impairments that would have limited their participation. Patients were allowed to use their prescribed medications as long as they were stable on the same dose for 2 mo prior to enrollment in the study and remained on the same dose throughout the study. The study was approved by the UCSD Human Research Protections program and San Diego Veterans Administration Healthcare System.
Study Design
After signed informed consent was received, participants meeting criteria were tested for cognitive performance using the Montreal Cognitive Assessment (MoCA)25 and were assessed by a neurologist (JCB) using the Unified Parkinson's Disease Rating Scale (UPDRS)26 and Hoehn and Yahr (H&Y)27,28 for staging of the disease. Additionally, all patients underwent a history and physical examination by a physician specializing in sleep medicine (JSL and JEM). All patients were scheduled for overnight baseline OSA assessment with PSG at the Gillin Laboratory for Sleep and Chronobiology (GSCRC), which was followed the next day by a four-nap multiple sleep latency test (MSLT). Patients with PD meeting criteria for OSA (AHI ≥ 10) were randomized in a 1:1 ratio using a randomized block design (block size = 4) into either the therapeutic CPAP (tCPAP) group, which received 6 w of therapeutic CPAP, or the placebo CPAP (pCPAP) group, which received 3 w of placebo CPAP followed by 3 w of therapeutic CPAP. Randomization was stratified on PD severity (H&Y score < 3 versus ≥ 3).
After randomization, all patients had 2 consecutive nights of PSG recordings. On the first night, a CPAP titration in which CPAP pressure was adjusted according to the patient's needs was conducted for those in the tCPAP group. A sham titration21 (for details see the following paragraphs) was conducted for the pCPAP group. On the second night, sleep was recorded while patients slept with their assigned CPAP. An MSLT was conducted on the day following the second PSG study. Patients were sent home with their assigned CPAP machine for 3 w. After the 3 w, patients returned for 2 additional consecutive nights of sleep recording. On the first night, sleep was recorded while patients slept with their assigned CPAP (therapeutic or placebo). An MSLT was conducted on the day following the first night. On the second night, those in the pCPAP group were switched to therapeutic CPAP and a titration was conducted. The next day, patients were sent home with their assigned CPAP machine (all had therapeutic CPAP at this point) for an additional 3 w. After the second 3-w period, all patients returned for 1 night of PSG with tCPAP, followed by an MSLT. This study used the PSG data from baseline, 3 w (night 1), and 6 w.
Polysomnographic Evaluation
PSG was recorded with the video-enabled Compumedics Somté (Charlotte, NC, USA). Electroencephalography (F4, C4, O1, or O2), electrooculography (left and right outer canthus), submental electromyography (EMG), respiratory effort (thoracic and abdominal piezoelectric bands), airflow (nasal pressure transducer), electrocardiogram, oximetry, and tibialis EMG were recorded.
Sleep recordings were scored by a scorer blinded to the clinical assessment, treatment condition, and questionnaire data. All PSG records were staged and scored according to accepted American Academy of Sleep Medicine (AASM) criteria.29 Apneas were scored when there was > 90% decrease in airflow amplitude from baseline lasting for ≥ 10 sec. Hypopneas were scored when there was a 50-90% decrease in amplitude lasting ≥ 10 sec, associated with an arousal or an oxygen desaturation ≥ 3%. Periodic limb movements (PLMs) was defined as a series of at least four consecutive movements with movement duration of 0.5-5 sec and onsets each 5-90 sec apart, and with an ≥ 8 μV increase in EMG voltage above resting EMG. The following sleep parameters were computed: %N1, %N2, %N3, %R sleep, sleep efficiency (SE; total sleep time divided by time in bed), AHI, %time oxygen saturation (SaO2) < 90%, arousal index (ArI; number of arousals per hour of sleep), and PLM index (PLMI; number of PLM per hour of sleep). An arousal was defined as any sudden increase in EEG frequency (to alpha or theta) that lasted < 15 but > 3 sec.
Objective Assessment of Daytime Sleepiness
A MSLT30 was performed to objectively assess daytime sleepiness. The MSLT was conducted the morning after an overnight PSG and the sleep recording sensors were left on the patients during the four “naps”. The patients were asked to go to bed and try to fall asleep at 08:00, 10:00, 12:00, and 02:00. Sleep was monitored and sleep onset latency was calculated for each nap. Outcome measures were the average sleep onset latency for all four naps (MSL) and the number of naps on which patients fell asleep in < 10 min.
CPAP Treatment
Patients in whom OSA was diagnosed (AHI ≥ 10) on the screening PSG received a REMstar® Plus M-series CPAP (Philips Respironics, Andover, MA) with a heated humidifier. The CPAP units (both real and placebo) were enabled to track treatment adherence with smart card technology that recorded date/time and hours of use.
To achieve blinding to assigned treatment, a CPAP nasal mask was modified with 8 to 12 quarter-inch drilled holes and a pressure reducer was placed in the CPAP tube at the output of the heated humidifier. This placebo mask was used in the placebo group with pressure set to 8 cm H2O to control for CPAP machine noise.21,22,31 These modifications allowed for adequate air exchange while preventing rebreathing. Pressure at the nose with the placebo CPAP varied from 0.5 cm H2O at end expiration to 0.0 cm H2O during inspiration. While to a significantly lesser degree than in the tCPAP group, patients were still able to feel a gentle breeze through the mask with the placebo CPAP apparatus.
