Abstract
Modulation of miR-33 and miR-122 has been proposed to be a promising strategy to treat dyslipidemia and insulin resistance associated with obesity and metabolic syndrome. Interestingly, specific polyphenols reduce the levels of these mi(cro)RNAs. The aim of this study was to elucidate the effect of polyphenols of different chemical structure on miR-33a and miR-122 expression and to determine whether direct binding of the polyphenol to the mature microRNAs (miRNAs) is a plausible mechanism of modulation. The effect of two grape proanthocyanidin extracts, their fractions and pure polyphenol compounds on miRNA expression was evaluated using hepatic cell lines. Results demonstrated that the effect on miRNA expression depended on the polyphenol chemical structure. Moreover, miR-33a was repressed independently of its host-gene SREBP2. Therefore, the ability of resveratrol and epigallocatechin gallate to bind miR-33a and miR-122 was measured using 1H NMR spectroscopy. Both compounds bound miR-33a and miR-122 and differently. Interestingly, the nature of the binding of these compounds to the miRNAs was consistent with their effects on cell miRNA levels. Therefore, the specific and direct binding of polyphenols to miRNAs emerges as a new posttranscriptional mechanism by which polyphenols could modulate metabolism.
INTRODUCTION
Mi(cro)RNAs are short (22 nt) double-stranded regulatory noncoding RNAs and have emerged as critical regulators of gene expression at the posttranscriptional level (1,2). To date, thousands of miRNAs have been discovered, and it is thought that these small molecules may regulate >60% of all gene transcripts (3). Specifically, miR-33a/b (4,5) and miR-122 (5) have emerged as key regulators of genes involved in lipid metabolism in liver. miR-122 is liver specific and plays a critical role in liver homeostasis (6,7), and its inhibition has been associated with the deregulation of genes playing key roles in the control of liver lipid metabolism, such as fatty acid synthase (FAS). miR-33a/b plays an important role in the regulation of cholesterol homeostasis, regulating the ATP-binding cassette transporters (ABC transporters) ABCA1 and ABCG1, in addition to its role in fatty acid β-oxidation. miR-33a and b are intronic of the sterol response element protein 2 (SREBF2) and 1 (SREBF1) genes, respectively, and they are simultaneously cotranscribed (8). Increasing evidence indicates that deregulation of these miRNAs is related to metabolic diseases. Moreover, the modulation of miR-33 and miR-122 has been proposed to be a promising strategy to treat dyslipidemia and insulin resistance associated with obesity and metabolic syndrome.
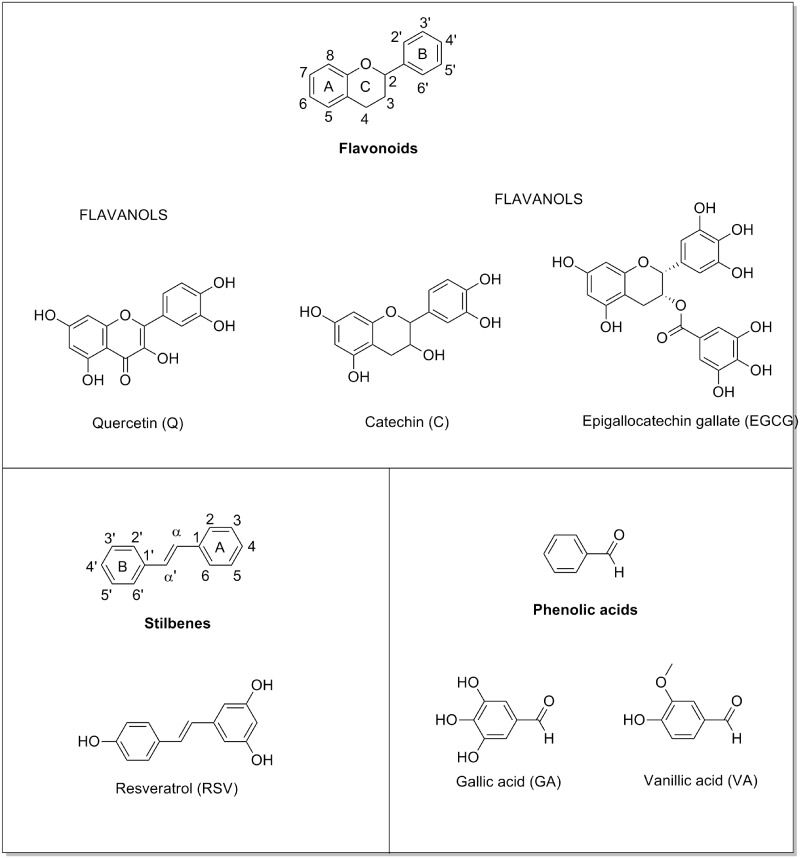
Dietary polyphenols are known to improve dyslipidemia (9,10) and insulin resistance (11–13) in rodents with metabolic syndrome. These compounds are present in most fruits and vegetables (14). The most abundant polyphenols in the human diet are the proanthocyanidins, a subclass of flavonoid. The basic structural skeleton of flavonoids contains 12 carbons composing two aromatic rings connected by a pyrone ring (Figure 1). Flavonoids are divided into six subclasses depending on the oxidation state of the pyrone ring and the connection of one aromatic ring with the pyrone ring: flavonols (quercetin, Q), flavanones, flavones, anthocyanidins, isoflavones and flavanols. Flavanols, such as epicatechin (EC), catechin (C) and epigallocatechin, can exist in a variety of oligomeric structures that differ in chain lengths, hydroxylation pattern, stereochemistries, interflavan linkages and whether they are esterified with gallic acid (GA) (15,16). Stilbenoids represent another type of polyphenols and include resveratrol (RSV), which is produced de novo by plants in response to stress factors, such as pathogen attack. The basic structural skeleton of RSV features a central carbon–carbon double bond conjugated with two phenolic moieties (17) (Figure 1). We have previously demonstrated that proanthocyanidis extracts repress miR-33 and miR-122 in liver of healthy (18) and obese (19) rats. However, the effect of each individual polyphenol and the exact mechanism by which these dietary compounds modulate miRNA expression remains unclear. Hence, the aim of this study was to elucidate the influence of the polyphenolic chemical structure on miR-33a and miR-122 expression in hepatic cells and to determine whether polyphenols can directly bind to mature miRNAs.
Figure 1.
Chemical structure of representative polyphenols.
MATERIALS AND METHODS
Chemicals and reagents
Grape seed proanthocyandin extract (GSPE) was kindly provided by Les Dérives Résiniques et Terpéniques (Dax, France), and its composition is described in Table 1. Grape pomace extract (GPE) and their monomeric (MF), dimeric (DF) and oligomeric (OF) fractions were kindly provided by Miguel Torres S.A. (Vilafranca del Penedès, Spain), and their compositions are also described in Table 1. (+)-C, (−)-EC, Q dehydrate, RSV, epigallocatechin gallate (EGCG), procyanidin B2, GA, vanillic acid (VA), m-hydroxyphenylacetic acid (m-HPAA) and 3′-hydroxybenzoic acid (HBA) were purchased from Sigma-Aldrich (Madrid, Spain). Organic solvents (high-performance liquid chromatography grade) were obtained from Scharlab (Barcelona, Spain) and Merck (Darmstadt, Germany).
