Abstract
Newborn screening (NBS) is a public health program aimed at identifying treatable conditions in presymptomatic newborns to avoid premature mortality, morbidity, and disabilities. Currently, every newborn in the Unites States is screened for at least 29 conditions where evidence suggests that early detection is possible and beneficial. With new or improved treatment options and development of high-throughput screening tests, additional conditions have been proposed for inclusion into NBS programs. Among those are several conditions with a strong neuronopathic component. Some of these conditions have already been added to a few national and international screening programs, whereas others are undergoing pilot studies to determine the test performance metrics. Here, we review the current state of NBS for 13 lysosomal storage disorders, X-adrenoleukodystrophy, Wilson disease, and Friedreich ataxia.
Keywords: dried blood spots, newborn screening, tandem mass spectrometry, immuno-quantification, lysosomal storage disorder, peroxisomal disorders
INTRODUCTION
In the early 1960s, Robert Guthrie developed an assay for the presymptomatic identification of phenylketonuria, an inborn error of amino acid metabolism which causes irreversible neurological damage unless treatment is initiated within the first few weeks of life. The assay was a simple and inexpensive bacterial inhibition assay for the detection of abnormally elevated concentrations of phenylalanine in blood that was collected from newborns by heel stick and dried on filter paper [Guthrie, 1963, 1996]. Since Guthrie’s pioneering studies, newborn screening (NBS) emerged as a public health program aimed at the identification of an increasing number of conditions for which early intervention can prevent premature mortality, morbidity, and disabilities. Each condition added to the screening panel required a dedicated screening assay until tandem mass spectrometry (MS/MS) was adapted for NBS in the 1990s. This technology allowed for rapid and simultaneous analysis of amino acid and acylcarnitine profiles, thereby facilitating the identification of more than 40 different inborn errors of amino acid, fatty acid, and organic acid metabolism from a single 3-mm dried blood spot (DBS) sample [Chace et al., 2002; Turgeon et al., 2008]. Over the next decade, the slow implementation of this new technology led to significant discrepancies of the number of conditions included in the various NBS programs. This situation was improved in 2006, following the recommendation by a Newborn Screening Expert Group convened by the American College of Medical Genetics (ACMG) that every newborn should be screened for at least 29 core conditions [Watson et al., 2006]. In acknowledgement of the fact that screening tests do not determine disease status—rather, they measure the analytes that, in most cases, are not specific for a particular disease—the ACMG report includes 25 more conditions which are either of uncertain clinical significance or are untreatable, but on the basis of screening results are considered in the differential diagnosis of the core conditions.
NBS FOR LYSOSOMAL STORAGE DISORDERS
Several lysosomal storage disorders (LSDs), including mucopolysaccharidosis type I (MPS I; alpha-L-iduronidase deficiency; OMIM #607014), Fabry disease (alpha-galactosidase A deficiency; OMIM #301500), Pompe disease (α-glucosidase [GAA] deficiency; OMIM #232300), and Krabbe disease (gal-actocerebrosidase deficiency; OMIM #245200) were also considered by the ACMG Newborn Screening Expert Group because of increasing availability of treatment options including enzyme replacement therapy and bone marrow transplantation, both of which appear to be particularly effective when initiated early in life [Escolar et al., 2005; Urbanelli et al., 2011]. Other therapeutic approaches, such as chemical chaperone therapy, substrate reduction therapy, gene therapy, and stop-codon read-through were used in clinical trials or were under development [Beck, 2010; Schiffmann, 2010; Urbanelli et al., 2011]. An additional argument for inclusion of at least some LSDs has been the relative prevalence of these conditions. The overall incidence of LSDs has been reported in one study to be as high as one in 5,200 [Sanderson et al., 2006] but pilot NBS studies in Italy and Taiwan for Fabry disease alone revealed an unexpectedly high incidence of one case in approximately 3,100 and 1,250 male newborns, respectively [Spada et al., 2006; Hwu et al., 2009], which is more than five times higher than that of phenylketonuria. Nevertheless, no LSD was considered appropriate for inclusion in NBS programs because a proven high-throughput NBS assay was not yet available at the time [Watson et al., 2006].
However, NBS for Pompe and then Fabry diseases was introduced in screening centers in Taiwan, which together cover approximately 90% of the country’s newborn population [Chien et al., 2008; Hwu et al., 2009; Lin et al., 2009]. All programs applied modified fluorometric assays based on the methods originally described by Chamoles and colleagues [Chamoles et al., 2001, 2004; Niizawa et al., 2005]. One program applied a tiered approach where samples with either low GAA (deficient in Pompe disease) or low α-galactosidase A (GLA; deficient in Fabry disease) were additionally tested for a control enzyme, neutral glucosidase (NAG) or β-galactosidase (GLB), respectively [Chien et al., 2008; Hwu et al., 2009]. If either GAA or GLA was deficient, and the respective ratio (NAG/GAA or GLB/GLA) was elevated, the patient was recalled for a repeat DBS analysis and, if still abnormal, confirmatory testing was initiated that included enzyme assays using white blood cells. Published data from one program’s pilot study for Pompe disease involved 132,538 newborns of which 1,101 required follow-up and four were eventually diagnosed with the disease (Table 1). For Fabry disease, data from two screening programs are available, indicating a false-positive rate (FPR) of 0.52% and a positive predictive value (PPV) of 7% for the two-tiered approach [Hwu et al., 2009], whereas FPR and PPV were only 1.42 and 3%, respectively, for the single-enzyme assay [Lin et al., 2009] (Table 1).
