Abstract
Background
Recent studies in HIV-infected men report an association between low vitamin D (25OH-D) and CD4 recovery on HAART. We sought to test this relationship in the Women’s Interagency HIV Study (WIHS).
Methods
We examined 204 HIV-infected women with advanced disease, who started HAART after enrollment in the WIHS. We measured vitamin D (25OH-D) levels about 6 months prior to HAART initiation. The relationship between CD4 recovery (defined as increases of ≥50, 100, and 200 cells at 6, 12, and 24 months) and exposure variables was examined using logistic regression models at 6, 12 and 24 months post-HAART initiation in unadjusted and adjusted analyses, and using multivariable longitudinal Generalized Estimating Equations (GEE). Vitamin D insufficiency was defined as 25OH-D levels at least 30 ng/ml.
Results
The majority were non-Hispanic black (60%) and had insufficient vitamin D levels (89%). In adjusted analyses, at 24 months after HAART, insufficient vitamin D level (OR 0.20, 95% CI 0.05–0.83) was associated with decreased odds of CD4 recovery. The undetectable viral load (OR 11.38, 95% CI 4.31–30.05) was associated with CD4 recovery. The multivariable GEE model found that average immune reconstitution attenuated significantly (P <0.01) over time among those with insufficient vitamin D levels compared with those with sufficient vitamin D levels.
Conclusion
Vitamin D insufficiency is associated with diminished late CD4 recovery after HAART initiation among US women living with advanced HIV. The mechanism of this association on late CD4 recovery may be late vitamin D-associated production of naive CD4 cells during immune reconstitution.
Keywords: antiretroviral therapy, HIV, immune reconstitution, vitamin D, women
Introduction
Vitamin D plays a role in overall health, and vitamin D deficiency has been linked to cellular immunity, cardiovascular disease, autoimmune disease, insulin resistance, depression, and impaired control of infections, such as tuberculosis [1–4]. The active form of vitamin D, 1,25-(OH)2D, has anti-inflammatory activity [5] and there are numerous studies describing its role in the regulation of human T-cell and antigen-presenting cell (APC) functions [6].
Cross-sectional studies in HIV-infected patients have reported high rates of vitamin D insufficiency and deficiency [7–11] and we found that 60% of participants in the Women’s Interagency HIV Study (WIHS) had vitamin D deficiency with African American race being a strong predictor for this deficiency [11]. In two small cross-sectional studies, vitamin D-deficient HIV patients had significantly lower CD4 counts [12,13]. In one study in HIV and Mycobacterium Avium Complex coinfected patients [12], there was a strong inverse correlation between TNF-α and 1,25(OH)2D and the authors concluded that this might further impair immune response and represent an important feature of the pathogenesis of HIV-related immunodeficiency. A proportion of individuals who start HAART fail to achieve adequate CD4 cell reconstitution despite sustained viral suppression, a recent study in HIV patients showed a correlation between vitamin D levels and CD4 T-cell recovery [14] but did not assess for associations over time. In our study, we aimed to determine the association of vitamin D insufficiency with immune recovery over time after HAART initiation in HIV-infected women.
Methods
The WIHS is a prospective cohort study of HIV-infected and uninfected at-risk women from six sites. Women are seen semi-annually for an interview and a physical exam with collection of blood and genital specimens. Informed consent was obtained from all participants in accordance with the US Department of Health and Human Services guidelines and the institutional review boards of participating institutions. The cohort was designed to reflect the demographics of the HIV epidemic among women in the United States [15].
Of the 1357 HIV-infected women who started HAART during the WIHS study, we found that 460 of these women had CD4 less than 200 at last pre-HAART visit. We examined HIV-infected women who started HAART during the WIHS study and who had CD4 data 6 and 12 months after HAART initiation (n = 348). The patients were then excluded if there was no available data for 6 and 12 month visits (n = 10) as well as if there were no vitamin D test results from last pre-HAART visit (n = 79). Women with the last preantiretroviral therapy (pre-ART) visit occurring prior to May 1, 1996 were excluded to ensure a cohort treated after the advent of HAART (n = 55). Patients who were started on any antiretroviral therapy after this date were included, even if it was not HAART therapy. A total of 204 HIV-infected women were included in the analysis. The primary outcome was CD4 count obtained at 6, 12 and 24 months after HAART initiation.
Vitamin D (25OH-D) testing was performed on stored sera from their last pre-HAART visit by Quest Diagnostics on frozen sera stored at −70°C using the liquid chromatography/mass spectroscopy/tandem spectroscopy (LC/MS/TS) method. The LC/MS/TS method is sensitive with the average inter-assay coefficient of variation percentage across the analytical range of 7%. Sufficient vitamin D was defined as more than 30 ng/ml and vitamin D insufficiency as less than or equal to 30 ng/ml [2].
