SUMMARY
Cancers arising in mucosal tissues account for a disproportionately large fraction of malignancies. IgG and the neonatal Fc receptor for IgG (FcRn) have an important function in the mucosal immune system which we have now shown extends to the induction of CD8+ T cell-mediated anti-tumor immunity. We demonstrate that FcRn within dendritic cells (DC) was critical for homeostatic activation of mucosal CD8+ T cells which drove protection against the development of colorectal cancers and lung metastases. FcRn-mediated tumor protection was driven by DC activation of endogenous tumor-reactive CD8+ T cells via the cross-presentation of IgG complexed antigens (IgG IC) as well as the induction of cytotoxicity-promoting cytokine secretion, particularly interleukin-12 (IL-12), both of which were independently triggered by the FcRn–IgG IC interaction in murine and human DC. FcRn thus has a primary role within mucosal tissues in activating local immune responses that are critical for priming efficient anti-tumor immunosurveillance.
Cancers arising at mucosal barrier sites, particularly the lung, large intestine (LI), stomach and cervix, account for a considerable fraction of human malignancies (Siegel et al., 2012). One contributing factor to the colon's susceptibility to malignant transformation is its immunosuppressive environment (MacDonald et al., 2011) which is necessary for tolerance towards microbial and dietary antigens but also results in dampened anti-cancer immune responses (Revaz and Nardelli-Haefliger, 2005; Saleh and Trinchieri, 2011). Identifying physiologic factors capable of countering this inherent downside of local tolerance is critical for understanding and manipulating carcinogenesis at this, and possibly other, mucosal sites.
The production and handling of IgG are critical components of mucosal immunity, particularly in the LI where IgG accounts for a large fraction of homeostatic mucosal immunoglobulin secretion (Kozlowski et al., 1997). The presence of IgG in the intestinal lumen is associated with the actions of the bidirectional IgG transport receptor, FcRn (neonatal Fc receptor for IgG), which is expressed lifelong in most murine and human endothelial, epithelial and hematopoietic cells (Claypool et al., 2004; Zhu et al., 2001). FcRn is uniquely capable of delivering IgG into the lumen and also retrieving lumenal IgG and IgG containing immune complexes (IgG IC) which are delivered into the local immune system of the lamina propria (LP) (Claypool et al., 2004; Yoshida et al., 2004). FcRn within antigen presenting cells such as dendritic cells (DC) also plays a critical role in the processing of antigens delivered as IgG IC and actively promotes major histocompatibility complex (MHC) class I and class II restricted T cell responses (Baker et al., 2011; Qiao et al., 2008) which can alternatively promote anti-bacterial IgG-driven colitis (Kobayashi et al., 2009) and protect from mucosal pathogens (Yoshida et al., 2006).
It is well accepted that cytotoxic CD8+ T cell-mediated responses are critical for efficient anti-tumor immunity (Pages et al., 2005) and FcRn has recently been shown to enable highly efficient cross-presentation of IgG-complexed antigens by CD8−CD11b+ DC (Baker et al., 2011). Given the abundance of both IgG and CD8−CD11b+ monocyte-derived DC in mucosal tissues, especially in the context of malignancy (Kozlowski et al., 1997; Ma et al., 2011; MacSween and Eastwood, 1980), we examined the role of FcRn in homeostatic CD8+ T cell responses and as an effector of anti-cancer immunosurveillance.
RESULTS
FcRn protects against the development of colorectal cancer
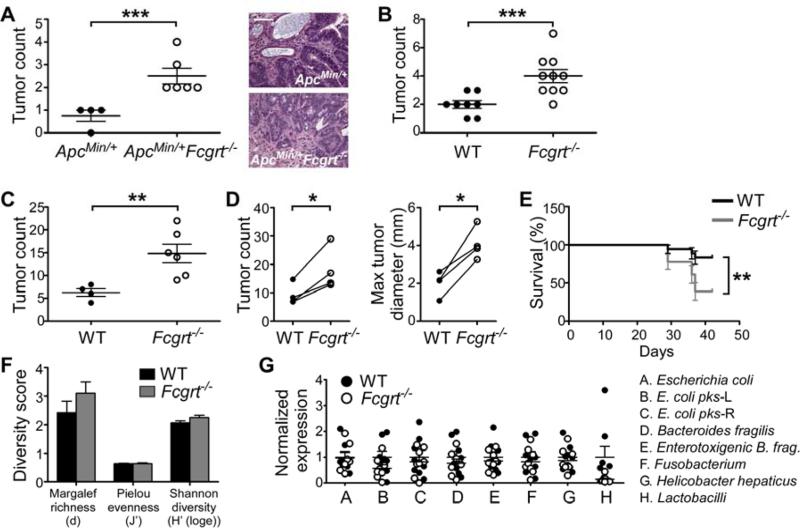
The majority of sporadic colorectal cancers (CRC) arise following a defined series of mutational events often involving inactivation of the adenomatous polyposis coli (APC) gene (Walther et al., 2009). We thus began by investigating whether FcRn could contribute to the development of CRC in ApcMin/+ mice which possess an abnormal copy of Apc and spontaneously develop large numbers of small intestinal adenomas (Saleh and Trinchieri, 2011). Typically, ApcMin/+ mice do not develop colonic lesions in the absence of further insults, such as the additional loss of a tumor suppressor gene (Aoki et al., 2003; Saleh and Trinchieri, 2011). However, ApcMin/+ mice crossed with mice deficient in FcRn (Fcgrt−/−) spontaneously developed significantly more LI tumors than their ApcMin/+ littermates (Figure 1A). Importantly, high grade dysplasia and local invasion through the LP were detected only in lesions from ApcMin/+Fcgrt−/− but not ApcMin/+ animals (Figures 1A and S1A). Of note, no differences were detected in the frequency of tumors in the small intestine (SI) (Figure S1B), where tumor development in ApcMin/+ mice does not depend on a second genetic event. We next investigated the role of FcRn in the development of CRC induced by the chronic exposure of a chemical carcinogen, azoxymethane (AOM), which, upon repeated administration, drives the development of colorectal malignancies (Meunier et al., 2009). We observed that Fcgrt−/− mice subjected to a standard regimen of AOM administration developed significantly more abundant and larger tumors (Figures 1B and S1C) than did WT littermates. These data demonstrate the importance of FcRn in determining susceptibility to the development of sporadic CRC.
Figure 1. FcRn protects against the development of colorectal cancer through a mechanism independent of intestinal microbiota.
(A) Large intestine (LI) tumor incidence at 5 months of age and representative tumor histology in ApcMin/+ and ApcMin/+Fcgrt−/− mice. Scale bar = 100 μm. (B) Tumor incidence in WT and Fcgrt−/− littermates treated with 8 doses of azoxymethane (AOM). (C) Tumor incidence in AOM/DSS-treated WT and Fcgrt−/− littermates. (D) Tumor incidence and maximum tumor diameter in WT and Fcgrt−/− littermates in each of four independent experiments with n ≥ 3 mice per group per experiment. (E) Percent survival of WT and Fcgrt−/− littermates treated with AOM/DSS. Significance was assessed by Logrank test. (F) Richness indices of microbiota associated with the distal LI of untreated 8-week old WT and Fcgrt−/− littermates, as revealed by T-RFLP analysis. n = 3-5 mice per group. (G) Abundance of specific microbial species in the distal LI of untreated 7-week old WT andFcgrt−/− littermates as assessed by qPCR. n = 9 mice per group. Representative results of two (A,B,E) or four (D) independent experiments each with n = 4-10 mice per group. All data represent mean ± s.e.m. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S1.
Knowing that inflammatory bowel disease is associated with a heightened risk of CRC and that inflammation plays an important role in driving even sporadic neoplasias (Coghill et al., 2012; Herrinton et al., 2012), we examined whether FcRn-mediated tumor protection extended to inflammation-associated CRC. We found that Fcgrt−/− mice treated with AOM and dextran sodium sulfate (AOM/DSS) (Figure S1D) (Wirtz et al., 2007) developed significantly larger and more abundant colorectal adenocarcinomas than WT littermates (Figures 1C,D and S1E). Additionally, at higher concentrations of DSS, Fcgrt−/− mice experienced significantly poorer survival rates compared to their WT littermates (Figure 1E), indicating that FcRn-mediated anti-tumor immunity is potent enough to influence disease outcome. The smaller initial weight loss in the Fcgrt−/− mice compared to WT controls (Figure S1F) was consistent with previous findings that Fcgrt−/− mice are protected from IgG-induced colitis (Kobayashi et al., 2009), suggesting that tumor development in the context of FcRn-deficiency is not simply due to increased inflammation.