Table 1.
FPRs (Detection Rates in Parenthesis) as Reported From Various NBS Pilot Studies (IL, WA, Austria) and a NBS Program in Taiwan
| Illinoisa (Digital Microfluidics) | Washingtonb (LC-MS/MS) | Austria (LC-MS/MS) | Taiwan (Fluorometry) | |
|---|---|---|---|---|
| Fabry | 0.05%b (1:1,144) | 0.005%b (1:16,667) | 0.055%b (1:3,859) | 0.87%b (1:1,2410 |
| Gaucher | 0.25%b (1:4,006) | – | 0.006%b (1:17,368) | – |
| Krabbe | – | – | – | – |
| MPS I | – | 0.005%b (1:30,000) | – | – |
| NPA/B | – | – | 0.003% (Not detected) | – |
| Pompe | 0.025% | 0.013%b (1:30,000) | 0.006%b (1:8,6840 | 0.83%b (1:33,135) |
PPV, 64%;
PPV, 50%;
PPV, 32%;
Data combined from two separate studies (PPV, 5%) [Hwu et al., 2009; Lin et al., 2009];
PPV, 9%;
PPV, 50%;
PPV, 40%;
PPV, 20%.;
PPV, 80%;
PPV, 0.36%.
New or improved analytical methods have been developed in recent years that not only allow for high-throughput screening but also for the simultaneous screening of several conditions. Particularly noteworthy are the approaches proposed by groups led by Michael Gelb (University of Washington, Seattle, WA), John Hopwood (Women and Children’s Hospital, Adelaide, Australia), and Advanced Liquid Logic (Cary, NC); each group applied different analytical platforms [Gelb et al., 2006; Meikle et al., 2006; Sista et al., 2011].
Based on Chamoles’ concept of determining enzyme activity in DBS, Gelb and coworkers developed an MS/ MS-based assay that allows for the simultaneous determination of the activity of several lysosomal enzymes [Gelb et al., 2006]. This method requires aliquotting of DBS extracts for separate 24-hr incubations with enzyme-specific substrates, followed by solid-phase extraction and pooling of aliquots for subsequent flow-injection MS/MS analysis to determine the concentration of each reaction product based on the intensity of internal standards. A modified version of this assay was implemented in 2006 by New York’s NBS program for the detection of Krabbe disease [Orsini et al., 2009]. To ensure as low as possible the number of false-positive results for this devastating neurodegenerative condition, a second tier molecular genetic assay that assesses the entire coding region of GALC, the gene for galactocerebrosidase, for mutations including a common 30-kb deletion was applied. This approach aimed to guarantee timely bone marrow transplantation among newborns with low galactocerebrosidase activity and at least one mutation detected. Among the first 550,000 newborns screened, 25 required clinical follow-up. A more recent report on now more than 1.2 million newborns screened indicates that early onset Krabbe disease was confirmed in four infants (1:300,000) and 24 cases (6 “high” and 18 “moderate” risk) are awaiting a final determination of being either simple carriers for Krabbe disease or affected with a later onset, milder variant of the condition [Orsini et al., 2011]. Data on the overall FPR, the PPV, the need for second tier molecular testing, and the impact on families confronted with abnormal screening results in their newborns have not been reported to date. However, the New York laboratory has also developed a method to measure psychosine in DBS which may be a possibly effective second tier assay to improve screening performance [Orsini et al., 2011].
To streamline the sample preparation steps, further modifications of the MS/MS-based method to include an online separation of analytes by liquid chromatography (LC) coupled to the tandem mass spectrometer (LC-MS/MS) were developed by the groups of Gelb [Spacil et al., 2011] and Kasper [Kasper et al., 2010]. The former assay for three LSDs (Pompe, Fabry, MPS I) is currently under evaluation with deidentified NBS samples in a prospective study in Washington [Scott et al., 2012]. Abnormal results are followed up by molecular genetic analysis to confirm or exclude a presumptive diagnosis. Preliminary data suggest an overall FPR of 0.023% (Table 1).
The assay developed by Kasper and colleagues for the measurement of four enzyme activities was validated in a prospective study of deidentified NBS samples in Austria [Mechtler et al., 2012]. Over a 6-month period, nearly 35,000 samples were tested for the enzymes deficient in Pompe, Fabry, Gaucher, and Niemann–Pick A/B diseases. When an abnormally low enzyme activity was measured, respective samples were submitted for molecular genetic analysis of the relevant gene to confirm the presumptive diagnosis. Based on their findings, the authors reported an overall FPR of 0.07% and a PPV of 40% (Table 1).
