Abstract
Study Objectives:
To examine whether subjective sleep quality and sleep duration moderate the association between age and telomere length (TL).
Design:
Participants completed a demographic and sleep quality questionnaire, followed by a blood draw.
Setting:
Social Neuroscience Laboratory.
Participants:
One hundred fifty-four middle-aged to older adults (age 45-77 y) participated. Participants were excluded if they were on immunosuppressive treatment and/or had a disease with a clear immunologic (e.g., cancer) component.
Interventions:
N/A.
Measurements and Results:
Subjective sleep quality and sleep duration were assessed using the Pittsburgh Sleep Quality Index (PSQI) and TL was determined using peripheral blood mononuclear cells (PBMCs). There was a significant first-order negative association between age and TL. Age was also negatively associated with the self-reported sleep quality item and sleep duration component of the PSQI. A significant age × self-reported sleep quality interaction revealed that age was more strongly related to TL among poor sleepers, and that good sleep quality attenuated the association between age and TL. Moreover, adequate subjective sleep duration among older adults (i.e. greater than 7 h per night) was associated with TL comparable to that in middle-aged adults, whereas sleep duration was unrelated to TL for the middle-aged adults in our study.
Conclusions:
The current study provides evidence for an association between sleep quality, sleep duration, and cellular aging. Among older adults, better subjective sleep quality was associated with the extent of cellular aging, suggesting that sleep duration and sleep quality may be added to a growing list of modifiable behaviors associated with the adverse effects of aging.
Citation:
Cribbet MR; Carlisle M; Cawthon RM; Uchino BN; Williams PG; Smith TW; Gunn HE; Light KC. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. SLEEP 2014;37(1):65-70.
Keywords: Aging, sleep quality, telomeres
INTRODUCTION
Sleep complaints constitute one of the most common difficulties facing middle-aged and older adults.1 Indeed, epidemiological studies suggest that most community-dwelling older adults have some concerns about their ability to fall asleep or stay asleep, with fewer than 20% reporting “rarely” or “never” having any sleep complaints.2 Older adults often take longer to fall asleep, wake more frequently during the night, and experience lower sleep efficiency than their younger counterparts.3 Importantly, these objective age-related changes in sleep across the life span are often associated with self-reports of poor sleep quality.4–6
One pathway through which both objective and subjective measures of sleep may be associated with health is inflammation. Sleep is closely linked to immune system changes via the sleep-wake cycle, with immune cells at their highest blood levels in the early evening and lowest in the morning,7 and disruption of the regular sleep-wake cycle appears to significantly affect immune function.8,9 Importantly, sleep deprivation has been found to induce increases in circulating levels of inflammatory markers, such as tumor necrosis factor-α (TNF-α),10 C-reactive protein (CRP),11 and interleukin-6 (IL-6),12 with significant elevations occurring after only 1 night of sleep loss.11 Additionally, experimentally induced sleep deprivation in the laboratory has also been associated with nuclear factor kappa- B cell (NF-κB) activation, a molecular pathway through which sleep disturbance may influence leukocyte inflammatory gene expression and inflammation-related disease risk.13 This body of literature demonstrates effects of sleep loss on a range of well-known markers of the acute inflammatory system, independent of actual injury or infection to the host. The combination of high prevalence of sleep problems among older adults, as well as the effects of sleep loss on inflammatory processes, may put older adults at increased risk for many chronic conditions, because aging alone is associated with low-grade elevation of inflammatory markers.14
In addition to increased inflammation during chronological aging, inflammation also appears to play a role in cellular aging. Immune system cells are under tremendous proliferative demand, requiring telomeres to remain intact. Telomeres are repetitive structures at the end of chromosomes that help to promote cellular stability.15,16 However, with each successive replication of the cell, telomeres shorten and when a critical threshold is met, the result is cellular senescence. In all mitotic tissues aside from germline tissue, telomere length (TL) declines with age.17,18 Telomere activity serves several critical genomic purposes, including the prevention of chromosomal fusions and unregulated cellular activity.19 Lymphocytes, an immune cell, are capable of upregulating the enzyme (telom-erase) that elongates telomeres, prolonging cellular life span.20 In addition, recent work demonstrates telomere shortening in monocytes is associated with increased synthesis of proinflammatory cytokines.21 Although the set point of TL appears to be genetically determined,22 it is not yet fully understood what drives age-associated telomere loss, although several factors have been identified (e.g., chronic stress and proliferation-induced shortening).20
A small but growing body of literature now demonstrates associations between sleep and TL.23–27 Of these studies, three provide evidence that self-reported sleep quality and sleep duration are related to TL. For example, Prather and colleagues found an association between self-reported sleep quality among middle-aged women and TL, such that women who reported poorer sleep quality had shorter leukocyte TL.26 This finding has recently been replicated in healthy men (Mage = 63.3, standard deviation = 5.6).27 In addition, Liang and colleagues found that long sleep duration was significantly associated with long TL among women younger than 50 years, but not among women older than 50 years.25 These findings raise the question of whether associations between sleep quality, sleep duration, and TL change as we age. That is, as we get older, do sleep duration and self-reported sleep quality differentially predict TL compared to a relatively younger population? These prior studies suggest that further study examining associations between sleep duration, self-reported sleep quality, and TL is warranted.