Medications
All patients were assessed for medication use (type, dose, frequency, time, reason, and duration of use). Dopaminergic therapy regimen highly differs between patients. Therefore, in order to allow comparisons among patients on different dopaminergic regimens, drug dosages were converted to Levodopa Dosage Equivalents (LDE) according to the formulae provided by Tomlinson et al.32
Data Analysis
The treatment groups (tCPAP and pCPAP) were compared at baseline using independent sample t-test for any differences in sleep parameters, clinical characteristics, medication use, and demographics to ensure appropriate comparisons.
Mixed models repeated-measures analyses33 were used to model the association between each of the sleep parameters (SE, %N1, %N2, %N3, %R, ArI, %time SaO2 < 90%, AHI, PLMI, and MSL), phases (baseline, 3 w, and 6 w) and group (tCPAP and pCPAP). Phase, group, and the group-by-time interaction were included in the model as fixed effects. A significant group-by-time interaction at 3 w would indicate efficacy of therapeutic CPAP over placebo CPAP. A paired sample t-test combining the 3 w of therapeutic CPAP in both groups was used to assess overall treatment effect of 3 w of therapeutic CPAP (treatment group: the first 3 w in the tCPAP group and the last 3 w in the pCPAP group; control group: the first 3 w in the pCPAP group). Within-tCPAP group analysis examined the effect of 3 w versus 6 w of therapeutic CPAP, with no difference between phases suggesting treatment maintenance. The composition arousals during the night (i.e., number of spontaneous arousals, respiratory arousals, and PLM arousals) were assessed in post hoc analyses. Statistical significance was defined as P < 0.05 (two-tailed); no corrections were made for multiple comparisons so as not to miss possible treatment effects; this is one of the first studies to examine CPAP effects on sleep architecture in PD in the context of a randomized trial. SPSS (version 17.0, SPSS, Chicago, IL) and SAS (version 9.1, SAS Institute, Cary, NC) were used for analyses.
RESULTS
Participant Characteristics
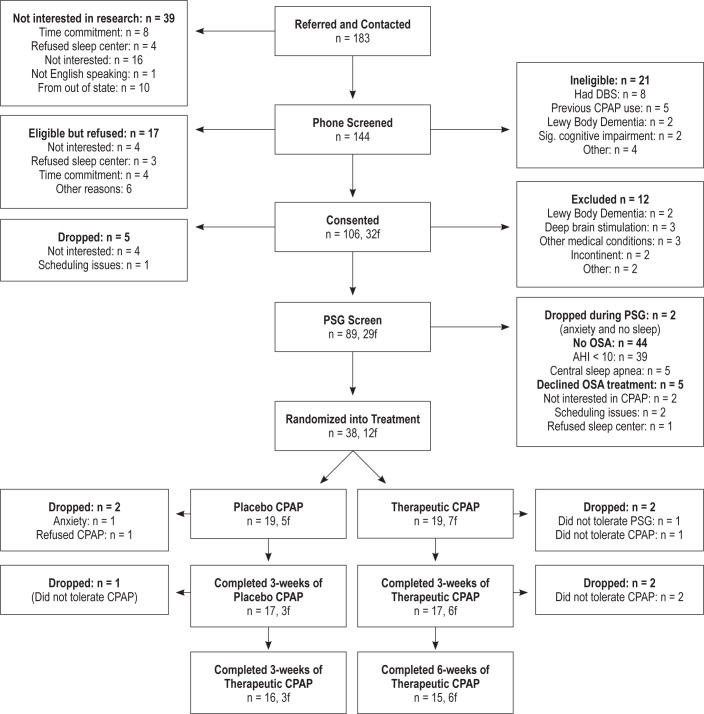
Study participation and treatment allocation is provided (Figure 1). A total of 38 patients with PD [12 females; mean age 66.7 ± 8.5 y; mean duration of PD symptoms 5.3 ± 4.9 y]) were randomized into the tCPAP group (n = 19) or pCPAP group (n = 19), 34 completed 3 w of treatment (tCPAP = 17 and pCPAP = 17), and 31 completed the 6 w (tCPAP = 15 and pCPAP = 16). There was no significant difference in attrition rates between the two groups. A total of seven patients were dropped after randomization (three from pCPAP and four from tCPAP), and these numbers are comparable between treatment arms and study phase. Importantly, in the mixed-model analysis, all 38 randomized participants are included.
Figure 1.
Consort table.
Of the 38 patients randomized into this study, 12 of them (31.6%) received levodopa monotherapy and 11 (28.9%) received dopamine agonist monotherapy, whereas 13 patients (34.2%) had a combination of levodopa with a dopamine agonist. Other PD medications included rasagiline (11 patients; 28.9%), selegiline (nine patients; 23.7%), amantadine (seven patients; 18.4%), and entacapone (five patients; 13.2%). Moreover, 12 patients (31.6%) took antidepressants and 3 (7.9%) took benzodiazepines. There were no significant differences in number of medication used or dosages between the groups.
CPAP Adherence
Partial adherence data (at 3 w) were available for 16 patients in the tCPAP group (94%) and 15 in the pCPAP group (88%). Complete adherence data (at 6 w of treatment) were available for 12 patients in the tCPAP group (80%) and 13 patients in the pCPAP group (81%). The missing adherence data were a result of equipment failure. There were no significant differences in treatment adherence between the groups at 3 w or 6 w and adherence did not differ between 3 and 6 w within groups. On average, the entire sample used CPAP for 5.2 ± 1.7 h per night for 88% of the nights during the 6-w study.