Statistical analyses
In univariate analysis, differences in categorical variables were assessed using the Pearson’s chi-square tests, or their nonparametric equivalent, and differences in continuous variables were assessed with the nonparametric Kruskal–Wallis test. The relationship between CD4 recovery and vitamin D was examined at 6, 12 and 24 months post-HAART initiation. The relationship between CD4 recovery and exposure variables was examined using logistic regression models at 6, 12 and 24 months post-HAART initiation in unadjusted and adjusted analyses, and with longitudinal Generalized Estimating Equations (GEE). In the logistic regression models, CD4 recovery was defined as an increase of 50 cells or less at 6 months, 100 cells or less at 12 months, and 200 cells or less at 24 months based on previous studies defining suboptimal recovery of CD4 count in HIV-infected patients [16–20]. CD4 count over time was measured as a continuous variable in the GEE analysis. Both analyses were adjusted for the following covariates in addition to vitamin D status: age, race/ethnicity, BMI, any history of previous antiretroviral use, baseline viral load, viral load at each time point and WIHS site location. A P-value less than 0.05 was considered significant. Statistical analyses were conducted using SAS version 9.2 and IBM SPSS Statistics 19.
Results
Table 1 shows the characteristics of the 204 HIV-infected women included in the study. The majority of women were non-Hispanic black (60%) and had pre-HAART vitamin D levels 30 ng/ml or less] (89%). Vitamin D2 levels were available for 169 of 204 patients, and were detectable (levels >3) in 29 of 169 (17%), whereas 10 (6%) had levels above 10. Women with vitamin D levels 30 ng/ml or less were more likely to be older than 38 years of age (94 vs. 85%) and be either overweight or obese (98 vs. 82%) compared with women with sufficient vitamin D levels. However, these groups did not differ in hepatitis C status, CD4 nadir, use of any antiretroviral therapy for any length of time prior to HAART, viral load at the visit prior to HAART initiation, or having an undetectable viral load 24 months after HAART initiation.
Table 1.
Participant demographic and clinical characteristics by vitamin D status, n = 204.
| Vitamin D status | |||
|---|---|---|---|
| >30 ng/ml | ≤30 ng/ml | P-value | |
| Total, n (%) | 22 (11) | 182 (89) | |
| Age, n (%) | |||
| 38 years or younger | 16 (15) | 89 (85) | 0.04 |
| Over 38 years | 6 (6) | 93 (94) | |
| Race/ethnicitya, n (%) | |||
| Non-Hispanic white | 8 (38) | 13 (62) | <0.0001 |
| Hispanic | 7 (17) | 35 (83) | |
| Non-Hispanic black | 4 (3) | 119 (97) | |
| Other | 3 (17) | 15 (83) | |
| Median BMI (kg/m3) (IQR) | 22.2 (21.2–22.9) | 25.3 (21.6–30.4) | 0.002 |
| Hepatitis C antibody status, n (%) | |||
| Negative | 17 (13) | 119 (87) | 0.26 |
| Positive | 5 (7) | 63 (93) | |
| CD4 nadir, n (%) | |||
| 50 cells/μl or more | 13 (10) | 123 (90) | 0.42 |
| Less than 50 cells/μl | 9 (13) | 59 (87) | |
| Antiretroviral naivea, n (%) | |||
| No | 19 (13) | 130 (87) | 0.20 |
| Yes | 3 (5) | 52 (95) | |
| Viral load last pre-HAARTa, n (%) | |||
| <100 000, cells/ul | 15 (11) | 116 (89) | 0.32 |
| 100 000+, cells/ul | 4 (6) | 58 (94) | |
| Undetectable viral load 24 months post-HAART visit, n (%) | |||
| No | 13 (11) | 108 (89) | 0.94 |
| Yes | 7 (11) | 56 (89) | |
Chi-square tests were utilized to examine the relationship between vitamin D status and categorical variables. The nonparametric Kruskal–Wallis test was used to examine the difference in median BMI by vitamin D status. Due to missing values, not all rows add up to n = 204.
Fisher’s exact test used where expected cell size was less than 5.