Hopwood and colleagues chose a different multiplex approach, taking advantage of the fact that in most LSDs the pathogenic mutations lead to a decreased amount of protein as well as low enzyme activity [Parkinson-Lawrence et al., 2006]. Applying microbead array technology, a multiplex assay system was developed for immune quantification of 11 proteins in a single-blood spot analysis [Meikle et al., 2006]. The goal was to enable the positive identification of 11 different conditions by overnight extraction of the proteins from a DBS, followed by a 16-hr incubation of the extract with capture beads and reporter antibodies. After washing, resuspension and short incubation in a buffer containing streptavidin–phycoerythrin conjugate, the captured proteins are fluorometrically quantified by comparison to a standard curve. This assay was recently modified by Hopwood’s group to include the measurement of 14 proteins to better delineate the same 11 conditions [Fuller et al., 2011] and by the authors’ laboratory to include 17 proteins to allow for the additional detection of Krabbe disease and nonlysosomal disorders, Wilson disease (WD; #277900), and Friedreich ataxia (FRDA; #229300) (Table 2).
Table 2.
Comparison of the Multiplexing Potential of Various NBS Assays Proposed for Specific LSDs, XALD, WD, and FRDA
| Condition | FIA-MS/MS | LC-MS/MSb | LC-MS/MSc | LC-MS/MSd | Immuno capture | DMe |
|---|---|---|---|---|---|---|
| Fabry disease | + | + | + | + | ||
| Gaucher disease | + | + | + | + | ||
| Krabbe disease | + | + | + | |||
| MLD | + | |||||
| MPS I | + | + | + | + | ||
| MPS II | + | + | ||||
| MPS IIIA | + | |||||
| MPS IIIB | + | |||||
| MPS IV | + | |||||
| MPS VI | + | + | ||||
| ML II/III | + | |||||
| NPA/B | + | + | + | + | ||
| Pompe disease | + | + | + | + | ||
| FRDA | + | |||||
| WD | + | + | ||||
| aCPa | + | + | ||||
| Menke diseasea | + | + | ||||
| AT-1 deficiencya | + | + | ||||
| XALD | + | + | ||||
| ZSDa | + | + | ||||
| Acyl-CoA oxidase def.a | + | + | ||||
| Bifunctional protein deficiencya | + | + | ||||
| Number of conditions | 10 | 9 | 4 | 4 | 17 | 5 |
Conditions not considered primary targets by proponents.
Abbreviations: aCP, aceruloplasminemia; AT-1, acetyl-CoA transporter; MLD, metachromatic leukodystrophy; MPS, mucopolysaccharidosis; ML, mucolipidosis; NPA/B, Niemann–Pick A/B; FRDA, Friedreich ataxia; WD, Wilson disease; XALD, X-adrenoleukodystrophy; ZSD, Zellweger spectrum disorders.
As recently reported, Hopwood and colleagues were able to correctly identify all but one patient; the false-negative result was observed in a patient with MPS II (iduronate-2-sulfatase deficiency; OMIM #309900) [Fuller et al., 2011]. Furthermore, the identification of patients with Gaucher disease (β-glucosidase deficiency; OMIM #230800) was less reliable when considering β-glucosidase alone, as opposed to additional measurement of chitotriosidase which typically is elevated in patients with this condition. However, because chitotriosidase is deficient in 6% of the healthy population, it may be necessary to measure additional proteins such as glucosylceramide and ceramide which are also abnormally abundant in Gaucher disease [Chamoles et al., 2002; Groener et al., 2008; Wajner et al., 2007].
The third multiplex approach to NBS for LSDs was developed by Advanced Liquid Logic (ALL) [Sista et al., 2011]. Also, following Chamoles’ basic concept of measuring activities of enzymes extracted from DBS with fluorometry, the novelty of ALL’s method lies in its ability to utilize minimal sample (DBS extract, 3.4 μL) and reagent volumes that are applied on disposable cartridges for 48 samples and analyzed on a small table-top instrument. Preparation of 48 samples can be accomplished within 1 hr and analysis requires 2.5 hr, making this assay the fastest among those described. This “digital microfluidics” (DM) assay was applied in a pilot study including several birthing centers in Chicago, IL. In total, 8,012 newborns were screened for Pompe, Fabry, and Gaucher diseases before the study was terminated after 5 months reportedly because of problems in scaling up the assay for high-throughput testing (Table 1) [Burton, 2012].
NBS FOR X-ADRENOLEUKODYSTROPHY
X-adrenoleukodystrophy (XALD) was also considered in the selection of conditions for the uniform NBS panel, but primarily because of the lack of a validated screening test, it was not included. There were also concerns about the available treatment options and about the manner of selecting patients for the different therapeutic interventions [Watson et al., 2006].
Adrenocortical insufficiency is one of the most frequent symptoms affecting XALD patients of all ages for which biochemical evidence can be apparent already in the first year of life [Dubey et al., 2005]. Adrenal steroid supplementation is an effective and life-saving approach for all those individuals who show biochemical abnormalities and subclinical signs of adrenocortical insufficiency. Mineralocorticoid replacement is not always required as is glucocorticoid therapy. Although hormone replacement therapy is mandatory in affected patients, it is well known that it affects neither the development nor the progression of neurological symptoms [Moser et al., 2000]. Hematopoietic stem cell transplantation (HSCT) is the only proven successful treatment for the cerebral form of XALD [Peters et al., 2004; Miller et al., 2011], either in the traditional approach or as vector for gene therapy [Cartier et al., 2009]. In fact, among inborn errors of metabolism, XALD is the second most common indication for HSCT. However, it is also well established that the timing of any intervention, as well as appropriate case selection, is extremely important. Similar to early onset Krabbe disease, a serious limitation of HSCT in ALD is the timing of the procedure. Once cerebral demyelination has started, HSCT not only is unlikely to be successful, but it may even accelerate neurologic decline, sometimes over a very short period of time [Cartier and Aubourg, 2010]. Accordingly, for HSCT to be successful, it must be performed while the patient has no neurological symptoms, no significant neuropsychological deficits, and very limited cerebral demyelination as determined by magnetic resonance imaging (MRI). These requirements reduce the number of subjects eligible for the procedure to mostly siblings of index cases. NBS for XALD has the potential of improving this situation as it would allow for the detection of patients prior to the onset of CNS involvement.