The purpose of the current study was to examine associations among age, subjective sleep quality, sleep duration, and telomere length in a sample of middle-aged and older adults. The first aim was to replicate prior findings of an association between age and TL. A second aim was to replicate recent findings demonstrating associations between subjective sleep quality and self-reported sleep duration with TL, with the prediction that poorer sleep quality and shorter sleep duration would be associated with shorter TL.26 Additionally, prior research suggests a U-shaped association between sleep duration and negative health effects.28 A third aim of the current study was to examine a nonlinear model. Chronological age is generally associated with poorer sleep quality as well as cellular aging, and poor sleep quality is linked to shorter TL. Thus, it was predicted that increasing age would be associated with shorter TL among those reporting shorter sleep duration and poorer sleep quality.
METHOD
Participants
As part of a larger study, 154 relatively healthy participants (89 men and 65 women) were included in this study. An additional 23 participants were studied, but for various reasons had no telomere data (e.g., blood draw or blood separation problems). Importantly, comparing these individuals with participants who had complete data on age—the only demographic factor related to telomere length—revealed no significant group differences (mean age = 60.1 versus 60.6, P > 0.70). We recruited a middle-aged to older adult sample (45 to 77 y [Mage = 60.1, standard deviation = 6.76]) to allow for greater variability in TL. Because many older adults are on some form of medication, we only excluded individuals who were on immunosuppressive treatment (e.g., corticosteroid therapy) and/or had current cancer or human immunodeficiency virus to address concerns about potential effects on peripheral blood mononuclear cells (PBMCs), which we used for the determination of TL. Corticosteroid therapy and treatment regimen are factors known to influence immune cell function.29 Because there was little evidence on the extent of such influences on TL determined from these cells at the time of the study, we conservatively used them as exclusion criteria.
Procedure
The current investigation was performed in accordance with the ethical standards of the University of Utah institutional review board. All eligible participants were scheduled for an appointment at the Social Neuroscience Laboratory, and following informed consent, were rechecked against the exclusion criteria. Participants then completed a demographic questionnaire (e.g., age, sex) and the Pittsburgh Sleep Quality Index (PSQI).30 Following completion of the questionnaires, approximately 20 cc of blood were drawn and treated with EDTA to prevent clotting. Telomere length was determined using PBMCs. DNA from the PBMCs was extracted from fresh cells, which were separated using density gradient centrifugation with Ficoll-Hypaque (GE Healthcare). Following the blood draw, participants were debriefed and received $75.00 for their participation.
Measures
Pittsburgh Sleep Quality Index
The PSQI30 assesses sleep habits during the previous month. The scale is composed of 19 items, which are used to derive seven component scores: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication, and daytime dysfunction. Component scores on the PSQI are summed to produce a global measure of subjective sleep quality score, with higher scores indicating poorer sleep quality. This instrument has demonstrated good reliability (alpha = 0.83) and validity.31 For the current study, the sleep duration component score and the item assessing sleep quality were used.