In the adjusted logistic regression analyses at 6 months, having an undetectable viral load 6 months post-HAART initiation was the only characteristic significantly associated with CD4 recovery more than 50 cells in the adjusted analysis [OR 8.89, 95% confidence interval (CI): 3.72–21.23]. At 12 months, being naive to any antiretroviral use (not just HAART) prior to HAART initiation (OR 2.56, 95% CI: 1.16–5.69) and having an undetectable viral load 12 months post-HAART initiation (OR 7.68, 95% CI: 3.46–17.03) were positively associated with CD4 recovery more than 100 cells in the adjusted analyses. At 24 months, vitamin D levels 30 ng/ml or less (OR 0.20, 95% CI 0.05–0.83) were associated with decreased odds of achieving a CD4 increase of more than 200 cells/μl, while having an undetectable viral load 24 months post-HAART initiation (OR 11.38, 95% CI 4.31–30.05) remained associated with an increased odds of achieving a CD4 increase of more than 200 cells/μl in the adjusted analysis at 24 months (Table 2). The mean change in CD4 from the pre-HAART visit until 24 months was +188 in the sufficient vitamin D group and +134 for the insufficient group. We also examined a subset of women with vitamin D levels less than 20 and found that their odds of achieving a CD4 increase of more than 200 cells/μl at 24 months was 0.21 (95% CI 0.05–0.91, P = 0.04) compared with women with sufficient vitamin D levels after adjusting for all other covariates in the model.
Table 2.
Multivariate analysis of factors associated with an increase in CD4 cell count of 200 cells/μl or greater at 24-months post-HAART visit, n = 167a.
| Odds ratio (95% CI) | |
|---|---|
| Age | |
| 38 years or younger | Ref |
| Over 38 years | 0.84 (0.35–2.00) |
| Race/ethnicity | |
| Non-Hispanic white | Ref |
| Hispanic | 0.23 (0.05–1.10) |
| Non-Hispanic black | 0.88 (0.24–3.20) |
| Other | 2.05 (0.41–10.34) |
| Vitamin D (250H-D) status | |
| >30 ng/ml | Ref |
| ≤30 ng/ml | 0.20 (0.05–0.83) |
| BMI (kg/m3) status | |
| Underweight/normal weight (<25 kg/m3) | Ref |
| Overweight/obese(≥25 kg/m3) | 0.93 (0.38–2.24) |
| Antiretroviral naive | |
| No | Ref |
| Yes | 1.28 (0.50–3.27) |
| Viral load last pre-HAART | |
| <100 000, cells/ul | Ref |
| 100 000+, cells/ul | 0.73 (0.27–1.95) |
| Undetectable viral load 24 months post-HAART visit | |
| No | Ref |
| Yes | 11.38 (4.31–30.05) |
The model is adjusted for all variables presented. CI, confidence interval; Ref, Reference category.
Women with missing data were excluded from the multivariate analysis.
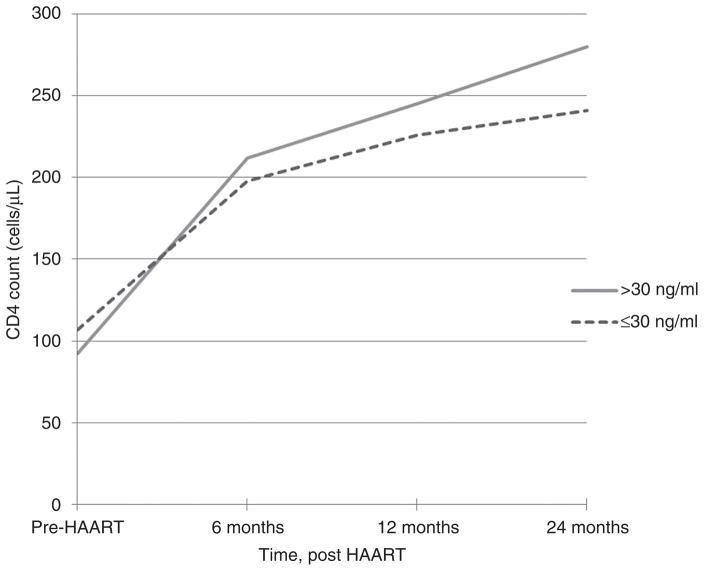
In the GEE model controlling for age, race/ethnicity, BMI, antiretroviral therapy history, viral load over time and WIHS site there was a significant and positive main effect of linear time on CD4 reconstitution (P <.01). There was no significant difference in CD4 count at baseline by vitamin D status. The difference in immune reconstitution between vitamin D groups was modeled by a quadratic time trend, which found a modest but significant (P <0.01) attenuation from the average positive curvilinear trend among those with vitamin D levels 30 ng/ml or less compared with those with sufficient vitamin D levels (Fig. 1).
