Chemotherapy results in transient or permanent ovarian failure in the majority of women. Prechemotherapy assessment of serum anti-Müllerian hormone may be useful for predicting postchemotherapy ovarian function. This finding has implications for decision making about adjuvant endocrine therapy in premenopausal women treated with chemotherapy.
Keywords: Breast cancer, Chemotherapy, Ovarian function, Anti-Müllerian hormone
Learning Objectives
Explain the association between clinical factors and postchemotherapy ovarian function.
Explain the association between biochemical markers and postchemotherapy ovarian function.
Discuss the role that age and anti-Müllerian hormone may play in prediction of postchemotherapy ovarian function status.
Abstract
Background.
Reproductive-aged women frequently receive both chemotherapy and endocrine therapy as part of their treatment regimen for early stage hormone receptor-positive breast cancer. Chemotherapy results in transient or permanent ovarian failure in the majority of women. The difficulty in determining which patients will recover ovarian function has implications for adjuvant endocrine therapy decision making. We hypothesized that pretreatment serum anti-Müllerian hormone (AMH) and inhibin B concentrations would predict for ovarian function following chemotherapy.
Methods.
Pre- and perimenopausal women aged 25–50 years with newly diagnosed breast cancer were enrolled. Subjects underwent phlebotomy for assessment of serum AMH, inhibin B, follicle-stimulating hormone, and estradiol prior to chemotherapy and 1 month and 1 year following completion of treatment. Associations among hormone concentrations, clinical factors, and biochemically assessed ovarian function were assessed.
Results.
Twenty-seven subjects were evaluable for the primary endpoint. Median age was 41. Twenty subjects (74.1%) experienced recovery of ovarian function within 18 months. Of the 26 evaluable subjects assessed prior to chemotherapy, 19 (73.1%) had detectable serum concentrations of AMH. The positive predictive value of a detectable baseline serum AMH concentration for recovery of ovarian function was 94.7%, and the negative predictive value was 85.7%. On univariate analysis, younger age and detectable serum AMH concentration at chemotherapy initiation were predictive of increased likelihood of recovery of ovarian function.
Conclusion.
Prechemotherapy assessment of serum AMH may be useful for predicting postchemotherapy ovarian function. This finding has implications for decision making about adjuvant endocrine therapy in premenopausal women treated with chemotherapy.
Implications for Practice:
Premenopausal women with breast cancer can develop ovarian failure when treated with chemotherapy. The ability to accurately predict ovarian function after chemotherapy has implications for both adjuvant endocrine therapy decision making and future fertility planning. We evaluated potential clinical predictors as well as blood markers of ovarian reserve. We identified an association between both younger age and higher serum anti-Müllerian hormone and increased likelihood of recovery of ovarian function following chemotherapy. This finding could have implications for younger women who are contemplating future fertility as well as older premenopausal women who are making decisions about adjuvant antihormonal treatment for breast cancer.
Introduction
Although most breast cancers diagnosed each year in the United States are in postmenopausal women, a substantial minority are premenopausal. Menstrual status has both survivorship and treatment implications. Younger women with breast cancer are more likely to be treated with chemotherapy, which can lead to cessation of ovarian function and consequent loss of fertility, decreased sexual activity, and higher risk of long-term adverse medical conditions, such as osteoporosis and coronary artery disease [1–3]. Chemotherapy causes temporary amenorrhea in the majority of women, and risk of permanent amenorrhea increases with age and with treatment regimen [4, 5]. Most studies have evaluated presence or absence of menses, rather than biochemical assessment, as a measure of ovarian function. Because women can have residual ovarian function in the absence of menses, and because factors such as adjuvant endocrine therapy can influence menses, the impact of chemotherapy on long-term ovarian function is unclear.
Ovarian function can be challenging to assess, especially in the postchemotherapy setting. Potential markers of ovarian reserve include biochemical markers and imaging assessments [6, 7]. Serum concentrations of anti-Müllerian hormone (AMH) and inhibin B both decrease with declining ovarian function, whereas follicle-stimulating hormone (FSH) increases [8]. Serum AMH concentration has been shown to be the best biochemical marker for assessment of decline in reproductive capacity in healthy women [8]. In addition, imaging with transvaginal ultrasound can be used to determine antral follicle count, which may correlate with residual ovarian function [9]. Limited data in chemotherapy-treated women with breast cancer have demonstrated that prechemotherapy serum AMH and inhibin B concentrations predicted for chemotherapy-related amenorrhea 1 year following chemotherapy [10]. Likewise, a second study demonstrated that serum AMH concentration, but not inhibin levels, was predictive for continued menses 4–5 years after chemotherapy administration [11].