Baseline Group Differences
Complete baseline characteristics of the patients with PD who were randomized are provided in Table 1. There were no significant differences between the two groups in any demographic measures, medications, or in screening measures of cognition, or disease severity. Additionally, there were no significant baseline differences between groups in sleep architecture (sleep stages), AHI, %time SaO2 < 90%, TST, wake after sleep onset (WASO), or sleep efficiency (SE). There was a significant difference at baseline between the groups in ArI, with the pCPAP group having significantly more arousals during the night (10.3 versus 5.7; P = 0.03). Differences in sleep parameters between the groups at the different phases are summarized in Table 2.
Table 1.
Demographics at baseline
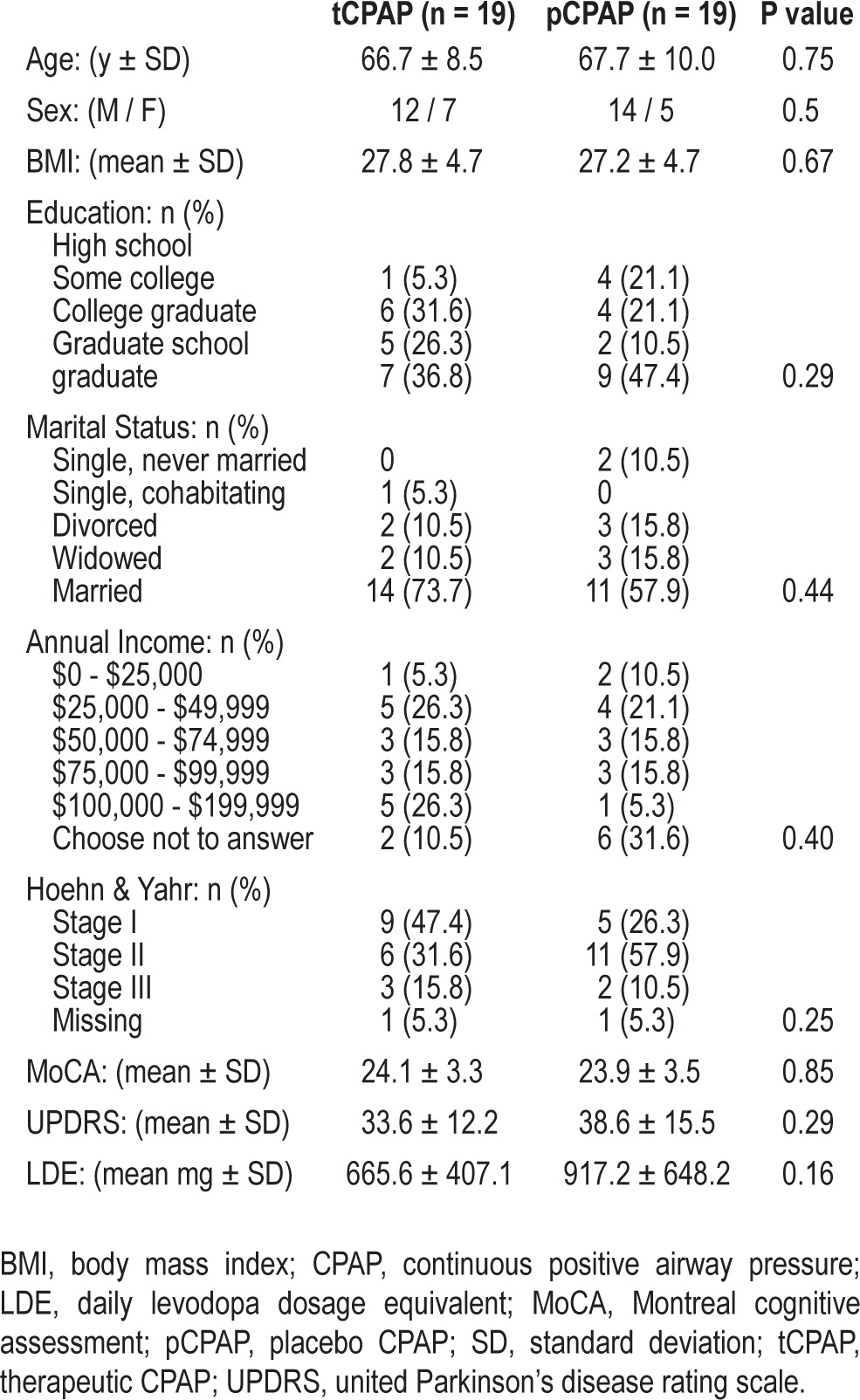
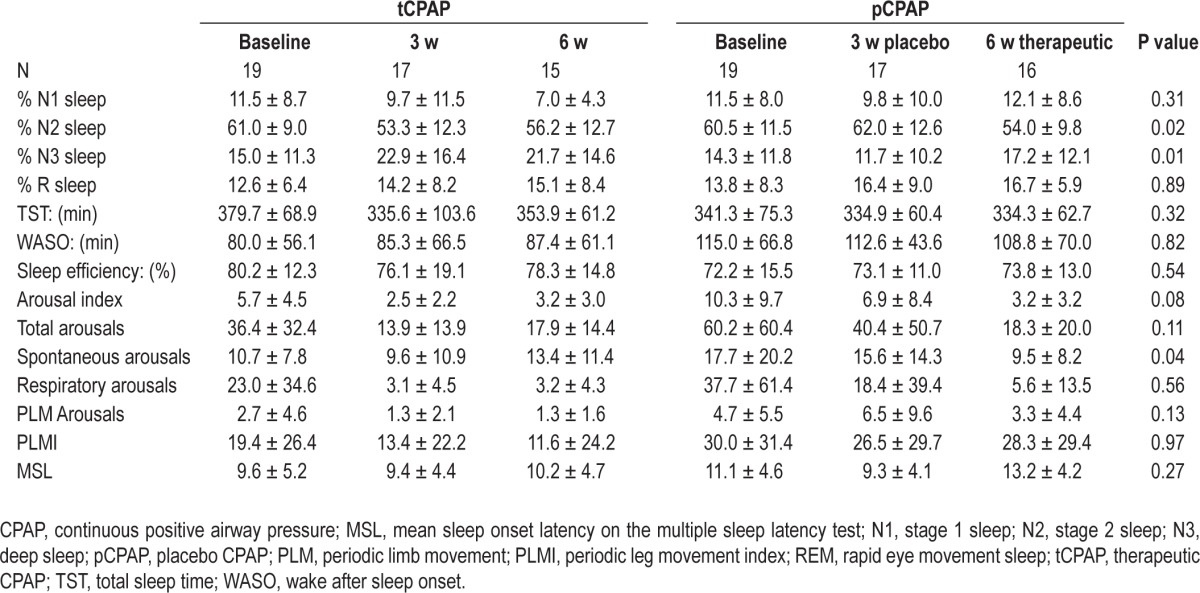
Table 2.
Sleep variables (P value of interaction term from the mixed models; means ± standard deviation)
Treatment Effect on OSA
Mixed-models analyses revealed a significant group-by-time interaction for AHI (F = 9.21, P < 0.001) and %time SaO2 < 90% (F = 4.08, P = 0.02) indicating efficacy of therapeutic CPAP over placebo. Compared to the pCPAP group (AHI = 20.00, SD = 14.3; %time SaO2 < 90% = 12.9%, SD = 14.9), the tCPAP group had significantly lower AHI (5.2, SD = 8.1, P = 0.01) and spent less %time SaO2 < 90% (1.2%, SD = 3.8, P < 0.01) at 3 w. The pCPAP showed no