Abstract
Background:
Arousal disorders may have serious health consequences.
Objective:
To develop a scale assessing the severity of arousal disorders (Paris Arousal Disorders Severity Scale, PADSS).
Setting:
University hospital.
Design:
Controlled study.
Participants:
Consecutive patients (older than 15 y), with sleepwalking (SW) and/or sleep terrors (ST), subjects with previous SW/ST, normal controls and patients with rapid eye movement sleep behavior disorder.
Intervention:
The self-rated scale listed 17 parasomniac behaviors (PADSS-A), assessed their frequency from never to twice or more per night (PADSS-B) and evaluated the consequences (PADSS-C: disturbed sleep, injuries, fatigue, and psychological consequences). The clinimetric properties and face validity of the scale were tested.
Results:
Half of the 73 patients with SW/ST (more men than women) had injured themselves or others, whereas 15% had concomitant sexsomnia and 23% had amnestic eating behaviors. The total PADSS score (range: 0-50) was 19.4 ± 6.3 (range: 8-36) in this group, 11.7 ± 5.9 in 26 subjects with previous SW/ST, 8.8 ± 3.2 in 26 patients with RBD, and 2.0 ± 3.5 in 53 normal controls (P < 0.05). The PADSS demonstrated high sensitivity (83.6%), specificity (87.8%), internal consistency, and test-retest reliability (0.79). The best cutoff for the total score was at 13/14. Exploratory factor analysis revealed two components: wandering and violence/handling. The complexity of behaviors emerging from N3 sleep (scored on videopolysomnography) positively correlated with scores for the PADSS-total, PADSS-A, PADSS-C, and the “violence/handling” factor.
Conclusion:
This scale had reasonable psychometric properties and could be used for screening and stratifying patients and for evaluating the effects of treatments.
Citation:
Arnulf I; Zhang B; Uguccioni G; Flamand M; Noël de Fontréaux A; Leu-Semenescu S; Brion A. A scale for assessing the severity of arousal disorders. SLEEP 2014;37(1):127-136.
Keywords: Arousal disorders, confusional arousal, parasomnia, sexsomnia, sleep related eating disorder, sleep terror, sleepwalking
INTRODUCTION
Arousal disorders are parasomnias that occur as an individual emerges from N3 sleep.1 They include sleepwalking (SW), sleep terrors (ST), and confusional arousals. The affected patients may scream, sit up in bed, look around, stand up, walk, run, or handle objects as if they were awake. However, their lack of full awareness and responsiveness, inappropriate behaviors, reduced mentation, and poor recollection of the event suggest that they are partly asleep. Recent functional brain imaging and intracerebral stereoelectroencephalographic data suggests a dissociated sleep-wake state during nonrapid eye movement (NREM) parasomniac episodes, combining slow wave activity in associative areas with motor and cingular arousal.2–4 Sleep related eating disorder and sexsomnia share some features with arousal disorders (such as frequent emergence from N3 sleep and partial awareness), although both disorders may also emerge from N2 or rapid eye movement (REM) sleep.1,5,6 All of these abnormal behaviors lead to significant risk that an affected individual may injure oneself or others and suffer from sleep disturbances. Many violent, even forensic, behaviors have been reported in individuals with arousal disorders. Patients with SW may wander from their homes, open the doors and windows, defenestrate, or drive automatically.7 These patients may try to escape as if to avoid an imminent danger, squeeze the arm or neck of a bed partner, and even murder or rape in some rare cases.5,8 Patients with ST may awaken their household members by crying out in terror, injure themselves as they get out of bed, and even sustain fatal injuries if they collide into furniture or break through a window.9 In addition, patients with SW have higher daytime sleepiness, fatigue, and a reduced quality of life compared to controls.10,11 Unlike REM sleep behavior disorder (RBD), the arousal disorders have received little attention. Indeed, they are often considered benign with a spontaneous disappearance during adolescence. However, although the condition is more common in children, it can exist at any age. SW and ST affect 9-14.7% of children from age 3 to 10 y, 3.8-7% at 11 y, and 1.2-3.3% at 13 y.12 In adults, the prevalence of SW is estimated between 2.5% and 4%,13,14 but one third of American adults have experienced some nocturnal wandering.15 Furthermore, most adults with SW (89% of men and 85% women) also experienced childhood somnambulism.13
Given this high propensity of sleep related injuries and the significant impact of arousal disorders on quality of life, it is crucial that these conditions be diagnosed and assessed. The diagnosis is mainly based on clinical history. The videopolysomnography (performed over 1 or 2 nights, often preceded by sleep deprivation) is not mandatory, but it is essential to observe abnormal behaviors emerging from N3 sleep, to identify potential triggers (e.g., noise, sleep disordered breathing, leg movements), and to rule out differential diagnoses such as nocturnal frontal lobe epilepsy and RBD. However, this test consumes time and resources. Therefore, screening tools may be used to identify subjects who are at a high risk for arousal disorders, evaluate their severity, identify the patients who require an intervention, and assess the benefit of treatment. Surprisingly, to the best of our knowledge, there is no available scale for assessing the diagnosis and severity of arousal disorders, although several instruments have been developed in RBD, a more recently identified parasomnia.16–20 Thus, the goal of this study was to develop and validate an easily applicable instrument for diagnosing, monitoring, and assessing the effects of treatment in arousal disorders.
METHODS
Development of the Scale
The Paris Arousal Disorders Severity Scale (PADSS, see Table 1) was developed after several expert consensus meetings. Two neurologists (IA and SLS) and two psychiatrists (AB and MF) experienced in managing patients with NREM parasomnias developed the scale with three parts, including an inventory of behaviors (PADSS-A), the frequency of episodes (PADSS-B), and the general consequences of the disorder (PADSS-C). For the behavioral part, the physicians listed the most problematic behaviors reported by their patients during parasomniac episodes. The behaviors were presented according to the pattern of complexity, ranging from behaviors that occur in the bed (screaming, sitting up) to standing up, walking in the room, going out of the room, and leaving the home. Within this spatial exploration, we paid particular attention to behaviors involving the windows with an increasing potential for danger (touching furniture around windows, stepping over windows, opening them) and behaviors involving the stairs due to the risk of falling. As for handling objects, we gradually listed behaviors from those with a reduced potential for danger, such as handling light objects, to those with a greater potential for danger (handling heavy objects, knives, or objects that may be used to light a fire). We systematically added two items related to amnestic eating behavior and sexsomnia, as these behaviors may be associated with arousal disorders.5,6 We chose to limit the answer to these items to three choices (“never,” “sometimes” and “often”) for simplicity and because patients had difficulty being more precise, i.e. using five anchors instead of three. Part B was developed as a classic gradient of the frequency of episodes as commonly reported by patients as nightly, weekly, monthly, and yearly. In part C, we listed the most common consequences of the episodes (and the patients' motives for seeking medical advice). We previously noted that in addition to disturbing their companion's sleep and causing harm to themselves and their companions, many patients with arousal disorders reported that they felt tired or sleepy during the daytime, and we found that the Epworth Sleepiness Scale (ESS) score was higher in these patients than in control patients.10 Additionally, patients also reported that they were ashamed or felt anxious about displaying these behaviors; hence, this finding was listed as a specific item on psychological consequences of the disorder. The scale was self-completed and measured as follows: dangerous behaviors (17 items with three possible answers: never = 0, sometimes = 1, often = 2), frequency of episodes (equal to or more than two episodes per night = 6, one per night = 5, equal to or more than 1 episode per week = 4, equal to or more than 1 episode per month = 3, equal to or more than 1 episode per year = 2, less than 1 episode per year = 1, never had any = 0), and consequences of the disorder (5 items with three response options: never = 0, sometimes = 1, often = 2). Although PADSS was aimed at being a severity scale, it appeared that normal controls sometimes screamed during the night, hence the option of choosing the items “less than one episode per year = 1” and “never = 0”. We first attempted to apply the scale over a lifetime and then decided to assess the period over the past year to reduce the bias of recall and the inclusion of simple dreaming-enacting behaviors which are common in the general population.21 Because the PADSS-B (frequency of episodes) is a categorical scale with large time intervals and not a numeric continuous scale, we transformed the answers in the tables from categories to continuous numbers (number of episodes/mo), using the following rule, which is based on the minimal value in each interval: six (equal to or more than two episodes/night) = 60 episodes/mo, five (at least one episode/night) = 30 episodes/mo, four (equal to or more than one episode/w, but less than one/night) = 4.3 episodes/ mo, three (equal to or more than one episode/mo, but less than one/w) = one/mo, two (equal to or more than one episode/y but less than one/mo) = 0.08/mo, one (less than one episode/y) and 0 (never) = 0/mo. We chose the minimal value of each interval, rather than the mean, to minimize bias of transformation. If patients report they have two or more episodes per night, the lower bound of the interval is two (i.e., 60 episodes per mo), but the upper bound is infinite; hence, there is no means of doing a mean value in the interval. As soon as the first interval is transformed to its lower bound, the same rule should be kept for subsequent intervals.