The biochemical marker of XALD is a high level of saturated very long chain fatty acids (VLCFA) in blood, particularly of hexacosanoic acid (C26:0). However, depending on the fiber source, VLCFA are present in variable amounts in the wax esters found in the filter paper used for NBS. Furthermore, contamination with VLCFA can occur via fingerprints, bacteria, and fungi [Jakobs et al., 1993; Johnson, 2000]. This prohibits the application of the well-established methods for free VLCFA quantitation in plasma to DBS. In 2006, it was shown that C26:0 lysophosphatidylcholine (C26:0 LPC) can be elevated in blood spots of patients affected with XALD and Zellweger spectrum disorders, as well as approximately 80% of females carrying one mutation in the ABCD1 gene, which is defective in XALD [Hubbard et al., 2009]. Furthermore, LPC can be measured by LC-MS/MS, but the liquid chromatographic separation of four LPCs (C20:0, C22:0, C24:0, and C26:0) required a 13-min per-sample analysis, rendering this approach ineffective for NBS [Hubbard et al., 2006]. Improvement of the assay allowed reduction of the analytical time, first to 6.5 min per sample and, more recently, to 2 min per sample, which is sufficient for high-throughput testing [Hubbard et al., 2009; Sandlers et al., 2012]. This assay is currently under review in a pilot study in Maryland, but data on test performance have not yet been reported. The latest version of the assay also allows for the extraction of acylcarnitines along with the LPCs which suggests a reduction of DBS sample requirements [Sandlers et al., 2012]. However, owing to the fact that acylcarnitines are typically analyzed simultaneously with amino acids, and from the same DBS extract, this assay does not constitute a real advantage. A combined extraction of acylcarnitines with LPC does not translate into a more efficient workflow and does not reduce the requirement for additional and costly LC-MS/MS equipment in any NBS laboratory that screens more than approximately 50,000 newborns per year. A different approach taken by the authors’ laboratory was to develop a FIA-MS/MS assay that not only allows for rapid measurement of LPCs in DBS but also simultaneously measures six lysosomal enzyme activities during the same analysis [Tortorelli S et al., manuscript in preparation] (Table 2).
NBS FOR WILSON DISEASE
Wilson disease (WD) is an autosomal recessive disorder of copper transport caused by mutations in ATP7B. It results in copper accumulation in the liver as well as in the central nervous system. The prevalence of WD is considered to be approximately 1 in 30,000 newborns and affected individuals usually present in the first or second decade of life with chronic hepatitis and cirrhosis or acute liver failure. Neuropsychiatric symptoms are more common in adults and include dystonia, tremor, personality changes, and cognitive impairment. The disease is progressive and ultimately fatal if untreated, and it is one of the most frequent causes of chronic liver disease in children [Mak and Lam, 2008]. The diagnosis is established based on high clinical suspicion and biochemical testing. Molecular genetic testing, such as full gene sequencing, is not a first line diagnostic test but an effective confirmatory test that also helps to identify affected siblings of probands, including those without definitive symptoms. WD is easily treatable using chelating agents and serious symptoms can be avoided if an early diagnosis is made. Patients with WD and liver cirrhosis or neurological deficits often have a poor prognosis. Given the availability of inexpensive and effective treatment of patients identified prior to the development of irreversible symptoms, population screening for WD has been considered and few pilot studies were published measuring ceruloplasmin (CP) in infants or children [Hahn et al., 2002; Mak and Lam, 2008]. Until recently, CP was considered a poor marker in neonates because it was thought to be physiologically low. Given this understanding, it is not surprising that WD has not been recommended for NBS by the ACMG Newborn Screening Expert Group. However, applying a sandwich ELISA assay, Hahn and coworkers were able to show that CP was markedly reduced in the original NBS blood spots of two patients with WD compared to matched controls [Kroll et al., 2006]. In an additional study, this assay was applied in the authors’ laboratory to screen 40,000 deidentified NBS samples prospectively and with a preliminary FPR of 0.3% (unpublished data). Among those samples with low CP levels, three cases were found to be carriers for ATP7B mutations. One case with immeasurable CP was found to carry a novel mutation. Whether these patients are truly simple carriers or actual WD patients, where the second mutation remains elusive, is impossible to ascertain, given that the study was conducted on deidentified specimens. Furthermore, it is possible that these patients may be affected with aceruloplasminemia (OMIM #604290), Menkes disease (OMIM #309400), or other inborn errors affecting copper metabolism that present with low CP concentrations, such as the recently described acetyl-CoA transporter (AT-1) deficiency owing to mutations in SLC33A1 (OMIM *603690) [Huppke et al., 2012]. An LC-MS/MS assay for the measurement of peptides derived from CP following trypsin digestion was reported in 2008 [deWilde et al., 2008]. However, due to the 7.5 min necessary for the chromatographic separation of CP-specific peptides, this method is not amenable to high-throughput testing because only eight samples could be analyzed per hour on a single LC-MS/MS system.