improvement with placebo at 3 w but improved significantly at 6 w after being switched to therapeutic CPAP. Additionally, the tCPAP group showed no additional improvement at 6 w in these variables, suggesting effects were maintained (Figure 2).
Figure 2.
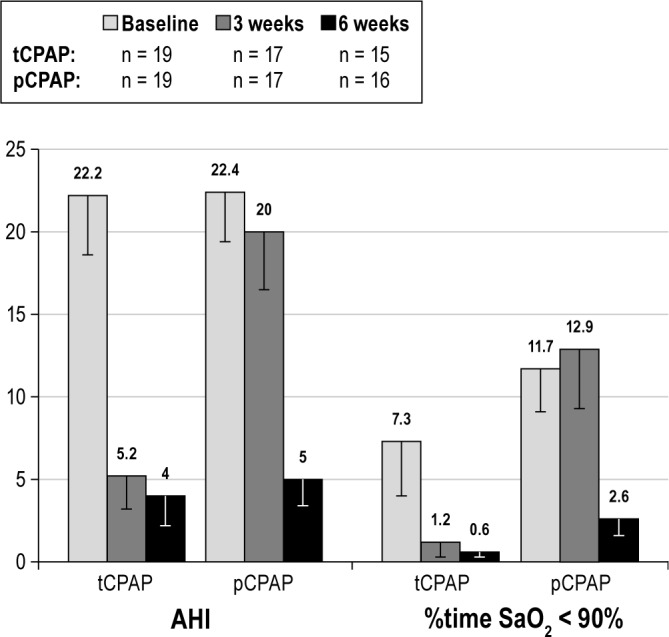
Mixed models findings: Group and time (study phase) differences in apnea-hypopnea index (AHI) and percent of sleep time spent below 90% O2 saturation (%time SaO2 < 90%) between patients with Parkinson disease in the therapeutic CPAP (tCPAP) and placebo CPAP (pCPAP) groups at baseline, at 3 w of treatment (either with tCPAP or pCPAP), and at 6 w (all receiving tCPAP at this time). Bars represent standard error of the mean.
Treatment Effect on SE and Sleep Architecture
Mixed models indicated significant group-by-time interactions for %N2 (F = 4.49, P = 0.015) and %N3 (F = 4.61, P = 0.014), indicating efficacy of therapeutic CPAP over placebo. Compared to the pCPAP group, at 3 w the tCPAP group showed significant decrease in %N2 (P = 0.048) and increase in %N3 (P = 0.025). The pCPAP group showed no improvement at 3 w with placebo but they did improve significantly at 6 w after being switched to tCPAP (Figure 2). The tCPAP group showed no additional improvement at 6 w in these variables, indicating the effects were maintained. Neither group showed significant changes in %N1 sleep (F = 1.21, P = 0.31) or %R sleep (F = 0.12, P = 0.89) throughout the study.