Table 1.
Paris Arousal Disorders Severity Scale, English version
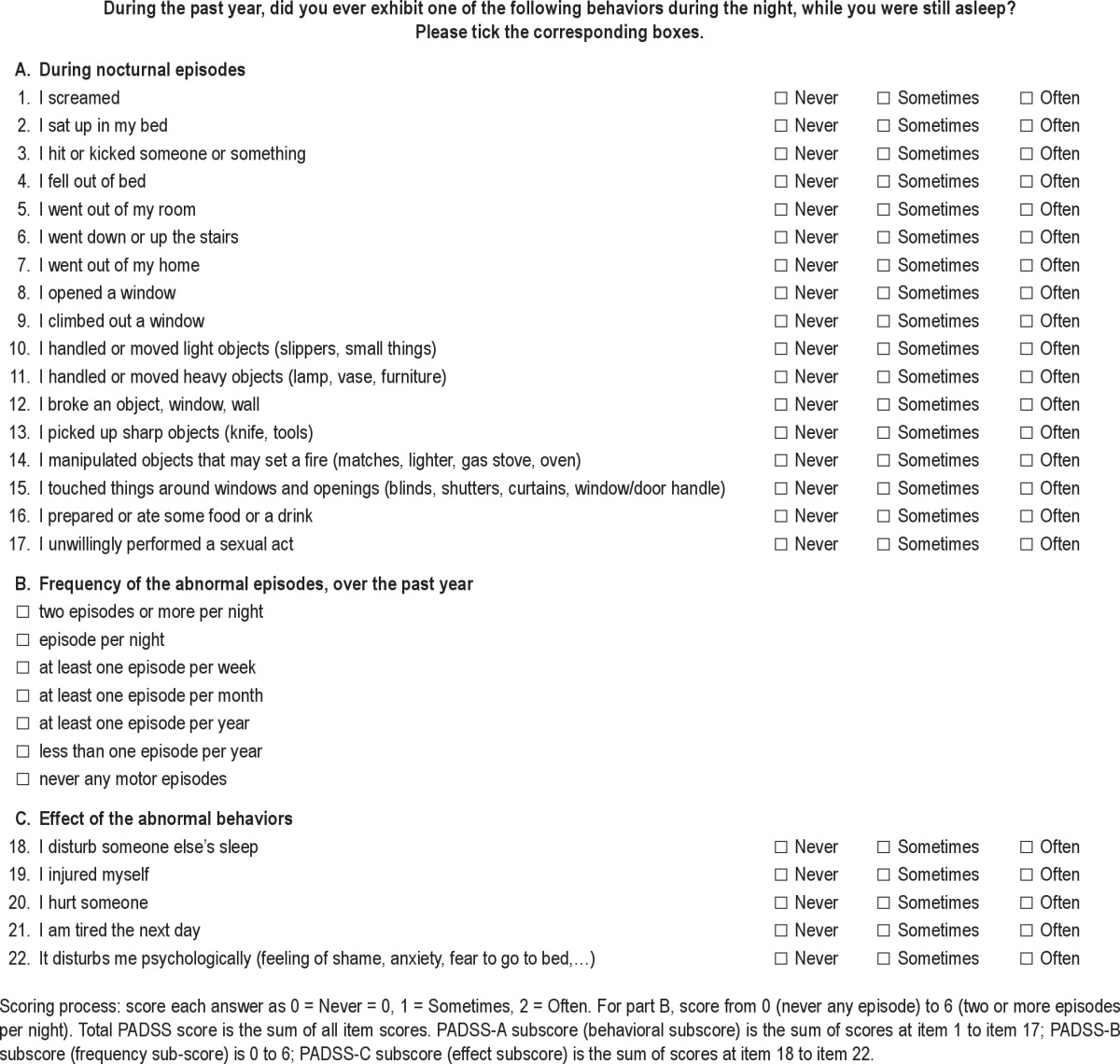
The study was conducted in two stages: piloting and validation. In the piloting stage, the draft version of the PADSS scale was tested on a sample of 15 patients with arousal disorders to evaluate the feasibility and comprehensibility of the scale. The item “I handled electronic objects (television, telephone, clock radio…)” was later added after observing patients who had disabled their alarm clocks while sleepwalking and hence were not able to wake up on time in the morning (which can be considered a deleterious consequence of the disorder). It appeared that this behavior was either rare or without serious consequences, so it was deleted in the final version of the PADSS. The total PADSS score ranged from 0 to 50.
Participants
The study was approved by the local ethics committee (CPP Paris IDF 6), and all participants gave their consent to take part in the study. To validate the PADSS, we consecutively recruited patients with sleepwalking or sleep terror (SW/ST) referred to the sleep disorder unit from January 2012 to December 2012. There was no patient overlap with our previous study on dreamlike mentations during SW and ST.10 The sleep disorder unit was located in a university hospital, and it received only adult patients and teenagers older than 15 y who were referred by general practitioners (this was mostly the case for patients with SW/ST) or by other specialists, mainly neurologists (this was mostly the case for patients with RBD). Because the instrument is a severity scale, and as we were aware that mild forms of SW and ST are common in the young adult population,21,22 we found it interesting to test the scale in a population of young adults who had never consulted a physician for sleep disorders but who had had some abnormal amnestic behaviors during the night. We expected lower ranges of severity, or alternatively to find quite serious behaviors that would have been overlooked (as sleepwalking is often considered as a seminormal behavior with no need for treatment in France). We therefore found, by word of mouth and face-to-face interview, subjects who had been former sleepwalkers or who had still presented abnormal behaviors during the night, but who would not have consulted any physician for this purpose. These patients were recruited between March and September 2012. To test if PADSS was specific for NREM rather than REM parasomnias, we administered it to a series of nondemented patients with RBD who were referred for the first time to the unit from July to October 2012. Eventually, we administered the PADSS to young, normal controls who were recruited among the families of the team, their friends, and medical students, with the goal of including subjects who were matched for age and sex. The control group consisted of the RBD group, the previous SW/ST group, and the healthy controls. All participants were interviewed in person by the sleep specialists.