Table 1.
Identified phenolic compounds and derivatives determined by reversed-phase HPLC-MS
| Phenolic compound | GSPE | GPE | MF | DF | OFa |
|---|---|---|---|---|---|
| Gallic acid | 15.1 ± 3.5 | 24.5 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Vanillic acid | 0.2 ± 0.05 | 0.03 ± 0.01 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Procyanidin dimerb | 82.2 ± 13.1 | 81.1 ± 5.5 | 5.0 ± 0.5 | 406.6 ± 50.9 | 0.0 ± 0.0 |
| Dimer B2b | 30.8 ± 8.3 | 25.8 ± 3.0 | 2.1 ± 0.5 | 133.5 ± 24.9 | 0.0 ± 0.0 |
| Catechin | 51.8 ± 14.9 | 97.5 ± 28.7 | 746.5 ± 30.1 | 10.4 ± 0.1 | 0.7 ± 0.02 |
| Epicatechin | 33.6 ± 12.7 | 37.7 ± 7.1 | 234.2 ± 5.4 | 4.2 ± 0.1 | 0.2 ± 0.0 |
| Gallate dimerb | 23.9 ± 7.4 | 34.5 ± 2.0 | 0.0 ± 0.0 | 107.7 ± 8.6 | 8.9 ± 2.4 |
| Epigallocatechin gallate | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 |
| Trimer C1 | 4.4 ± 1.0 | 4.8 ± 0.4 | 0.0 ± 0.0 | 19.4 ± 7.9 | 0.5 ± 0.1 |
| Epicatechin gallatec | 10.3 ± 5.5 | 3.3 ± 2.9 | 0.0 ± 0.0 | 17.2 ± 8.1 | 0.0 ± 0.0 |
| Quercetin | 0.2 ± 0.0 | 5.6 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Resveratrol | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
aFraction enriched in procyanidin oligomers with a mean degree of polymerization of 3.7, mean molecular weight of 1232 and percentage galloylation of 31%, as estimated by thioacidolysis and HPLC and described by Torres et al. (29).
bQuantified using the calibration curve of procyanidin B2.
cQuantified using the calibration curve of epigallocatechin gallate.
Values are expressed as mg compound/g extract.
Extracts characterization by high-performance liquid chromatography–mass spectrometry
Phenolic composition of extracts was quantified by high-performance liquid chromatography–mass spectrometry (HPLC-MS). Instrumental equipment included an Agilent 1200 HPLC series coupled to 6120 TOF mass detector (Agilent Technologies). Chromatographic separation was carried out in a Zorbax SB-Aq column (3.5 μm, 150 mm × 2.1 mm internal diameter) equipped with a Zorbax SB-C18 pre-Column (3.5 μm, 15 mm × 2.1 mm internal diameter), both from Agilent. Data analysis was done by using Masshunter software. Individual compounds were quantified by means of a six-point calibration curve by using standards (Supplementary TableS1).
Cell cultures
FAO cells, rat hepatoma cell line (ECACC, code 85061112), were grown in Nutrient Mixture F12 Coon’s Modification (F6636-10X1L, Sigma-Aldrich, Madrid, Spain) supplemented with gentamicin (50 μg/ml) (LONZA, Barcelona, Spain), polymyxin B (50 μg/ml) (Sigma-Aldrich, Madrid, Spain) and 10% fetal bovine serum (BioWhittaker, Cologne, Germany) in a humidified incubator with 5% CO2 at 37°C. HepG2 cells, human hepatocarcinoma cell line (ATCC, LGC Promochen, HB8065, Salisbury, UK), were grown in Dulbecco’s Modified Eagle Medium (Lonza, Barcelona, Spain) supplemented with 10% fetal bovine serum (Lonza, Barcelona, Spain), 0.1 mM nonessential amino acids (Sigma, Madrid, Spain), 100 U/ml penicillin and 100 mg/ml streptomycin (Lonza, Barcelona, Spain), 2 mM glutamine (Lonza, Barcelona, Spain) and 25 mM 4-(2)-Hydroxyethyl)piperazine-1-ethanesulfonic acid (Sigma, Madrid, Spain) in a humidified incubator with 5% CO2 at 37°C.
Once cells arrived to 80% of confluence, the media were replaced with serum-depleted media supplemented with 100 µM oleic acid (MERCK, Germany) and 40 µM bovine serum albumin (fatty acid free, Sigma-Aldrich, Madrid, Spain) for 15 h. Further, cells were treated with 25 mg/l of GSPE, GPE or MF, DF, OF or with 50 μM of Q, C, EC, RSV, EGCG, B2, HBA, m-HPPA, VA or GA. Cells were cultured for 1 h for miRNAs analysis or for 5 h for mRNA and proteins analysis. A stock solution in ethanol of extracts and compound were prepared previously to cell cultures studies to obtain in media a final ethanol concentration <0.05%.
RNA extraction
Cells were washed twice with phosphate buffered saline, and total RNA, containing small RNA species, was extracted using the mi/mRNA extraction kit (miRNA kit, E.Z.N.A., Omega Bio-tek, Norcros, USA) according to the manufacturer’s protocol. The RNA quality was checked using a NanoDrop 1000 Spectrophotometer (Thermo Scientific).
microRNA quantification by real-time qRT-PCR
Reverse transcription was performed using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Madrid, Spain). miRNA-specific reverse-transcription primers were provided by TaqMan® MicroRNA Assay (Applied Biosystems, Madrid, Spain). For the reverse transcription, a My Gene L Series Peltier Thermal Cycler (Long Gene) was used. The reaction was performed at 16°C for 30 min; 42°C for 30 min and 85°C for 5 min. Total RNA final concentration was 2.5 ng/µl (17.5 ng). A total of 1.33 µl of this diluted cDNA was used in a subsequent quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) amplification using TaqMan Universal PCR master mix (Applied Biosystems, Madrid, Spain) and the respective specific probe provided in the TaqMan® MicroRNA Assay (Applied Biosystems). Specific Taqman probes were as follows: microRNA-122 (miR-122: hsa-mir-122), 5′UGGAGUGUGACAAUGGUGUUUG-3′, and microRNA-33 (miR-33: hsa-mir-33), 5′- GUGCAUUGUAGUUGCAUUG-3′. The results were normalized with U6 small nuclear RNA (U6 snRNA), 5′-GTGCTCGCTTCGGCAGCACATATACTAAAATTGGAACGATACAGAGAAGATTAGCATGGCCCCTGCGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTT-3′, that was used as an endogenous control. Amplification was performed using the ABI Prism 7300 sodium dodecyl sulphate (SDS) Real-Time PCR system (Applied Biosystems, Madrid, Spain) at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The fold change in the miRNA level was calculated by the log 2 scale of the equation 2−ΔΔCt, where ΔCt = Ct miRNA − Ct U6 and ΔΔCt = ΔCt treated samples − ΔCt untreated controls.
