Abstract
Curcumin, a dietary polyphenol, has preventive and therapeutic potential against several diseases. Because of the chronic nature of many of these diseases, sustained-release dosage forms of curcumin could be of significant clinical value. However, extreme lipophilicity and instability of curcumin are significant challenges in its formulation development. The objectives of this study were to fabricate an injectable microparticle formulation that can sustain curcumin release over a 1-month period and to determine its chemopreventive activity in a mouse model. Microparticles were fabricated using poly(D, L-lactide-co-glycolide) polymer. Conventional emulsion solvent evaporation method of preparing microparticles resulted in crystallization of curcumin outside of microparticles and poor entrapment (~1%, w/w loading). Rapid solvent removal using vacuum dramatically increased drug entrapment (~38%, w/w loading; 76% encapsulation efficiency). Microparticles sustained curcumin release over 4 weeks in vitro, and drug release rate could be modulated by varying the polymer molecular weight and/or composition. A single subcutaneous dose of microparticles sustained curcumin liver concentration for nearly a month in mice. Hepatic glutathione-s-transferase and cyclooxygenase-2 activities, biomarkers for chemoprevention, were altered following treatment with curcumin microparticles. The results of these studies suggest that sustained-release microparticles of curcumin could be a novel and effective approach for cancer chemoprevention.
Keywords: polymeric systems, sustained release, formulation, controlled delivery, poly(lactic/glycolic) acid, cancer chemoprevention, microparticles, curcumin
INTRODUCTION
Curcumin, a polyphenol derived from the root of Curcuma longa, is widely used in traditional medicine and as a food spice. Over the last two decades, numerous studies have established the preventive and therapeutic benefits of curcumin in neurological, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases.1–4 Curcumin mediates its anti-inflammatory effects through the downregulation of inflammatory transcription factors (nuclear factor kappa B), enzymes (cyclooxygenase-2 and 5-lipooxygenase), and cytokines (tumor necrosis factor alpha, interleukin-1, and interleukin-6).3 Despite this considerable potential, the broad usefulness of curcumin in systemic diseases is diminished by its poor pharmacokinetic profile. Curcumin has low oral bioavailability (<1%) because of poor gastrointestinal absorption and extensive hepatic first-pass metabolism.5 In addition, curcumin has a short half-life and the metabolites are less active.6
Given the chronic nature of the pathologies in which curcumin is useful, delivery systems that can provide sustained systemic concentrations of the drug would be highly valuable. However, curcumin is most effective in the 0.5–50 μM dose range,1 which, combined with the short half-life, necessitates the administration of large doses (e.g., 4–8 g/day orally).7 This requires formulations with high curcumin loading. In addition, curcumin is highly lipophilic and unstable in solution,8 making formulation development and characterization extremely challenging.
Our group is pursuing the goal of developing an injectable sustained-release delivery system of curcumin as a novel approach to cancer chemoprevention. Our recent study showed that a single dose of curcumin microparticles formulated using poly (D, L-lactide-co-glycolide) (PLGA) polymer could sustain curcumin blood levels for a period of 4 weeks, and effectively inhibit tumor growth in a mouse model of breast cancer.9 Microparticles represent a simple yet effective system for sustaining systemic delivery of drugs. Many hydrophobic drugs have been encapsulated in PLGA microparticles using oil-in-water emulsion solvent evaporation technique.10 The rate of solvent removal strongly influences the characteristics of microparticles prepared by solvent evaporation, and depends on temperature, pressure, solubility characteristics of the polymer, and the solvent used.11 Perhaps the greatest formulation challenge using this technique is to fabricate particles with high drug loading. Previous studies with other hydrophobic drugs have reported loading values of 10%–20% (w/w).12 In this paper, we report the development of highly loaded (~40%, w/w), sustained-release microparticle formulation of curcumin. In addition to in vitro characterization, microparticles were evaluated for their ability to sustain liver concentrations of curcumin and to alter specific biomarkers for chemoprevention in a mouse model.
Other groups have reported the encapsulation of curcumin in PLGA-based nanoparticles.13–15 Previous studies have shown that nanoparticles can enhance the availability of the encapsulated drug at the target site. Yallapu et al.13 concluded that therapeutic efficacy of curcumin could be enhanced using PLGA nanoparticle formulations, and tumor-targeted delivery of curcumin is feasible by coupling of anti-cancer antibody to nanoparticles. Nanoparticles were also shown to improve oral bioavailability of curcumin. Shaikh et al.16 demonstrated that curcumin entrapped in nanoparticles had at least a ninefold increase in oral bioavailability compared with curcumin administered with piperine as absorption enhancer. However, nanoparticles typically demonstrate low drug loading and often release the drug within a few days to weeks. For example, Anand et al.14 reported a curcumin loading of 4 μg/mg in PLGA nanoparticles (0.4%, w/w). As our goal was to develop a highly loaded formulation (>30%, w/w) that could also release the drug over a 1-month period, we investigated microparticles rather than nanoparticles for sustained curcumin delivery.
MATERIALS AND METHODS
Materials
Curcumin [molecular weight (MW) 368.38; ≥94% curcuminoid content], polyvinyl alcohol (average MW 30–70 kDa; PVA), Tween 80, N-acetyl- L-cysteine (NAC), and butylated hydroxytoluene (BHT) were purchased from Sigma (St. Louis, Missouri). PLGA (lactide-to-glycolide ratios of 50:50 and 75:25, and average MW ranging from ~13 to ~120 kDa) and poly(D, L-lactide) (~65 kDa; PLA) were purchased from Durect Corporation (Pelham, Alabama). Six-well Transwell® inserts were from Corning (Lowell, Massachusetts). Glutathione-s-transferase (GST) and cyclooxygenase (COX) activity assay kits were from Cayman Chemical Company (Ann Arbor, Michigan).