The patients with SW/ST had to meet the criteria of the International Classification of Sleep Disorders (ICSD), which defined sleepwalking as1: (1) a history of ambulation during sleep; (2) the persistence of sleep or impaired judgment during ambulation; and (3) the disturbance could not be better explained by another sleep, medical, mental or neurological disorder, or by medication/drug use. Sleep terror was defined as (1) a history of a sudden episode of terror occurring during sleep, usually initiated by a cry or loud scream with sympathetic and behavioral manifestations of intense fear; (2) difficulty in arousing the person, or mental confusion when awakened from an episode, or complete or partial amnesia of the episode, or dangerous or potentially dangerous behaviors; and (3) the disturbance could not be better explained by another sleep, medical, mental, or neurological disorder, or by medication/drug use. In addition to these clinical criteria, we observed at least one of the following features in all patients in the videopolysomnography (although these features were neither totally sensitive nor specific, they were supportive in the context of a systematic study): (1) at least one cortical arousal during N3 sleep was associated with an abnormal motor behavior suggesting surprise, confusion or fear (e.g., startling, sitting up in the bed, or looking around surprised); or (2) sudden cortical arousals during N3 sleep. The patients with RBD met the international RBD criteria, including (1) a clinical history of complex, vigorous, violent, or injurious behavior during sleep that was frequently associated with dream mentation; and (2) enhanced chin muscle tone during REM sleep; or (3) simple or complex behaviors on video during REM sleep. In both disorders, patients with sleep apnea syndrome (apnea-hypopnea index greater than 15) were not included. Patients with arousal disorders and idiopathic RBD were drug naïve, which means that patients with RBD or arousal disorders triggered by drugs were not included in the study. Patients with RBD associated with parkinsonism received their usual dopaminergic treatment. The patients with SW/ST and RBD completed the scale on the evening before the diagnostic video-polysomnography. Nocturnal frontal lobe epilepsy was ruled out by (1) reviewing the patient's personal and family history; combined with (2) the absence of epileptic activity on the extended EEG during the 20 h (two periods of 10 h) of sleep monitoring (especially at sleep-wake transitions, visualized with a 20-sec period); (3) a nonstereotyped aspect of the behavioral and motor episodes; (4) the absence of head version or dystonic postures or movements; and (5) the exclusive occurrence of motor events on emerging from N3 (and never from other sleep stages). The presence of enacted dreaming during SW/ST also provided evidence against frontal lobe epilepsy.
Sleep Monitoring
The videopolysomnography was performed on two consecutive nights preceded by a mild sleep deprivation (sleep restricted to 4 h on the night preceding the monitoring). It included eight electroencephalograph (EEG) leads (Fp1; C3; T3; O1; Fp2; C4; T4, O2) acquired in a monopolar referential montage and displayed on screen as bipolar, with sleep stages scored on the Fp1/C3, C3/A2 and C3/O1 channels, two electro-oculograms (EOG) (left inferior epicanthus/right mastoid; right superior epicanthus/right mastoid), three surface electromyographs (EMG, levator menti, right and left tibialis anterior), nasal pressure, tracheal sounds, chest and abdomen movements via contention belts, electrocardiogram (EKG), pulse oximeter, position, as well as synchronized ambiance sounds and infrared video. The patients with RBD mostly stayed for 1 night and underwent the same monitoring procedure as the patients with SW/ST, except that the EEG channels were restricted to the Fp1/C3, C3/A2 and C3/O1 channels. Two nurses supervised the monitors during the night. They reported their observation on a sheet log, but they were instructed not to interfere with the patient's sleep, unless the patient was in danger, asked for help, or lost several important leads. The recordings were exclusively scored by experienced neurologists. The eight EEG channels were examined on 20-sec epochs because the scorers were trained to recognize epileptic activity at this slower speed, whereas sleep was scored using 30-sec epochs. All respiratory events were measured, including apnea, hypopnea, and flow limitation (defined as a plateau on nasal pressure lasting at least 10 sec and followed by an arousal).
The analysis of videopolysomnography included the number of sudden awakenings from stage N3 sleep during the 2 nights, as well as the corresponding observed behaviors on the video, scored by a unique scorer (MF). We based this new complexity analysis on our experience and that of others.23 The analysis of the videopolysomnography included the number of sudden awakenings from stage N3 sleep during the 2 nights, as well as the corresponding observed behaviors on the video. We counted 0 if no vocalization occurred during these awakenings and 1 if vocalization occurred at least once. We used a similar code for raising the head (0 or 1), sitting (0 or 1), standing up (0 or 1), observing a sympathetic activation (0 or 1, determined as a sudden doubling of the heart rate together with a vasoconstriction on the pulse oximeter), handling objects (0 or 1), or exhibiting any behavior (0 or 1), leading to a total behavior complexity ranging from 0 to 6.
Statistical Analysis
We used descriptive statistics, including the mean ± standard deviation for the quantitative variables and percentages for the qualitative variables. The psychometric properties of the questionnaire were assessed by calculating the sensitivity and specificity for each threshold with a 95% confidence interval (CI). The receiver operating characteristics (ROC) curve of the test was used to evaluate the discriminative capacity of the scale. The internal consistency was assessed using the Cronbach alpha coefficient. The test-retest reliability was analyzed by Spearman rank correlations. An exploratory factor analysis was performed to determine the structural validity of the scale. The initial criterion for the extraction of factors was based on an eigenvalue exceeding one and the scree plot inspection. After performing kurtosis, skewness, and Kolmogorov-Smirnov 1-sample tests for checking the normality of the distribution, the between-group comparisons of the demographic and clinical characteristics were performed using the Mann-Whitney U test (two groups), the Wilcoxon rank test (paired comparisons) and the Kruskal-Wallis test (more than two groups) for data without normal distribution and the Student t-test and the chi-square test for those with normal distribution. In case of continuous measures in the four subgroups, we performed analysis of variance (ANOVA) next, if significant by post hoc test (chi-square tests) with adjusted P values (significant below 0.008). The correlation between quantitative variables was performed using the Student t-test and Pearson test, and the correlation between qualitative variables was performed using the Spearman test. All statistical analyses were conducted using Statistical Package for Social Science 16.0 (SPSS, Chicago, IL, USA).