mRNA quantification by quantitative reverse transcriptase polymerase chain reaction
mRNA levels were evaluated by reverse transcription performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). For the reverse transcription, a My Gene L Series Peltier Thermal Cycler (Long Gene) was used. The reaction was performed at 25°C for 10 min, 37°C for 120 min and 85°C for 5 s. The final total RNA concentration used was 25 ng/µl (3125 ng). We used 5 µl of this diluted cDNA solution for subsequent quantitative RT-PCR amplification using TaqMan Universal PCR master mix (Applied Biosystems). Specific Taqman probes were used for each gene: Abca1 (Rn00710172_m1), Fasn (Rn00569117_m1) and Srebp2 (Rn01502638_m1). The results were normalized to cyclophilin (PPIA: Rn00690933_m1), which was used as an endogenous control. Amplification was performed using the ABI Prism 7300 SDS Real-Time PCR system (Applied Biosystems) with a protocol of 50°C for 2 min, 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. The fold change in the mRNA level was calculated by the log 2 scale using the equation 2 − ΔΔCt, where ΔCt = Ct miRNA − Ct U6 and ΔΔCt = ΔCt treated samples − ΔCt untreated controls.
Western blot analysis
Proteins were extracted using radioimmunoprecipitation buffer (RIPA: 100 nM Tris–HCl, pH 7.4 (300 nM NaCl), 10% Tween, 10% Na-Deoxycholate). Equal amounts of proteins, 20 µg of HepG2 cells, were resolved on 7.5 and 5% Tris–glycine polyacrylamide minigels, for ABCA1 and FAS, respectively, and transferred to polyvinylidene fluoride (PVDF) membranes (Immun-Blot PVDF Membrane for Protein Blotting, BR05814503, Bio-Rad Laboratories, UK) using a tank-transfer system. Membranes were blocked with 5% skimmed milk in Tris-buffered saline (TBS) and incubated with primary antibodies in TBS containing 0.05% Tween-20 at 4°C overnight. Primary antibodies were used at the following dilutions: Abca1 at 1:1000 (ab18180, abcam, Cambridge, UK), Hsp90 at 1:1000 (610419, BD Biosciences, Franklin Lakes, NJ, USA), Fas at 1:5000 (ab128870, abcam). Secondary antibodies were used at the following dilutions: secondary antibody to mouse IgG-H&L (Horseradish peroxidase) (ab6728, abcam) was used at 1:5000 and secondary antibody to rabbit (NA934V, Amersham, Buckinghamshire, UK) was used at 1:10000 in 5% skimmed milk in TBS containing 0.05% Tween-20. Signals were revealed using an enhanced chemiluminescence reagent (ECL PlusWestern Blotting Detection System, RPN2132, Amersham), and digital images were taken with a Chemi XL1.4 Camera (Syngene, Cambridge, UK), which permits the semiquantification of the band intensity. Hsp90 was used as an endogenous protein control.
1H nuclear magnetic resonance spectroscopy
All compounds were reconstituted in 600 μl of D2O/H2O or deuterated methanol CD3OD. 1H nuclear magnetic resonance (NMR) spectra were measured at a 600.20 MHz frequency using an Avance III-600 Bruker spectrometer equipped with an inverse TCI 5 mm cryoprobe®. For the 1D aqueous spectra, 1D Nuclear Overhauser Effect Spectroscopy with a spoil gradient (noesygppr1d) was used. Solvent presaturation was applied during recycling delay (RD = 5 s) and mixing time (tm = 100 ms) to suppress residual water. A total of 256 transients were collected across 12 kHz spectral width at 300 K into 64 k data points, and exponential line broadening of 0.3 Hz was applied before Fourier transformation. The sequence of the two miRNAs used were as follows: hsa-miR-122: 5′-ugg agu gug aca aug gug uuu g-3′ and hsa-miR-33a: 5′-gug cau ugu agu ugc auu gca -3′, which were purchased at Biomers (Ulm, Germany). miRNAs concentration was 50 µM and polyphenols concentrations were 15, 30 or 50 µM to get stoichiometric ratios polyphenol:miRNA of 0.3:1; 0.6:1; 1:1, respectively. Data were processed with TopSpin 2.1 (Bruker Biospin, Falladen, Switzerland).
Statistical analysis
The results are reported the mean ± SEM of three independent experiments for the gene expression analyses in vitro. Group means were compared with independent-samples Student’s t-test (P ≤ 0.05) using SPSS software (SPSS, Inc., Chicago, IL, USA).
RESULTS
Phenolic characteristics of grape extracts
Two different grape proanthocyanidin extracts (GSPE and GPE) and their MF, DF and OF fractions were used to evaluate their effect on miR-33a and miR-122 expression. Previously, the extracts and fractions were characterized using reversed-phase HPLC-MS (Table 1). The results indicated that GPE is richer than GSPE in nearly all of the analyzed phenolic compounds. For example, GPE contains more monomeric forms, such as ∼46% more C and 10% more EC, than GSPE; GPE also contains 30% more gallate dimer. Regarding other types of flavonols, such as Q, GPE contains ∼100% more Q than GSPE; GA and gallate forms of flavanols, such as EGCG, are also present in GPE at levels that are 40 and 60% higher, respectively, than those in GSPE; GPE also contains RSV, whereas GSPE does not. Conversely, GSPE is richer in VA, containing 85% more VA than GPE; GSPE is 16% richer in B2 and 68% richer in epicatechin gallate. Moreover, the MF is rich in the monomeric flavanols C and EC; the DF fraction is rich in dimeric procyanidins; and the OF fraction is rich in oligomers with a polymerization degree >4.