Mounting evidence indicates that certain intestinal microbes play a role in promoting the development of CRC (Arthur and Jobin, 2011; Arthur et al., 2012). We thus profiled the intestinal microbiota in our WT and Fcgrt−/− littermate mice in order to determine whether FcRn was exerting tumor protection through the regulation of gut microbial composition. Terminal restriction fragment length polymorphism (T-RFLP) analysis of the overall microbial community composition and diversity from WT and Fcgrt−/− littermates revealed no significant differences in either post-weaning, eight week old mice or pre-weaning, two week old mice (Figures 1F and S1G,H) in any of three separate intestine-associated tissue compartments (proximal LI, distal LI and feces). However, regardless of genotype, the microbiota were found to differ between these three tissue sites, thereby confirming that our analysis had sufficient power to detect differences in microbial composition (Figures S1I,J). In order to exclude differences in specific organisms previously associated with CRC development (Arthur and Jobin, 2011), we also assessed the abundance of these microbes in a separate cohort of seven week old mice using genus or species specific qPCR and found no significant differences in either the distal LI (Figure 1G) or feces (Figure S1K) of WT and Fcgrt−/− littermates. Together, these data demonstrate that FcRn does not protect against colorectal tumor development by regulating intestinal microbial diversity or decreasing the presence of tumor-promoting microbes.
FcRn promotes the retention and activation of tumor protective CD8+ T cells in the large intestine
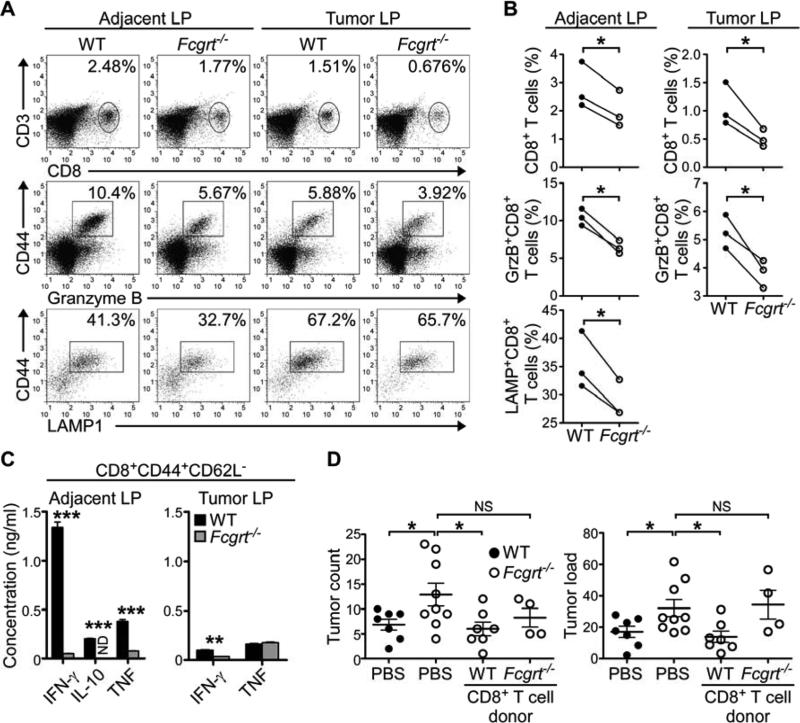
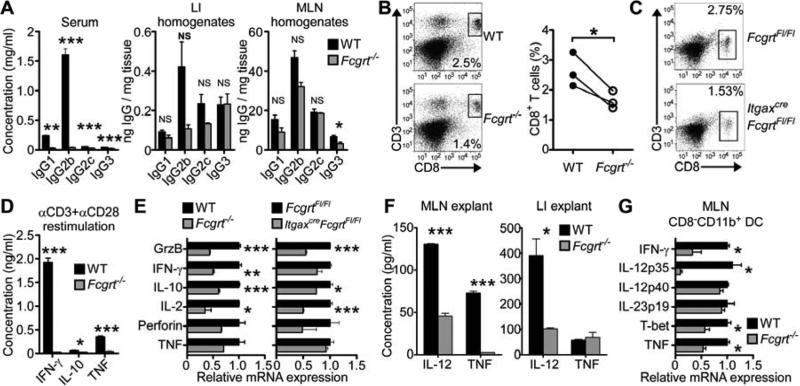
In seeking to better understand the nature of FcRn-driven anti-tumor immunity, we examined the immunological composition of LP lymphocytes (LPL) in both dissected tumors and macroscopically tumor-free adjacent tissue. While no differences were noted in the numbers of CD4+ T cells, natural killer (NK) cells or macrophages (Figure S2A), significantly greater numbers of CD8+ T cells were consistently found both within tumor tissue and adjacent tissue of WT AOM/DSS-treated mice in comparison to their Fcgrt−/− littermates (Figures 2A,B and S2B). This same deficiency in CD8+ T cell infiltration into the tumor microenvironment of FcRn-deficient mice was also seen in ApcMin/+Fcgrt−/− animals in comparison to their ApcMin/+ littermates (Figure S2C) as well as in Fcgrt−/− mice treated with AOM alone (Figure S2D). Furthermore, a greater percentage of the CD8+ T cells from AOM/DSS treated WT animals expressed intracellular granzyme B or surface lysosomal associated membrane protein-1 (LAMP1) (Figures 2A,B) than did those from their Fcgrt−/− littermates. We confirmed this using anti-CD3 and anti-CD28 restimulation of sorted effector CD8+CD44+CD62L− T cells from the LI of AOM/DSS-treated mice (Figure 2C). In response to this, CD8+ T cells from Fcgrt−/− tumor-bearing mice secreted only small amounts of interferon-γ (IFN-γ), tumor necrosis factor (TNF) and interleukin-10 (IL-10), the latter of which has recently been shown to be critical for efficient cytotoxic CD8+ T cell-mediated anti-viral and anti-tumor immunity (Mumm et al., 2011; Zhang and Bevan, 2011). While no differences were seen in the rates of CD8+ T cell proliferation or apoptosis, as assessed by Ki-67 and annexin V staining, respectively (Figure S2E), both upregulation of CD103, an integrin associated with T cell retention (Le Floc'h et al., 2007), and increased expression of activation-associated CD44 on CD62L+CD8+ T cells were observed in CD8+ T cells infiltrating the LP of WT but not Fcgrt−/− littermates (Figure S2F). This was specific for the tumor-associated tissues as these differences were not observed in the mesenteric lymph nodes (MLN) (Figure S2F) and is notable because the presence of high numbers of CD8+CD44+CD62L+ cells bearing an effector memory (TEM) cell phenotype has been associated with improved prognosis in human CRC patients (Pages et al., 2005). These data are thus most consistent with a role for FcRn in driving anti-tumor immunity by promoting the retention and activation of cytotoxic T cells having homed to the LI.
Figure 2. FcRn drives the activation and retention of tumor-reactive cytotoxic CD8+ T cells which confer tumor protection.
(A) Frequency of CD8+ T cells in the lamina propria lymphocyte (LPL) fraction of tumor and adjacent LI tissue in WT and Fcgrt−/− littermates (upper panels) following AOM/DSS treatment. Cytotoxic potential of cells within the CD3+CD8+ gate was assessed by intracellular staining for granzyme B (middle panels) or surface staining of LAMP1 (lower panels). (B) Mean CD8+ T cell frequency and cytotoxic potential in WT and Fcgrt−/− mice, as assessed by flow cytometry, in each of three independent experiments. (C) Cytokine secretion of sorted effector CD8+ CD44+CD62L− cells from the LP of tumor and adjacent tissue of AOM/DSS treated WT and Fcgrt−/− mice following 24 h restimulation with anti-CD3 and anti-CD28. (D) Tumor incidence and tumor load (sum of the diameters of all tumors) in recipient mice adoptively transferred with CD8+ T cells from WT or Fcgrt−/− AOM/DSS-treated donors. Significance was assessed by Mann-Whitney test. Representative results of three independent experiments with n ≥ 4 mice per group per experiment. All data represent mean ± s.e.m. NS = not significant. ND = not detected. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S2.
We next sought to confirm that FcRn-mediated activation of CD8+ T cells was critical for its tumor protective function using adoptive transfer of CD8+ T cells isolated from both the MLN and LP of AOM/DSS-treated donors in order to minimize the number of T cells likely to have been exposed to a tolerizing tumor microenvironment (Chen and Mellman, 2013). Transfer of CD8+ T cells from WT donors, but not from Fcgrt−/− donors, into Fcgrt−/− recipients decreased tumor frequency and total neoplastic colon surface area (tumor load) (Grivennikov et al., 2012) to quantities similar to those observed in WT mice and significantly less than seen in PBS treated Fcgrt−/− animals (Figure 2D). Similar experiments performed with adoptively transferred CD4+ T cells revealed that CD4+ T cells are not sufficient for FcRn-mediated tumor protection (Figure S2G). Rather, our data are consistent with activation of cytotoxic CD8+ T cells being a primary mechanism by which FcRn-driven tumor immune surveillance operates.