RESULTS
Demographic and Clinical Characteristics of Participants
Patients with Arousal Disorders
Seventy-three patients with SW/ST and 98 control subjects participated in the validation study (Table 2). The 73 patients suffered from isolated SW (n = 24, 33% of the sample), isolated ST (n = 4, 5%) and a combination of SW and ST (n = 45, 62%). Sleep talking was reported by 63 of 73 patients (86%). The ESS score ranged from 0 to 20 (mean 9.4 ± 5) and was greater than or equal to 11 in 41% of the patients. The arousal disorder had started during childhood in 38 patients (52% of the sample), during adolescence in 8 (11%), between 20 and 30 y old in 7 (9.6%), after 30 y old in 9 (12%), whereas 10 patients (14%) did not know when the arousal disorder had started. The arousal disorder had recently worsened in all patients, which was the reason for referral to the hospital. Thirty-nine patients (28 men, 72% and 11 women, 28%) had experienced an episode severe enough to hurt themselves (n = 34, 47% of the sample) or their bed partner (n = 20, 27% of the sample). The injuries included a bone fracture while kicking in the wall (n = 1) laceration to oneself (n = 3), hematoma while climbing a bookshelf (n = 1), falling out of the bed, dropping objects, opening a window, falling out of a window from several floors resulting in multiple pelvis fractures or a forearm fracture (n = 2) or climbing on the roof (n = 1). The dangerous behaviors directed toward the bed partners included grabbing and pushing the partners violently, strangling them (n = 4), hitting them (n = 4), scratching them (n = 1), projecting them into the air (n = 1) and pushing them out of the bed (n = 3). Although it was not the motive for referral, sexsomnia was reported by 11 patients (15% of the sample) and eating or drinking food during amnestic episodes was reported by 17 patients (23% of the sample). Nightmarish mental content combined with the sensation of a vital threat was associated with some SW/ST episodes in 53% of patients, including the feeling of a dangerous intruder (sometimes a murderer) entering the room (n = 6), collapse of the walls, ceiling, or house or being buried alive (n = 5), crocodile, bugs, and spiders (n = 3), choking (n = 5), an urgent need to flee or sensation of being chased (n = 3), baby falling out of the bed or suffocating (n = 1), being in a running car or a falling lift without a brake (n = 1), and replaying a recent event that had happened during the day in real life or on TV (n = 4). A male patient enacted the event of saving his niece from being drowned (an event that had already happened the previous day) and defenestrated. A female patient dreamed of a sand tsunami soon after the Fukushima tsunami and climbed over a bookshelf. A young mother thought she observed her baby had drowned in a milk perfusion bag after she had experienced a difficult parturition, and a male patient reenacted the movie ‘Saving Private Ryan’ after he observed it on television and crawled under a fictitious barbed wire fence.
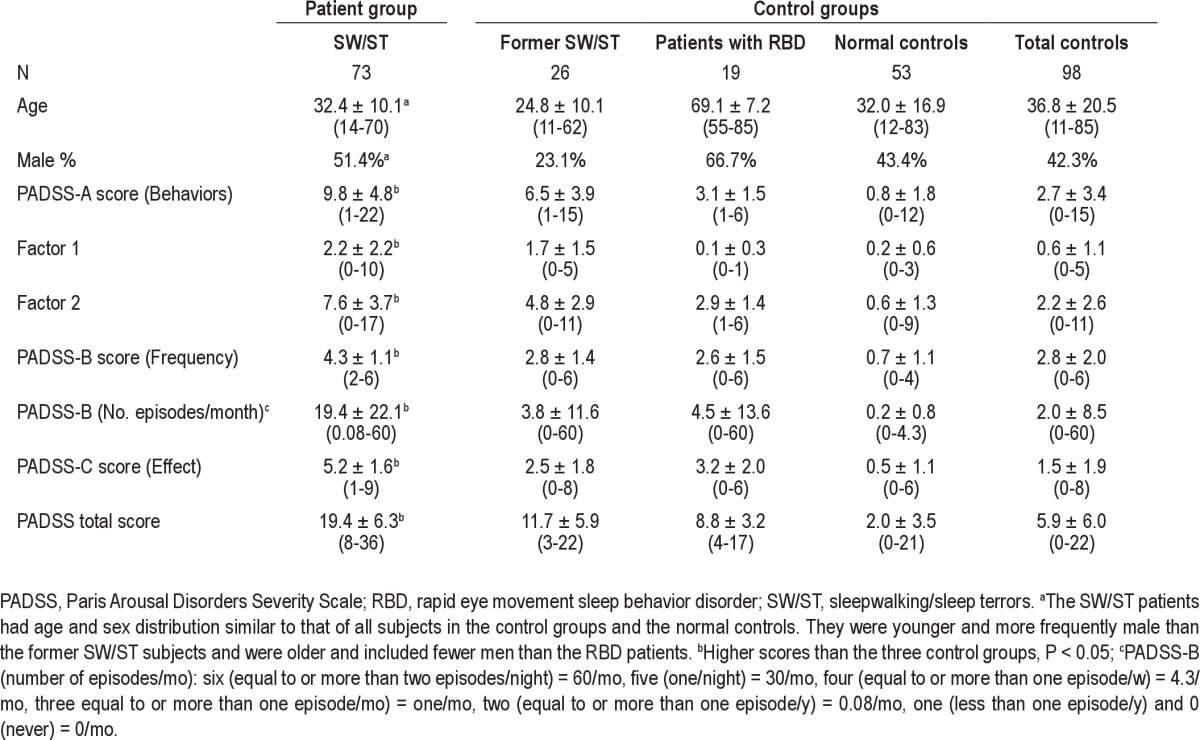
Table 2.
Demographic characteristics and Paris Arousal Disorders Severity Scale scores in patients with sleepwalking and sleep terrors and controls
In videopolysomnography, the patients with SW/ST had a median of five awakenings from N3 sleep (range 0-16, 95% confidence interval: [4.2-5.8], all emerging from the former NREM sleep stage 4) during the first night and five awakenings from N3 sleep during the second night (range 0-12, 95% confidence interval: [4.3-5.7]). Abnormal behaviors were observed when emerging from N3 arousals in 61 patients, no behaviors when emerging from N3 arousals in 10 patients, and missing data (video problems) for two patients.
Control Groups
The control group consisted of 26 subjects who reported previous SW/ST in the interview but who had never sought medical advice, 19 nondemented patients with RBD (six with Parkinson disease, eight with idiopathic RBD, one with multiple systemic atrophy, and one with likely early dementia with Lewy bodies), and 53 normal controls.
The age (P < 0.001, F = 51.1, df = 3, ANOVA) and sex distribution (P = 0.023, χ2 = 9.58, df = 3, chi-square test) were different between the four groups, but patients with SW/ST had a similar age (P = 1, post hoc test) and sex distribution as the normal controls. Although the age in these groups was similar, there were more men in the current SW/ST group than in the former SW/ST group (P = 0.007, χ2 = 6.42, df = 1, chi-square test). As expected, the patients with RBD were older (P < 0.0001, post hoc test) and more frequently male compared with the other groups (P = 0.023, χ2 = 9.58, df = 3, chi-square test).
Acceptability of the PADSS
The patients and subjects completed the scale within 3.6 ± 1.1 min (range 2-7 min). Only four patients with SW/ST (5.5%) did not fully complete all items and left question marks in front of one to four items. Therefore, 94.5% of the patients completed 100% of the items. The unanswered items were “I disturbed someone else's sleep” by a woman sleeping alone (hence the question mark), “I handled light, heavy and sharp objects” (not known by one patient), and “I used the stairs”, an item that seemed to have been missed by two patients.