Formulation of Curcumin-Loaded Microparticles
Curcumin-loaded microparticles were prepared using a modification of the emulsion solvent evaporation method.12,17 Curcumin (10–20 mg) and polymer (20–30 mg) were solubilized in a 10:1 mixture of chloroform (1–1.5 mL) and methanol (0.1–0.15 mL). Methanol was used to aid solubilization of curcumin in chloroform. This solution was emulsified in 2% (w/v) aqueous PVA solution (6 mL) by homogenization (Tissuemizer; Fisher Scientific, Pittsburgh, Pennsylvania) at 5000 rpm for 5 min or by vortex mixing (Digital Vortex Mixer; VWR, West Chester, Pennsylvania) at 1000 rpm for 2 min. The emulsion was stirred at 500 rpm for 18 h under ambient conditions, then in a dessicator under vacuum for 1 h to evaporate the organic solvents. Alternatively, the emulsion was directly subjected to high vacuum (710 mm Hg) at 4° C using a rotary evaporator (Laborta 4001 Efficient Rotaevaporator, Heidolph, Schwabach, Germany), which resulted in rapid (<20 min) and complete removal of the organic solvents. Microparticles were recovered by centrifugation (5810R; Eppendorf, Westbury, New York) at 800 × g for 10 min, washed twice with endotoxin-free water (<0.005 EU/mL, deionized, distilled, 0.1 μm sterile filtered, 50 mL) to remove unencapsulated curcumin and excess PVA, and lyophilized (Free-zone 4.5®; Labconco, Kansas City, Missouri). In some cases, microparticles were washed twice with 10% (w/v) Tween 80 in endotoxin-free water (50 mL), then twice with endotoxin-free water (50 mL), and lyophilized.
Microparticle Characterization
Mean diameter of microparticles was determined using an optical microscope. Microparticles (~1 mg/0.5 mL) were dispersed in distilled water by sonication (Model 3000; Misonix, Farmingdale, New York) at 3 W for 60 s. A drop of this dispersion was placed on a glass slide and examined under a microscope (Eclipse TS100; Nikon Instruments, Melville, New York) at 400× magnification. Diameters of 500 particles in several different fields were measured using Adobe Photoshop (Adobe Systems, San Jose, California), and the number average particle size was calculated.
The morphology and surface of microparticles was examined using scanning electron microscopy (SEM). Microparticles were placed on a double stick carbon tape over aluminum stubs and carbon coated. The samples were observed under an electron microscope (JSM 6500F; JEOL, Peabody, Massachusetts) at 1000× or 5000× magnification.
Drug loading was determined by extracting microparticles (2 mg) with 2 mL methanol for 18 h (Labquake Shaker; Barnstead Thermolyne, Dubuque, Iowa). The methanolic extract was centrifuged at 20,000 × g for 15 min and curcumin concentration in the supernatant was determined by high-performance liquid chromatography (HPLC; System Gold® 126 Solvent Module and 508 Autosampler, Beckman Coulter, Fullerton, California) using a C-18 column (Ultrasphere ODS, 250 mm × 4.6 mm i.d., 5 μm particle size; Beckman Coulter). A 60/40 (v/v) mixture of acetonitrile and ammonium acetate (10 mm, adjusted to pH 4.0 with glacial acetic acid) was used as mobile phase at a flow rate of 1 mL/min. Curcumin was detected using a PDA detector (System Gold® 168 Detector) at a wavelength of 430 nm. The retention time of curcumin under these conditions was 5.8 min. The standard curve was linear over 25–1000 ng/mL, with a correlation coefficient of r2 = 0.998. Drug loading in microparticles (%, w/w, n = 3) was defined as the amount of curcumin in 100 mg of microparticles. Drug encapsulation efficiency was defined as the percent of added curcumin that was encapsulated in microparticles.
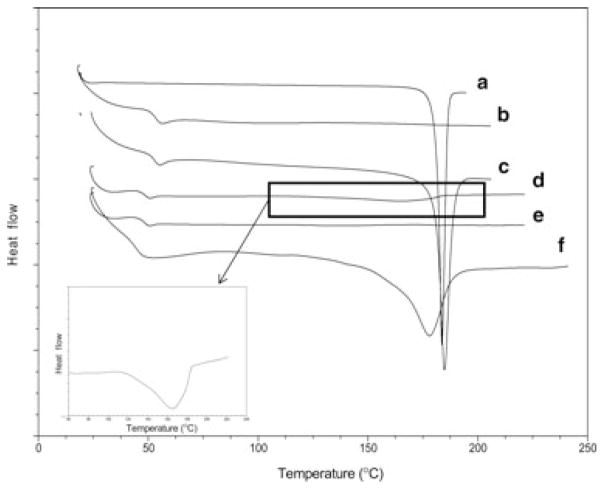
Heating curves of curcumin and microparticles were obtained using a differential scanning calorimeter (MDSC, Model 2920, TA Instruments, New Castle, Delaware) equipped with a refrigerated cooling accessory. A 4–5 mg sample was packed in a non-hermetically crimped aluminum pan, and heated under dry nitrogen purge. Samples were heated from 25°C to 225°C at a heating rate of 20°C/min, except for curcumin microparticles prepared by rapid solvent removal, where a heating rate of 50°C/min was used to prevent recrystallization during the DSC run. DSC heating curves were analyzed using Universal Analysis 2000 software (TA Instruments).
In Vitro Release of Curcumin
Initial studies focused on optimizing the media for determining curcumin release. The maximum solubility of curcumin in plain aqueous buffer was reported to be 11 ng/mL.18 We determined the solubility of curcumin in different concentrations of Tween 80 (1%–10% w/v) in phosphate-buffered saline (0.15 M, pH 7.4; PBS). The goal was to achieve a solubility of at least 2 μg/mL, which ensured both sink conditions and detectability by HPLC (limit of detection ~25 ng/mL) under the release study conditions. Following this, solution stability of curcumin was determined in the presence or absence of 0.1% (w/v) NAC or 0.01% (w/v) BHT or both. PBS containing 10% (w/v) Tween 80 was used as control. Samples were placed in an incubator shaker (C24 Incubator Shaker; New Brunswick Scientific, Edison, New Jersey) set at 100 rpm and 37°C. Curcumin concentration in the buffer was monitored using HPLC.
The release study was performed in a six-well plate containing Transwell® inserts with a pore size of 400 nm. PBS containing 10% (w/v) Tween 80, 0.1% (w/v) NAC, and 0.01% (w/v) BHT was used as the release buffer. Curcumin-loaded microparticles (equivalent to 2 μg curcumin), suspended in 1 mL of the release buffer, were placed on top of the Transwell® insert and 3 mL of the release buffer was added to the bottom of the well. The plates with the inserts were sealed with Parafilm®, and placed in an incubator shaker set at 100 rpm and 37°C. At various time intervals over 28 days, the entire release buffer in the bottom chamber was removed and replaced with fresh release buffer. Curcumin concentration in the release buffer at each time point was determined by HPLC. A control experiment performed with curcumin solution (instead of microparticles) added to the top chamber confirmed rapid (<8 h) equilibration of curcumin between the two chambers.