FcRn-dependent cross-priming by dendritic cells induces effective anti-tumor CD8+ T cell responses
In light of our recent demonstration that FcRn in CD8−CD11b+ DC, in which the acidic endosomal and phagosomal pH favors FcRn–IgG binding, drives the cross-presentation of IgG IC-delivered antigens and the resulting activation of CD8+ T cells (Baker et al., 2011), we sought to determine whether FcRn-dependent cross-priming by DC was required for its anti-tumor effects. Given that this mechanism would necessitate the presence of tumor-reactive IgG to form IC and that tumor-reactive IgG has previously been documented in human CRC (Auer et al., 1988; Kijanka et al., 2010), we first confirmed the presence of these effector molecules in our model. Both ELISA (Figures 3A and S3A) and Western blotting (Figure S3B) assays using IgG-depleted tumor epithelium lysates from AOM/DSS-treated mice as the source of antigen verified that tumor-reactive IgG was present in the serum as well as in MLN and LI tissue homogenates of AOM/DSS-treated WT and Fcgrt−/− littermates but not non-tumor bearing controls. We also noted increases of similar magnitude in anti-phosphatidylserine and anti-cardiolipin IgG, which have been shown to promote the formation of IC containing apoptotic bodies or mitochondria released by dying tumor cells (Kepp et al., 2009), in the serum of both WT and Fcgrt−/− littermates (Figure S3C). Moreover, both tumor-reactive IgG (Figures 3A and S3A) and total IgG (Figure S3D) were also present at similar levels in the MLN and intestinal tissues of AOM/DSS-treated WT and Fcgrt−/− mice. Thus, although FcRn is critically important in protecting circulating IgG from catabolism and our Fcgrt−/− mice were predictably systemically hypogammaglobulinemic (Figure S3D) (Roopenian et al., 2003), this was not the case in tissues for either total IgG (Figure S3D) or tumor-specific IgG (Figure 3A) where local IgG production by resident plasma cells is likely sufficient to normalize tissue IgG levels. Thus, the extreme susceptibility of Fcgrt−/− mice to tumor development cannot simply be attributed to a local deficiency in the IgG ligand for FcRn.
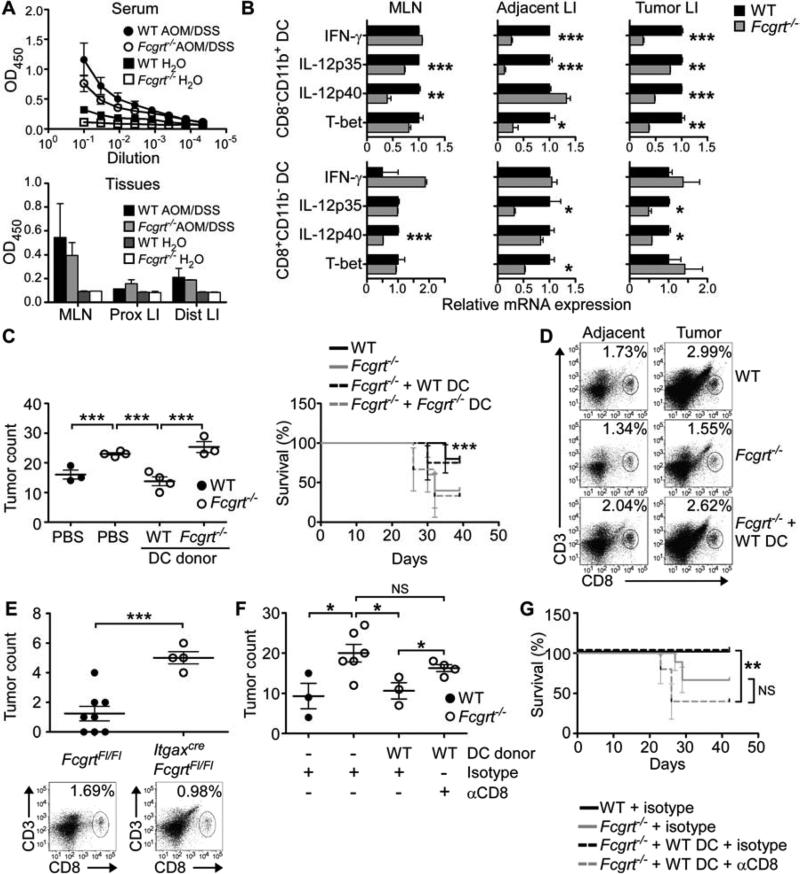
Figure 3. CD8−CD11b+ DC utilize FcRn to efficiently prime protective anti-tumor CD8+ T cell responses.
(A) Tumor antigen-specific IgG in the serum or MLN and LI homogenates of AOM/DSS treated WT or Fcgrt−/− mice. ELISA plates coated with lysates from tumor epithelium were probed with dilutions of serum or tissue homogenates from tumor bearing mice. (B) Transcript profiles of sorted CD8−CD11b+ and CD8+CD11b− DC subsets isolated from the indicated tissue compartment of AOM/DSS-treated WT and Fcgrt−/− littermates. (C) Tumor incidence and survival in Fcgrt−/− recipients adoptively transferred with DC from the MLN and LP of AOM/DSS-treated WT or Fcgrt−/− donors. Endpoint survival was assessed using a Chi-Squared test. (D) CD8+ T cell frequency in the LI LP following transfer of WT DC to AOM/DSS-treated Fcgrt−/− recipients. (E) Tumor incidence and LI LP CD8+ T cell frequency in ItgaxcreFcgrtFl/Fl mice and their littermate FcgrtFl/Fl controls upon treatment with AOM/DSS. (F-G) Tumor incidence (F) and survival (G) of CD8+ T cell-depleted Fcgrt−/− mice adoptively transferred with WT DC. CD8+ T cells were depleted by chronic i.p. administration of anti-CD8 antibody (or isotype control). Representative results of three (B-E) or two (A,F) independent experiments with n = 3-6 mice per group per experiment. All data represent mean ± s.e.m. NS = not significant. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S3.
Having confirmed the presence of IgG capable of binding tumor antigens in both WT and Fcgrt−/− mice, we verified that there were no differences in the distribution of DC subsets or DC FcγR expression in the LI LP of WT and Fcgrt−/− littermates (Figure S3E). This was particularly important given that FcRn-dependent cross-presentation requires FcγR for the initial IgG IC internalization (Baker et al., 2011). Evaluation of the functional characteristics of sorted CD8−CD11b+ and CD8+CD11b− DC from the MLN, adjacent, and tumor tissues of AOM/DSS-treated mice revealed that DC from Fcgrt−/− mice were significantly deficient in the production of cytokines (IFN-γ, IL-12) and transcription factors (T-bet) known to drive effective cytotoxic T cell-mediated immunity (Figure 3B) (Garrett et al., 2009; Gerosa et al., 1996; Trinchieri, 2003; Zhang and Bevan, 2011). These differences were greatest in the CD8−CD11b+ DC subset which are highly efficient at FcRn-dependent cross-presentation (Baker et al., 2011). Furthermore, analysis of whole tissue transcripts taken from the tumor and adjacent tissue of tumor-bearing mice (Figures S3F-H) clearly demonstrated decreased transcripts at the tissue level of these pro-cytotoxicity cytokines in FcRn-deficient animals. These data thus indicate that FcRn within DC is required for the establishment of a tissue level cytokine environment within the LI that is conducive to effective CD8+ T cell activation.
In order to demonstrate that FcRn-sufficient DC were directly involved in driving anti-tumor immunity, we first conducted a series of adoptive transfer experiments. Fcgrt−/− mice receiving CD8−CD11b+ DC (Figure S3I) from AOM/DSS treated WT donors developed significantly fewer tumors than control PBS-treated Fcgrt−/− mice or Fcgrt−/− mice given Fcgrt−/− DC (Figure 3C) despite equivalent homing and persistence of donor DC from both genotypes (Figure S3J). Furthermore, administration of WT DC, but not Fcgrt−/− DC, protected Fcgrt−/− recipients from AOM/DSS-induced mortality (Figure 3C). Of considerable interest, the transfer of even a small number of FcRn-sufficient WT CD8−CD11b+ DC was able to normalize the infiltration of CD8+ T cells into adjacent and tumor LI tissue of Fcgrt−/− mice (Figure 3D), thereby confirming that no primary defect in CD8+ T cells is operating in Fcgrt−/− mice. Ex vivo assays on DC isolated from the MLN of Fcgrt−/− recipients 7 days after the transfer of WT DC further confirmed that FcRn-dependent cross-priming capacity had been restored (Figure S3K). We next validated these findings using mice bearing a floxed Fcgrt gene (FcgrtFl/Fl) (Montoyo et al., 2009) which were bred with Itgaxcre animals in order to specifically delete FcRn in DC (Figure S3L). Treatment of ItgaxcreFcgrtFl/Fl mice with AOM/DSS induced significantly more colorectal tumors than were found in their FcgrtFl/Fl littermates (Figure 3E). Of note, ItgaxcreFcgrtFl/Fl mice were also deficient in infiltration of the LI LP with CD8+ T cells (Figure 3E), thereby further supporting our hypothesis that FcRn specifically within DC could orchestrate CD8+ T cell activation within the intestine. We confirmed this by performing simultaneous DC transfer and CD8+ T cell depletion experiments which revealed that removal of CD8+ T cells from Fcgrt−/− recipients of WT CD8−CD11b+ DC undergoing AOM/DSS treatment abrogated the improvement in tumor incidence and cancer survival conferred by the WT DC (Figures 3F,G). These data thus confirm that an important mechanism of FcRn-mediated tumor protection is DC-dependent activation of CD8+ T cells via cross-priming and conditioning of the cytokine environment.