The different composition of grape extracts and their fractions differentially influenced miR-33a and miR-122 expression in hepatic cells
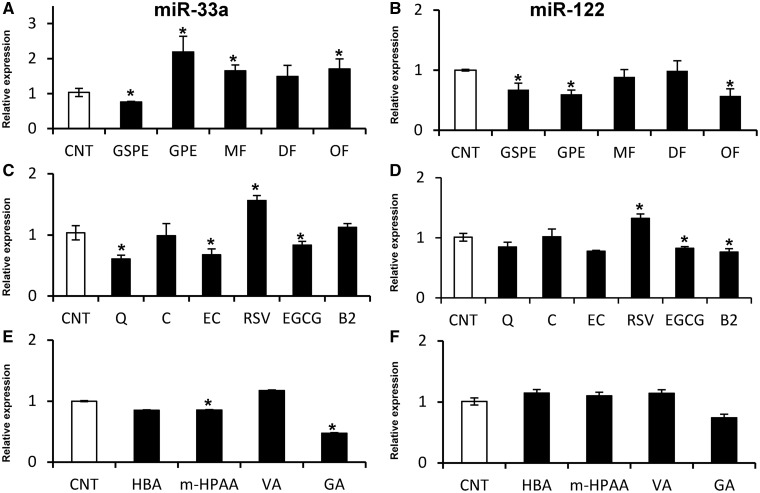
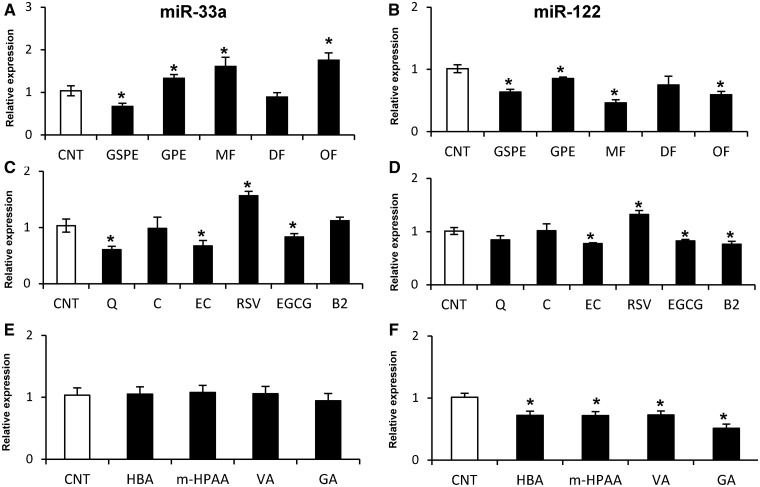
The effect of GSPE, GPE and their MF, DF and OF fractions on miR-33a and miR-122 expression in rat (Fao cells; Figure 2A and B) and human (HepG2 cells; Figure 3A and B) hepatic cell lines was investigated after 1 h of treatment. The effects on miR-122 and miR-33a exerted by the two extracts were similar in Fao and HepG2 cells. However, although GSPE reduced both miR-122 and miR-33a levels, GPE reduced miR-122 but increased miR-33a expression. The expression of miR-122 and miR-33a was not altered by the DF fraction in either Fao or HepG2 cells. Conversely, the OF fraction displayed a similar effect as GPE, decreasing miR-122 and increasing miR-33a levels in both cell lines. Although the DF and OF fractions demonstrated a similar effect on miR-33a and miR-122 expression in Fao and HepG2 cells, the MF fraction demonstrated a different effect on miR-122 expression, which depended on the cell type. Hence, the MF fraction reduced miR-122 levels in HepG2 cells, whereas the expression in Fao cells was not altered. Nevertheless, miR-33a expression was increased by the MF in both cell lines. These results demonstrated that the different composition of the grape extracts differentially influence miR-33a and miR-122 expression in hepatic cells, indicating that the molecular structure of each polyphenol induced different effects on the expression of these miRNAs.
Figure 2.
Effect of polyphenolic extracts, pure compounds and microbial metabolites on the levels of miR-33a and miR-122 in rat hepatoma Fao cells. Fao cells were treated with 25 mg/l of polyphenolic extracts and fractions (A, B), 50 μM of pure compounds (C, D) and 50 μM of microbial metabolites (E, F) for 1 h. miRNA levels were determined by qRT-PCR and normalized to U6 snRNA levels. All values represent the mean of three independent experiments. White bars represent the control group (CNT), and black bars represent the treated group. An asterisk denotes a significant difference between control cells and treated cells (P < 0.05; Student’s t-test). Abbreviations: CNT, control cells; GSPE, grape seed proanthocyanidin extract; GPE, grape pomace extract; MF, monomeric fraction; DF, dimeric fraction; OF, oligomeric fraction; Q, quercetin; C, catechin; EC, epicatechin; RSV, resveratrol; EGCG, epigallocatechin gallate; B2, dimer B2; HBA, 3′-hydroxybenzoic acid; m-HPAA, m-hydroxyphenylacetic acid; VA, vanillic acid; and GA, gallic acid.
Figure 3.
Effect of polyphenolic extracts, pure compounds and microbial metabolites on the levels of miR-33a and miR-122 in human hepatoma HepG2 cells. HepG2 cells were treated with 25 mg/l of polyphenolic extracts and fractions (A, B), 50 μM of pure compounds (C, D) and 50 μM of microbial metabolites (E, F) for 1 h. Experimental details, symbols and abbreviations are as in Figure 2.
Flavonoids repressed and RSV increased miR-33a and miR-122 expression in hepatic cells
To elucidate which polyphenol or class of polyphenol from the extracts is primarily responsible for modulating miR-33a and miR-122 expression, Fao and HepG2 cells were treated with different phenolic compounds present in GSPE and/or GPE for 1 h (Figure 2C and D and Figure 3C and D). The flavanol C and the flavonol Q were evaluated as two compounds present in GPE at higher levels than in GSPE, whereas the flavanols EC, EGCG and B2 were evaluated as three different compounds present in both GSPE and GPE at a similar concentration. Additionally, the stilbene RSV was evaluated as a compound only present in GPE and not in GSPE. All compounds demonstrate a similar effect on Fao and HepG2 cell lines, demonstrating an identical tendency in modulating these two miRNAs. Regarding the four studied flavanols, although C did not demonstrate any effect on miR-33a and miR-122 expression, EC and EGCG decreased miR-33a and miR-122 levels. Finally, B2 also decreased miR-122 expression but did not alter miR-33a expression. Similarly, Q repressed miR-33a expression but did not affect miR-122 expression. Interestingly, RSV increased the levels of these two miRNAs in contrast to all of the other evaluated compounds. In summary, all evaluated flavonoids, with the exception of C, repressed miR-33a and miR-122 expression in a similar fashion as GSPE. In contrast, the stilbene RSV, which is only present in GPE, increased the expression of these two miRNAs, which is more similar to the upregulation of miR-33a expression by GPE.
Microbial metabolites of polyphenolic extracts induced a different modulation of miR-33a and miR-122 expression in Fao cells compared with HepG2 cells
We analyzed four different phenolic acids as colonic microbial metabolites of proanthocyanidins. For this purpose, the effect of GA, VA, m-HPAA and HBA was investigated in Fao and HepG2 cells after 1 h of treatment (Figure 2E and F; Figure 3E and F). In contrast to the nonmetabolized compounds, the microbial metabolite phenolic acids demonstrated a different effect on Fao cells compared with HepG2 cells. All compounds repressed miR-122 expression in HepG2 cells, whereas no effect was observed in Fao cells. In contrast, miR-33a expression was not affected by the metabolites in HepG2 cells, whereas m-HPAA and GA downregulated miR-33a expression in Fao cells. Therefore, it appears that the HepG2 cells are more sensitive to microbial metabolites than Fao cells.