In Vivo Evaluation of Curcumin Microparticles
Animal experiments were carried out in compliance with protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Six-week-old BALB/c female mice (Charles River Laboratories, Wilmington, Massachusetts) were injected with a single dose of curcumin-loaded microparticles in the subcutaneous space near the neck. Microparticles (equivalent to 29.1 mg curcumin) were dispersed in 0.5 mL PBS prior to injection. Untreated mice were used as controls. Animals were euthanized at various time points (n = 6 per time point), and liver samples were harvested for analyzing GST and COX-2 activities, and curcumin concentration. Liver samples were weighed, homogenized in 1 mL of cold potassium phosphate buffer (100 mm, pH 7.0), and centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were again centrifuged at 10,000 × g for 60 min at 4°C. The resulting supernatants were assayed for GST and COX-2 activities using commercially available assay kits. For drug analysis, liver homogenates were lyophilized and extracted with 3 mL of diethyl ether. The ether extracts were evaporated in a water bath at 37°C and reconstituted with 0.3 mL mobile phase. Hydroxybenzophenone was used as the internal standard. Drug concentration in the extracts was determined by LC–MS/MS (Agilent 1100; Agilent Technologies, Palo Alto, California, coupled to Finnigan TSQ Quantum Discovery Max triple quadrupole detector; Thermo Electron, San Jose, California). Separation was achieved on a C-18 column (Zorbax SB-18, 150 mm × 0.5 mm i.d., 5 μm particle size, Agilent) using a 60/40 (v/v) mixture of acetonitrile and ammonium acetate (10 mm, adjusted to pH 4.0 with glacial acetic acid) as the mobile phase (flow rate 10 μL/min). Samples were analyzed in positive ion mode. Curcumin and hydroxybenzophenone (internal standard) were monitored using single reaction monitoring of the 369.2 to 285.1 and 199.2 to 121.1 transitions, respectively. Retention times of hydroxybenzophenone and curcumin under these conditions were 3.8 and 4.8 min, respectively. The standard curve was linear over 1–1000 ng/mL, with a correlation coefficient of r2 = 0.997. The chromatographic data were acquired and analyzed using Xcaliber software (Thermo Scientific). Curcumin concentrations were normalized to wet liver weights.
Statistical Analysis
Liver concentrations, GST, and COX-2 activities were represented as mean ± standard error (SE). Differences in GST and COX-2 activities between treated and untreated animals were determined using ANOVA followed by post hoc Dunnett’s multicomparison test. A p value of less than 0.05 was considered significant.
RESULTS
Microparticle Characterization
Microparticles loaded with curcumin were characterized for size, morphology, surface characteristics, and drug loading. Results from optical microscopy studies indicated that microparticles prepared using homogenization had an average diameter of 3.6 ± 1.2 μm (Table 1, Fig. 1a) and those prepared using vortexing had an average diameter of 18.1 ± 9.2 μm (Table 1, Fig. 2a). No aggregation of microparticles was observed following lyophilization.
Table 1.
Effect of Formulation Variables on Size and Curcumin Loading in PLGA Microparticles
| Technique | Drug: Polymera Ratio | Size Reduction Method | Washing Solvent | Mean Diameter (μm) ± SD | Theoretical Loading (mg/100 mg Microparticles) | Curcumin Loading (mg/100 mg Microparticles) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|---|---|
| ESE | 1:3 | Homogenization | Water | 3.4 ± 1.6 | 25 | 13.4 ± 6.2 | 53.6 ± 24.6 |
| ESE | 1:3 | Homogenization | 10% Tween 80 | 3.6 ± 1.2 | 25 | 0.8 ± 0.1 | 3.2 ± 0.4 |
| ESE | 1:3 | Vortex | 10% Tween 80 | 18.1 ± 9.2 | 25 | 0.7 ± 0.1 | 2.8 ± 0.3 |
| RSR | 1:3 | Vortex | 10% Tween 80 | 19.9 ± 9.8 | 25 | 21.2 ± 1.7 | 84.8 ± 6.9 |
| RSR | 1:1 | Vortex | 10% Tween 80 | 20.8 ± 9.0 | 50 | 33.6 ± 0.7 | 67.2 ± 1.5 |
Polymer used was PLGA 50:50 ~120 kDa.
ESE, emulsion solvent evaporation; RSR, rapid solvent removal.
Figure 1.
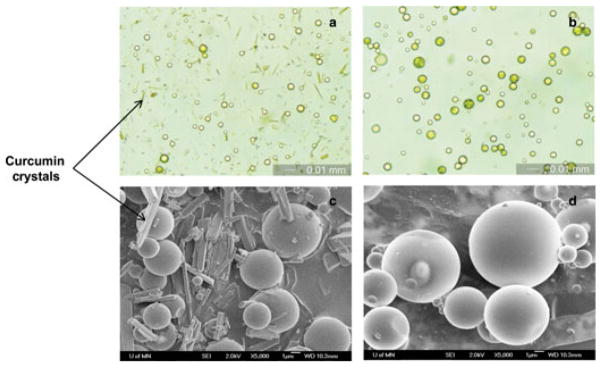
Optical microscopy (a and b) and SEM (c and d) images of curcumin-loaded PLGA microparticles prepared by conventional solvent evaporation and homogenization technique. (a and c) After washing with water. (b and d) After washing with 10% (w/v) Tween 80. For (a and b), magnification is 400× and bar is 10 μm. For (c and d), magnification is 5000× and bar is 10 μm.
Figure 2.