Fig. 1. Mean CD4 count (cells/μl) among women with normal (>30 ng/ml) and insufficient or deficient vitamin D (≤ 30 ng/ml), before HAART initiation and 6, 12, and 24 months post-HAART initiation.
In univariate analysis of variance (ANOVA), difference in mean CD4 by vitamin D status is nonsignificant (F = 0.639, P = 0.424); difference in mean CD4 by time point is significant (ANOVA F = 14.92, P <0.001), and vitamin D by time interaction is nonsignificant (F = 0.358, P = 0.783).
Discussion
Vitamin D homeostasis plays an essential role in overall health [2,21]. Vitamin D receptors (VDR) have a broad distribution that includes activated T and B lymphocytes. Although the most well known and studied effects of low vitamin D levels have been on the musculoskeletal system, recent investigations show that vitamin D is linked to cellular immunity, cardiovascular disease, autoimmune disease, insulin resistance, and in the control of infections, such as tuberculosis [22–28].
Our study found that vitamin D insufficiency is associated with late CD4 recovery after HAART initiation. Our results extend the findings of Ross et al. [14], which reported that among 149 HIV-infected patients, greater vitamin D level was associated with greater CD4 T-cell restoration after HAART initiation (P < 0.01). This analysis examined change in CD4 count (defined as current CD4 T-cell count at time of evaluation minus nadir CD4 T cell count) and did not perform longitudinal analysis controlling for time varying covariates. The GEE model is a much more robust way to control for the effect of time on immune reconstitution. Although vitamin D has been reported to have seasonal variations, a study by our group in 108 HIV infected women which looked at two separate time periods 3 years apart suggests that in the absence of active pharmacologic interventions, vitamin D deficiency will persist among the majority of them (O.A., unpublished data).
There may be biological mechanisms, which explain the effect of vitamin D insufficiency on late CD4 cell recovery after HAART initiation. During CD4 cell recovery, a rapid increase in the number of memory T cells within 1–2 weeks of starting HAART and continuing over the first 3 months to a year occurs due to expansion of preexisting clones, redistribution of T cells sequestered in lymphoid tissues, or reduction in apoptotic cell death. A slower second stage occurs in naive T cells, which may be due to de-novo T-cell synthesis from the thymus or redistribution of T cells from tissue to blood [11–14]. This results in the long-term rise in CD4 count. This long-term rise is related to the level of virologic control in each patient. Our study findings suggest that vitamin D insufficiency could be related to production of naive T cells.
In addition, recent studies have shown that vitamin D is crucial for the activation of immune defenses [29,30]. Vitamin D is closely involved in the functioning of T and B lymphocytes in the adaptive immune system by regulating the activation of lymphocytes directly and by effects on APC. Vitamin D also has well known effects on the innate immune system [31]. One particular study described the requirement for vitamin D binding to the VDR in order to activate the gene for phospholipase C-γ1, which in turn is required for T-cell activation by the classical T-cell antigen receptor (TCR) signaling pathway [29]. An alternative pathway used by naive T cells due to their low expression of VDR involves signaling through the mitogen-activated protein kinase 38, which results in VDR upregulation and subsequent signaling through the classical pathway. Thus, this study indicated vitamin D requirement for activation of both naive and memory T cells [25] and the difference in activation between memory and naive T cells. Late immune reconstitution that is dependent on de-novo synthesis of naive T cells from the thymus may therefore be affected by vitamin D deficiency.
Limitations of our study include that we were under-powered to examine more detailed associations due to the low representation of some of the baseline demographic characteristics and the small percentage of vitamin D-sufficient patients. Although dichotomous representations of these variables were adjusted for in the multivariable model, future studies should recruit larger samples of women with sufficient vitamin D levels. In addition, our criteria for immune reconstitution at each of the time points, while chosen from data in the literature [16–20], may have been too restrictive. However, our additional analysis of CD4 recovery over time continued to show an effect of pre-HAART vitamin D levels on CD4 recovery.
Finally, we did not have vitamin D levels at each of the time points and instead used pre-HAART vitamin D levels. Although we have only pre-HAART vitamin D levels, other large epidemiologic studies have used baseline vitamin D to assess outcomes up to 10 years later [32–34]. In a separate WIHS analysis, we found that vitamin D levels did not differ significantly over a 3-year period in the absence of interventions (OA, unpublished data). Future studies on this issue should measure vitamin D levels at multiple time points.
Conclusion
Vitamin D insufficiency is associated with impaired late CD4 recovery on HAART in the WIHS cohort. The mechanism of this association CD4 recovery may be impaired late vitamin D-associated production of naive CD4 cells during immune reconstitution, however this merits further exploration.