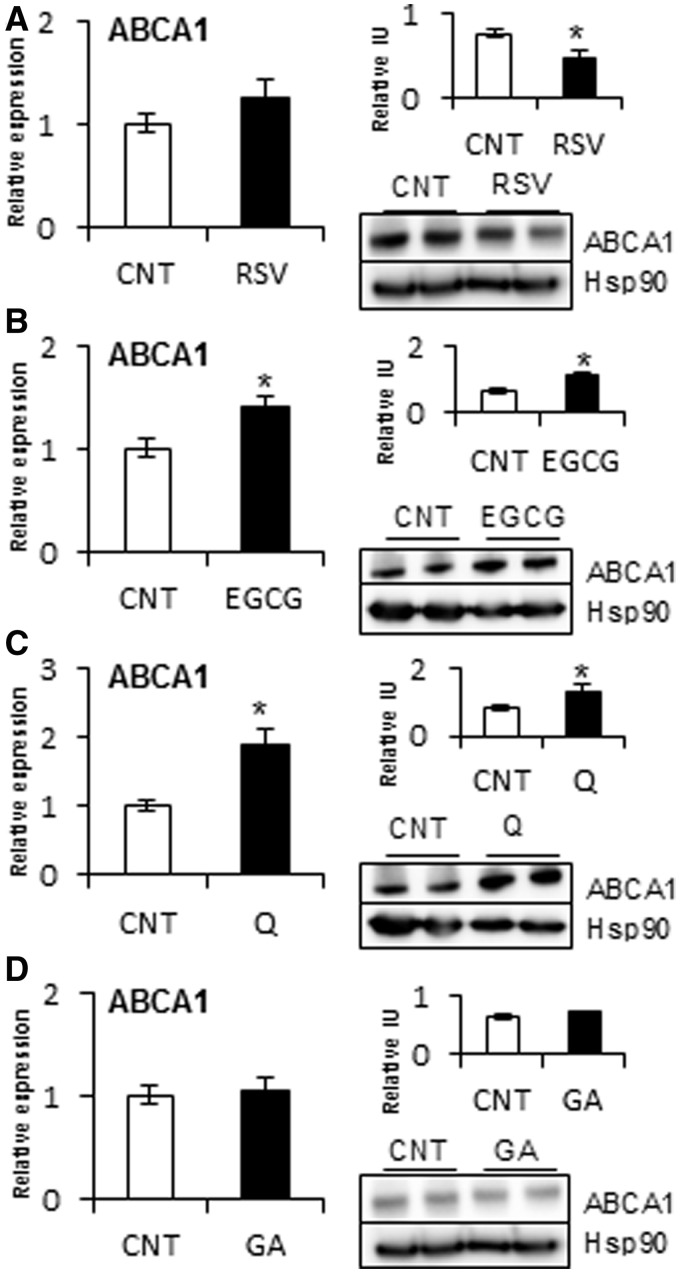
RSV, EGCG, Q and GA modulated ABCA1 and FAS according to the modulation of miR-33a and miR-122 in HepG2 cells
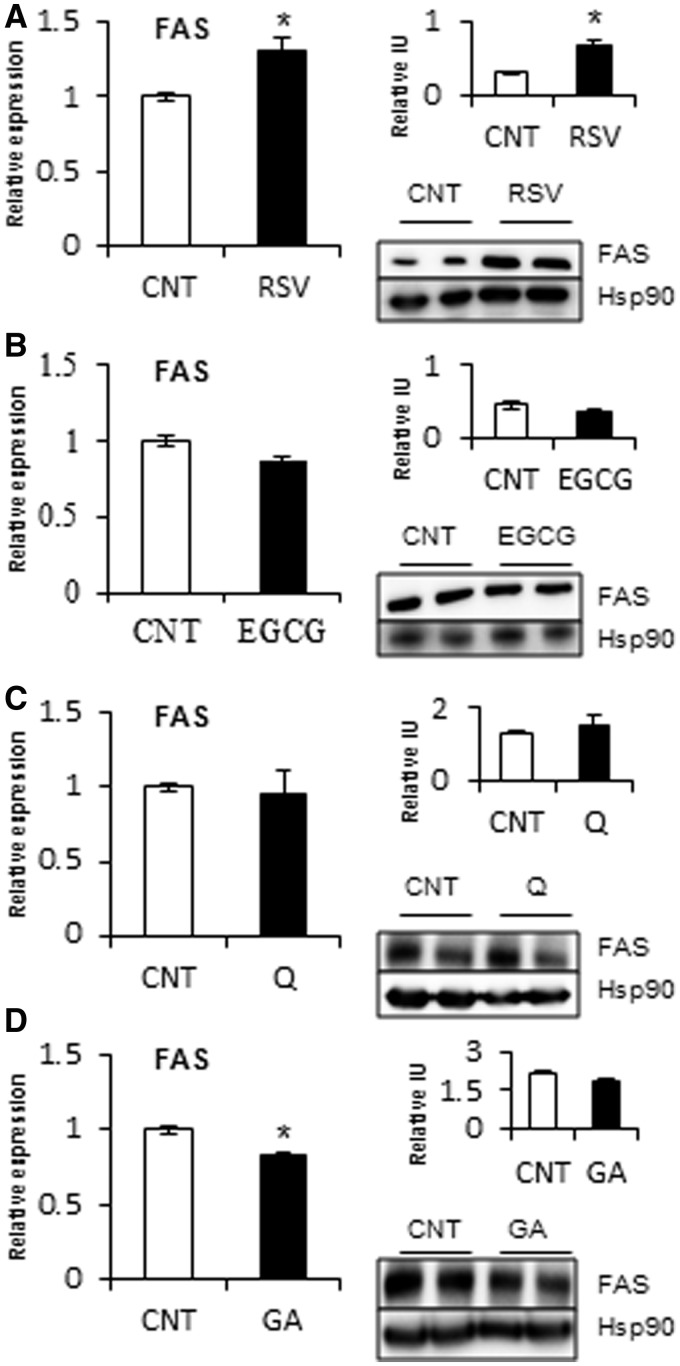
Of the phenolic compounds that affected miR-33a and miR-122 expression, we selected RSV, EGCG, Q and GA as representative of each class of polyphenol. Moreover, each of these selected compounds demonstrated a different effect on miRNA expression. Of the selected compounds, we analyzed whether the target genes of miR-33a and miR-122, ABCA1 and FAS, respectively, were also modulated by treating HepG2 cells with these compounds for 5 h (Figures 4 and 5). The results indicated that RSV, which increased miR-33a and miR-122 expression, did not alter ABCA1 mRNA levels but significantly decreased ABCA1 protein levels at 5 h. Consistent with increased miR-122 expression, RSV treatment produced an increase in FAS mRNA and protein levels. Conversely, EGCG treatment, which decreased miR-33a and miR-122 levels, increased ABCA1 mRNA and protein levels. However, the level of FAS mRNA was not altered by EGCG, which only slightly decreased FAS protein levels, although this was not significant. Q, which only decreased miR-33a expression, increased ABCA1 mRNA and protein levels without any modification of FAS mRNA and protein levels. In contrast, GA treatment, which decreased miR-122 expression, displayed a decrease in FAS mRNA, with no significant modification in FAS protein levels. Therefore, flavonoids, RSV and phenolic acids correspondingly modulated the target genes ABCA1 and FAS with their modulation of miR-33a and miR-122 expression, respectively, in HepG2 cells.
Figure 4.