PADSS Scores
The mean total PADSS score for all subjects was 11.7 ± 9.1 (range 0-36). Patients with SW/ST had a significantly higher total PADSS score (range 8-36) than the control group (range 0-22, P < 0.0001, z = -9.4, Mann-Whitney U test) and the three control subgroups (former SW/ST: range 3-22, RBD: range 4-17, normal controls: range 0-21) (P < 0.0001, z = -9.7, Kruskal-Wallis test). Additionally, they had significantly higher scores for subscores PADSS-A (P < 0.0001, z = -8.7, Mann-Whitney U test), PADSS-B (frequency, P < 0.0001, z = -9.1), and PADSS-C (P < 0.0001, z = -9.4) (Table 2). The distribution of the PADSS total score in the various groups is shown in Figure 1, and the distribution of subscores PADSS-A, -B, and -C are shown in Figures S1, S2, and S3 in the supplemental material.
Figure 1.
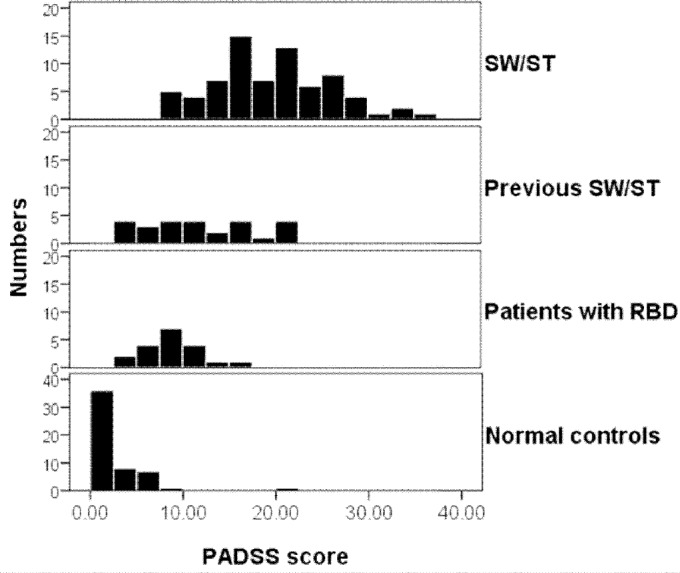
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS score (x axis, ranging from 0 to 50) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ST, the patients with RBD, and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.
Face Validity of the PADSS
The face validity of the PADSS was tested in comparison with the ESS and to the complexity of the episodes in videopolysomnography. The ESS scores correlated positively with the answers to item 21 (“I am tired the next day”), with Spearmen rho = 0.264, P = 0.027. The answer “never” was associated with a mean 5.3 ± 4.4 (range 0-13) ESS score, “sometimes” was associated with a 9.4 ± 5.6 score (range 0-17), and “often” was associated with a 9.8 ± 4.7 (range 0-20).
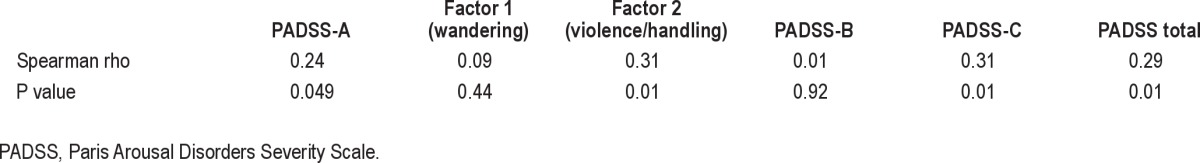
In the videopolysomnography, the complexity of behaviors emerging from N3 sleep (scored from 0 to 6) correlated positively with the scores for the PADSS-total, PADSS-A, and PADSS-C as well as with factor 2 (violence/handling) in PADSS-A, but not with scores for the PADSS-B (Table 3). However, item 2, “I sat up in bed”, was not associated with more frequent sitting behaviors in the videopolysomnography. Men and women had similar scores on the PADSS (P = 0.259, Z = 1.128, Mann-Whitney U test).
Table 3.
Correlation between the complexity of the behaviors emerging from N3 sleep in videopolysomnography and the PADSS scores (n = 71)
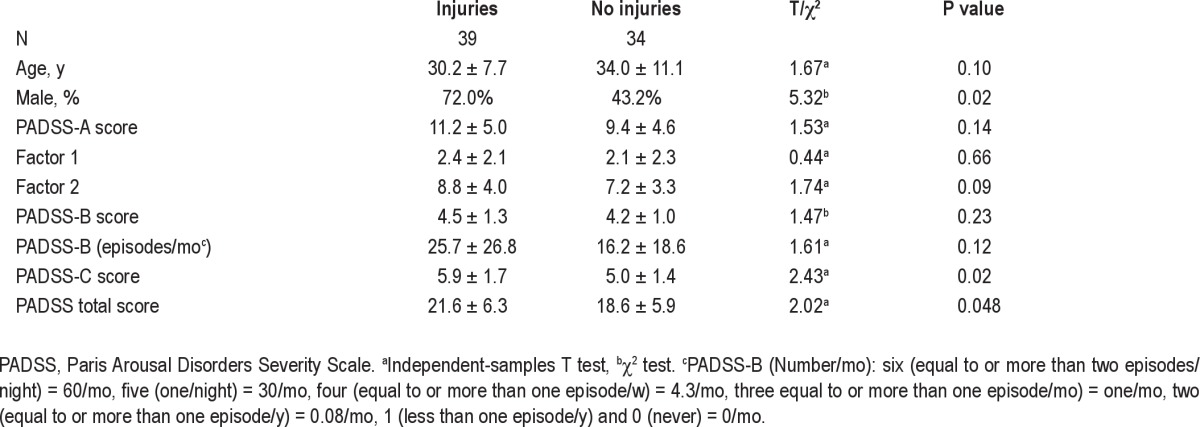
The 39 patients (53% of the sample) with a history of violent episodes (hurting themselves or their bed partner) had higher total PADSS scores (21.6 ± 6.3 versus 18.6 ± 5.9, Mann-Whitney U test) and a greater effect of the disorder (PADSS-C, 5.9 ± 1.7 versus 5.0 ± 1.4) than the 34 patients (47% of the sample) without a history of violent episodes, but there were no significant differences in the behavioral inventory (PADSS-A) and frequency of episodes (PADSS-B). More men (72%) reported violent episodes than women (28%) whereas as many men (43.2%) as women (56.8%) reported nonviolent episodes (Table 4).
Table 4.
Paris Arousal Disorders Severity Scale scores in patients with and without injuries (to self or others) during sleepwalking/sleep terrors
Construct Validity of the PADSS
The value of the Kaiser-Meyer-Olkin measure of sampling adequacy was 0.68 and the Bartlett test of sphericity yielded a significance value of less than 0.001, rendering an exploratory factor analysis adequate for the PADSS scale. The two-, three-, and four-factor models had been applied to explore the construction of PADSS, and the two-factor model was the best. Varimax-rotated two-factor solution for the PADSS scale was illustrated in Table 5. The two-factor model accounted for a substantial part of scale variance (i.e., 50.3%), with the item loading ranging from 0.469 to 0.747. Seven items (Q5-Q7, Q13, Q14, Q16, and Q17) loaded on factor 1, which delineated most wandering behaviors, including eating and performing a sexual act. The rest of the items (Q1-Q4, Q8-12, and Q15) were loaded on factor 2, which delineated most violent and handling behaviors.