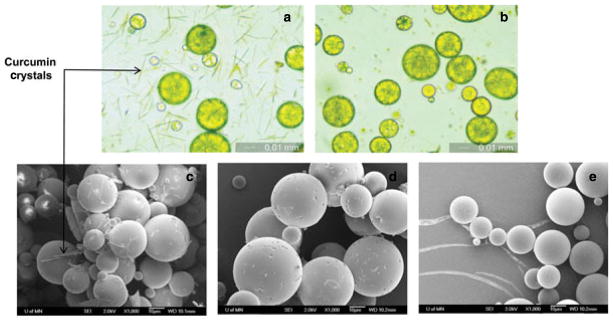
Optical microscopy (a and b) and SEM (c and d) images of curcumin-loaded PLGA microparticles prepared by conventional solvent evaporation and vortexing technique. (a and c) after washing with water. (b and d) after washing with 10% (w/v) Tween 80. For (a and b), magnification is 400× and bar is 10 μm. For (c and d), magnification is 1000× and bar is 1 μm. Panel e shows SEM image of blank PLGA microparticles prepared by conventional solvent evaporation and vortexing after washing with water (1000× magnification; bar is 1 μm).
Scanning electron microscopy studies indicated that microparticles had a spherical morphology; however, a large number of crystals were observed (Figs. 1c and 2c). Nondrug-loaded microparticles did not have such crystals (Fig. 2e) indicating that these crystals were probably curcumin. A melting peak in the DSC heating curve (Fig. 3d) further confirmed that those were indeed curcumin crystals. Curcumin loading in microparticles prepared by homogenization technique was found to be approximately 13% (w/w) (Table 1). An additional washing step with 10% Tween 80 in the formulation step eliminated the crystals and reduced the drug loading to approximately 1% (w/w) (Table 1). Similar loss in drug loading following Tween 80 wash was observed for microparticles prepared by vortex method. Elimination of curcumin crystals with Tween 80 wash was established by optical microscopy (Figs. 1b and 2b), SEM (Figs. 1d and 2d), and by the loss of melting peak in the DSC heating curve (Fig. 3e).
Figure 3.
DSC heating curves of (a) curcumin, (b) blank PLGA microparticles, (c) physical mixture of curcumin and blank PLGA microparticles, (d) curcumin-loaded PLGA microparticles prepared by conventional solvent evaporation and washed with water, (e) curcumin-loaded PLGA microparticles prepared by conventional solvent evaporation and washed with 10% (w/v) Tween 80, and (f) curcumin-loaded PLGA microparticles prepared by rapid solvent removal and washed with 10% (w/v) Tween 80. Inset shows the DSC heating curve of (d) at a greater magnification for clarity.
When the organic solvents were removed rapidly using high vacuum during the manufacturing process, curcumin loading in microparticles increased to approximately 34% (w/w) (Table 1). Microparticles prepared using this technique had a smooth, spherical morphology (Fig. 4a), and an average diameter of 20.8 ± 9.0 μm (Table 1, Fig. 4b). DSC studies showed that curcumin was present in microparticles in crystalline form, as indicated by a melting peak (Fig. 3f), but no crystals were found on the outside of microparticles (Fig. 4a). Microparticles appeared dark orange-brown as a result of high drug loading (Fig. 4b). It was interesting to note that the melting peaks for curcumin shifted in the case of microparticles (Figs. 3d and 3f) compared with that of pure curcumin (Fig. 3a). It is possible that the formulation conditions (emulsification, freeze drying, etc.) affect the crystal structure of curcumin, causing a shift in the melting peak.
Figure 4.
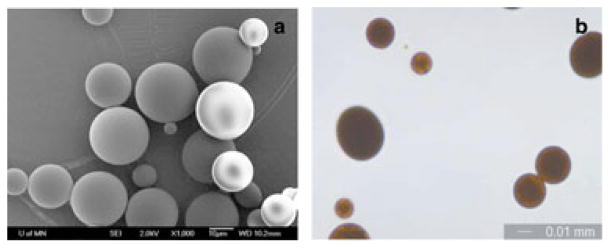
(a) Scanning electron microscopy image of curcumin-loaded microparticles prepared by rapid solvent removal process. 1000× magnification. (b) Optical microscopy image of curcumin-loaded microparticles prepared by rapid solvent removal process. 400× magnification. Bar is 10 μm.
To maximize drug loading using the rapid solvent removal process, the effect of polymer lactide-to-glycolide ratio (50:50 to 100:0) and molecular weight (from ~13 to ~120 kDa) and organic to aqueous phase volume ratio on drug loading was investigated (Table 2). Increasing the molecular weight of polymer increased the encapsulation efficiency from 53% to 67% (w/w). Increasing the glycolide content of the polymer increased the encapsulation efficiency from 40% to 66% (w/w). Increasing the organic to aqueous phase volume ratio increased the encapsulation efficiency from 67% to 76% (w/w).
Table 2.
Effect of Formulation Variables on Curcumin Loading in Microparticles
| Effect of | Molecular Weight (kDa) | Lactide Content (%) | Phase Volume Ratio | Mean Diameter (μm) ± SD | Theoretical Loading (mg/100 mg Microparticles) | Curcumin Loading (mg/100 mg Microparticles) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|---|---|
| Molecular weight | ~120 | 50 | 0.19 | 20.8 ± 9.0 | 50 | 33.6 ± 0.7 | 67.2 ± 1.5 |
| ~13 | 50 | 0.19 | 21.1 ± 7.9 | 50 | 26.7 ± 1.2 | 53.4 ± 2.4 | |
| Lactide content | ~65 | 75 | 0.19 | 24.5 ± 9.0 | 50 | 33.2 ± 0.5 | 66.4 ± 1.1 |
| ~65 | 100 | 0.19 | 22.8 ± 9.3 | 50 | 20 ± 2.5 | 40 ± 5 | |
| Organic phase volume ratio | ~120 | 50 | 0.19 | 20.8 ± 9.0 | 50 | 33.6 ± 0.7 | 67.2 ± 1.5 |
| ~120 | 50 | 0.28 | 21.5 ± 8.9 | 50 | 38.1 ± 0.9 | 76.2 ± 1.9 |
In Vitro Release of Curcumin
In the initial optimization studies, we determined that 10% (w/v) Tween 80 was required to achieve the target curcumin solubility of 2 μg/mL in PBS. As shown in Figure 5, curcumin was highly unstable in this medium, with about 60% of the initial concentration degrading in 5 days. Addition of NAC or BHT decreased the degradation rate of curcumin. When a combination of NAC and BHT was used, degradation of curcumin was significantly decreased, with only 10% degrading in 5 days. On the basis of the results of this optimization study, PBS containing 10% Tween 80, 0.1% NAC, and 0.01% BHT was used as the release buffer.