Effect of RSV, EGCG, Q and GA on the mRNA and protein levels of FAS in HepG2 cells. HepG2 cells were treated with 50 μM of RSV (A), EGCG (B), Q (C) and GA (D) for 1 h to analyze FAS mRNA levels and for 5 h to analyze FAS protein levels. mRNAs levels were determined by qRT-PCR and normalized to PPIA mRNA levels. Proteins were extracted with RIPA buffer and analyzed by Western blot. Protein levels were normalized to an endogenous protein, Hsp90. Relative intensity units were obtained by dividing the intensity of the band of the target protein by that of the endogenous protein. All of the values represent the means of two independent experiments. An asterisk denotes a significant difference between control cells and treated cells (P < 0.05; Student’s t-test). Abbreviations: EGCG, epigallocatechin gallate; GA, gallic acid; Q, quercetin; and RSV, resveratrol.
Figure 5.
Effect of RSV, EGCG, Q and GA on the mRNA and protein levels of ABCA1 in HepG2 cells. HepG2 cells were treated with 50 μM of RSV (A), EGCG (B), Q (C) and GA (D) for 1 h to analyze ABCA1 mRNA levels and for 5 h to analyze ABCA1 protein levels. Experimental details, symbols and abbreviations are as in Figure 4.
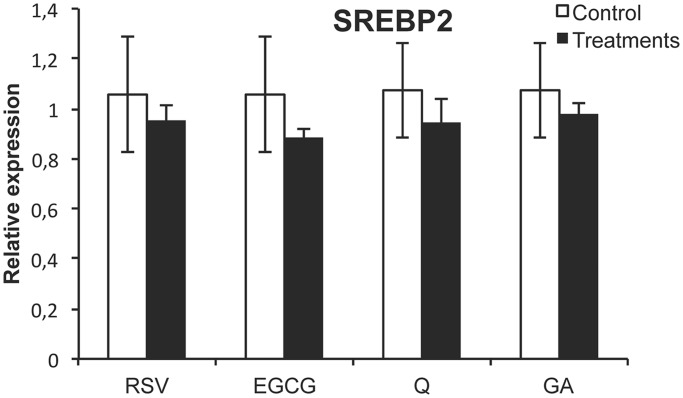
Polyphenols modulate miR-33a levels without deregulation of the SREBP2 host gene
To establish the mechanism by which polyphenols modulate miRNA levels, we studied two potential approaches. First, we evaluated whether miR-33a is indirectly modulated by the deregulation of its host gene SREBP2 because they are coexpressed. For this purpose, we studied the expression of SREBP2 in HepG2 cells after 1-h treatment with RSV, EGCG, Q and GA. The results indicated that the mRNA levels of SREBP2 were not modified by any of these compounds (Figure 6). Therefore, this points out that the mechanism by which polyphenols modulated miR-33a was independent of its host gene regulation.
Figure 6.
SREBP2 mRNA levels after 1 h RSV, EGCG, Q and GA treatment in HepG2 cells. HepG2 cells were treated with 50 μM of RSV, EGCG, Q and GA for 1 h to analyze mRNA levels of SREBP2. mRNAs levels were determined by RTqPCR and normalized to PPIA mRNA levels. All the values are the means of three independent experiments. Asterisk denotes significant difference between control cells and treated cells (P < 0.05; Student’s t-test). Abbreviations: RSV, resveratrol; EGCG, epigallocatechin gallate; Q, quercetin; GA, gallic acid.
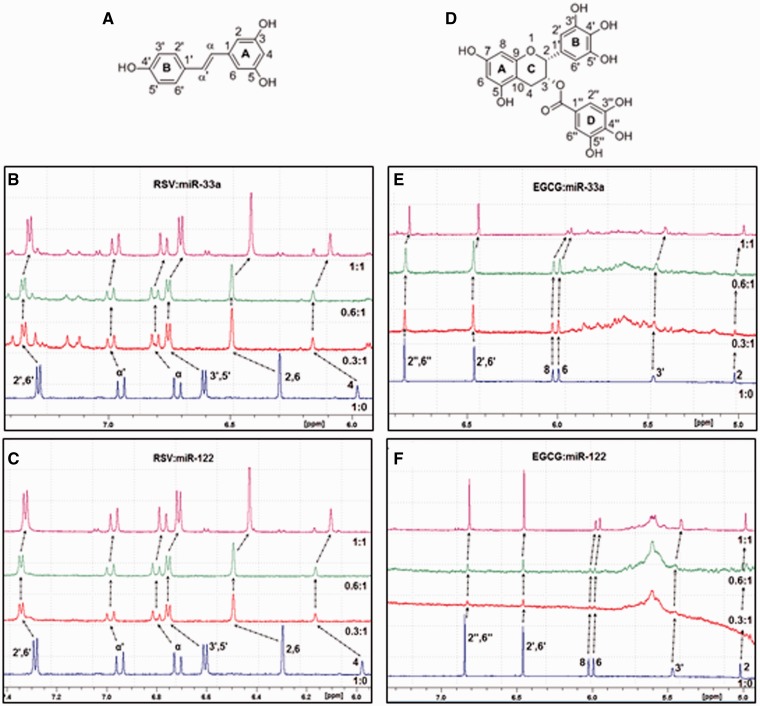
RSV and EGCG directly bind to miR-33a and miR-122 as evidenced by 1H NMR spectroscopy
The second approach to elucidate the mechanism of miRNAs modulation by polyphenols was to evaluate whether polyphenols can directly and specifically bind to miRNAs. 1H NMR spectroscopy is a useful technique to probe the interactions of small molecules with nucleic acids (20). As RSV and EGCG demonstrated major and opposite effects on the modulation of the miRNAs, we selected these compounds to determine and characterize their binding to the miRNAs. For this purpose, a 1H NMR titration experiment was performed with an increasing concentration of either RSV or EGCG in the presence of each miRNA. The results exhibited 1H chemical shift displacements (ΔδHz = δfree − δcomplex) and broad signals for most of the protons of both RSV and EGCG in solution with miR-33a or miR-122. Thus, this indicated a direct interaction of RSV and EGCG with both miR-33a and miR-122 (Table 2). Interestingly, when either of these miRNAs was present in solution with RSV, all of the protons were deshielded, whereas with EGCG, the protons were shielded (Figure 7). Furthermore, the chemical shift displacements were larger in the presence of RSV than in the presence of EGCG. Specifically, when RSV was present in solution with either miR-33a or miR-122, the deshielding effect and broad signals were similar for both miRNAs. At a 0.3:1 RSV:miRNA-33a ratio, all RSV protons were significantly deshielded, whereas at a 0.6:1 ratio, the signals were not further displaced downfield. Interestingly, at a 1:1 ratio, the protons were displaced upfield compared with those in the spectrum for a 0.6:1 ratio and resembled the free RSV spectrum. The most deshielded protons were H2, H6 and H4. When EGCG was present in solution with miR-122, the chemical shift displacement upfield was larger than with miR-33a. Furthermore, on increasing concentrations of EGCG relative to miR-33a, a larger shielding effect was observed, whereas with miR-122 at a 0.3:1 EGCG:miR-122 ratio, the shielding effect was identical to that observed for a 1:1 ratio (Table 2). Collectively, these results indicate a stronger interaction of EGCG with miR-122 than with miR-33a. In contrast to RSV, all of the protons of EGCG demonstrated a similar chemical shift displacement and broad signals. Therefore, all this together indicated that EGCG and RSV directly and differently bond to mature miRNAs.