Table 5.
Rotated factor loadings for the Paris Arousal Disorders Severity Scale

We further analyzed the mean scores of the two factors as extracted from the factor analysis. The patients with SW/ST had significantly higher scores for both factor 1 (wandering) and factor 2 (violence/handling) than the control group (factor 1: 2.2 ± 2.2 versus 0.6 ± 1.1; factor 2: 7.6 ± 3.7 versus 2.2 ± 2.6, P < 0.001, respectively). The difference was also significant between the patients with SW/ST and the three control subgroups (Table 2).
Criteria Validity of the PADSS
We performed two ROC analyses with the total PADSS score and the PADSS-A subscore. The ROC analyses indicated that both the total scale and the PADSS-A had good diagnostic accuracy. The best cutoff for the total scale (score ranging 0-50) was located at 13/14 with a sensitivity of 83.6% and a specificity of 87.8% (area under curve [AUC] = 0.93), whereas the best cutoff for the PADSS-A (score range 0-34) was located at 5/6 with a sensitivity of 83.6% and a specificity of 83.7% (AUC = 0.89) (Figure 2). The positive predictive value was 83.6%, and the negative predictive value was 87.8%. The cutoff for the total PADSS (13/14) was further analyzed between the patients with SW/ST and the three control subgroups (Tables 5 and 6).
Figure 2.
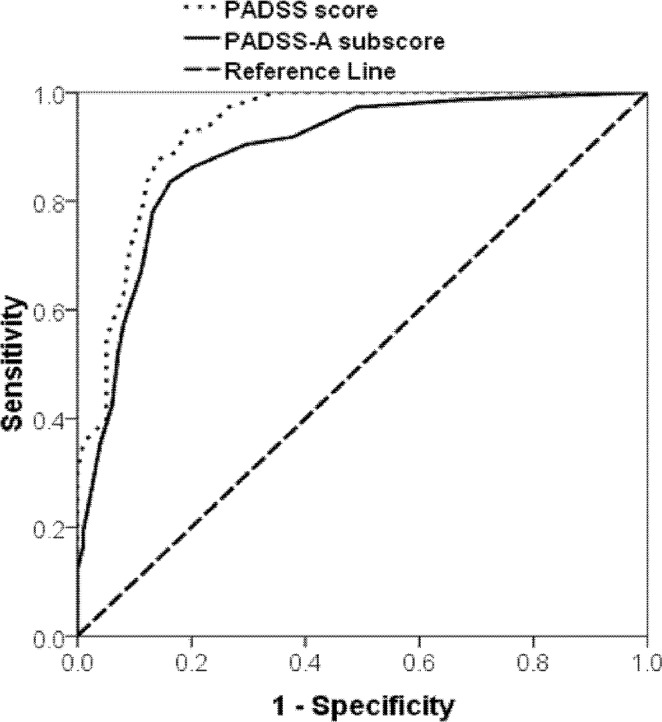
Receiver operating curve (ROC) for the PADSS as a diagnostic instrument. The best cutoff for the overall scale (PADSS score, blue line) was located at 13/14 with a sensitivity of 83.6% and a specificity of 87.8% (AUC = 0.93). The best cutoff for the PADSS-part A (list of behaviors, green line) was located at 5/6 with a sensitivity of 83.6% and a specificity of 83.7% (AUC = 0.89). PADSS, Paris Arousal Disorders Severity Scale.
Table 6.
Sensitivity, specificity, positive predictive value, and negative predictive value at the cutoff point for PADSS (cutoff score of 13/14)
Clinimetric Properties, Internal Consistency, and Test-Retest Reliability
The internal consistency of the PADSS, estimated by Cron-bach alpha coefficients, was 0.79 for the overall scale, 0.77 for PADSS-A, and 0.71 and 0.75 for factor 1 (wandering) and factor 2 (violence/handling), respectively, in the PADSS-A. The scale was retested in 25 patients with an SW/ST (34.2% of the sample) at an interval of 59 ± 47 days (range 13-198 days). The test-retest coefficients were 0.79 (Pearson correlation, P < 0.001) for the total PADSS score, 0.71 (Pearson correlation, P < 0.001) for the PADSS-A score, 0.88 (Spearmen correlation, P < 0.001) for the PADSS-B score, and 0.66 (Pearson correlation, P < 0.001) for the PADSS-C score. The Wilcoxon signed rank test did not reveal any significant differences between the scores of the patients at time 1 and time 2 for the PADSS (21.7 ± 5.7 and 19.2 ± 5.1, respectively), PADSS-A (11.8 ± 4.0 and 9.7 ± 3.6, respectively), PADSS-B (4.4 ± 1.1 and 4.4 ± 1.2, respectively), and PADSS-C scores (5.6 +1.8 and 5.1 ± 1.8, respectively). The PADSS-B test-retest difference was 0.04 ± 0.55.
DISCUSSION
The aim of this study was to develop a valid and reliable self-administered scale for arousal disorders, the PADSS. During the development of this scale, several groups of patients and subjects were included to calibrate the severity (patients with SW/ST versus subjects with former, mild SW/ST) and the specificity (patients with NREM versus REM parasomnias, patients with arousal disorders versus normal controls matched for age and sex). The resulting scale demonstrated robust psychometric properties and consistent subscales, suggesting that this instrument could provide a useful tool for monitoring and measuring the clinical symptoms and severity of arousal disorders in future patient surveys and intervention studies.
Subjects completed the PADSS within less than 5 min, and only four patients with SW/ST did not fully complete all items. These results suggested that the PADSS was acceptable and easily understandable, which would improve the face validity and the consistency estimates of the PADSS. For the overall scale, subscales, and two factors in the PADSS-A, the high internal consistency was evidenced by the coefficient alpha of more than 0.70, and the high consistency over time was evidenced by test-retest coefficients of more than 0.70. The face validity of the PADSS could be supported by the complexity of the episodes observed by videopolysomnography and the ESS obtained during the clinical evaluation. Both measures positively correlated with the related content in the PADSS. Furthermore, patients with violent episodes had higher overall PADSS scores and higher PADSS-C subscores than patients without violent episodes, which showed that violent episodes had more impact on the patients' personal lives. We noticed that the score distributions for the overall scale and subscales in the patients with SW/ ST were approximately normal with no evidence of ceiling and floor effects, which guaranteed that the severity of the arousal disorders could be evaluated efficiently.
The factor analysis of PADSS revealed a highly stable two-factor structure. Both factors corresponded to the essential clinical features, as suggested by the literature. Factor 1 (wandering) was characterized as the aspects of wandering around a room or house and some behaviors that first required some ambulation (getting food, cooking, and possibly performing a sexual act, although this behavior mostly happened in the bed). Because picking up sharp objects (knives and tools) and manipulating objects that could cause a fire could be part of a cooking procedure, these two items (item 13 and item 14) were also loaded in factor 1. Factor 2 reflected the violent or handling behaviors, which are typical in arousal disorders. We were surprised by the relatively high frequencies of sexsomnia (15%) and sleep related eating behaviors (23%) found in this sample, especially because these were not the reasons for referral to the clinic and these behaviors were discovered during the scale completion. The inventory portion of the scale (PADSS-A) has the advantage of systematically asking these two embarrassing questions about sex and eating behaviors, which may not otherwise be mentioned during the medical interview. It would be interesting to assess the PADSS in patients specifically referred for sleep related eating behavior and for sexsomnia to look for concomitant behaviors suggestive of arousal disorders.