FcRn within DC enables activation of endogenous CD8+ T cells towards defined cognate tumor antigens
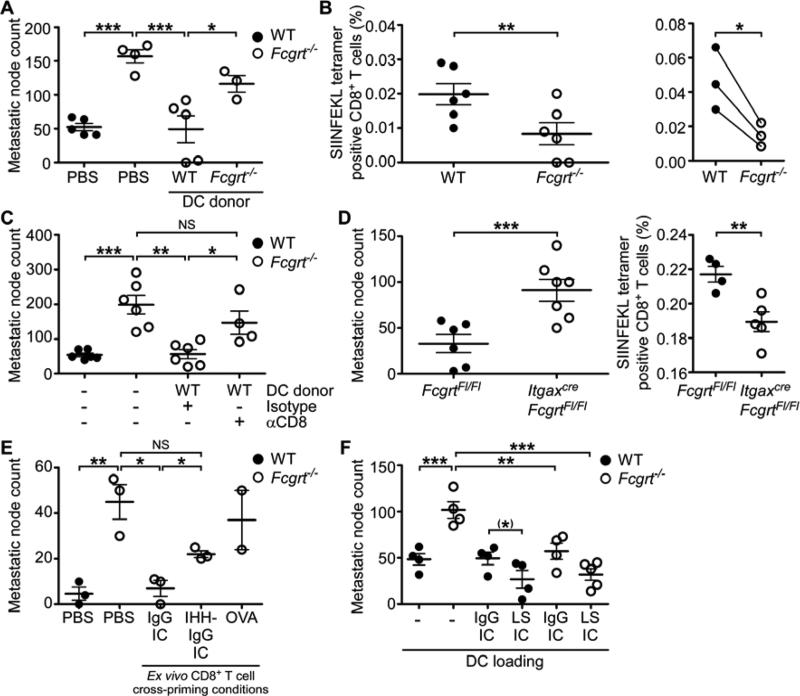
The limitations of our CRC models to examine antigen specificity led us to seek an alternative approach in order to investigate the link between tumor antigen specific IgG and CD8+ T cell responses. We turned to a pulmonary metastasis model using a melanoma cell line (B16) expressing the OVA antigen (OVA-B16) (Falo et al., 1995). Knowing that FcRn is highly expressed in the lung (Spiekermann et al., 2002), we first verified that the lungs of WT mice were enriched in CD8+ T cells in comparison to those of their Fcgrt−/− littermates (Figure S4A). These data extend the range of FcRn-regulated mucosal CD8+ T cell responses to a second site which is frequently affected by cancer (Siegel et al., 2012) and is known to engage in both FcRn-dependent immune responses (Yoshida et al., 2004) and immunological crosstalk with the intestine (Keely et al., 2011). Subsequent to i.v. administration of OVA-B16, we observed a rise in anti-OVA IgG in lung homogenates and serum from both WT and Fcgrt−/− littermates (Figures S4B,C). This is consistent with our findings of equivalent numbers of anti-OVA IgG-secreting B cells in the LN and spleens of WT and Fcgrt−/− mice (Figure S4D) and confirms that there is no defect in the local production of tumor antigen-specific IgG in FcRn deficient animals. As expected, WT mice developed considerably fewer pulmonary nodules than Fcgrt−/− mice and subcutaneous vaccination at a distant site with WT DC, but not Fcgrt−/− DC conferred protection from pulmonary metastatic seeding to Fcgrt−/− recipients. (Figure 4A). Using SIINFEKL/H-2kb tetramer staining, we established that a greater proportion of endogenous CD8+ T cells with OVA tumor antigen specificity arose in the lungs of WT mice receiving OVA-B16 tumor cells than in their Fcgrt−/− littermates (Figure 4B). In order to confirm that DC-based, FcRn-mediated tumor protection was dependent upon activation of CD8+ T cells, we chronically administered a depleting anti-CD8 antibody to Fcgrt−/− recipients immunized with WT CD8− CD11b+ DC and given OVA-B16. Whereas the transfer of WT DC significantly decreased the incidence of metastatic pulmonary nodules in Fcgrt−/− recipients, this protection was abrogated by depletion of CD8+ T cells (Figure 4C). We further demonstrated that the main locus of FcRn-mediated tumor immunosurveillance was the DC by showing that ItgaxcreFcgrtFl/Fl mice developed greater numbers of pulmonary nodules than did their FcgrtFl/Fl littermates and were less efficient in driving the expansion of tumor specific CD8+ T cells (Figure 4D). These findings identify both endogenously arising tumor-reactive IgG and cognate endogenously derived CD8+ T cells as important components of the mechanism by which DC exert FcRn-dependent tumor immune surveillance.
Figure 4. FcRn drives the induction of endogenous tumor-reactive CD8+ T cells and can be therapeutically targeted.
(A) Incidence of pulmonary metastatic nodules formed by i.v. administered OVA-expressing B16 melanoma cells (OVA-B16) in WT or Fcgrt−/− mice or Fcgrt−/− mice pre-immunized with WT or Fcgrt−/− DC. (B) Frequency of endogenously occurring OVA-specific CD8+ T cells in WT and Fcgrt−/− metastasis-bearing mice. Left panel demonstrates results from individual animals in a single experiment. Right panel shows the results of three independent experiments each with n = 3-6 mice per group. (C) Frequency of pulmonary metastases from mice treated as in (A) and given either a CD8+ T cell-depleting antibody or isotype control. (D) Frequency of pulmonary metastatic nodules and OVA-specific CD8+ T cells in the lungs of FcgrtFl/Fl and ItgaxcreFcgrtFl/Fl littermates. (E) Incidence of pulmonary metastatic nodules in WT or Fcgrt−/− mice or Fcgrt−/− mice adoptively transferred with OVA-specific CD8+ T cells primed ex vivo by DC loaded with OVA-containing IgG IC, FcRn non-binding IHH-IgG IC or soluble OVA. (F) Incidence of pulmonary nodules in OVA-B16-treated WT and Fcgrt−/− mice pre-immunized with WT DC loaded ex vivo with OVA-containing IC formed with IgG or enhanced FcRn-binding LS-IgG. Representative results of three (A,B,D) or two (C,E,F) independent experiments with n = 3-6 mice per group per experiment. All data represent mean ± s.e.m. NS = not significant. (*) p = 0.09, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S4.
We next sought to demonstrate that targeting FcRn-mediated cross-presentation with a single IgG-complexed tumor antigen could be a viable and attractive strategy for anti-tumor immunotherapy. In order to do so, we made use of a non-FcRn binding IHH-IgG, which contains three point mutations in the Fc domain that disable FcRn, but not Fcγ receptor, binding (Baker et al., 2011) and an enhanced FcRn binding LS-IgG, which contains the ‘LS’ mutation (M428L/N434S) known to increase FcRn binding while maintaining pH dependency (Claypool et al., 2004; Zalevsky et al., 2010). When OVA-reactive OT-I CD8+ T cells were stimulated ex vivo by WT DC primed with OVA-containing IgG or IHH-IgG IC and adoptively transferred to Fcgrt−/− recipient mice, only CD8+ T cells primed by IgG IC-pulsed DC protected against the development of pulmonary metastases (Figure 4E). Furthermore, immunizing mice with DC loaded with OVA-containing LS-IgG IC conferred significantly greater protection from metastasis development than did immunization with IgG IC loaded DC (Figure 4F). This is consistent with our finding that LS-IgG IC was more potent than native IgG IC at inducing cross-priming of low dose antigen in vitro (Figure S4E). Collectively, these data demonstrate that targeting the immunostimulatory potential of FcRn using complexes formed from a single defined tumor antigen and IgG or FcRn-binding-enhanced IgG is a tractable and effective anti-tumor therapeutic approach.