NBS FOR FRIEDREICH ATAXIA
Friedreich ataxia (FRDA) is an autosomal recessive disease estimated to affect 1:40,000 individuals. Clinically, it is characterized by progressive ataxia of all extremities, cerebellar dysarthria, absent reflexes in the lower extremities, sensory loss, and pyramidal signs [Harding, 1981; Koeppen, 2011]. Hypertrophic cardiomyopathy is found in most patients and represents the most frequent cause of premature death. Diabetes mellitus develops in approximately 30% of patients. Onset of symptoms can vary from childhood to adulthood. The FRDA locus encodes a precursor poly-peptide that is imported by the mitochondria and processed to the mature protein designated frataxin [Koeppen, 2011]. In patients with FRDA, frataxin is reduced to <30% of normal [Campuzano et al., 1997]. FRDA is currently diagnosed by molecular genetic methods. Effective therapy is not yet available, but several clinical treatment trials are underway, examining the potential of antioxidants (i.e., idebenone), iron chelators (i.e., deferiprone) as well as strategies to increase frataxin levels by inducing gene expression [Schulz et al., 2009; Tsou et al., 2009]. As is the case with other progressive conditions, there is reason to believe that initiation of treatment as early as possible has the greatest benefit and likelihood of reducing, if not preventing morbidity and mortality. Given the fact that most patients are homozygous for an expansion of a GAA trinucleotide repeat in intron 1 of the gene that encodes frataxin, molecular genetic testing appears to be a reasonable approach to NBS for FRDA. Unfortunately, molecular genetic testing for FRDA is patented (Athena Diagnostics) and the methodology used for DNA analysis is not amenable to incorporate into a multiplex assay. Therefore, our laboratory has developed and validated an immunocapture assay that measures the presence of frataxin in DBS and is included in the multiplex assay for the abovementioned LSDs.
MAYO CLINIC AND NBS FOR LSD AND OTHER CONDITIONS
With different analytical platforms proposed for the identification of LSDs, XALD, and WD from blood spots, the question arises as to which method should be used for high-throughput NBS. Unfortunately, a straightforward answer is not available. The choice may depend on whether the intension is to screen for one or more conditions, whether a high-complexity testing platform can be accommodated by the laboratory, and whether there is sufficient space and funding available. Moreover, and perhaps most importantly, the choice may depend on the analytical robustness, capacity, and performance of a particular assay for a condition or group of conditions. For instance, based on the assay performance, it may be necessary to apply second tier testing to reduce the FPR in a manner similar to Krabbe screening in New York [Kemper et al., 2010]. Any additional test, however, adds to the laboratory’s cost, space requirements, and turnaround time. As NBS panels are likely to expand to include additional conditions, the methods that can detect multiple conditions from a single blood spot with a single analysis are more desirable as they make better use of the limited number of blood spots available for screening. The MS/MS-based screening method proposed by Gelb’s group, the immunoquantification method proposed by Hopwood’s group, and ALL’s digital microfluidics assay are multiplex methods. The advantage of the immunoquantification over the other two methods may be the larger number of conditions currently included in the analysis. Both the immunoquantification and the ALL methods are characterized by lower equipment costs and space requirement, and, perhaps most importantly, the low complexity of the technology applied. The latter may allow for consistent and reliable results between screening laboratories as it is less dependent on user experience, which has been an obstacle of excellent performance in MS/MS-based NBS [Rinaldo et al., 2006; McHugh et al., 2011].
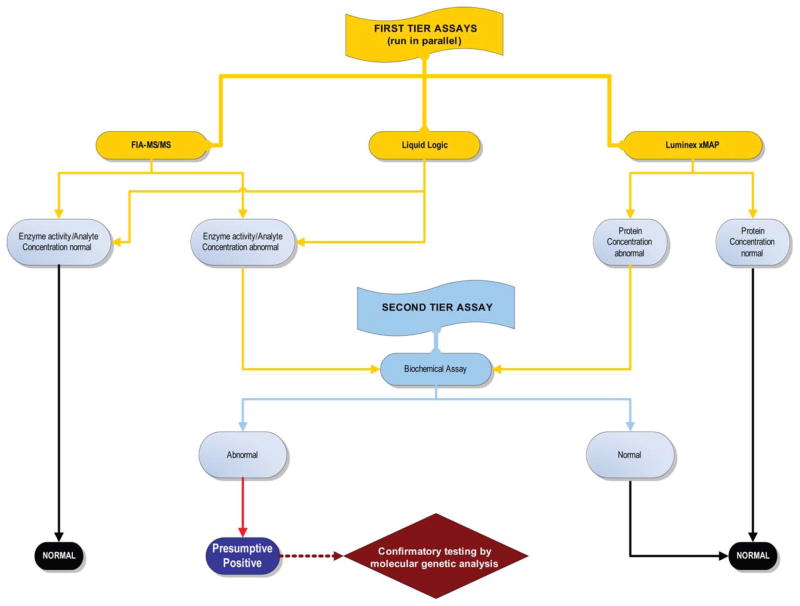
To determine the most effective strategy for NBS for LSDs, XALD, WD, and FRDA, in September 2011, Mayo Clinic’s Biochemical Genetics Laboratory began a comprehensive, prospective study to screen 100,000 deidentified NBS samples over a 2-year period using three multiplex methods (immunoquantification, FIA-MS/MS, and digital microfluidics) in parallel. Our goal is to identify all patients who could, in theory, benefit from early initiation of treatment while achieving the lowest possible FPR. The latter seems important because the studies on the impact of presumptive positive results revealed that, while long-term outcome is improved for truly affected patients, those who receive false-positive results may be at higher risk of dysfunctional parent–child relationships [Waisbren et al., 2003; Timmermans and Buchbinder, 2010; Schmidt et al., 2012]. Certainly, this would hold true where conditions such as LSDs are concerned as they are not only associated with potentially severe outcomes but may also require invasive and/or highly expensive lifelong treatment. Therefore, our study’s goal is to develop an effective and efficient NBS approach for these conditions by comparison of the different screening technologies (Table 2 and Fig. 1). All samples with abnormal results will be further evaluated by specific fluorometric enzyme assays and/or molecular genetic analysis to confirm or exclude abnormal screening results.