Acknowledgments
M.A. and O.M.A. had significant contributions to the writing of this article, analysis of data, and submission. B.L. and J.B.-M. performed all statistical analysis. The article was extensively reviewed, edited, and approved for submission by all other co-authors, who are part of the Women’s Interagency HIV Study and had patients involved in the study.
Data in this article were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Study was presented in part at the Conference on Retroviruses and Opportunistic Infections, Seattle WA, 2012.
References
- 1.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxy vitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 4.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyVitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 5.Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxy vitamin D3: a novel immunoregulatory hormone. Science. 1984;224:1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- 6.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French AL, Adeyemi OM, Agniel DM, Evans CT, Yin MT, Anastos K, Cohen MH. The association of HIV status with bacterial vaginosis and vitamin D in the United States. J Womens Health (Larchmt) 2011;20:1497–1503. doi: 10.1089/jwh.2010.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vescini F, Cozzi-Lepri A, Borderi M, Re MC, Maggiolo F, De Luca A, et al. Prevalence of hypoVitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 9.Mueller NJ, Fux CA, Ledergerber B, Elzi L, Schmid P, Dang T, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2011;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2011;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeyemi OM, Agniel D, French AL, Tien PC, Weber K, Glesby MJ, et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr. 2011;57:197–204. doi: 10.1097/QAI.0b013e31821ae418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haug CJ, Aukrust P, Lien E, Muller F, Espevik T, Froland SS. Disseminated mycobacterium avium complex infection in AIDS: immunopathogenic significance of an activated tumor necrosis factor system and depressed serum levels of 1,25 dihydroxy vitamin D. J Infect Dis. 1996;173:259–262. doi: 10.1093/infdis/173.1.259. [DOI] [PubMed] [Google Scholar]
- 13.Haug C, Muller F, Aukrust P, Froland SS. Subnormal serum concentration of 1,25-Vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. J Infect Dis. 1994;169:889–893. doi: 10.1093/infdis/169.4.889. [DOI] [PubMed] [Google Scholar]
- 14.Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16:555–563. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 16.Teixeira L, Valdez H, McCune JM, Koup RA, Badley AD, Hellerstein MK, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 17.Tuboi SH, Brinkhof MW, Egger M, Stone RA, Braitstein P, Nash D, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45:52–59. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 18.Nakanjako D, Kiragga A, Ibrahim F, Castelnuovo B, Kamya MR, Easterbrook PJ. Sub-optimal CD4 reconstitution despite viral suppression in an urban cohort on antiretroviral therapy (ART) in sub-Saharan Africa: frequency and clinical significance. AIDS Res Ther. 2008;5:23. doi: 10.1186/1742-6405-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48:328–337. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 20.Grabar S, Le Moing V, Goujard C, Leport C, Kazatchkine MD, Costagliola D, Weiss L. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 21.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 22.Chiu KC, Chu A, Go VL, Saad MF. HypoVitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 23.Chandra G, Selvaraj P, Jawahar MS, Banurekha VV, Narayanan PR. Effect of vitamin D3 on phagocytic potential of macrophages with live Mycobacterium tuberculosis and lymphoproliferative response in pulmonary tuberculosis. J Clin Immunol. 2004;24:249–257. doi: 10.1023/B:JOCI.0000025446.44146.52. [DOI] [PubMed] [Google Scholar]
- 24.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 25.Karnchanasorn R, Ou HY, Chiu KC. Plasma 25-hydroxy vitamin D levels are favorably associated with beta-cell function. Pancreas. 2011:863–868. doi: 10.1097/MPA.0b013e31823c947c. [DOI] [PubMed] [Google Scholar]
- 26.Choi AI, Lo JC, Mulligan K, Schnell A, Kalapus SC, Li Y, et al. Association of vitamin D insufficiency with carotid intima-media thickness in HIV-infected persons. Clin Infect Dis. 2011;52:941–944. doi: 10.1093/cid/ciq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med. 2011;155:820–826. doi: 10.7326/0003-4819-155-12-201112200-00004. [DOI] [PubMed] [Google Scholar]
- 28.Pelajo CF, Lopez-Benitez JM, Kent DM, Price LL, Miller LC, Dawson-Hughes B. 25-HydroxyVitamin D levels and juvenile idiopathic arthritis: is there an association with disease activity? Rheumatol Int. 2011 doi: 10.1007/s00296-011-2287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation ofhuman T cells. Nat Immunol. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 30.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88:441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxy vitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2011;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]