Dendritic cell FcRn enables homeostatic CD8+ T cell activation and Tc1 cytokine secretion in the LI
Our discovery that FcRn-mediated anti-tumor immunity in the LI occurs in the absence of preexisting inflammation suggested that FcRn might be playing an active role in intestinal immunosurveillance before the onset of cancer development, We thus investigated whether FcRn regulates CD8+ T cell activation in the LI under homeostatic conditions. Similar to our observation in AOM/DSS-treated mice, and despite the well-known differences in circulating IgG concentrations (Roopenian et al., 2003), similar quantities of IgG were present in the LI and MLN tissue of both WT and Fcgrt−/− littermates under steady-state conditions (Figure 5A). These results confirmed that the susceptibility of Fcgrt−/− mice to tumor development could not be attributed to homeostatic local IgG deficiency. In spite of these comparable tissue IgG amounts, however, the LI LP of WT mice contained greater quantities of CD8+ T cells, but not other lymphocyte subsets, relative to that observed in Fcgrt−/− mice (Figure 5B). A similar deficiency in CD8+ T cell infiltration into the LI LP was present in untreated ItgaxcreFcgrtFl/Fl mice compared to their littermate controls (Figure 5C). Moreover, CD8+ T cells from the LI LP of WT mice not only secreted more IFN-γ, IL-10 and TNF upon restimulation in comparison to T cells obtained from Fcgrt−/− littermates (Figure 5D) but also expressed more activation and cytotoxicity associated cytokines when assessed immediately after isolation (Figure 5E). Adoptive transfer of congenic CD8+ T cells into WT and Fcgrt−/− recipients indicated that not only did a greater number of transferred T cells accumulate in the LI LP of WT mice 10 days after transfer but that these also upregulated significantly more of the activation marker CD44 (Figure S5A), consistent with our findings of deficient CD8+CD44+CD62L+ T cells in Fcgrt−/− tumor-bearing mice (Figure S2F). In contrast, CD8+ T cells from WT or Fcgrt−/− donors transferred to congenic WT recipients homed equally well to the LI LP (Figure S5B), thereby confirming that the effect of FcRn is within the local tissue microenvironment rather than being intrinsic to the T cells.
Figure 5. FcRn within DC enables homeostatic CD8+ T cell activation and IL-12 production in the LI.
(A) IgG isotype content of the serum and LI or MLN homogenates in untreated WT and Fcgrt−/− littermates. (B) CD8+ T cell frequency of the LI LPL fraction of untreated WT and Fcgrt−/− littermates in a single experiment (left panels) or across three independent experimental repeats (right panel). (C) Frequency of CD8+ T cells in the LPL fraction of FcgrtFl/Fl and ItgaxcreFcgrtFl/Fl littermates. (D) Cytokine secretion by CD8+ T cells sorted from LI LP of untreated WT and Fcgrt−/− mice following 24 h restimulation with anti-CD3 and anti-CD28. (E) Transcript profiles of CD8+ T cells sorted from LI LP of untreated littermate control mice. (F) Cytokine secretion from 24 h tissue explant cultures of the MLN and LI of untreated WT and Fcgrt−/− mice. (G) Transcript profiles of sorted CD8−CD11b+ DC from the MLN of untreated littermates. Representative results of three independent experiments with n = 3-5 mice per group per experiment. All data represent mean ± s.e.m. NS = not significant. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S5.
Given that efficient CD8+ T cell activation requires an appropriate cytokine environment, we next examined the local tissue cytokine milieu of Fcgrt−/− mice under homeostatic conditions. Tissue explant cultures (Figure 5F) and analysis of tissues RNA transcripts (Figure S5C) indicated that in the absence of FcRn, the MLN and LI were deficient in their ability to produce cytotoxicity-promoting IL-12 and TNF. By examining the cytokine profiles of sorted DC from the MLN of untreated WT and Fcgrt−/− littermates, we observed a similar dependence for FcRn on the expression of IFN-γ, IL-12p35, T-bet and TNF, but not IL-23p19, in CD8−CD11b+ DC (Figure 5G). This suggests that FcRn within the CD8−CD11b+ subset of tissue-associated DC is responsible for establishing a cytokine milieu conducive to CD8+ T cell activation and of thus promoting tumor immunosurveillance in the LI. Similarly, CD4+ T cells from the LI of untreated Fcgrt−/− mice were deficient in secretion of Th1 cell-associated cytokines upon anti-CD3 and anti-CD28 restimulation despite being present in equal amounts in WT and Fcgrt−/− mice (Figures S5D,E). These data suggest that IgG IC ligation of FcRn in DC contributes to the establishment of homeostatic Th1 and T cytotoxic-1 (Tc1) cell polarization as well as CD8+ T cell function in the LI.
Multimeric IgG IC ligation of FcRn in DC induces the production of IL-12
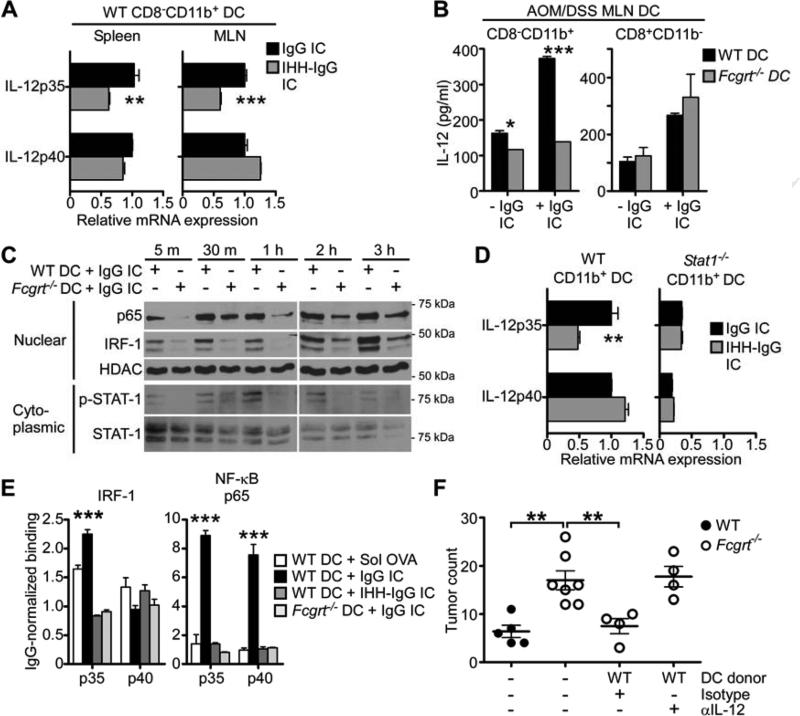
Knowing that IL-12 is a potent enhancer of CD8+ T cell-mediated immunity (Gerosa et al., 1996; Trinchieri, 2003) and having consistently observed greater quantities of IL-12 in DC, particularly the CD8−CD11b+ subset, from the mucosal tissues of WT compared to Fcgrt−/− littermates, we next investigated the effects of ligation of FcRn by IgG IC or FcRn non-binding IHH-IgG IC on IL-12 secretion. Incubation of WT CD8−CD11b+ DC from the MLN and spleen of untreated WT mice with FcRn binding IgG IC, but not IHH-IgG IC, led to the direct induction of IL-12p35 transcripts (Figure 6A). Similarly, IgG IC stimulation of WT but not Fcgrt−/− CD8−CD11b+ DC isolated from the MLN of AOM/DSS treated mice resulted in increased IL-12 secretion (Figure 6B). Mechanistically, we observed that stimulation of Fcgrt−/− DC with IgG IC led to considerably less phosphorylation of the Th1 cell-associated transcription factor STAT-1 (Antonios et al., 2010) than was seen in WT DC (Figure 6C). We confirmed that STAT-1 activation was an important component of FcRn-induced IL-12 production by treating CD8−CD11b+ DC isolated from Stat1−/− mice with IgG IC or IHH-IgG IC and observed that, as for IHH-IgG IC, IgG IC failed to induce IL-12p35 in the absence of STAT-1 (Figure 6D). We further showed that stimulation of WT CD8−CD11b+ DC with IgG IC led to significantly greater nuclear translocation and IL-12p35 promoter binding of both interferon regulatory factor-1 (IRF-1) and NF-κB p65 than was observed in Fcgrt−/− DC or upon stimulation with IHH-IgG IC (Figures 6C,E) and confirmed that this was MYD88 independent (Figures S6A,B). IgG IC ligation of FcRn in DC is thus able to directly induce the production of the potent Th1 and Tc1 cell-associated cytokine IL-12.
Figure 6. IgG IC ligation of FcRn in CD8−CD11b+ DC induced IL-12 production via activation of a signaling cascade.