Fig. 1.
Testing strategy of a NBS study using deidentified samples to determine an effective screening approach for the conditions listed in Table 2. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
CONCLUSIONS
Although NBS studies for LSDs, XALD, WD, and FRDA are planned and conducted, the discussion of which of these conditions should be included in a NBS panel is likely to continue on all levels. Patient support groups continue to provide their input and influence on the state and federal level. The Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC; http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/index.html), which is tasked with advising the US Secretary of the Department of Health and Human Services on the composition of the universal NBS panel, already received and reviewed proposals for inclusion of Krabbe, Pompe, Fabry, and Niemann–Pick A/B diseases. None of these conditions has yet been endorsed because of concerns that still need to be addressed, such as the cost of a successful screening strategy (high sensitivity, high PPV, low FPR), the risks and cost of treatment, and questions about the appropriate time to initiate treatments in patients with milder or late-onset disease variants. Nevertheless, New York has already added Krabbe disease in 2006, and three other states (Illinois, Missouri, and New Mexico) have taken action independently as they are required by their state’s law to start NBS for up to five LSDs within the next few years. These activities should help provide an answer to the question whether screening for these conditions can be more widely recommended. Given these realities, the ACMG has already formed a workgroup to develop follow-up guidelines (ACTion sheets) and algorithms for the laboratory evaluation of each LSD suggested for NBS [Wang et al., 2011].
Acknowledgments
Grant sponsor: The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services; Grant number: #HHSN275201000017C; Grant sponsor: The Newborn Screening Translational Research Network; Grant number: #HHSN275200800001C 01; Grant sponsor: The Friedreich Ataxia Research Association (FARA).
The authors acknowledge a generous gift from The Legacy of Angels Foundation.
References
- Beck M. Therapy for lysosomal storage disorders. IUBMB Life. 2010;62:33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- Burton B, Charrow J, Angle B, et al. A pilot newborn screening study for lysosomal storage disorders (LSD) in Illinois. Mol Genet Metab. 2012;105:S23–S24. [Google Scholar]
- Burton BK. Newborn screening for Pompe disease: an update, 2011. Am J Med Genet C Semin Med Genet. 2012;160:8–12. doi: 10.1002/ajmg.c.31315. [DOI] [PubMed] [Google Scholar]
- Campuzano V, Montermini L, Lutz Y, et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet. 1997;6:1771–1780. doi: 10.1093/hmg/6.11.1771. [DOI] [PubMed] [Google Scholar]
- Cartier N, Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:857–862. doi: 10.1111/j.1750-3639.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Chace DH, Kalas TA, Naylor EW. The application of tandem mass spectrometry to neonatal screening for inherited disorders of intermediary metabolism. Annu Rev Genomics Hum Genet. 2002;3:17–45. doi: 10.1146/annurev.genom.3.022502.103213. [DOI] [PubMed] [Google Scholar]
- Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308:195–196. doi: 10.1016/s0009-8981(01)00478-8. [DOI] [PubMed] [Google Scholar]
- Chamoles NA, Blanco M, Gaggioli D, et al. Gaucher and Niemann-Pick diseases—enzymatic diagnosis in dried blood spots on filter paper: retrospective diagnoses in newborn-screening cards. Clin Chim Acta. 2002;317:191–197. doi: 10.1016/s0009-8981(01)00798-7. [DOI] [PubMed] [Google Scholar]
- Chamoles NA, Niizawa G, Blanco M, et al. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clin Chim Acta. 2004;347:97–102. doi: 10.1016/j.cccn.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Chien YH, Chiang SC, Zhang XK, et al. Early detection of pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39–45. doi: 10.1542/peds.2007-2222. [DOI] [PubMed] [Google Scholar]
- deWilde A, Sadilkova K, Sadilek M, et al. Tryptic peptide analysis of ceruloplasmin in dried blood spots using liquid chromatography-tandem mass spectrometry: application to newborn screening. Clin Chem. 2008;54:1961–1968. doi: 10.1373/clinchem.2008.111989. [DOI] [PubMed] [Google Scholar]