Mixed models did not reveal a significant group-by-time interaction in ArI (F = 2.65, P = 0.079). However, there was a significant time effect for ArI (F = 8.92, P < 0.001), suggesting significant changes in average arousals per hour of sleep between 3 w and 6 w (Table 2) for both groups. There were no significant group-by-time interactions for SE, TST, or WASO (all P > 0.05) as determined by the mixed-model analysis.
The post hoc assessment of types of arousals revealed that for both groups, more than 60% of arousals at baseline were respiratory arousals. However, after 3 w tCPAP, respiratory arousals significantly decreased and accounted for 26% of arousals. CPAP treatment did not significantly decrease spontaneous arousals or PLM arousals in either group. After 3 w of tCPAP with the resulting reduction of respiratory arousals, arousals in combined groups were composed of 60.5% spontaneous arousals and 13.5% PLM arousals, resulting in 1.6 h of WASO.
Overall Treatment Effect of CPAP at 3 Weeks
As shown in Table 3, paired sample analyses revealed that 3 w of tCPAP resulted in overall significant decreases in AHI (t = 8.05, P < 0.001), %time SaO2 < 90% (t = 3.87, P = 0.001), and ArI (t = 3.4, P = 0.002). In addition, the paired sample analysis also revealed that 3 w of tCPAP resulted in significant decrease in %N2 (t = 4.17, P < 0.001) and increase in %N3 (t = -4.77, P < 0.001) but no significant changes in %N1 or %R sleep, and SE.
Table 3.
Improvement in nighttime sleep after 3 w of therapeutic CPAP for all subjects (paired sample; mean ± standard deviation)
Treatment Effect on Daytime Sleepiness
Mixed-models analyses revealed no significant group-by-time interaction for MSL (F = 1.34, P = 0.27). However, paired sample analyses revealed that 3 w of tCPAP resulted in an overall significant decrease in sleepiness as measured objectively by MSL (t = -2.69, P = 0.011) (Figure 3). Additionally, prior to CPAP treatment, patients fell asleep on average on 2.5 ± 1.2 naps in < 10 min compared to 1.9 ± 1.4 naps after 3 w of therapeutic CPAP (P = 0.027).
Figure 3.
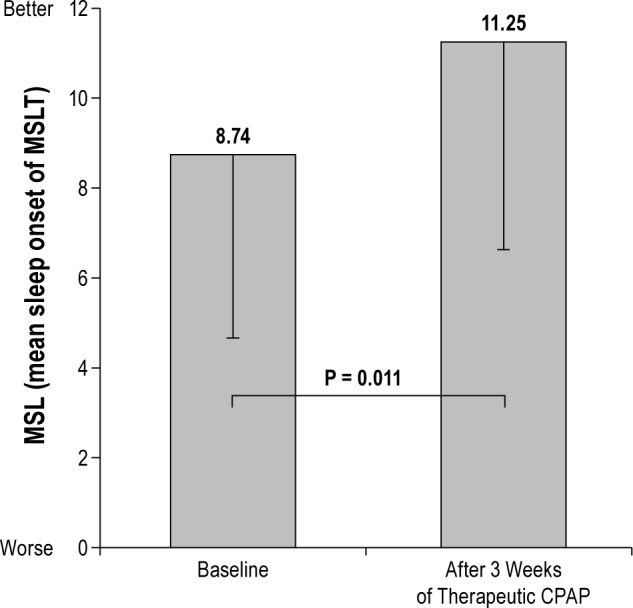
Objective sleepiness (MSL) differences before continuous positive airway pressure (CPAP) treatment (baseline) and after 3 w of therapeutic CPAP (P value from paired sample analysis; n = 32). MSLT, multiple sleep latency test.
DISCUSSION
In this group of patients with PD and OSA, therapeutic CPAP resulted in successful treatment of OSA (decrease in AHI and %time SaO2 < 90%) as well as a deepening of sleep (decrease in %N2 and increase in %N3) when compared to those receiving placebo CPAP. These positive effects were seen after 3 w of treatment and were maintained at 6 w. Importantly, these results also showed that 3 w of CPAP treatment resulted in statistical and clinically significant decreases in sleepiness. Furthermore, this study demonstrated that patients with PD were able to use the CPAP apparatus despite any motor dysfunction they may have experienced and were able to tolerate CPAP, using the CPAP for an average of 5 h a night, no different than the average use of many sleep disorders clinic patients.34–36
Specifically, this study showed that after 3 w of tCPAP treatment, AHI was decreased to nonclinically significant levels, sleep deepened (decrease in %N2 and increase in %N3), and nocturnal oxygen desaturation improved (%time SaO2 < 90%). Although there were no significant interactions in overall nighttime ArI, paired analysis showed significantly fewer Arls during the night after 3 w of tCPAP, which contributes to improved sleep by allowing for more continuous and less disturbed sleep. Interestingly, deepening sleep and decreasing number of arousals during the night did not result in improvements in either WASO or TST. Both groups had extended periods of WASO and although total arousals were reduced for both groups with 3 w of tCPAP, this did not translate to shorter WASO or longer TST. Difficulty staying asleep is commonly reported by patients with PD and studies reported up to 44% of patients with PD with multiple nocturnal awakenings.2,37 We believe that the lack of effect of CPAP on WASO or TST demonstrates the multifaceted etiology of sleep disturbances in PD. Future clinical studies targeting the multiple forms of arousals are necessary in order to determine if multifaceted sleep therapy will help reduce WASO or increase TST in patients with PD.