Telomere Length
DNA was extracted from isolated PBMCs using the reagents and protocols contained in Qiagen's Gentra Puregene Blood Kit, Catalog # 158389, with the exception that the red blood cell lysis step was skipped, as it was unnecessary due to prior removal of RBCs by centrifugation. Relative TLs were then determined for the PBMCs using quantitative polymerase chain reaction (PCR). For each DNA sample, we measured the factor by which the sample differed from a reference DNA sample in its ratio of telomere repeat copy number to single copy gene copy number. This ratio is proportional to the average TL (T/S ratio).32 These assays were run in several batches, and each batch consisted of DNA samples from 96 participants per quantitative PCR run. The interassay geometric mean of the coefficient of variation for this assay was 3.13%, based on examining the variation in the mean T/S ratio across days.33 The intra-assay geometric mean of the coefficient of variation was 5.22%. This was determined by examining variations in the T/S of samples across triplicate repeat measurements of the samples, collected in the same run.33 Outliers were determined within triplicate measurements of T/S for a given sample: when PCR amplification of either the telomere target and/or the single copy gene target failed for one of the three replicates, that replicate was removed from the analysis. When amplification of two or more of the three replicates of a sample failed, the assay was repeated in triplicate for that DNA sample. The Shapiro-Wilk test of normality, which is appropriate for this sample size, was 0.98, indicating a high degree of normality in the resulting T/S ratio data.
Overview of Analyses
To characterize the sample with respect to age, sleep duration, self-reported sleep quality, and telomere length, means, standard deviations and zero-order correlations among these variables were calculated.
Separate regression analyses examined independent associations among age, sleep duration (PSQI component score), subjective sleep quality (PSQI item), and TL. All models included covariates known to be associated with telomere length (sex; body mass index; average weekly alcohol consumption; average weekly tobacco use; average weekly exercise; use of hormone replacement therapy; and use of hypertension, cholesterol, and diabetes medications). All variables were centered to minimize multicollinearity.34
To examine the moderating effects of sleep duration and self-reported sleep quality on the association between age and TL, separate regression models that included first-order effects and the cross product terms (e.g., age × sleep quality) were conducted, adjusting for the covariates just listed. In order to probe significant interactions, separate regression models were restructured on high and low values (one standard deviation above and below the mean) of sleep duration and sleep quality, respectively.34
Finally, because prior research suggests a U-shaped association between sleep duration and negative health effects,28 a nonlinear model was also examined. Significant interactions were probed by restructuring the regression model one standard deviation above and below the means of age and sleep duration.34
RESULTS
Primary Analyses
Means, standard deviations, and correlations among the study variables are presented in Table 1. Age was significantly inversely correlated with the sleep duration component of the PSQI and the PSQI item about self-reported sleep quality. The well-known association between age and TL was replicated (b = -0.011, t = -3.83, P = 0.0002).
Table 1.
Means, standard deviations, and zero-order correlations among study variables
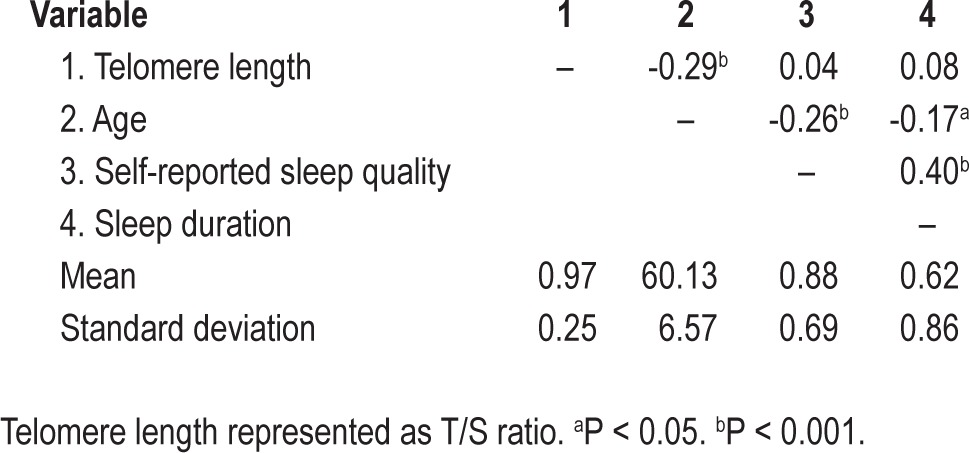
Because prior research on sleep in older adults suggests that sleep duration and sleep quality are important for health,3–6 we examined the PSQI sleep duration component and self-reported sleep quality item. In all regression models, variables were centered to minimize multicollinearity. In separate regression models that included the PSQI sleep duration component and the PSQI sleep quality item scores age remained a significant predictor of TL (P < 0.001), above and beyond the following covariates: sex, body mass index, average weekly alcohol consumption, average weekly tobacco use, average weekly exercise, use of hormone replacement therapy, use of hypertension, cholesterol, diabetes medications.