Figure 5.
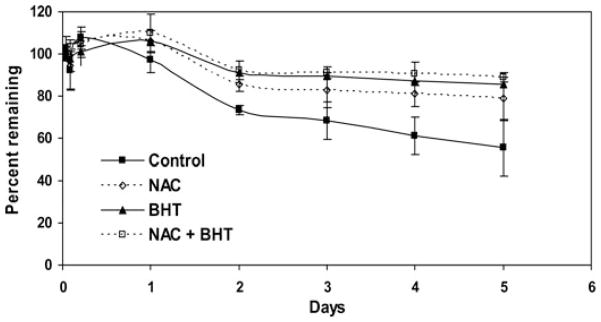
Solution stability of curcumin in the presence of antioxidants. Control—PBS containing 10% Tween 80; NAC—PBS containing 10% Tween 80 and 0.1% NAC; BHT—PBS containing 10% Tween 80 and 0.01% BHT; and NAC + BHT—PBS containing 10% Tween 80, 0.1% NAC and 0.01% BHT. Curcumin concentrations were quantified by HPLC. Data shown are mean ± SD, n=3.
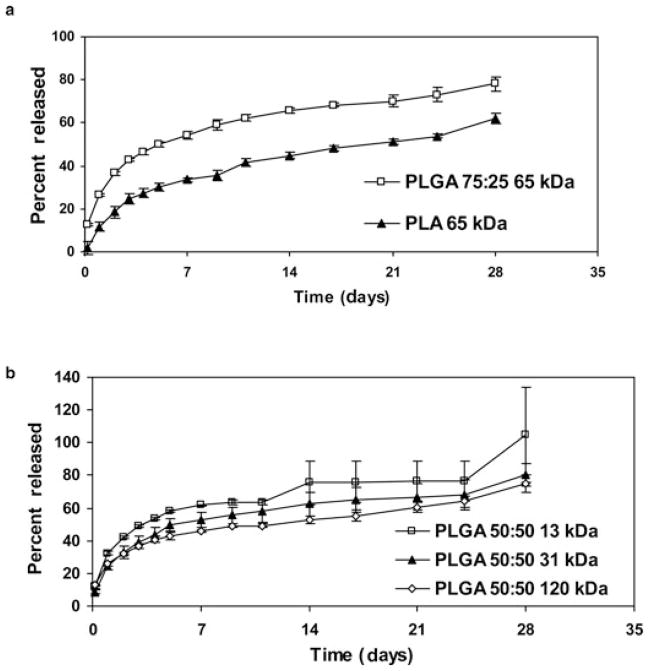
Polymers of different lactide-to-glycolide ratios and molecular weights resulted in microparticles with varying drug release rates. In general, a small burst release was observed in the initial 24 h, followed by a relatively constant release over the remaining duration of the study. Rate of curcumin release from these microparticles could be modulated from about 60% to 100% over 28 days by changing the lactide-to-glycolide ratio (Fig. 6a) or polymer molecular weight (Fig. 6b).
Figure 6.
In vitro release of curcumin from microparticle formulations that vary in polymer lactide-to-glycolide ratio (a), and polymer molecular weight (b). Curcumin concentrations were quantified by HPLC. Data shown are mean ± SD, n=3.
Microparticles Sustain Curcumin Concentration and Alter Biomarkers In Vivo
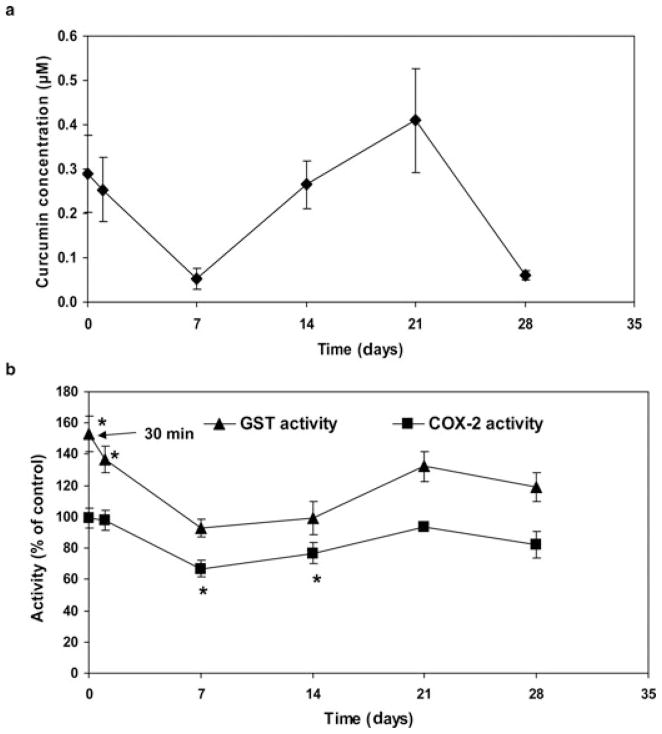
Microparticles prepared from higher molecular weight PLGA (50:50; 120 kDa) were chosen for in vivo studies. A single subcutaneous dose of curcumin microparticles sustained the hepatic concentration of curcumin over the 4-week study period (Fig. 7a). GST activity in liver samples of mice treated with curcumin microparticles was found to be significantly higher than that in untreated mice (849 ± 116 nm/min/g) at 30 min (153.4 ± 11.32% of untreated control; p < 0.05) and 24 h (136.9 ± 8.33%; p < 0.05; Fig. 7b), and correlated with liver concentrations of curcumin. COX-2 activity in liver samples of mice treated with curcumin microparticles was found to be lower than that in untreated mice (133 ± 10 nm/min/g), with the difference reaching statistical significance (p < 0.05) on day 7 (66.8 ± 5.41% of control) and day 14 (76.8 ± 6.88%; Fig. 7b). Although the differences in activities were not statistically significant at other time points, the overall activity profiles of both GST and COX-2 appeared to follow the changes in the liver concentration of curcumin over the 4 weeks of the study.
Figure 7.
(a) Liver concentrations of curcumin following a single dose of curcumin microparticles. (b) Hepatic GST and COX-2 activities following a single dose of curcumin microparticles. GST and COX-2 activities were determined using commercial assay kits, whereas curcumin concentrations were quantified by LC–MS/MS. Data shown are mean ± SE, n=6. *p < 0.05.