There were also no significant interactions in overall sleep latency on MSLT; however, paired analysis showed significantly longer sleep latencies on MSLT after 3 w of therapeutic CPAP, suggesting less daytime sleepiness. Additionally, patients fell asleep less frequently in less than 10 min, which is the suggested clinical cutoff in four-nap MSLT,30 highlighting the clinical significance of this finding. Because daytime sleepiness is a major problem in patients with PD, these data add to the positive effect of treating OSA.
Sleep disturbances such as sleep fragmentation, difficulty staying asleep, and daytime sleepiness are the most common nonmotor symptoms of PD,3,37–39 and sleep fragmentation is a quintessential characteristic of OSA.40 OSA is strongly associated with cardiac arrhythmias, nocturnal hypertension, nocturia, nighttime confusion, and neuropsychological impairment.15–17,41 Decreasing sleep fragmentation and accompanying hypoxemia in OSA may help prevent the negative consequences of untreated OSA such as impaired cognition and autonomic regulation.15–17 Decrease in slow wave sleep has also been indicated in homeostatic regulation42 and memory processes,43 both of which may be impaired in PD and are considered as nonmotor symptoms of the disease. Therefore, OSA treatment in this patient population is an important and viable method of treatment because this study also demonstrated that the patients were able to adequately use and tolerate the CPAP. A previous study by Cochen De Cock et al.13 questioned the clinical relevance of OSA in patients with PD; in their study, AHI did not correlate with hypertension, stroke, or heart disease nor did it correlate with daytime sleepiness, nocturia, or cognitive impairment. They further stated that benefit of CPAP treatment in this population “remains to be demonstrated”. Because these types of data are not available in our study, future analyses and studies will determine if treating OSA in PD will also improve other symptoms or conditions. However, the data presented here strongly suggest a positive effect of CPAP treatment on sleep and sleep disturbances in patients with PD who also have OSA. Our study showed that CPAP treatment for patients with PD and OSA results in deepening sleep and aligns with previous findings in our laboratory showing that tCPAP in patients with Alzheimer disease and OSA also resulted in deeper sleep.21 Previous studies with different nondemented adult populations showed a similar effect of deeper sleep with CPAP treatment.44–50
To our knowledge, this is the first study that used a randomized placebo-controlled trial to investigate the effect of CPAP treatment on sleep in patients with PD and OSA, as well as the first study to assess the effect of CPAP treatment on daytime sleepiness, both significant strengths of this study. In non-PD populations, there have been multiple studies that used a placebo-controlled design with either use of oral placebo,51–55 different counseling therapies,56,57 or subtherapeutic CPAP pressure.22,31,58–63 These studies showed mixed results, which may have resulted from the placebo methodology used. For example, use of subtherapeutic pressure may bias results, as low pressure used (ranging 1-3 cm H2O) may have some positive effect.61–63 In this study we used a pressure that did not exceed 0.5 cm H2O and thus prevents possible effects of partial treatment. Studies using identical placebo methodology have shown no therapeutic effect using this placebo apparatus, which is also evident in our study.21,22 A study by Rodway et al.64 compared PSG measures with versus without the placebo CPAP using similar placebo methodology and some differences between the groups were reported, but with small effect sizes and minimal clinical significance. They concluded that their findings support the use of placebo CPAP in clinical trials.
An additional strength of this study was availability of treatment adherence data, which demonstrated that patients with PD were able and willing to use CPAP. Adequate CPAP adherence has been defined as routine use of CPAP for more than 4.5 h of use per night.34 Our sample used their CPAP on 88% of the days of which it was assigned to them for an average of 5.2 h per night. Additionally, there were no group differences in CPAP adherence between groups, which strengthens the validity of these findings.
Published reports regarding the prevalence of OSA in PD are mixed, with rates estimated to be between 20-60%; it is not clear whether OSA is more common in PD than in the general older population.10–14,65,66 In our study, OSA was diagnosed in close to 50% of our sample (43 of 87). This rate is similar to rates reported in other studies of older adults.67,68 Although our study was not restricted to patients with PD who complain of poor sleep, the mere fact that this was a sleep study may have biased this rate. Nonetheless, this high rate indicates the widespread occurrences of the disorder and thus the need to assess for OSA in this population.
This study had several limitations. One significant limitation was the small sample size. The study included a total of six overnight PSG assessments, five of them occurring over a 6-w period with multiple visits and assessments at either the patient's home or in the laboratory. This was a significant logistical challenge for some patients, particularly those who did not drive, who tired easily, or required caregiver assistance throughout the day and night. We found that some patients were apprehensive or unwilling to participate due to this burden. The study was thus likely underpowered to detect small improvements in sleep architecture as a result of tCPAP over placebo. Additionally, our study assessed only the short-term effect (3 and 6 w) of tCPAP treatment. Future studies assessing the long-term effect of CPAP treatment on incidents such as stroke, heart disease, excessive daytime sleepiness, and cognitive impairment will further indicate clinical relevance of OSA in this population. Due to the multiple overnight visits required in this protocol, we were not able to include a habituation night and thus this study may be biased by first-night effects that may have resulted in sleep architecture changes at baseline. However, the significant interaction at 3 w from the mixed models indicates that changes were due to CPAP treatment even if they were biased to a certain degree by first-night effects. Another limitation was the generalizability of these findings due to the stringent inclusion/exclusion criteria that included primarily patients with mild to moderate PD. For example, it is unclear if the positive results from our study are generalizable to patients with more severe PD or those who received deep brain stimulation. Another limitation is that we did not have any measures of pharyngeal dysfunction with which to demonstrate that these particular patients (who were, at most, moderately afflicted, with a Hoehn & Yahr rating of I and II) also experiencing upper airway pathophysiology. Future research studies might consider using motor function measurements as post-treatment outcomes. Despite such limitations, this study was successful in showing positive and beneficial effects of tCPAP over placebo on OSA and on sleep.