To examine the moderating effect of sleep duration and sleep quality on the association between age and TL, separate regression models that included centered first-order effects and the cross product terms (age × sleep quality item; age × sleep duration component score) were conducted. One significant interaction emerged. There was a significant interaction between age and self-reported sleep quality (b = -0.011, t = -2.42, P < 0.05, ΔR2 = 0.04) above and beyond sex, body mass index, average weekly alcohol consumption, average weekly tobacco use, average weekly exercise, use of hormone replacement therapy, and use of medications for hypertension, cholesterol, and diabetes. No other main effects or interactions of these PSQI scores emerged. In order to probe the significant age × self-reported sleep quality interaction, the regression model was restructured on high and low values (one standard deviation above and below the mean) of self-reported sleep quality (item).34 As displayed in Figure 1 (which displays plotted values one standard deviation above and below the mean of the PSQI item about self-reported sleep quality), follow-up analyses revealed that age was more strongly negatively related to TL among those with poor self-reported sleep quality (b = -0.021, P ≤ 0.0001) than those with better self-reported sleep quality (b = -0.005, P = 0.24), suggesting that sleep quality attenuated the effect of age on TL.
Figure 1.
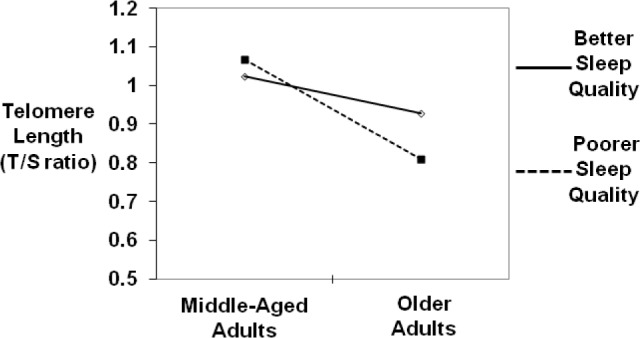
Subjective sleep quality attenuates the effect of age on telomere length.
Finally, given prior research suggesting that both high and low sleep duration may have negative health effects (i.e., a curvilinear effect),28 a nonlinear association between the component score of sleep duration and TL was examined in a regression model that included sex, age, the linear sleep duration term (component score), and a quadratic sleep duration term. There was a significant association between the quadratic sleep duration term and telomere length (b = 0.05, P < 0.05) above and beyond sex; body mass index; average weekly alcohol consumption; average weekly tobacco use; average weekly exercise; use of hormone replacement therapy; use of hypertension, cholesterol, and diabetes medications; and chronological age. Moreover, age moderated the association between the quadratic sleep duration term and TL (b = 0.013, t = 3.61, P < 0.001, ΔR2 = 0.08). In order to probe the significant interaction, the regression model was restructured one standard deviation above and below the means for both age and the quadratic sleep duration term.34 Analysis of simple slopes revealed that sleep duration was unrelated to TL for the middle-aged adults in our sample (P > 0.10). However, adequate sleep duration (i.e., > 6.9 h/night) in older adults was positively associated with TL (b = 0.25, P < 0.01). Moreover, it appears that the benefits of adequate sleep duration begin around age 60 y (b = 0.06, P < 0.05). Although the simple slope for older adults with low sleep duration (< 5 h/night) was also significant (b = 0.12, P < 0.01), very few older adults reported sleep duration this low (n = 4) and the predicted TL in this group was well below that of the middle-aged participants. Figure 2 shows mean TL values based on the range of values for the sleep duration component on the PSQI and on values one standard deviation above and one standard deviation below the mean of age (Mage = 60.57 y). TL for older adults with adequate sleep duration (i.e., > 6.9 h/ night) is comparable to that of the middle-aged group. Thus, adequate sleep duration in older adults appears to be associated with attenuated aging, as indicated by TL.