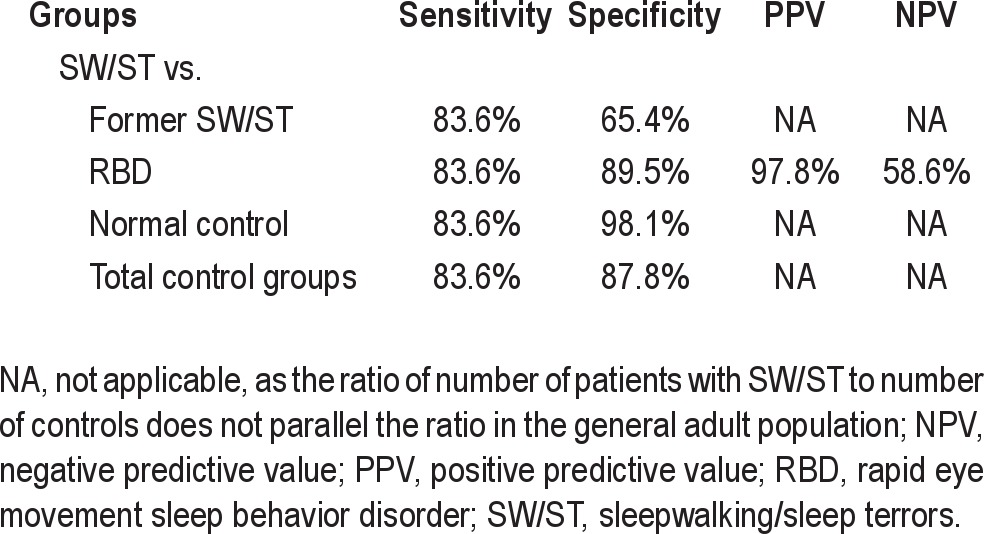
When combined with frequency of episodes (part B) and the general consequences of episodes (part C), the overall PADSS was better able to differentiate arousal disorders than relying only on the behavioral episodes (part A). The best cutoff score for the overall PADSS (range 0-50) was found at 13/14 and had high sensitivity (83.6%) and specificity (87.8%). We further assessed this cutoff in the three control subgroups. The specificity in the patients with RBD (89.5%) and the normal control patients (98.1%) was satisfactory, so high PADSS scores could substantially differentiate the RBD and did not reflect the characteristics of the RBD. The patients with RBD mostly reported the first four behavioral items, which were related to screaming, kicking, sitting up, and falling out of bed, but they responded negatively with regard to ambulatory and handling behaviors. As a consequence, the NPV of patients with RBD (58.6%) was low. Standing up and walking are indeed exceptional behaviors in patients with RBD,18,24 unless they are affected by a parasomnia overlap disorder, i.e., a combination of RBD and arousal disorders.25 In this last case, it may be interesting to combine an RBD severity scale plus the PADSS, as the RBD scales do not evaluate ambulatory and handling behaviors.16,17,19,20
However, the specificity (65.4%) of the scale in subjects with previous SW/ST was low, which meant that one third of the subjects with previous SW/ST would be judged as patients with SW/ST using the 13/14 cutoff. In the recruitment process, by word of mouth and face-to-face interviews, we recruited subjects who had been former sleepwalkers or who still exhibited behaviors during the night, but who had never consulted a physician for this purpose. This clinical impression was supported by the results of the PADSS. Some of these patients may have mild SW/ST. We were surprised to find that significant amnestic behaviors had been overlooked, as sleepwalking is, at least in France, often considered as a seminormal behavior with no need for treatment.
A number of limitations in this study should be acknowledged. First, all patients with SW/ST in this study were adolescents and adults, and no children were included, because our hospital was not allowed to receive children younger than age 15 y. It is well known that SW and ST are more common in children than in adults.12 Therefore, the main population (children) of patients with SW and ST was not analyzed here. As a result, the validity and reliability of the PADSS in children could not be evaluated, and studies should be performed in the future to address this issue, unless a scale more specific for childhood sleepwalking is developed. Also, subjects with history of SW/ST were recruited by word of mouth, whereas other methods such as face-to-face interview, epidemiological sampling, or advertisement could be preferred. Second, the patients themselves completed the PADSS, without any help from their bed partners. Some patients lived alone, and the bed partners were frequently absent during the interviews with these young adults. We commonly observed a contrast between middle-aged patients with RBD, who frequently attended the clinic as retired couples, and young patients with SW/ST, who frequently attended the clinic alone, possibly because the bed partners worked and could not take the day off to accompany these patients. The procedure that we used here (self-assessment) had the advantage of being consistent and could be used in single patients, but the partner- versus patient-completed scales should be contrasted in the future. In a study that developed an RBD questionnaire, the scores based on the patients' self-reports were slightly lower compared to those provided by both patients and bed partners.20 This phenomenon could also exist for the PADSS. As frontal lobe epilepsy is a differential diagnosis for arousal disorders, the PADSS should also be assessed in a sample of epileptic patients to assess its specificity.23 These limitations need to be explored in further studies.
In summary, we developed a valid and reliable self-administered scale for arousal disorders (PADSS). This scale could benefit our clinical experience and research in several fields. As a screening tool, the scale could be applied by general practitioners to patients referred for nocturnal violence and abnormal behaviors to recognize arousal disorders and evaluate their severity. This application would guarantee that patients receive the proper diagnosis and intervention in time and that the medical resources would not be wasted. As a severity scale, the PADSS could help doctors to assess the effects of treatments, such as drugs and intervention. Notably, no drug has yet been approved for SW and ST, and large, controlled trials of drugs and interventions (such as hypnosis) are surprisingly lacking for a disorder that may expose patients and their partners to serious injuries and forensic consequences.26 The test-retest properties of the PADSS were excellent over a timeline of 2 mo, which suggests that this instrument could be used for assessing changes over time. Eventually, it is conceivable that the scale may be used for genotype-phenotype studies in the general population. Several studies in monozygotic or dizygotic twins, as well as in families, have shown that a large part of the phenotypic variance of arousal disorders could be attributed to genetics.13,27–31 In these studies, the diagnosis of SW or ST was defined by a “yes” or “no”. The current study, as well as several studies in the general population,15 or in some selected populations of students or postpartum mothers,21,22 showed that the spectrum of disorders included some mild, tolerable forms, both in terms of the associated behaviors and the frequency of episodes, as well as some very severe forms. The PADSS could describe a wider phenotypic spectrum than a “yes/no” explanation and could help identify genetic allelic variants associated with these disorders.
DISCLOSURE STATEMENT
The work was supported by the Xu Guangqi 2012 grant from the French Research Ministry to Dr. Arnulf and Dr. Zhang. Dr. Arnulf received an Inserm-APHP interface grant. The study had no other financial support, no industry sponsoring, and did not used any drug. Dr. Arnulf has participated in speaking engagements and consulted for for UCB Pharma, Jazz, the American Academy of Sleep Medicine, and Movement Disorder Society; and has received grants for her team from the Kleine-Levin Syndrome Foundation and from the Fondation NRJ -Institut de France. Dr. Leu-Semenscu and Dr. Brion have participated in speaking engagements with UCB Pharma. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
Drs. Arnulf and Zhang equally contributed to this work.