In summary, this study provided evidence that therapeutic CPAP can be tolerated by patients with PD and results in improvement in OSA, nighttime oxygenation level, and in sleep architecture (particularly by deepening sleep) and in daytime sleepiness. These findings increase the clinical importance of OSA recognition and treatment in populations with neurode-generative disease such as PD.
DISCLOSURE STATEMENT
This was not an industry supported study. Support for the study was provided by NIA AG08415, NIH UL1RR031980, UCSD Stein Institute for Research on Aging, and Department of Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH). Dr. Ancoli-Israel is a consultant and/or on the scientific advisory board for Ferring Pharmaceuticals Inc., Merck, NeuroVigil, Inc., and Purdue Pharma LP. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
CPAP machines and masks were donated by Philips Respironics. The authors thank Mark Mahowald for his support and advice, the neurologists in San Diego County for referring their patients, and the study patients and caregivers who volunteered their time to help in this research.
REFERENCES
- 1.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson's disease. Clin Neuropharmacol. 1988;11:512–9. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gjerstad M, Wentzel-Larsen T, Aarsland D, Larsen J. Insomnia in Parkinson's disease: frequency and progression over time. J Neurol Neurosurg Psychiatry. 2007;78:476–9. doi: 10.1136/jnnp.2006.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Healy DG, Schapira AH National Institute for Clinical Excellence. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 4.Findley L, Eichhorn T, Janca A, et al. Factors impacting on quality of life in Parkinson's disease: Results from an international survey. Mov Disord. 2002;17:60–7. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- 5.Findley L, Aujla M, Bain PG, et al. Direct economic impact of Parkinson's disease: a research survey in the United Kingdom. Mov Disord. 2003;18:1139–45. doi: 10.1002/mds.10507. [DOI] [PubMed] [Google Scholar]
- 6.Bergonzi P. L-dopa plus dopa-decarboxylase inhibitor: Sleep organization in Parkinson's syndrome before and after treatment. Acta Neurol Belg. 1975;75:5–10. [PubMed] [Google Scholar]
- 7.Friedman A. Sleep pattern in Parkinson's disease. Acta Medica Polona. 1980;21:193. [PubMed] [Google Scholar]
- 8.Lavie P, Bental E, Goshen H, Sharf B. REM ocular activity in Parkinsonian patients chronically treated with levodopa. J Neural Trans. 1980;47:61–7. doi: 10.1007/BF01256640. [DOI] [PubMed] [Google Scholar]
- 9.Mouret J. Differences in sleep in patients with Parkinson's disease. Electroencephalog Clin Neurophysiol. 1975;38:653–7. doi: 10.1016/0013-4694(75)90168-6. [DOI] [PubMed] [Google Scholar]
- 10.Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson's disease and sleepiness an integral part of PD. Neurology. 2002;58:1019–24. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- 11.Zoccolella S, Savarese M, Lamberti P, Manni R, Pacchetti C, Logroscino G. Sleep disorders and the natural history of Parkinson's disease: The contribution of epidemiological studies. Sleep Med Rev. 2011;15:41–50. doi: 10.1016/j.smrv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Norlinah M, Afidah K, Noradina A, et al. Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism Relat Disord. 2009;15:670–4. doi: 10.1016/j.parkreldis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Cochen De Cock V, Abouda M, Leu S, et al. Is obstructive sleep apnea a problem in Parkinson's disease? Sleep Med. 2010;11:247–52. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Maria B, Sophia S, Michalis M, et al. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med. 2003;97:1151–7. doi: 10.1016/s0954-6111(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 16.Shepard JW., Jr Hypertension, cardiac arrhythmias, myocardial infarction, and stroke in relation to obstructive sleep apnea. Clin Chest Med. 1992;13:437–58. [PubMed] [Google Scholar]
- 17.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–7. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 18.Shpirer I, Miniovitz A, Klein C, et al. Excessive daytime sleepiness in patients with Parkinson's disease: a polysomnography study. Mov Disord. 2006;21:1432–8. doi: 10.1002/mds.21002. [DOI] [PubMed] [Google Scholar]
- 19.Braga-Neto P, Pereira da Silva-Júnior F, Sueli Monte F, de Bruin PF, de Bruin V. Snoring and excessive daytime sleepiness in Parkinson's disease. J Neurol Sci. 2004;217:41–5. doi: 10.1016/j.jns.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–81. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooke JR, Ancoli-Israel S, Liu L, et al. Continuous positive airway pressure deepens sleep in patients with Alzheimer's disease and obstructive sleep apnea. Sleep Med. 2009;10:1101–6. doi: 10.1016/j.sleep.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loredo JS, Ancoli-Israel S, Kim E-J, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep. 2006;29:564–71. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev. 2007;11:99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson G, Angus J. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;45:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Holstein N, editors. Recent developments in Parkinson's disease. Vol. 2. Plurham Park, NJ: Macmillian Healthcare Information; 1987. pp. 153–63. [Google Scholar]
- 27.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998;50 doi: 10.1212/wnl.50.2.318. 318 and 16 pages following. [DOI] [PubMed] [Google Scholar]