Table 2.
1H chemical shifts of RSV and EGCG in the presence and absence of miR-33a and miR-122
| Polyphenol:miR-33a |
Polyphenol:miR-122 |
||||||
|---|---|---|---|---|---|---|---|
| Protons | δ (ppm) free | Δδ (Hz) | Δδ (Hz) | Δδ (Hz) | Δδ (Hz) | Δδ (Hz) | Δδ (Hz) |
| 0.3:1 | 0.6:1 | 1:1 | 0.3:1 | 0.6:1 | 1:1 | ||
| RSV protons | |||||||
| 2′, 6′ | 7.29 | −36.01 | −36.01 | −18.00 | −36.01 | −36.01 | −24.00 |
| α' | 6.95 | −30.01 | −30.01 | −18.00 | −24.00 | −24.00 | −12.00 |
| α | 6.73 | −54.01 | −54.01 | −30.01 | −54.01 | −54.01 | −30.01 |
| 3′, 5' | 6.60 | −90.03 | −90.03 | −60.02 | −90.03 | −90.03 | −66.02 |
| 2, 6 | 6.30 | −114.03 | −114.03 | −72.02 | −114.03 | −114.03 | −78.02 |
| 4 | 5.98 | −108.03 | −108.03 | −66.02 | −114.03 | −114.03 | −72.02 |
| EGCG protons | |||||||
| 2'', 6'' | 6.85 | 6.00 | 12.00 | 18.00 | 48.01 | 48.01 | 48.01 |
| 2′, 6′ | 6.46 | 0 | 6.00 | 6.00 | 42.01 | 42.01 | 42.01 |
| 8 | 6.03 | 6.00 | 12.00 | 18.00 | 36.01 | 36.01 | 36.01 |
| 6 | 5.99 | 0 | 6.00 | 18.00 | 42.01 | 42.01 | 42.01 |
| 3′ | 5.47 | 12.00 | 18.00 | 24.00 | −42.01 | −42.01 | −42.01 |
| 2 | 5.02 | 6.00 | 12.00 | 18.00 | n. d. | n. d. | n. d. |
ΔδHz = δfree-δcomplex.The concentration of the miRNAs was 50 µM, and the concentrations of the polyphenols were 15, 30 and 50 µM to obtain polyphenol:miRNA ratios of 0.3:1, 0.6:1 and 1:1, respectively.
EGCG, epigallocatechin gallate; and RSV, resveratrol.
Figure 7.
1H NMR spectra of RSV and EGCG in the presence of miR-33a and miR-122. (A) The chemical structure of RSV. (B) 1H NMR spectra of RSV alone or in solution with mature miR-33a. (C) 1H NMR spectra of RSV alone or in solution with mature miR-122. (D) The chemical structure of EGCG. (E) 1H NMR spectra of EGCG alone or in solution with mature miR-33a. (F) 1H NMR spectra of EGCG alone or in solution with mature miR-122. The miRNA concentration was 50 µM, and the concentrations of the polyphenols were 15, 30 and 50 µM to obtain the polyphenol:miRNA ratios of 0.3:1, 0.6:1 and 1:1, respectively. Abbreviations: EGCG, epigallocatechin gallate; and RSV, resveratrol.
DISCUSSION
Because plant extracts are complex mixtures of different polyphenolic compounds of different chemical structures, which range from monomers to oligomeric forms, we attempted to elucidate whether specific compounds in the extracts were able to deregulate specific miRNAs. Furthermore, although some of the miRNAs whose expression was deregulated by these dietary compounds serve important functions in regulating metabolism [reviewed in (21)], most of the studies are based in cancer.
We recently established a new mechanism by which GSPE exerts its hypolipidemic effect by downregulating miR-33a and miR-122 and correspondingly increasing the miR-33a target gene ABCA1 and repressing the miR-122 indirect target gene FAS in the liver (18). Moreover, several studies based on the effect of polyphenols and polyphenol-rich extracts on miRNAs expression indicated that each polyphenol targets specific miRNAs in liver (19,22,23). Hence, this suggests that the type of polyphenol influence the miRNA’s modulation. Therefore, the first aim of this study was to elucidate the influence of the polyphenolic chemical structure on miR-33a and miR-122 expression in hepatic cells. For this purpose, we studied the expression of miR-33a and miR-122 in hepatic cells after treatment with two grape proanthocyanidin extracts of different polyphenolic composition and polyphenols representative of different classes. The polyphenol doses used (50 µM) in this experiment were pharmacological. Moreover, the studies were realized using hepatic cells of human (HepG2 cells) and rats (Fao cells) to determine whether the polyphenol effect on miRNAs is specie-dependent.
We demonstrated that GSPE or GPE induced a different modulation of miR-33a and miR-122 expression, most likely due to their different composition of polyphenols. Furthermore, when cells were treated with MF or oligomeric (DF and OF) proanthocyanidins, miR-33a and miR-122 were differentially deregulated, hence, indicating that the polyphenol size differentially influenced the expression of these miRNAs. All kinds of flavonoids studied repressed or did not affect miR-33a and miR-122 expression. Nevertheless, RSV, a nonflavonoid, increased the expression of these miRNAs. Flavonoids consist of two aromatic rings connected to a pyrone ring, which can be esterified with a GA or other multiple substitutions generating different classes of flavonoids. In contrast, RSV features a central carbon–carbon double bond conjugated with two phenolic moieties. Hence, it could be suggested that the chemical structure of the polyphenols influence the modulation of miR-33a and miR-122 expression in hepatic cells. As RSV was present in GPE but not in GSPE, it might be hypothesized that the upregulation of these two miRNAs by GPE may be due to the presence of RSV.
After absorption, dietary polyphenols undergo phase II enzymatic conjugations in the small intestine and/or in the liver (24). Furthermore, most dietary polyphenols can reach the colon in which the enzymes produced by the gut bacteria can convert them into different low molecular weight metabolites such as phenolic acids that later can be absorbed in situ (25). Therefore, we also analyzed different phenolic acids as colonic microbial metabolites of proanthocyanidins. In contrast to nonmetabolized compounds, which demonstrated an identical tendency to modify the miRNA levels in HepG2 cells compared with Fao cells, a different effect of microbial metabolites was observed in Fao cells compared with HepG2 cells. Thus, it can be suggested that the human hepatic cells are more sensitive to microbial metabolites than rat hepatic cells.
Moreover, most classes of polyphenols studied correspondingly modify ABCA1 and FAS mRNA and protein levels in HepG2 cells with modulation of miR-33a and miR-122 levels, respectively. Therefore, these results indicate that miRNA modulation by polyphenols could be behind the hypolipidemic effect of some polyphenols.