- Dubey P, Raymond GV, Moser AB, et al. Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr. 2005;146:528–532. doi: 10.1016/j.jpeds.2004.10.067. [DOI] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- Fuller M, Tucker JN, Lang DL, et al. Screening patients referred to a metabolic clinic for lysosomal storage disorders. J Med Genet. 2011;48:422–425. doi: 10.1136/jmg.2010.088096. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Turecek F, Scott CR, et al. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J Inherit Metab Dis. 2006;29:397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groener JE, Poorthuis BJ, Kuiper S, et al. Plasma glucosylceramide and ceramide in type 1 Gaucher disease patients: correlations with disease severity and response to therapeutic intervention. Biochim Biophys Acta. 2008;1781:72–78. doi: 10.1016/j.bbalip.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Guthrie R. Phenylketonuria screening programs. N Engl J Med. 1963;269:52–53. [Google Scholar]
- Guthrie R. The introduction of newborn screening for phenylketonuria. A personal history. Eur J Pediatr. 1996;155:S4–S5. doi: 10.1007/pl00014247. [DOI] [PubMed] [Google Scholar]
- Hahn SH, Lee SY, Jang YJ, et al. Pilot study of mass screening for Wilson’s disease in Korea. Mol Genet Metab. 2002;76:133–136. doi: 10.1016/s1096-7192(02)00026-4. [DOI] [PubMed] [Google Scholar]
- Harding AE. Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intra-familial clustering of clinical features. Brain. 1981;104:589–620. doi: 10.1093/brain/104.3.589. [DOI] [PubMed] [Google Scholar]
- Hubbard WC, Moser AB, Liu AC, et al. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) method. Mol Genet Metab. 2009;97:212–220. doi: 10.1016/j.ymgme.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Hubbard WC, Moser AB, Tortorelli S, et al. Combined liquid chromatography-tandem mass spectrometry as an analytical method for high throughput screening for X-linked adrenoleukodystrophy and other peroxisomal disorders: preliminary findings. Mol Genet Metab. 2006;89:185–187. doi: 10.1016/j.ymgme.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Huppke P, Brendel C, Kalscheuer V, et al. Mutations in SLC33A1 cause a lethal autosomal-recessive disorder with congenital cataracts, hearing loss, and low serum copper and ceruloplasmin. Am J Hum Genet. 2012;90:61–68. doi: 10.1016/j.ajhg.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu WL, Chien YH, Lee NC, et al. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A) Hum Mutat. 2009;30:1397–1405. doi: 10.1002/humu.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs C, van den Heuvel CM, Stellaard F, et al. Diagnosis of Zellweger syndrome by analysis of very long-chain fatty acids in stored blood spots collected at neonatal screening. J Inherit Metab Dis. 1993;16:63–66. doi: 10.1007/BF00711316. [DOI] [PubMed] [Google Scholar]
- Johnson DW. A rapid screening procedure for the diagnosis of peroxisomal disorders: quantification of very long-chain fatty acids, as dimethylaminoethyl esters, in plasma and blood spots, by electrospray tandem mass spectrometry. J Inherit Metab Dis. 2000;23:475–486. doi: 10.1023/a:1005612214179. [DOI] [PubMed] [Google Scholar]
- Kasper DC, Herman J, De Jesus VR, et al. The application of multiplexed, multidimensional ultra-high-performance liquid chromatography/tandem mass spectrometry to the high-throughput screening of lysosomal storage disorders in newborn dried bloodspots. Rapid Commun Mass Spectrom. 2010;24:986–994. doi: 10.1002/rcm.4496. [DOI] [PubMed] [Google Scholar]
- Kemper AR, Knapp AA, Green NS, et al. Weighing the evidence for newborn screening for early-infantile Krabbe disease. Genet Med. 2010;12:539–543. doi: 10.1097/GIM.0b013e3181e85721. [DOI] [PubMed] [Google Scholar]
- Koeppen AH. Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303:1–12. doi: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll CA, Ferber MJ, Dawson BD, et al. Retrospective determination of ceruloplasmin in newborn screening blood spots of patients with Wilson disease. Mol Genet Metab. 2006;89:134–138. doi: 10.1016/j.ymgme.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Lin HY, Chong KW, Hsu JH, et al. High incidence of the cardiac variant of Fabry Disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–456. doi: 10.1161/CIRCGENETICS.109.862920. [DOI] [PubMed] [Google Scholar]
- Mak CM, Lam CW. Diagnosis of Wilson’s disease: a comprehensive review. Crit Rev Clin Lab Sci. 2008;45:263–290. doi: 10.1080/10408360801991055. [DOI] [PubMed] [Google Scholar]
- McHugh DM, Cameron CA, Abdenur JE, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med. 2011;13:230–254. doi: 10.1097/GIM.0b013e31820d5e67. [DOI] [PubMed] [Google Scholar]
- Mechtler TP, Stary S, Metz TF, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet. 2012;379:335–341. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Grasby DJ, Dean CJ, et al. Newborn screening for lysosomal storage disorders. Mol Genet Metab. 2006;88:307–314. doi: 10.1016/j.ymgme.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118:1971–1978. doi: 10.1182/blood-2011-01-329235. [DOI] [PubMed] [Google Scholar]
- Moser HW, Loes DJ, Melhem ER, et al. X-Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31:227–239. doi: 10.1055/s-2000-9236. [DOI] [PubMed] [Google Scholar]