(A) Induction of IL-12p35 upon ex vivo stimulation of WT CD8−CD11b+ DC from the spleen or MLN with IgG IC or FcRn non-binding IHH-IgG IC for 6 h. (B) IL-12 secretion after 24 h IgG IC stimulation of CD8−CD11b+ and CD8+CD11b− DC sorted from the MLN of AOM/DSS-treated WT and Fcgrt−/− mice. (C) Phosphorylation of STAT-1 and nuclear translocation of IRF-1 and NF-κB p65 upon IgG IC stimulation of DC isolated from WT or Fcgrt−/− mice. (D) IL-12 transcript production by WT or Stat-1−/− CD8−CD11b+ DC following stimulation with IgG or IHH-IgG IC for 6 h. (E) Binding of IRF-1 and NF-kB p65 to the promoters of IL-12p35 and IL-12p40 upon stimulation of WT or Fcgrt−/− DC with IgG IC or IHH-IgG IC for 4 h. (F) Tumor incidence in mice adoptively transferred with WT DC and treated with a neutralizing anti-IL-12 antibody or isotype control. Representative results of three (A-E) or one (F) independent experiments with n = 3-7 mice per group per experiment. All data represent mean ± s.e.m. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S6.
In order to demonstrate that FcRn-mediated induction of IL-12 by DC contributes to the ability of this receptor to promote anti-tumor immune surveillance, we next performed IL-12 neutralization experiments in which Fcgrt−/− mice adoptively transferred with WT DC were subjected to AOM/DSS treatment in the presence of a neutralizing anti-IL-12 antibody or isotype control (Wysocka et al., 1995). Whereas WT DC were able to decrease the incidence of colorectal tumors in Fcgrt−/− recipients down to the numbers seen for WT control mice, this protection was completely abrogated when mice were treated with anti-IL-12 (Figure 6F). However, in vitro co-culture assays demonstrated that FcRn-dependent CD8+ T cell priming was independent of IL-12 production (Figure S6C), indicating that the cross-presenting and cytokine inducing functions of FcRn are independent. Collectively, these data indicate that FcRn-driven DC-mediated tumor protection results from a dual ability to promote cross-presentation of IgG IC-delivered antigen to CD8+ T cells as well as to induce secretion of IL-12 which, notably, can augment the cytotoxic capacity of T cells once primed.
FcRn expressing DC predict survival in human CRC and secrete FcRn-dependent IL-12
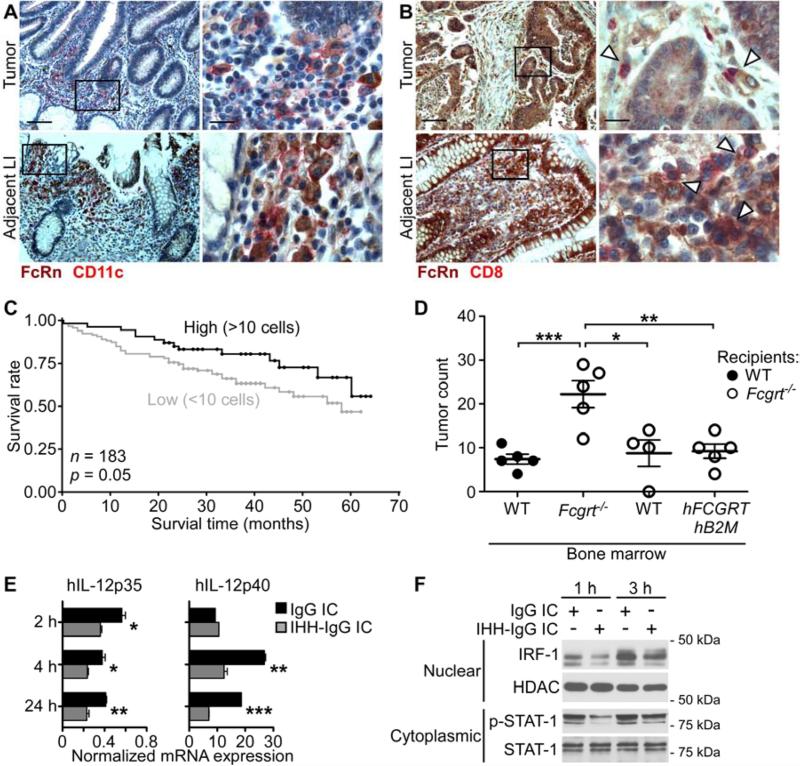
To examine whether our observations in mice were relevant to the development of human CRC, we evaluated the presence of FcRn-expressing DC in 50 matched cases of human CRC and adjacent normal tissue utilizing immunohistochemical staining for FcRn and CD11c. As shown in Figures 7A and S7A, FcRn+CD11c+ cells were clearly present in the stroma of both tumor LI (upper panels) and adjacent normal LI (lower panels) of CRC patients. Furthermore, a direct interaction of FcRn+ stromal cells with CD8+ T cells was observed in both tumor LI (upper panels) and CRC-adjacent normal LI (lower panels) (Figures 7B and S7B). Importantly, the frequency of FcRn+CD11c+ DC correlated positively with the presence of CD8+ T cells in the CRC-adjacent normal stroma (Figure S7C). In order to determine if the presence of FcRn+CD11c+ cells in the tumor microenvironment had an impact on patient survival, we stained a well characterized tissue microarray of 183 human CRC cases for these cells and analyzed their impact on disease outcome (Karamitopoulou et al., 2011). Kaplan-Meier survival curves indicated that patients with ≥ 10 FcRn+CD11c+ cells per punch had significantly longer survival times over a 70 month follow up than did those with < 10 FcRn+CD11c+ cells (Figure 7C). Furthermore, increasing numbers of FcRn+CD11c+ cells were found to have a positive effect on patient survival in univariate proportional hazard analysis (p = 0.0333), an effect which was maintained in a multivariable analysis (p = 0.0388) when adjusting for the indicated clinical parameters (Figure S7D). Collectively, these studies demonstrate that FcRn-expressing DC localize to both the CRC and CRC-associated adjacent microenvironment, correlate with the infiltration of CD8+ T cells into the tumor tissue and predict improved prognosis for CRC patients.
Figure 7. FcRn expressing DC predict survival in human CRC and secrete IL-12 upon FcRn stimulation.
(A) Double immunohistochemical staining of FcRn+CD11c+ DC in the stroma of CRC (upper panels) and CRC-adjacent normal LI (lower panels). FcRn = brown, CD11c = red. Scale bar left panels = 100 μm. Scale bar right panels = 20 μm. (B) Colocalization of FcRn+ DC (brown) and CD8+ T cells (red) in stroma of CRC (upper panels) and CRC-adjacent normal LI (lower panels). Arrowheads indicate areas of colocalization. (C) Kaplan Meier survival curves of 183 patients with high (≥10 per core) and low (≤ 10 per core) tumor infiltration by CD11c+FcRn+ cells. (D) Incidence of tumors in chimeric mice treated with AOM/DSS. WT recipients were reconstituted with WT bone marrow. Fcgrt−/− recipients were reconstituted with Fcgrt−/−, WT or hFCGRT-hB2M-mFcgrt−/− bone marrow. Representative result of two independent experiments with n = 4-5 mice per group per experiment. (E) hIL-12p35 and hIL-12p40 transcript expression in hMoDC upon stimulation with FcRn-binding (IgG IC) or FcRn non-binding (IHH-IgG IC) immune complexes. (F) Nuclear translocation of IRF-1 and phosphorylation of STAT-1 in hMoDC upon stimulation with IgG IC or IHH-IgG IC. Data in panels A-B are representative of a total of 50 matched CRC and adjacent normal LI pairs. Data in panels E-F are representative of six donors processed in pairs in each of three independent experiments. All data represent mean ± s.e.m. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. See also Figure S7.
In order to demonstrate a direct causative link between human FcRn and anti-tumor immunosurveillance, we generated chimeric mice in which irradiated Fcgrt−/− recipients were reconstituted with bone marrow from donors that were either WT, Fcgrt−/− or expressed human FcRn and β2-microglobulin (β2M) on an Fcgrt−/− background (hFCGRT-hB2M-mFcgrt−/−) and thus possess only the human form of the receptor (Roopenian et al., 2003). When the chimeras were subjected to AOM/DSS treatment, Fcgrt−/− mice reconstituted with Fcgrt−/− bone marrow developed far greater numbers of colorectal tumors than did Fcgrt−/− mice reconstituted with either WT or hFCGRT-hB2M-mFcgrt−/− bone marrow (Figure 7D). Furthermore, CD8+ T cell infiltration in the LI LP of tumor-bearing mice was restored to WT levels in Fcgrt−/− mice possessing human-FcRn expressing hematopoietic cells (Figure S7E). Thus, human FcRn is equally as capable of orchestrating anti-tumor immunosurveillance as is its murine ortholog.