Knowledge prior to chemotherapy administration of the likelihood of ovarian function recovery following chemotherapy has implications for planning for individual patients; for example, those who wish to maintain fertility may desire to undergo embryo or oocyte cryopreservation [12]. For those who do not desire future fertility, knowledge of permanent loss of ovarian function could lead to earlier implementation of preventive measures for menopause-related disorders such as osteoporosis and coronary artery disease. Finally, postchemotherapy ovarian function has direct implications for choice of adjuvant endocrine therapy for hormone receptor-positive (HR-positive) breast cancer.
Treatment of HR-positive breast cancer with adjuvant endocrine therapy has been shown to improve disease-free and overall survival rates. In postmenopausal women, aromatase inhibitors (AIs) have been shown to be more effective than tamoxifen [13]; however, in women who are premenopausal at the time of diagnosis, AIs may cause a paradoxical rise in estrogen levels resulting from reactivation of ovarian function. Consequently, even in women who have developed chemotherapy-induced ovarian failure, tamoxifen is the standard of care [14, 15].
Taken together, these factors highlight the clinical importance of assessment of ovarian function in pre- or perimenopausal women with HR-positive breast cancer who are treated with chemotherapy because this information directly affects endocrine therapy decision making. We hypothesized that low prechemotherapy serum concentrations of AMH and inhibin B would predict for lack of recovery of ovarian function following chemotherapy. In order to address this hypothesis, we prospectively evaluated a cohort of premenopausal women ranging from 25 to 50 years of age who were initiating chemotherapy for early stage breast cancer.
Materials and Methods
Subjects
This report is derived from a prospective registry study performed at the University of Michigan Comprehensive Cancer Center. Women aged 25–50 years who had a menstrual cycle within 3 months prior to study entry, who were diagnosed with stage I–III breast cancer, and who were scheduled to receive neoadjuvant or adjuvant chemotherapy were enrolled in the registry between December 2007 and December 2008 (ClinicalTrials.gov identifier NCT00644683). Prior cytotoxic chemotherapy for any reason, bilateral oophorectomy, hysterectomy, and pelvic radiation were prohibited. Subjects who were planning to receive concomitant gonadotropin-releasing hormone agonist therapy during chemotherapy were ineligible. The clinical protocol was approved by the University of Michigan institutional review board, and all subjects provided written informed consent prior to undergoing any protocol-directed procedures. Total planned accrual was 28 subjects, with 7 in each age cohort (25–34 years, 35–39 years, 40–44 years, 45–50 years).
Study Design
After enrollment, each subject underwent phlebotomy, completed a past medical and gynecological history questionnaire, and initiated treatment with her treating physician’s choice of adjuvant or neoadjuvant chemotherapy regimen. Phlebotomy was repeated 14–42 days and 12–15 months following completion of chemotherapy. Subjects underwent follow-up 6 months and 12–15 months following completion of chemotherapy, by telephone and in person, respectively, to collect information about recurrence of menses and concomitant medications. Subjects were instructed to notify the clinic if they experienced resumption of menses between clinic visits. Clinical records were reviewed to determine whether patients experienced resumption of menses following the 12- to 15-month study visit.
Laboratory Analyses
AMH, inhibin B, and FSH assays were performed by the Clinical Ligand Assay Satellite Service laboratory at the University of Michigan School of Public Health. Serum AMH and inhibin B concentrations were measured using the AMH Gen II ELISA (A73818; Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com) and Inhibin B Gen II ELISA (A81301; Beckman Coulter), respectively, according to the manufacturer’s instructions. For AMH, the minimum detectable concentration was 0.16 ng/mL, the upper level of quantification (LOQ) was 22.5 ng/mL, and the inter- and intra-assay coefficients of variation (CVs) were 7.1% and 2.9%, respectively. For inhibin B, the minimal detectable concentration was 10 pg/mL, the upper LOQ was 1,000 pg/mL, and the inter- and intra-assay CVs were 4.3% and 2.8%, respectively. Serum FSH concentrations were measured using a two-site chemiluminescence (sandwich) assay [16, 17], which has a minimum detectable concentration of 0.3 mIU/mL and an upper LOQ of 200 mIU/mL. Inter- and intra-assay CVs were 8.1% and 3.5%, respectively. Serum estradiol concentrations were measured using a gas chromatography tandem mass spectroscopy assay (InVentiv Health Clinical, Princeton, NJ, http://www.inventivhealthclinical.com), which has a minimal detectable concentration of 0.625 pg/mL [18].
Statistical Analysis
The primary endpoint was recovery of ovarian function within 18 months of completion of adjuvant or neoadjuvant chemotherapy. Ovarian function was defined as recurrence of menses or serum estradiol concentration > 10 pg/mL. Logistic regression was used to evaluate associations between clinical and biochemical factors. In univariate analysis, recovery of ovarian function was the response variable and was tested against each potential covariate (age at study enrollment; body mass index; smoking status; use of endocrine therapy; and serum concentrations of AMH, estradiol, FSH, and inhibin B). The multivariate analysis included only those factors that were significant in the univariate analysis. In both the univariate and multivariate analyses, AMH was evaluated as a dichotomous variable, divided into detectable levels (≥0.16 ng/mL) versus undetectable levels (<0.16 ng/mL). Values of p < .05 were considered statistically significant.