- Niizawa G, Levin C, Aranda C, et al. Retrospective diagnosis of glycogen storage disease type II by use of a newborn-screening card. Clin Chim Acta. 2005;359:205–206. doi: 10.1016/j.cccn.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Orsini JJ, Caggana M, Martin M, et al. Determination of psychosine in dried blood spots of Krabbe diseased patients. Mol Genet Metab. 2011;102:S32. [Google Scholar]
- Orsini JJ, Morrissey MA, Slavin LN, et al. Implementation of newborn screening for Krabbe disease: population study and cutoff determination. Clin Biochem. 2009;42:877–884. doi: 10.1016/j.clinbiochem.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Parkinson-Lawrence E, Fuller M, Hopwood JJ, et al. Immunochemistry of lysosomal storage disorders. Clin Chem. 2006;52:1660–1668. doi: 10.1373/clinchem.2005.064915. [DOI] [PubMed] [Google Scholar]
- Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- Rinaldo P, Zafari S, Tortorelli S, et al. Making the case for objective performance metrics in newborn screening by tandem mass spectrometry. Ment Retard Dev Disabil Res Rev. 2006;12:255–261. doi: 10.1002/mrdd.20130. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Green A, Preece MA, et al. The incidence of inherited metabolic disorders in the West Midlands, UK. Arch Dis Child. 2006;91:896–899. doi: 10.1136/adc.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandlers Y, Moser AB, Hubbard WC, et al. Combined extraction of acyl carnitines and 26:0 lysophosphatidylcholine from dried blood spots: prospective newborn screening for X-linked adrenoleukodystrophy. Mol Genet Metab. 2012;105:416–420. doi: 10.1016/j.ymgme.2011.11.195. [DOI] [PubMed] [Google Scholar]
- Schiffmann R. Therapeutic approaches for neuronopathic lysosomal storage disorders. J Inherit Metab Dis. 2010;33:373–379. doi: 10.1007/s10545-010-9047-0. [DOI] [PubMed] [Google Scholar]
- Schmidt JL, Castellanos-Brown K, Childress S, et al. The impact of false-positive newborn screening results on families: a qualitative study. Genet Med. 2012;14:76–80. doi: 10.1038/gim.2011.5. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Boesch S, Burk K, et al. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5:222–234. doi: 10.1038/nrneurol.2009.26. [DOI] [PubMed] [Google Scholar]
- Scott CR, Elliott S, Spacil Z, et al. A pilot program screening for Fabry, Pompe, and MPS I in a newborn screening laboratory: the first 60,000 samples. Mol Genet Metab. 2012;2:S56–S57. [Google Scholar]
- Sista RS, Eckhardt AE, Wang T, et al. Digital microfluidic platform for multiplexing enzyme assays: implications for lysosomal storage disease screening in newborns. Clin Chem. 2011;57:1444–1451. doi: 10.1373/clinchem.2011.163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacil Z, Elliott S, Reeber SL, et al. Comparative triplex tandem mass spectrometry assays of lysosomal enzyme activities in dried blood spots using fast liquid chromatography: application to newborn screening of Pompe, Fabry, and Hurler diseases. Anal Chem. 2011;83:4822–4828. doi: 10.1021/ac200417u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada M, Pagliardini S, Yasuda M, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans S, Buchbinder M. Patients-in-waiting: living between sickness and health in the genomics era. J Health Soc Behav. 2010;51:408–423. doi: 10.1177/0022146510386794. [DOI] [PubMed] [Google Scholar]
- Tsou AY, Friedman LS, Wilson RB, et al. Pharmacotherapy for Friedreich ataxia. CNS Drugs. 2009;23:213–223. doi: 10.2165/00023210-200923030-00003. [DOI] [PubMed] [Google Scholar]
- Turgeon C, Magera MJ, Allard P, et al. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin Chem. 2008;54:657–664. doi: 10.1373/clinchem.2007.101949. [DOI] [PubMed] [Google Scholar]
- Urbanelli L, Magini A, Polchi A, et al. Recent developments in therapeutic approaches for lysosomal storage diseases. Recent Pat CNS Drug Discov. 2011;6:1–19. doi: 10.2174/157488911794079127. [DOI] [PubMed] [Google Scholar]
- Waisbren SEP, Albers SMD, Amato SMD, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. J Am Med Assoc. 2003;290:2564–2572. doi: 10.1001/jama.290.19.2564. [DOI] [PubMed] [Google Scholar]
- Wajner A, Michelin K, Burin MG, et al. Comparison between the biochemical properties of plasma chitotriosidase from normal individuals and from patients with Gaucher disease, GM1-gangliosidosis, Krabbe disease and heterozygotes for Gaucher disease. Clin Biochem. 2007;40:365–369. doi: 10.1016/j.clinbiochem.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Wang RY, Bodamer OA, Watson MS, et al. Lysosomal storage diseases: diagnostic confirmation and management of presymptomatic individuals. Genet Med. 2011;13:457–484. doi: 10.1097/GIM.0b013e318211a7e1. [DOI] [PubMed] [Google Scholar]
- Watson MS, Lloyd-Puryear MA, Mann MY, et al. Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8:1S–252S. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]