DISCUSSION
Curcumin has been shown to prevent the development of cancers of skin, stomach, duodenum, colon, liver, pancreas, lungs, prostrate, and breast in rodents.4 Curcumin exerts its anticancer effect by interfering with multiple cellular pathways and tissue responses, including inflammation, cell cycle, apoptosis, proliferation, survival, invasion, angiogenesis, and metastasis.4 However, its poor oral bioavailability and short half-life necessitate frequent systemic administration of large doses to achieve effective plasma and tissue concentrations. A sustained-release microparticle formulation of curcumin offers the advantages of enhanced and sustained tissue availability, and reduced dosing frequency. PLGA was used in the formulation of curcumin microparticles because of its safety profile, biodegradability, and sustained-release properties. PLGA microparticles are currently approved by the FDA for use in other indications.19
The emulsion solvent evaporation technique has been used successfully in the past for the preparation of PLGA microparticles encapsulating hydrophobic drugs such as chlorpromazine,20 diazepam, progesterone,12 and paclitaxel.17 Microparticles prepared using this technique had a curcumin loading of approximately 13% (w/w), which is consistent with loading values observed for other drugs.12,21 However, we found that drug loading was highly variable, even within samples from the same batch and was not reproducible. This was probably because a significant amount of curcumin had precipitated as crystals outside of microparticles (Figs. 1a and 2a).
Previously, Bodmeier and McGinity12 washed microparticles with 75% ethanol to remove unencapsulated progesterone crystals without extracting progesterone from microparticles. Tsung and Burgess22 washed microparticles with 2% isopropanol to remove unencapsulated dexamethasone. We evaluated several solvents for this purpose, including ethanol and isopropanol (not shown); however, only 10% Tween 80 eliminated curcumin crystals completely (Figs. 1b and 2b). Washing with 10% Tween 80 reduced the drug loading to approximately 1% (w/w) (Table 1). This further confirmed that a significant fraction of the drug was not encapsulated inside microparticles.
Curcumin exerts its pharmacological effects in the micromolar concentration range.1 To sustain this concentration over an extended period of time and to minimize the amount of microparticle dose, formulations with high drug loading are necessary. Several methods have been reported to improve drug loading in polymeric microparticles, including increasing the particle size and the drug–polymer ratio.23–26 Our preliminary studies indicated that increasing the particle size from approximately 4 to 20 μm or changing the drug–polymer ratio from 1:3 to 1:1 increased curcumin loading only marginally. In the emulsion solvent evaporation method, slow diffusion of the organic solvent into the external aqueous phase followed by its evaporation at the air–water interface causes the polymer to precipitate and entrap the drug.27,28 Low drug loading is attributed to diffusion of the drug along with the organic solvent into the aqueous phase, followed by precipitation of the drug when the organic solvent evaporates. Rapid solvent removal allows the polymer to precipitate quickly, entrapping most of the drug within the polymer matrix.29 Applying reduced pressure30,31 or increasing the temperature32–34 can expedite solvent removal. Elevated temperature, however, can result in low particle yield, larger particle size, decreased encapsulation efficiency, and coarser morphology.33 We identified that rapid removal (< 20 min) of organic solvents using a rotary vacuum evaporator increased curcumin loading in microparticles from approximately 1% (w/w) to nearly 34% (w/w). This technique resulted in microparticles that were spherical in shape, had a smooth surface, and were in the injectable size range of 10–30 μm.
Polymers with different lactide-to-glycolide ratios (50:50 to 100:0) and molecular weights (from~13 to ~120 kDa), and varying organic to aqueous phase volume ratios were investigated to maximize drug loading. In general, higher molecular weight polymers tend to precipitate more quickly due to their lower aqueous solubility, resulting in greater drug entrapment.35 Similarly, increasing the glycolide content in the polymer reduces polymer solubility in the organic solvent, causing the polymer to precipitate quickly and resulting in greater encapsulation efficiency.36 Increasing organic to aqueous phase volume ratios resulted in increased amount of methanol in the organic phase and decreased the polymer solubility in the organic phase.37 This probably led to faster polymer precipitation and hardening, resulting in increased drug loading (up to 38%, w/w). Size appeared to be unaffected by any of the formulation parameters tested. This is expected because size has a greater dependence on the stirring rate, and the nature and concentration of emulsifier used in the oil-in-water emulsification process.38–40
Evaluating the in vitro release of curcumin from microparticles was a challenge because of the drug’s low solubility and poor stability. Solubility studies indicated that 10% (w/v) Tween 80 in PBS was required to achieve the target solubility of 2 μg/mL. The high concentration of Tween 80 used in this study could have possibly affected the particle integrity and polymer degradation rate but was required for maintaining sink conditions. The very low solubility of curcumin required a large volume of Tween 80-free release medium, which reduced curcumin concentrations in the medium below the detection limits of our analytical method. Curcumin is highly unstable in physiologic buffers because of rapid oxidation.8,41 Stability can be improved by lowering the pH or by adding glutathione, NAC, and ascorbic acid.8 We tested NAC at 0.1% (w/v) and BHT at 0.01% (w/v) as water-soluble and oil-soluble antioxidants, respectively. The oil-soluble antioxidant was expected to protect cur-cumin that was solubilized within the hydrophobic core of Tween 80 micelles, whereas the water-soluble antioxidant was expected to protect curcumin that was dissolved in PBS. Addition of both NAC and BHT significantly improved the stability of curcumin in the release medium (Fig. 5).
Dialysis membranes are commonly used for evaluating drug release from microparticles in vitro.42 Surfactants like Tween 80 can improve the aqueous solubility of hydrophobic drug molecules by micellar solubilization.43 At concentrations above the CMC (0.013–0.016 mg/mL), Tween 80 forms micelles that are 5–20 nm in size.44 Furthermore, these micelles increase in size when loaded with a drug.45 Tween 80 micelles, thus, cannot pass through the small pore diameters of dialysis membranes (~3 nm for 100,000 MW cutoff). Our preliminary studies with curcumin solution indicated that a membrane with a pore size of more than 50 nm was necessary to achieve rapid equilibrium between the donor and receptor compartments (not shown). We, therefore, used Corning Transwell® inserts with an average pore diameter of 400 nm as a novel and convenient tool for determining curcumin release from microparticles.