Figure 2.
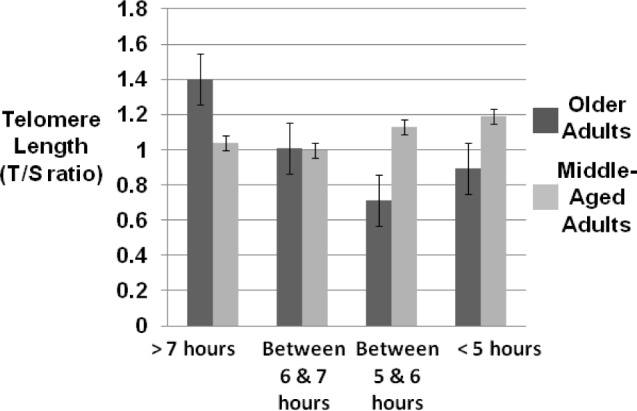
Mean telomere length among middle-aged and older adults by hours of sleep per night (Pittsburgh Sleep Quality Index component sleep duration).
DISCUSSION
In this study we examined associations between chronological age, cellular aging, sleep duration, and subjective sleep quality in a sample of middle-aged and older men and women. Consistent with prior research and our first aim, we found an inverse association between age and TL. For our second aim, we did not find the PSQI item of self-reported sleep quality to significantly predict TL on its own. However, it did moderate the association between age and TL, suggesting that among older adults, better sleep quality was significantly associated with longer TL; this pattern was not found for younger adults in this sample. Similar to findings by Jackowska and colleagues, results of the current study indicated a nonlinear association between the component of sleep duration and TL, such that both high and low sleep durations were associated with shorter TL.27 This nonlinear effect varied with age such that there was evidence of longer TL for older adults with adequate sleep duration; however sleep duration was unrelated to TL for the middle-aged adults in our sample.
The current study replicates and extends recent findings on the links between aging and TL. However, the current study differs from prior studies in important ways. First, the current study is the first to use both men and women in examining the association between PSQI measures of sleep quality and sleep duration with TL. Next, in contrast to findings of Liang and colleagues, results of the current study showed the sleep duration component was significantly associated with TL among older adults compared to middle-aged adults.25 We interpret current results to suggest that getting an adequate amount of sleep in older adulthood is potentially buffering deleterious effects of TL shortening that normally occur with age; thus, sleep is demonstrated to be a restorative process at the cellular level in older adults. Future research should examine the circumstances that shape how sleep duration influences cellular aging. These general findings are consistent with prior work showing the importance of subjective sleep quality and duration as predictors of TL.
TL, a marker of cellular aging, has been related to other psychosocial factors known to affect health, such as chronic stress,35 divorce,36 and childhood trauma.37 Thus, the results of this investigation may have implications for understanding the mechanisms through which daily metabolic insults such as stress affect health in older adults.38 Within this general framework, stress exposure, stress reactivity, stress recovery, and restoration are component processes by which stress adversely affects health, and sleep is considered to be a restorative process.39,40 Inadequate sleep is associated with adverse health consequences including impaired immune functioning,41 susceptibility to the common cold,42 metabolic syndrome,43,44 inflammatory processes,45 coronary artery calcification,46 coronary heart disease,47 and type 2 diabetes.48 Moreover, among middle-aged and older adults, sleeping less than 7 h or more than 8 h is associated with poor health outcomes28 and increased mortality rates.49 The data from this study highlight the potential positive effect of better sleep quality and longer sleep duration, especially for older adults.