SUPPLEMENTAL MATERIAL
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS-A score (behaviors, x axis, ranging from 0 to 34) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ST, the patients with RBD and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS-B score (frequency, x axis, ranging from 0 to 6) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ST, the patients with RBD and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS-C score (consequences of SW/ST, x axis, ranging from 0 to 10) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ ST, the patients with RBD and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–5. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 3.Terzaghi M, Sartori I, Tassi L, et al. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep. 2009;32:409–12. doi: 10.1093/sleep/32.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terzaghi M, Sartori I, Tassi L, et al. Dissociated local arousal states underlying essential clinical features of non-rapid eye movement arousal parasomnia: an intracerebral stereo-electroencephalographic study. J Sleep Res. 2012;21:502–6. doi: 10.1111/j.1365-2869.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 5.Schenck CH, Arnulf I, Mahowald MW. Sleep and sex: what can go wrong? A review of the literature on sleep related disorders and abnormal sexual behaviors and experiences. Sleep. 2007;30:683–702. doi: 10.1093/sleep/30.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brion A, Flamand M, Oudiette D, Voillery D, Golmard JL, Arnulf I. Sleep-related eating disorder versus sleepwalking: a controlled study. Sleep Med. 2012;13:1094–101. doi: 10.1016/j.sleep.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Schenck CH, Mahowald MW. A polysomnographically documented case of adult somnambulism with long-distance automobile driving and frequent nocturnal violence: parasomnia with continuing danger as a noninsane automatism. Sleep. 1995;18:765–72. doi: 10.1093/sleep/18.9.765. [DOI] [PubMed] [Google Scholar]
- 8.Pressman MR. Disorders of arousal from sleep and violent behavior: the role of physical contact and proximity. Sleep. 2007;30:1039–47. doi: 10.1093/sleep/30.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry. 1989;146:1166–73. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 10.Oudiette D, Leu S, Pottier M, Buzare MA, Brion A, Arnulf I. Dreamlike mentations during sleepwalking and sleep terrors in adults. Sleep. 2009;32:1621–7. doi: 10.1093/sleep/32.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez R, Jaussent I, Scholz S, Bayard S, Montplaisir J, Dauvilliers Y. Functional impairment in adult sleepwalkers: a case-control study. Sleep. 2013;36:345–51. doi: 10.5665/sleep.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laberge L, Tremblay R, Vitaro F, Montplaisir J. Development of parasomnias from childhood to early adolescence. Pediatrics. 2000;106:67–74. doi: 10.1542/peds.106.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Hublin C, Kaprio J, Partinen M, Heikkila K, Koskenvuo M. Prevalence and genetics of sleepwalking: a population-based twin study. Neurology. 1997;48:177–81. doi: 10.1212/wnl.48.1.177. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, Guilleminault C, Priest RG. Night terrors, sleepwalking, and confusional arousals in the general population: their frequency and relationship to other sleep and mental disorders. J Clin Psychiatry. 1999;60:268–76. doi: 10.4088/jcp.v60n0413. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon MM, Mahowald MW, Dauvilliers Y, Krystal AD, Leger D. Prevalence and comorbidity of nocturnal wandering in the U.S. adult general population. Neurology. 2012;78:1583–9. doi: 10.1212/WNL.0b013e3182563be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comella C, Nardine T, Diederich N, Stebbins G. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson's disease. Neurology. 1998;51:526–9. doi: 10.1212/wnl.51.2.526. [DOI] [PubMed] [Google Scholar]
- 17.Boeve B, Silber M, Ferman T, Smith G. Validation of a questionnaire for the diagnosis of REM sleep behavior disorder. Sleep. 2002;25:A486. Abstract Supplement. [Google Scholar]
- 18.Scaglione C, Vignatelli L, Plazzi G, et al. REM sleep behaviour disorder in Parkinson's disease: a questionnaire-based study. Neurol Sci. 2005;25:316–21. doi: 10.1007/s10072-004-0364-7. [DOI] [PubMed] [Google Scholar]
- 19.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 20.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Med. 2010;11:43–8. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen T, Svob C, Kuiken D. Dream-enacting behaviors in a normal population. Sleep. 2009;32:1629–36. doi: 10.1093/sleep/32.12.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen T, Paquette T. Dream-associated behaviors affecting pregnant and postpartum women. Sleep. 2009;30:1162–9. doi: 10.1093/sleep/30.9.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derry C, Harvey A, Walker M, Duncan J, Berkovic S. NREM arousal parasomnias and their distinction from nocturnal frontal lobe epilepsy: a video EEG analysis. Sleep. 2009;32:1637–44. doi: 10.1093/sleep/32.12.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–52. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 25.Schenck C, Boyd J, Mahowald M. A parasomnia overlap disorder involving sleepwalking, sleep terrors, and REM sleep behavior disorder in 33 polysomnographically confirmed cases. Sleep. 1997;20:972–81. doi: 10.1093/sleep/20.11.972. [DOI] [PubMed] [Google Scholar]
- 26.Harris M, Grunstein R. Treatments for somnambulism in adults: Assessing the evidence. Sleep Med Rev. 2009;13:295–7. doi: 10.1016/j.smrv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Bakwin H. Sleep-walking in twins. Lancet. 1970;2:446–7. doi: 10.1016/s0140-6736(70)90058-9. [DOI] [PubMed] [Google Scholar]
- 28.Kales A, Soldatos CR, Bixler EO, et al. Hereditary factors in sleepwalking and night terrors. Br J Psychiatry. 1980;137:111–8. doi: 10.1192/bjp.137.2.111. [DOI] [PubMed] [Google Scholar]
- 29.Abe K, Oda N. Contributions of genetic studies to clinical psychiatry. Jpn J Psychiatry Neurol. 1991;45:819–23. doi: 10.1111/j.1440-1819.1991.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 30.Gregory A. A genetic decomposition of the association between parasomnias and dyssomnias in 8-year-old twins. Arch Pediatr Adolesc Med. 2008;162:299–304. doi: 10.1001/archpedi.162.4.299. [DOI] [PubMed] [Google Scholar]
- 31.Licis A, Desruisseau D, Yamada K, Duntley S, Gurnett C. Novel genetic findings in an extended family pedigree with sleepwalking. Neurology. 2011;76:49–52. doi: 10.1212/WNL.0b013e318203e964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS-A score (behaviors, x axis, ranging from 0 to 34) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ST, the patients with RBD and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS-B score (frequency, x axis, ranging from 0 to 6) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ST, the patients with RBD and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.
Distribution (frequency histograms, y axis displaying the relative frequency within each group) of the total PADSS-C score (consequences of SW/ST, x axis, ranging from 0 to 10) in the different groups, ranging from the upper to the lower values, the patients with sleepwalking and/or sleep terror (SW/ST), the subjects with former SW/ ST, the patients with RBD and the normal control patients. PADSS, Paris Arousal Disorders Severity Scale; RBD, rapid eye movement sleep behavior disorder.