Drug release from PLGA microparticles can be varied from a few days to several weeks by varying the polymer composition (the lactide-to-glycolide ratio) and the molecular weight.46 A higher release rate from a lower molecular weight polymer is due to lower glass transition temperatures (Tg) and greater chain mobility of the polymer. Similarly, higher glycolide content in the polymer allows greater water permeability and faster drug release.47 The physical state of the drug inside microparticles can also influence the release rate. At high drug loading (>30% w/w), hydrophobic drugs exist in insoluble crystalline form, whereas at low drug loading, they exist as molecular dispersions.20,23 In general, when the drug is molecularly dispersed in the polymer, diffusion of the drug through the polymer controls the release rate. When the drug exists in crystalline form, dissolution of the drug in polymer controls the release rate. Release is often reduced at high drug loading because the drug has to first dissolve in polymer before diffusing out of microparticles.23,35 DSC studies established that curcumin was present in crystalline form in microparticles (Fig. 3). Thus, the observed biphasic curcumin release from PLGA microparticles can be explained as follows: an initial burst, attributable to the rapid release of drug molecules close to the surface,48–50 followed by pseudo zero-order release, attributable to the presence of precipitated drug in the polymer matrix.
For in vivo biomarker studies, the formulation with the highest drug loading (~38%, w/w, Table 2) was used. GST, a phase II enzyme that detoxifies carcinogens, is regarded as an important component of endogenous defense mechanism against carcinogenesis.51 COX-2, on the contrary, is a key mediator in the inflammation cascade, and has been implicated in tumorigenesis.52 Induction of GST and inhibition of COX-2 induction can thus serve as useful biomarkers of chemopreventive activity of curcumin.53,54 Our results suggest that hepatic levels of curcumin achieved with the microparticle formulation in the initial 24 h were adequate to induce GST activity (Fig. 7b). A similar correlation between curcumin liver concentration and GST activity was reported by Sharma et al.54 However, inhibition of COX-2 induction was not found to be significant until day 7 (Fig. 7b). The reason for the delay in inhibition of COX-2 activity is not clear. Further studies are needed to address this issue. The biomarker studies confirm that microparticles not only sustain curcumin concentrations in vivo but also that released curcumin retains chemopreventive activity.
CONCLUSIONS
Poor aqueous solubility and instability are challenges in curcumin formulation development. Using optimized manufacturing and in vitro release procedures, we fabricated PLGA microparticles with high cur-cumin loading and demonstrated sustained release of curcumin in vitro. Furthermore, a single subcutaneous dose of these microparticles resulted in sustained systemic exposure of curcumin in mice. Hepatic GST and COX-2 activities closely correlated with curcumin concentrations in the liver achieved with microparticles.
Acknowledgments
We thank Dr. John Nelson (Institute of Technology Characterization Facility, University of Minnesota) for assistance with SEM studies, and Sunny Bhardwaj and Dr. Raj Suryanarayanan for help with DSC studies. Funding from the NIH (CA 141996) and Grant-In-Aid program of the University of Minnesota.
References
- 1.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 2.Strimpakos AS, Sharma RA. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10(3):511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 6.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemo-preventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064. [PubMed] [Google Scholar]
- 7.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 8.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 9.Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. Injectable sustained release microparticles of curcumin: A new concept for cancer chemoprevention. Cancer Res. 2010;70(11):4443–4452. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282(1–2):1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Arshady R. Preparation of biodegradable microspheres and microcapsules: 2. Polylactides and related polyesters. J Control Release. 1991;17(1):1–21. [Google Scholar]
- 12.Bodmeier R, McGinity JW. The preparation and evaluation of drug-containing poly(dl-lactide) microspheres formed by the solvent evaporation method. Pharm Res. 1987;4(6):465–471. doi: 10.1023/a:1016419303727. [DOI] [PubMed] [Google Scholar]
- 13.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351(1):19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79(3):330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867–3875. [PubMed] [Google Scholar]
- 16.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37(3–4):223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Wang YM, Sato H, Horikoshi I. Preparation and characterization of poly(lactic-co-glycolic acid) microspheres for targeted delivery of a novel anticancer agent taxol. Chem Pharm Bull (Tokyo) 1997;44(10):1935–1940. doi: 10.1248/cpb.44.1935. [DOI] [PubMed] [Google Scholar]
- 18.Tonnesen HH, Masson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int J Pharm. 2002;244(1–2):127–135. doi: 10.1016/s0378-5173(02)00323-x. [DOI] [PubMed] [Google Scholar]
- 19.Okada H. One- and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv Drug Deliv Rev. 1997;28(1):43–70. doi: 10.1016/s0169-409x(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Price JC. Microencapsulation and dissolution properties of a neuroleptic in a biodegradable polymer, poly(d,l-lactide) J Pharm Sci. 1985;74(1):21–24. doi: 10.1002/jps.2600740106. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Chen L, Gu W, Xu Z, Gao Y, Li Y. In vitro and in vivo evaluation of donepezil-sustained release microparticles for the treatment of Alzheimer’s disease. Biomaterials. 2007;28(10):1882–1888. doi: 10.1016/j.biomaterials.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Tsung MJ, Burgess DJ. Preparation and characterization of gelatin surface modified PLGA microspheres. AAPS PharmSci. 2001;3(2):E11. doi: 10.1208/ps030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao S, Shi Y, Li L, Xu J, Schaper A, Kissel T. Effects of process and formulation parameters on characteristics and internal morphology of poly(d,l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur J Pharm Biopharm. 2008;68(2):214–223. doi: 10.1016/j.ejpb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Conti B, Genta I, Modena T, Pavanetto F. Investigation on process parameters involved in polylactide-co-glycolide microspheres preparation. Drug Dev Ind Pharm. 1995;21:615–622. [Google Scholar]