As shown in Table 1, the older adults in this study reported better sleep quality (PSQI sleep quality item) and slept longer (PSQI sleep duration component) than their middle-aged counterparts. These findings are inconsistent with the view that older adults report more sleep complaints and experience a decline in sleep quality over the life span.2–6 However, this sample was composed of relatively healthy middle-aged and older adults, and results of the current study are consistent with prior research indicating that sleep complaints among older adults are not the result of age per se, but are often secondary to physical health problems.50–52 Moreover, the results of the current study are consistent with findings from a recent population-based study of subjective sleep quality across the life span showing a decline in sleep quality until age 60 y, followed by a transient increase in sleep quality from age 60 y to 66 y, then a plateau in sleep quality after age 66 y.53 Although the finding that the older adults in this study reported better sleep quality than their middle-aged counterparts is in contrast to the view that sleep quality declines with age, these results suggest a growing need for a more differentiated description of changes in sleep quality during old age.
There are some limitations to the current study. First, the participants were a relatively healthy community sample of older adults, so generalizations to chronically ill populations and other age groups should be made cautiously. Future research should examine the association between TL and self-reported sleep quality in the context of diagnosed sleep disorders, such as insomnia and obstructive sleep apnea (OSA), especially in light of evidence that OSA in adults has been linked to shorter TL,54 whereas OSA in children has been associated with longer TL.55 Thus, more work examining connections between OSA and TL is needed before attempting to understand the role self-reported sleep quality may play in that relationship. Additionally, the data are cross-sectional in nature, limiting the ability to draw causal inference. Moreover, only 2% to 3% of the total variance in TL was explained by interactions between age and the individual item of sleep quality, indicating a fairly modest effect. Longitudinal investigations may increase our understanding of how poor sleep quality affects cellular aging throughout adulthood. Although self-reported sleep quality is important to examine and figures prominently in the development of chronic sleep disturbance, investigating these associations using objective indices of sleep (e.g., actigraphy and polysomnography) will be important. Relating indices of cellular aging to circadian and homeostatic processes may also help to clarify the mechanism through which sleep affects cellular aging.
Despite these limitations, the current study provides evidence for an association between subjective sleep quality, sleep duration, and cellular aging processes among middle-aged and older adults. These data suggest that sleep and subjective sleep quality and sleep duration can be added to a growing list of modifiable health behaviors linked to shorter TL.56–61 Thus, interventions aimed at improving modifiable aspects of sleep may help to lessen the adverse effects associated with cellular aging.
DISCLOSURE STATEMENT
This was not an industry supported study. Support for this research was provided by grant number R21 AG029239 (Dr. Uchino, PI) from the National Institute on Aging and Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources. Work was performed in the psychology department at the University of Utah. Dr. Cawthon has financial interests in and serves on the Scientific Advisory Board of Telome Health, Inc. Dr. Cawthon asserts that none of the above constitutes a conflict of interest regarding the current manuscript, as he was blinded with regard to age, sex, and all sleep quality-related data on the research participants. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
Matthew R. Cribbet, PhD and McKenzie Carlisle, PhD are co-first authors.