We lastly sought to confirm that the intracellular mechanisms we had demonstrated in mouse DC were also operative in their human equivalents using human monocyte-derived DC (hMoDC), which are phenotypically akin to the murine CD8−CD11b+ DC subset which engage in efficient FcRn-dependent cross-priming (Figures S7F,G) (Baker et al., 2011; Collin et al., 2011). As shown in Figure 7E, stimulation of hMoDC by IgG IC leads to greater production of both IL-12p35 and IL-12p40 than stimulation with non-FcRn-binding IHH-IgG IC. Furthermore, IgG IC induced greater phosphorylation of STAT-1 and nuclear translocation of IRF-1 after both 1 h and 3 h than did IHH-IgG IC (Figures 7F and S7H). Together, these data support the importance of human FcRn function in DC in enabling anti-tumor immunity through its ability to regulate IL-12 production and CD8+ T cell activation.
DISCUSSION
The findings presented here identify a key physiological role for FcRn-mediated cross-priming in driving homeostatic activation of CD8+ T cells and in CD8+ T cell-mediated anti-tumor immune surveillance in the LI and lung. We have clearly established in multiple tumor models that genetic deletion of FcRn increases susceptibility to carcinogenesis at these mucosal sites. Transfer of CD8+ T cells primed under FcRn-sufficient conditions or of WT FcRn-bearing DC was capable of rescuing FcRn-deficient animals from both a high tumor burden and tumor-induced mortality. This depended upon IgG IC-mediated ligation of FcRn within mucosal DC which enabled the priming of tumor antigen-specific endogenous CD8+ T cells via its dual induction of antigen cross-presentation and the production of immune-enhancing cytokines such as IL-12. We have also confirmed the human relevance of our findings by demonstrating that FcRn-expressing human DC respond to IgG IC stimulation with the production of IL-12, that FcRn+ DC localize to the CRC microenvironment where their ability to induce anti-tumor immunity contributes to improved patient survival, and that human FcRn in hematopoietic cells can substitute for its mouse ortholog in protecting against the development of CRC. Our findings are furthermore consistent with a model in which IgG IC binding to FcRn within mucosal DC directs not only the intracellular trafficking of IgG IC but also the previously unrecognized organization of a signaling cascade which enhances the secretion of cytotoxicity-promoting cytokines. These features of FcRn biology uniquely enable potent anti-tumor immunosurveillance which requires only small amounts of antigen and is capable of overcoming the immunoregulatory environment characteristic of intestinal and, potentially, other mucosal tissues (MacDonald et al., 2011). Moreover, our demonstration that FcRn-deficiency does not result in decreased quantities of tissue-associated IgG but rather diminished numbers and function of CD8+ T cell under homeostatic conditions further suggests that a major function of FcRn in tissues is in the regulation of cell mediated immunity rather than the protection of IgG from catabolism which is observed systemically.
An important prerequisite for FcRn-mediated tumor protection is the presence of IgG capable of recognizing a tumor antigen and initiating a cascade of FcRn-dependent anti-tumor responses which will feed in to the recently described “Cancer-Immunity Cycle” (Brichory et al., 2001; Chen and Mellman, 2013; Desmetz et al., 2011). The presence of appreciable quantities of endogenous IgG autoantibodies reactive or cross-reactive towards altered or abnormally expressed tumor antigens is well documented and these are likely to serve as the initiators for FcRn-mediated tumor protection. Specifically, the accelerated release of tumor-associated antigens, either alone or as part of cellular debris or apoptotic bodies, which is caused by increased rates of tumor cell death (Kepp et al., 2009), will promote the formation of immune complexes with endogenous tumor-reactive or phospholipid-specific autoantibodies. Subsequently, the concomitant induction of IL-12 production resulting from FcRn ligation by IgG IC can be expected to further amplify local FcRn-dependent anti-tumor immune responses since IL-12 is a potent inducer of humoral immunity (Metzger, 2010). A key physiological role for FcRn within DC therefore appears to be the integration of humoral and cellular adaptive immune responses capable of targeting mucosal malignancies and, undoubtedly, a host of intracellular microbial infections.
Several intriguing aspects of FcRn biology emerge from our work. The first of these is that FcRn-dependent immune regulation is operative under homeostatic conditions and is critical for establishing baseline colonic CD8+ T cell activation and function. We predict that such homeostatic responses are largely directed at microbial antigens given that IgG with antibacterial specificities are abundantly present in the intestine (Macpherson et al., 1996) and that the pathways described here may also play a critical role in immune surveillance against acute and chronic microbial infections (Yoshida et al., 2006) Secondly, our work highlights the differential role played by FcRn in different body compartments. Whereas FcRn is critical for maintaining IgG persistence within the circulatory system (Roopenian et al., 2003), our observations indicate that within tissues, FcRn is predominantly involved in the regulation of local immune responses. In addition to the inadequate immune activation seen in the intestines of Fcgrt−/− mice, this idea is supported by our findings that Fcgrt−/− mice were only minimally deficient in tissue IgG quantities where equal amounts of IgG producing cells were able to compensate for the lack of FcRn-mediated IgG protection. Finally, we have demonstrated the feasibility and effectiveness of targeting FcRn-mediated anti-cancer immunosurveillance pathways using a single defined tumor antigen in complex with native IgG or IgG engineered for enhanced FcRn binding. In addition to enabling antigen specific CD8+ T cell mediated immunity after tumor onset, such therapies also have the potential to promote tumor immunosurveillance in healthy high risk individuals by enhancing the baseline cytotoxic potential of the intestine towards common tumor antigens. While a large body of knowledge exists pertaining to the dynamics of FcRn–IgG interaction (Vaughn et al., 1997) and the ability to engineer IgG with increased affinity for FcRn is currently available (Mi et al., 2008; Zalevsky et al., 2010), targeting of FcRn has yet to be exploited by current DC-based vaccination strategies (Tacken et al., 2007) despite the growing interest in DC antibody vaccines (Palucka and Banchereau, 2013).
The pleiotropic nature of FcRn and its wide-reaching influence on normal physiology remain poorly understood. Whereas the main function of FcRn systemically is the protection of monomeric IgG from catabolism, a major role in tissues, particularly mucosal tissues replete with IgG, appears to be one of immunological activation upon ligation by multimeric IgG IC. To this effect, FcRn participates in the organization not only of an antigen presentation cascade but also of a signaling cascade that is associated with innate effector immune function. As shown here, a major consequence of this role for FcRn is the efficient induction of anti-tumor immunity. These studies show that FcRn functions in anti-tumor immunosurveillance through the induction (via IL-12) and instruction (via cross-presentation) of CD8+ T cells. Developing a greater understanding of the nuances of FcRn-modulated immune activation, particularly at the tissue level where FcRn in dendritic cells promotes the immunogenic catabolism of IgG-complexed antigens, holds considerable promise for the development of new therapies against mucosal diseases.
EXPERIMENTAL PROCEDURES
Mice and tumor models
Fcgrt−/− mice (Roopenian et al., 2003), deficient in FcRn, on a C57BL/6 background were originally purchased from The Jackson Laboratory. FcgrtFl/Fl mice were a kind gift of Dr. E. Sally Ward (University of Texas Southwestern Medical Center) (Montoyo et al., 2009). hFCGRT-hB2M-mFcgrt−/− mice have been described previously (Yoshida et al., 2004). Additional mouse strains are described in Supplemental Experimental Procedures. All procedures were approved by the Harvard Medical Area Standing Committee on Animals. AOM, AOM/DSS, Apcmin/+ and lung metastasis tumor models were performed using previously described protocols (LeibundGut-Landmann et al., 2008; Meunier et al., 2009; Wirtz et al., 2007) and are described fully in the Supplemental Experimental Procedures.
Microbiota analysis
Analysis of the microbiota was conducted as outlined in Supplemental Experimental Procedures using previously published methods (Uronis et al., 2011).
Human DC and tissue experiments
Human leukopacks were obtained from the Kraft Family Blood Donor Center of the Dana-Farber Cancer Institute and Brigham and Women's Hospital. hMoDC were derived as previously described (Zeissig et al., 2010) for 5 days in 1000 U/ml hGM-CSF and 500 U/ml hIL-4. During the final 24 h of culture, 100 U/ml IFN-γ was added. IgG and IHH-IgG stimulations were carried out as described above. One set of human CRC tissue micro-arrays containing 50 samples of matched tumor and adjacent normal tissue from the same donors were obtained from BioMax USA. A second TMA containing multiple punches from each of 220 patients and for which survival data was available has previously been described (Karamitopoulou et al., 2011). Tissue was stained using the EnVision G2 Doublestain System, Rabbit/Mouse (DAB+/Permanent Red) Kit from Dako following heat-induced epitope retrieval in 10mM citrate, 1mM EDTA, 0.05% Tween (pH 6.0). Primary antibodies were anti-hFCGRT (HPA012122, Sigma Aldrich), anti-hCD11c (Novocastra) and anti-hCD8 (Dako) all of which were used at 1/50. Experiments were performed under Brigham and Women's Hospital Review Board approval.