Currently, the molecular mechanism by which polyphenols modulate miRNA levels is unknown. Therefore, as a second objective of this study we evaluated how polyphenols modulate miR-33a and miR-122 levels. For this, first we determined whether polyphenols repress or increase miRNA expression by transcription modulation. Some miRNAs, like miR-33 but not miR-122, are intronic of genes, and polyphenols that modify host gene expression would also affect the miRNA levels. Herein, we demonstrated that polyphenols modify miR-33a levels without altering the levels of its host gene SREBP2, although they are simultaneously coexpressed. This suggests that polyphenols modify miR-33 levels by a posttranscriptional mechanism. There is evidence that polyphenols can bind to mRNAs and proteins (26,27). Therefore, it is possible that polyphenols also bind to miRNAs or to some component involved in miRNA biogenesis, such as Dicer or the RNA-induced silencing complex. Using 1H NMR spectroscopic studies, RSV and EGCG showed to directly bind to miR-33a and miR-122. Specifically, RSV binds equally to miR-33a and miR-122 primarily through an A ring interaction, whereas EGCG binds more strongly to miR-122 than to miR-33a through an interaction with all of the rings in the molecule. Moreover, RSV binds more strongly to these miRNAs than EGCG and through a different type of interaction because the protons in RSV were deshielded, whereas the protons in EGCG were shielded. These results are consistent with the opposing effects of RSV and EGCG on miRNA modulation. Altogether, the results suggest that polyphenols modulate miR-33 and miR-122 levels by direct binding, which could modify the miRNAs degradation or stability and hence change their levels. However, while EGCG is reported to have steric impediment, which result in its native form circulating in vivo, RSV is well known to undergo a strong phase II intestinal and hepatic metabolism. In particular, derivatives of RSV show an opposite biological behavior toward specific receptors (28).
In conclusion, the effect on miRNA expression depended on the polyphenol chemical structure and the modulation of miR-33a was independent of the expression of its host gene, SREBP2. RSV and EGCG bound directly to miR-33 and miR-122. The binding of the studied polyphenols to miRNAs was specific, and the molecular structure of the polyphenol will determine the nature of the binding to the specific miRNA. Interestingly, the nature of the binding of these compounds to the miRNAs was consistent with their effects on cell miRNAs levels. Hence, these results suggest that the interaction of polyphenols with miRNAs influences the polyphenol functionality by altering the binding of the miRNAs to the seed sequence of their target genes. Therefore, the specific and direct binding of polyphenols to miRNAs emerges as a new posttranscriptional mechanism by which polyphenols could modulate metabolism.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Spanish Government [AGL 2008–00387/ALI]; European Union Seventh Framework Programme FP7 2007–2013 [244995] (BIOCLAIMS Project). Funding for open access charge: Spanish Government [AGL 2008–00387/ALI].
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr Miguel Angel Rodríguez Gómez for NMR technical support.
REFERENCES
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramírez CM, Goedeke L, Fernández-Hernando C. “Micromanaging” metabolic syndrome. Cell Cycle. 2011;10:3249–3252. doi: 10.4161/cc.10.19.17558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu S, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Invest. 2012;122:2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl Acad. Sci. USA. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quesada H, del Bas JM, Pajuelo D, Diaz S, Fernandez-Larrea J, Pinent M, Arola L, Salvado MJ, Blade C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. (Lond.) 2009;33:1007–1012. doi: 10.1038/ijo.2009.136. [DOI] [PubMed] [Google Scholar]
- 10.Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2011;59:11862–11871. doi: 10.1021/jf2029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J. Am. Coll. Nutr. 2009;28:355–361. doi: 10.1080/07315724.2009.10718097. [DOI] [PubMed] [Google Scholar]
- 12.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006;136:2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 13.Rivera L, Moron R, Sanchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese zucker rats. Obesity (Silver Spring) 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Chung SJ, Song WO, Chun OK. Estimation of daily proanthocyanidin intake and major food sources in the US diet. J. Nutr. 2011;141:447–452. doi: 10.3945/jn.110.133900. [DOI] [PubMed] [Google Scholar]
- 15.Hackman RM, Polagruto JA, Zhu QY, Sun B, Fujii H, Keen CL. Flavanols: Digestion, absorption and bioactivity. Phytochem. Rev. 2008;7:195–208. [Google Scholar]
- 16.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 17.Chang X, Heene E, Qiao F, Nick P. The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in vitis cell. PLoS One. 2011;6:e26405. doi: 10.1371/journal.pone.0026405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baselga-Escudero L, Blade C, Ribas-Latre A, Casanova E, Salvado MJ, Arola L, Arola-Arnal A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012;56:1636–1646. doi: 10.1002/mnfr.201200237. [DOI] [PubMed] [Google Scholar]
- 19.Baselga-Escudero L, Arola-Arnal A, Pascual-Serrano A, Ribas-Latre A, Casanova E, Salvado MJ, Arola L, Blade C. Chronic administration of proanthocyanidins or docosahexaenoic acid reversess the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS One. 2013;8:e69817. doi: 10.1371/journal.pone.0069817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furtig B, Richter C, Wohnert J, Schwalbe H. NMR spectroscopy of RNA. Chembiochem. 2003;4:936–962. doi: 10.1002/cbic.200300700. [DOI] [PubMed] [Google Scholar]
- 21.Bladé C, Baselga-Escudero L, Salvadó MJ, Arola-Arnal A. miRNAs, polyphenols, and chronic disease. Mol Nutr Food Res. 2012;57:58–70. doi: 10.1002/mnfr.201200454. [DOI] [PubMed] [Google Scholar]
- 22.Milenkovic D, Deval C, Gouranton E, Landrier JF, Scalbert A, Morand C, Mazur A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS One. 2012;7:e29837. doi: 10.1371/journal.pone.0029837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arola-Arnal A, Blade C. Proanthocyanidins modulate MicroRNA expression in human HepG2 cells. PLoS One. 2011;6:e25982. doi: 10.1371/journal.pone.0025982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dall'Asta M, Calani L, Tedeschi M, Jechiu L, Brighenti F, Del Rio D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition. 2012;28:197–203. doi: 10.1016/j.nut.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Monagas M, Urpi-Sarda M, Sanchez-Patan F, Llorach R, Garrido I, Gomez-Cordoves C, Andres-Lacueva C, Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 26.Kuzuhara T, Sei Y, Yamaguchi K, Suganuma M, Fujiki H. DNA and RNA as new binding targets of green tea catechins. J. Biol. Chem. 2006;281:17446–17456. doi: 10.1074/jbc.M601196200. [DOI] [PubMed] [Google Scholar]
- 27.Xiao J, Kai G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012;52:85–101. doi: 10.1080/10408398.2010.499017. [DOI] [PubMed] [Google Scholar]
- 28.Ruotolo R, Calani L, Fietta E, Brighenti F, Crozier A, Meda C, Maggi A, Ottonello S, Del Rio D. Anti-estrogenic activity of a human resveratrol metabolite. Nutr. Metab. Cardiovasc. Dis. 2013;23:1086–1092. doi: 10.1016/j.numecd.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Torres JL, Varela B, Garcia MT, Carilla J, Matito C, Centelles JJ, Cascante M, Sort X, Bobet R. Valorization of grape (Vitis vinifera) byproducts. antioxidant and biological properties of polyphenolic fractions differing in procyanidin composition and flavonol content. J. Agric. Food Chem. 2002;50:7548–7555. doi: 10.1021/jf025868i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.