- 25.Cavalier M, Benoit JP, Thies C. The formation and characterization of hydrocortisone-loaded poly((+/−)-lactide) microspheres. J Pharm Pharmacol. 1986;38(4):249–253. doi: 10.1111/j.2042-7158.1986.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosilio V, Benoit JP, Deyme M, Thies C, Madelmont G. A physicochemical study of the morphology of progesterone-loaded microspheres fabricated from poly(D,L-lactide-co-glycolide) J Biomed Mater Res. 1991;25(5):667–682. doi: 10.1002/jbm.820250509. [DOI] [PubMed] [Google Scholar]
- 27.Tice TR, Gilley RM. Preparation of injectable controlled-release microcapsules by a solvent-evaporation process. J Control Release. 1985;2:343–352. [Google Scholar]
- 28.McGinity JW, O’Donnell PB. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28(1):25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 29.Bodmeier R, McGinity JW. Solvent selection in the preparation of poly (DL-lactide) microspheres prepared by the solvent evaporation method. Int J Pharm. 1988;43:179–186. [Google Scholar]
- 30.Izumikawa S, Yoshikawa S, Aso Y, Takeda Y. Preparation of poly (l-lactide) microspheres of different crystalline morphology and effect of crystalline morphology on drug release rate. J Control Release. 1991;15:133–140. [Google Scholar]
- 31.Chung TW, Huang YY, Liu YZ. Effects of the rate of solvent evaporation on the characteristics of drug loaded PLLA and PDLLA microspheres. Int J Pharm. 2001;212(2):161–169. doi: 10.1016/s0378-5173(00)00574-3. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki Y, Onuki Y, Yakou S, Takayama K. Effect of temperature-increase rate on drug release characteristics of dextran microspheres prepared by emulsion solvent evaporation process. Int J Pharm. 2006;324(2):144–151. doi: 10.1016/j.ijpharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Yang CY, Tsay SY, Tsiang RC. Encapsulating aspirin into a surfactant-free ethyl cellulose microsphere using non-toxic solvents by emulsion solvent-evaporation technique. J Microencapsul. 2001;18(2):223–236. doi: 10.1080/026520401750063937. [DOI] [PubMed] [Google Scholar]
- 34.Bodmeier R, McGinity JW. Polylactic acid microspheres containing quinidine base and quinidine sulphate prepared by the solvent evaporation technique. II. Some process parameters influencing the preparation and properties of microspheres. J Microencapsul. 1987;4(4):289–297. doi: 10.3109/02652048709021821. [DOI] [PubMed] [Google Scholar]
- 35.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Johansen P, Men Y, Audran R, Corradin G, Merkle HP, Gander B. Improving stability and release kinetics of microencapsulated tetanus toxoid by co-encapsulation of additives. Pharm Res. 1998;15(7):1103–1110. doi: 10.1023/a:1011998615267. [DOI] [PubMed] [Google Scholar]
- 37.Coombes AG, Scholes PD, Davies MC, Illum L, Davis SS. Resorbable polymeric microspheres for drug delivery–production and simultaneous surface modification using PEO-PPO surfactants. Biomaterials. 1994;15(9):673–680. doi: 10.1016/0142-9612(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 38.Boisdron-Celle M, Menei P, Benoit JP. Preparation and characterization of 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers. J Pharm Pharmacol. 1995;47(2):108–114. doi: 10.1111/j.2042-7158.1995.tb05760.x. [DOI] [PubMed] [Google Scholar]
- 39.Wakiyama N, Juni K, Nakano M. Preparation and evaluation in vitro of polylactic acid microspheres containing local anesthetics. Chem Pharm Bull (Tokyo) 1981;29(11):3363–3368. doi: 10.1248/cpb.29.3363. [DOI] [PubMed] [Google Scholar]
- 40.Jalil R, Nixon JR. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: Problems associated with preparative techniques and release properties. J Microencapsul. 1990;7(3):297–325. doi: 10.3109/02652049009021842. [DOI] [PubMed] [Google Scholar]
- 41.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–494. [PubMed] [Google Scholar]
- 42.Zidan AS, Sammour OA, Hammad MA, Megrab NA, Hussain MD, Khan MA, Habib MJ. Formulation of anastrozole microparticles as biodegradable anticancer drug carriers. AAPS PharmSciTech. 2006;7(3):61. doi: 10.1208/pt070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinko PJ, Martin AN. Physical pharmacy-physical chemical principles in the pharmaceutical sciences. 5. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 44.Turk M, Lietzow R. Stabilized nanoparticles of phytosterol by rapid expansion from supercritical solution into aqueous solution. AAPS PharmSciTech. 2004;5(4):e56. doi: 10.1208/pt050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attwood D, Ktistis G, McCormick Y, Story MJ. Solubilization of indomethacin by polysorbate 80 in mixed water-sorbitol solvents. J Pharm Pharmacol. 1989;41(2):83–86. doi: 10.1111/j.2042-7158.1989.tb06398.x. [DOI] [PubMed] [Google Scholar]
- 46.Alexis AF. Factors affecting the degradation and drug release mechanism of poly(lactic) acid and poly [(lactic acid)-co-(glycolic acid)] Polymer Int. 2005;54:36–46. [Google Scholar]
- 47.Jamshidi K, Hyon SH, Ikada Y. Thermal characterization of polylactides. Polymer. 1988;29:2229–2234. [Google Scholar]
- 48.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 49.Igartua M, Hernandez RM, Esquisabel A, Gascon AR, Calvo MB, Pedraz JL. Influence of formulation variables on the in-vitro release of albumin from biodegradable microparticulate systems. J Microencapsul. 1997;14(3):349–356. doi: 10.3109/02652049709051138. [DOI] [PubMed] [Google Scholar]
- 50.Jeffery H, Davis SS, O’Hagan DT. The preparation and characterization of poly(lactide-co-glycolide) microparticles. II. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm Res. 1993;10(3):362–368. doi: 10.1023/a:1018980020506. [DOI] [PubMed] [Google Scholar]
- 51.Sharma RA, Manson MM, Gescher A, Steward WP. Colorectal cancer chemoprevention: Biochemical targets and clinical development of promising agents. Eur J Cancer. 2001;37(1):12–22. doi: 10.1016/s0959-8049(00)00326-9. [DOI] [PubMed] [Google Scholar]
- 52.Sharma RA. Translational medicine: Targetting cyclo-oxygenase isozymes to prevent cancer. QJM. 2002;95(5):267–273. doi: 10.1093/qjmed/95.5.267. [DOI] [PubMed] [Google Scholar]
- 53.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18(44):6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 54.Sharma RA, Ireson CR, Verschoyle RD, Hill KA, Williams ML, Leuratti C, Manson MM, Marnett LJ, Steward WP, Gescher A. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. 2001;7(5):1452–1458. [PubMed] [Google Scholar]