- 28.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson's disease. Mov Disord. 2004;19:1020–8. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 29.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 31.Farré R, Hernández L, Montserrat JM, Rotger M, Ballester E, Navajas D. Sham continuous positive airway pressure for placebo-controlled studies in sleep apnoea. Lancet. 1999;353:1154. doi: 10.1016/S0140-6736(99)01056-9. [DOI] [PubMed] [Google Scholar]
- 32.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 33.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of longitudinal data. 2nd ed. New York, NY: Oxford University Press Inc; 2002. [Google Scholar]
- 34.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149:149–54. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 35.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 36.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea. A population-based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 37.Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord. 1998;13:895–9. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 38.Tandberg E, Larsen JP, Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson's disease: a community-based study. Mov Disord. 1999;14:922–7. doi: 10.1002/1531-8257(199911)14:6<922::aid-mds1003>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Borreguero D, Larrosa O, Bravo M. Parkinson's disease and sleep. Sleep Med Rev. 2003;7:115–29. doi: 10.1053/smrv.2002.0229. [DOI] [PubMed] [Google Scholar]
- 40.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 41.Covassin N, Neikrug AB, Liu LQ, et al. Relationships between clinical characteristics and nocturnal cardiac autonomic activity in Parkinson's disease. Auton Neurosci. 2012;171:85–8. doi: 10.1016/j.autneu.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13:480–7. doi: 10.1016/0166-2236(90)90081-k. [DOI] [PubMed] [Google Scholar]
- 43.Van Der Werf YD, Altena E, Schoonheim MM, et al. Sleep benefits subsequent hippocampal functioning. Nature Neurosci. 2009;12:122–3. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 44.Bonsignore G, Marrone O, Bellia V, Giannone G, Ferrara G, Milone F. Continuous positive airway pressure improves the quality of sleep and oxygenation in obstructive sleep apnea syndrome. Italian J Neurol Sci. 1987;8:129–34. doi: 10.1007/BF02337586. [DOI] [PubMed] [Google Scholar]
- 45.Lamphere J, Roehrs T, Wittig R, Zorick F, Conway W, Roth T. Recovery of alertness after CPAP in apnea. Chest. 1989;96:1364–7. doi: 10.1378/chest.96.6.1364. [DOI] [PubMed] [Google Scholar]
- 46.Fietze I, Quispe-Bravo S, Hansch T, Rottig J, Baumann G, Witt C. Arousals and sleep stages in patients with obstructive sleep apnoea syndrome: Changes under nCPAP treatment. J Sleep Res. 1997;6:128–33. doi: 10.1046/j.1365-2869.1997.00029.x. [DOI] [PubMed] [Google Scholar]
- 47.Collard P, Dury M, Delguste P, Aubert G, Rodenstein DO. Movement arousals and sleep-related disordered breathing in adults. Am J Respir Crit Care Med. 1996;154:454–9. doi: 10.1164/ajrccm.154.2.8756822. [DOI] [PubMed] [Google Scholar]
- 48.Verma A, Radtke RA, VanLandingham KE, King JH, Husain AM. Slow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep quality. Sleep Med. 2001;2:215–23. doi: 10.1016/s1389-9457(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 49.Brillante R, Cossa G, Liu PY, Laks L. Rapid eye movement and slow-wave sleep rebound after one night of continuous positive airway pressure for obstructive sleep apnoea. Respirology. 2012;17:547–53. doi: 10.1111/j.1440-1843.2012.02147.x. [DOI] [PubMed] [Google Scholar]
- 50.Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: Contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11:552–7. doi: 10.1016/j.sleep.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Engleman H, Martin S, Douglas N, Deary I. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 52.Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in” non-dippers”. Sleep. 1996;19:378–81. doi: 10.1093/sleep/19.5.378. [DOI] [PubMed] [Google Scholar]
- 53.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:114–9. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–5. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 56.Ballester E, Badia JR, Hernandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 57.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157:858–65. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 58.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 59.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 60.Hack M, Davies RJ, Mullins R, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000;55:224–31. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Profant J, Ancoli-Israel S, Dimsdale JE. A randomized, controlled trial of 1 week of continuous positive airway pressure treatment on quality of life. Heart Lung. 2003;32:52–8. doi: 10.1067/mhl.2003.8. [DOI] [PubMed] [Google Scholar]
- 63.Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest. 1999;116:1545–9. doi: 10.1378/chest.116.6.1545. [DOI] [PubMed] [Google Scholar]
- 64.Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33:260. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diederich NJ, Vaillant M, Leischen M, et al. Sleep apnea syndrome in Parkinson's disease. A case-control study in 49 patients. Mov Disord. 2005;20:1413–8. doi: 10.1002/mds.20624. [DOI] [PubMed] [Google Scholar]
- 66.Trotti LM, Bliwise DL. No increased risk of obstructive sleep apnea in Parkinson's disease. Mov Disord. 2010;25:2246–9. doi: 10.1002/mds.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of seep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]