REFERENCES
- 1.National Sleep Foundation. How much sleep do adults need? Washington, DC: National Sleep Foundation; 2010. [Accessed April 18, 2011]. http://www.sleepfoundation.org/article/white-papers/how-much-sleep-do-adults-need. [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 4.Cochen V, Arbus C, Soto M, et al. Sleep disorders and their impacts on healthy, dependent, and frail older adults. J Nutr Health Aging. 2009;13:322–9. doi: 10.1007/s12603-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 5.Unruh ML, Redline S, An M-W, et al. Subjective and objective sleep quality and aging in the Sleep Heart Health Study. J Am Geriatr Soc. 2008;56:1218–27. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 6.Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56:503–10. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- 7.Redwine L, Dang J, Irwin M. Cellular adhesion molecule expression, nocturnal sleep, and partial sleep deprivation. Brain Behav Immun. 2004;18:333–40. doi: 10.1016/j.bbi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65:S244–52. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 11.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Bio Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–29. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahse R, Fiedler W, Ernst G. Telomeres and telomerase: biological and clinical importance. Clin Chem. 1997:708–14. [PubMed] [Google Scholar]
- 16.Saretzki G, Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci. 2002;959:24–9. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 17.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 18.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan SRWL, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–21. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews NP, Fuji H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvert PA, Liew T, Gorenne I, et al. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol. 2011;31:2157–64. doi: 10.1161/ATVBAHA.111.229237. [DOI] [PubMed] [Google Scholar]
- 22.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: A twin study of three age groups. Am J Hum Genet. 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Barcelo A, Pierola J, Lopez-Escribano H, et al. Telomere shortening in sleep apnea syndrome. Respir Med. 2010;104:1225–9. doi: 10.1016/j.rmed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Lee S, Bhattacharjee R, Khalyfa A, Kheirandish-Gozal L, Gozal D. Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest. 2010;138:91–9. doi: 10.1378/chest.09-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang G, Schemhammer E, Qi L, Gao X, De Vivo I, Han J. Association between rotating night shifts, sleep duration, and telomere length in women. PLoS One. 2011;6:e23462. doi: 10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prather AA, Puterman E, Lin J, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011;2011:721390. doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackowska M, Hamer M, Carvalho LA, et al. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II Cohort Study. PLoS One. 2012;7:e47292. doi: 10.1371/journal.pone.0047292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2012;71:1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Abbas AK, Lichman AHH. Basic immunology updated edition: functions and disorders of the immune system. Philadelphia, PA: Elsevier Health Sciences; 2010. [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York, NY: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 32.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiken L, West S. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc; 1991. [Google Scholar]
- 35.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;7:17312–15. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mainous AG, Everett CJ, Diaz VA, et al. Leukocyte telomere length and marital status among middle-aged adults. Age Ageing. 2011;40:73–8. doi: 10.1093/ageing/afq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiecolt-Glaser JK, Glaser R. Stress and immunity: age enhances the risks. Curr Dir Psych Sci. 2001;10:18–21. [Google Scholar]
- 39.Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain Behav Immun. 2004;18:114–9. doi: 10.1016/j.bbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Williams PG, Smith TW, Gunn HE, Uchino BN. Personality and stress: individual differences in exposure, reactivity, recovery, and restoration. Handbook of stress science: Biology, psychology, and health. In: Contrada R, Baum A, editors. New York, NY: Springer; 2011. pp. 231–245. [Google Scholar]
- 41.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–5. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–7. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall MH, Muldoon MF, Jennings R, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 45.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 48.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 49.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 50.Foley DJ, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 51.McCrae CS, Rowe RA, Tierney CG, Dautovich ND, Definis AL, McNamara JP. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:82–9. doi: 10.1093/geronb/60.4.p182. [DOI] [PubMed] [Google Scholar]
- 52.Vitello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: Clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–9. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 53.Lemola S, Richter D. The course of subjective sleep quality in middle and old adulthood and its relation to physical health. J Gerontol B Psychol Sci Soc Sci. 2013;68:721–9. doi: 10.1093/geronb/gbs113. [DOI] [PubMed] [Google Scholar]
- 54.Barcelo A, Pierola J, Lopez-Escribano H, et al. Telomere shortening in sleep apnea syndrome. Respir Med. 2010;104:1225–9. doi: 10.1016/j.rmed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Kim J, Lee S, Bhattacharjee R, Khalyfa A, Kheirandish-Gozal L, Gozal D. Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest. 2010;138:91–9. doi: 10.1378/chest.09-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ. 2004;2004:pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- 57.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 58.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham heart study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 59.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2006;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 60.van der Harst P, de Boer RA, Samani NJ, et al. Telomere length and outcome in heart failure. Ann Med. 2010;42:36–44. doi: 10.3109/07853890903321567. [DOI] [PubMed] [Google Scholar]
- 61.Astrup AS, Tarnow L, Jorsal A, et al. Telomere length predicts all-cause mortality in patients with type 1 diabetes. Diabetologia. 2010;53:45–8. doi: 10.1007/s00125-009-1542-1. [DOI] [PubMed] [Google Scholar]