Biochemical methods
Flow cytometry, RNA analysis, IgG quantification, ChIP, Western blotting, ELISpot and in vitro co-culture experiments were conducted as outline in Supplemental Experimental Procedures and as previously described (Baker et al., 2011).
Statistical analyses
All data are expressed as mean ± s.e.m. Unless otherwise specified, data was analyzed using two-tailed unpaired Student's t tests. Significance of results across independent experiments was assessed by pairwise Student's t test. As indicated where relevant, non-normally distributed data was assessed using Mann-Whitney test and survival for mouse experiments was evaluated using Logrank test or Chi-Squared test. The human survival analysis was performed with the Kaplan–Meier method and the two curves were compared with the log rank test. Subsequently, FcRn+CD11c+ status was entered into uni- and multivariate Cox regression analysis. Hazard ratios (HR) and 95% confidence intervals (CI) were used to determine the prognostic effect of FcRn+CD11c+ cell numbers on survival time. All analyses were carried out using GraphPad Prism software (GraphPad Software, Inc.).
Supplementary Material
Highlights.
FcRn in colonic DC confers homeostatic CD8+ T cell activation and tumor protection
IgG immune complex cross-linking of FcRn in DC promotes Th1/Tc1 cytokine secretion
FcRn protects serum IgG but promotes immunogenic catabolism of tissue IgG complexes
Increased FcRn+CD11c+ DC in human colorectal tumors predicts improved survival
ACKNOWLEDGEMENTS
The authors would like to thank Jennifer Cusick and Michal Pyzik for expert editorial assistance as well as Son Huynh, Victoria Thiele, Samantha Torquato, Mario Sablon, and Victoria G. Aveson for excellent technical assistance. FcgrtFl/Fl mice were a kind gift of Dr. E. Sally Ward (University of Texas Southwestern Medical Center). OVA-expressing B16 melanoma cells (OVA-B16) were a generous gift of Dr. Kenneth Rock (University of Massachusetts Medical School). IL-12 neutralizing antibody was a kind gift of Dr. Giorgio Trinchieri (National Cancer Institute). This work was supported by the Canadian Institutes of Health Research (K.B); the Deutsche Forschungsgemeinschaft (RA 2040/1-1) (T.R.); the Crohn's & Colitis Foundation of America (M.B.F.); the High Pointe Foundation (R.S.B.); the National Institutes of Health T32 DK007737 (J.C.A.); DK73338 and DK47700 (C.J.); DK53056, DK053162, DK088199 and DK044319 (R.S.B.) and the Harvard Digestive Diseases Center NIH P30DK034854 (W.I.L. and R.S.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antonios D, Rousseau P, Larange A, Kerdine-Romer S, Pallardy M. Mechanisms of IL-12 Synthesis by Human Dendritic Cells Treated with the Chemical Sensitizer NiSO4. J. Immunol. 2010;185:89–98. doi: 10.4049/jimmunol.0901992. [DOI] [PubMed] [Google Scholar]
- Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/delta716 Cdx2+/− compound mutant mice. Nat. Genet. 2003;35:323–330. doi: 10.1038/ng1265. [DOI] [PubMed] [Google Scholar]
- Arthur JC, Jobin C. The struggle within: Microbial influences on colorectal cancer. Inflamm. Bowel Dis. 2011;17:396–409. doi: 10.1002/ibd.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer IO, Grosch L, Hardorfer C, Roder A. Ulcerative colitis specific cytotoxic IgG-autoantibodies against colonic epithelial cancer cells. Gut. 1988;29:1639–1647. doi: 10.1136/gut.29.12.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Qiao S-W, Kuo TT, Aveson VG, Platzer B, Andersen J-T, Sandlie I, Chen Z, de Haar C, Lencer WI, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9927–9932. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichory F, Beer D, LeNaour F, Giordano T, Hanash S. Proteomics-based Identification of Protein Gene Product 9.5 as a Tumor Antigen That Induces a Humoral Immune Response in Lung Cancer. Cancer Res. 2001;61:7908–7912. [PubMed] [Google Scholar]
- Chen Daniel S., Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, Lencer WI, Blumberg RS. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol. Biol. Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill AE, Newcomb PA, Poole EM, Hutter CM, Makar KW, Duggan D, Potter JD, Ulrich CM. Genetic Variation in Inflammatory Pathways Is Related to Colorectal Cancer Survival. Clin. Cancer Res. 2012;17:7139–7147. doi: 10.1158/1078-0432.CCR-11-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Bigley V, Haniffa M, Hambleton S. Human dendritic cell deficiency: the missing ID? Nat. Rev. Immunol. 2011;11:575–583. doi: 10.1038/nri3046. [DOI] [PubMed] [Google Scholar]
- Desmetz C, Mange A, Maudelonde T, Solassol J. Autoantibody signatures: progress and perspectives for early cancer detection. J. Cell Mol. Med. 2011;15:2013–2024. doi: 10.1111/j.1582-4934.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falo LD, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med. 1995;1:649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, Lord GM, Glickman JN, Glimcher LH. Colitis-Associated Colorectal Cancer Driven by T-bet Deficiency in Dendritic Cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–389. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- Karamitopoulou E, Zlobec I, Panayiotides I, Patsouris ES, Peros G, Rallis G, Lapas C, Karakitsos P, Terracciano LM, Lugli A. Systematic analysis of proteins from different signaling pathways in the tumor center and the invasive front of colorectal cancer. Hum. Pathol. 2011;42:1888–1896. doi: 10.1016/j.humpath.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2011;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Curr. Opin. Oncol. 2009;21:71–76. doi: 10.1097/CCO.0b013e32831bc375. [DOI] [PubMed] [Google Scholar]
- Kijanka G, Hector S, Kay EW, Murray F, Cummins R, Murphy D, MacCraith BD, Prehn JHM, Kenny D. Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut. 2010;59:69–78. doi: 10.1136/gut.2009.178574. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Qiao SW, Yoshida M, Baker K, Lencer WI, Blumberg RS. An FcRn-dependent role for anti-flagellin immunoglobulin G in pathogenesis of colitis in mice. Gastroenterology. 2009;137:1746–1756. e1741. doi: 10.1053/j.gastro.2009.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc'h A, Jalil A, Vergnon I, Chansac BLM, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- Ma Y, Aymeric L, Locher C, Kroemer G, Zitvogel L. The dendritic cell-tumor crosstalk in cancer. Curr. Opin. Immunol. 2011;23:146–152. doi: 10.1016/j.coi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of Homeostasis and Inflammation in the Intestine. Gastroenterology. 2011;140:1768–1775. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSween JM, Eastwood SL. Immunoglobulins associated with human tumours in vivo: igg concentrations in eluates of colonic carcinomas. Br. J. Cancer. 1980;42:503–509. doi: 10.1038/bjc.1980.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DW. Interleukin-12 as an adjuvant for induction of protective antibody responses. Cytokine. 2010;52:102–107. doi: 10.1016/j.cyto.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier C, Cai J, Fortin A, Kwan T, Marquis JF, Turbide C, Van Der Kraak L, Jothy S, Beauchemin N, Gros P. Characterization of a major colon cancer susceptibility locus (Ccs3) on mouse chromosome 3. Oncogene. 2009;29:647–661. doi: 10.1038/onc.2009.369. [DOI] [PubMed] [Google Scholar]
- Mi W, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, Ward ES. Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. J. Immunol. 2008;181:7550–7561. doi: 10.4049/jimmunol.181.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2788–2793. doi: 10.1073/pnas.0810796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm John B., Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al. IL-10 Elicits IFN-gamma-Dependent Tumor Immune Surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9337–9342. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revaz V, Nardelli-Haefliger D. The importance of mucosal immunity in defense against epithelial cancers. Curr. Opin. Immunol. 2005;17:175–179. doi: 10.1016/j.coi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA. Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Uronis JM, Arthur JC, Keku T, Fodor A, Carroll IM, Cruz ML, Appleyard CB, Jobin C. Gut microbial diversity is reduced by the probiotic VSL#3 and correlates with decreased TNBS-induced colitis. Inflamm. Bowel Dis. 2011;17:289–297. doi: 10.1002/ibd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn DE, Milburn CM, Penny DM, Martin WL, Johnson JL, Bjorkman PJ. Identification of critical IgG binding epitopes on the neonatal Fc receptor. J. Mol. Biol. 1997;274:597–607. doi: 10.1006/jmbi.1997.1388. [DOI] [PubMed] [Google Scholar]
- Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat. Rev. Cancer. 2009;9:489–499. doi: 10.1038/nrc2645. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IWL, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S, Dougan SK, Barral DC, Junker Y, Chen Z, Kaser A, Ho M, Mandel H, McIntyre A, Kennedy SM, et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J. Clin. Invest. 2010;120:2889–2899. doi: 10.1172/JCI42703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan Michael J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.