Results
Change in Ovarian Function With Chemotherapy
A total of 29 subjects were enrolled into four age cohorts: 25–34 years (n = 6), 35–39 years (n = 8), 40–44 years (n = 7), and 45–50 years (n = 8) (supplemental online Table 1). After signing the informed consent document, one subject in the youngest cohort was determined to be ineligible because she received gonadotropin-releasing hormone agonist therapy prior to initiation of chemotherapy. Twenty-five of the 27 eligible subjects (92.6%) reported being premenopausal at the time of enrollment. Two subjects reported being perimenopausal, defined as having irregular menses in the setting of previously regular menses. Baseline clinical and laboratory characteristics of enrolled subjects are listed in Tables 1 and 2.
Table 1.
Patient characteristics (n = 28)
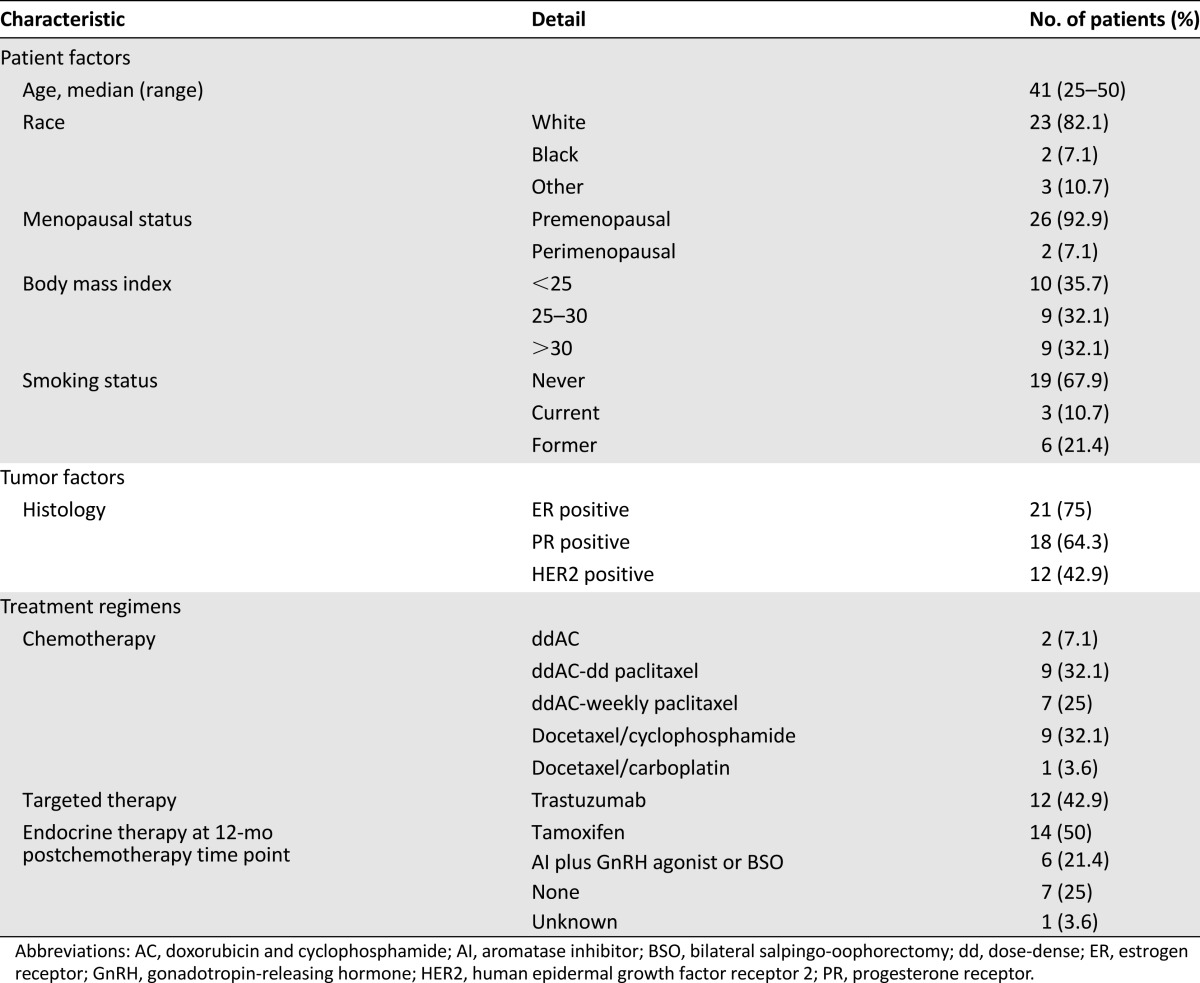
Table 2.
Markers of ovarian reserve prior to and following chemotherapy in evaluable subjects
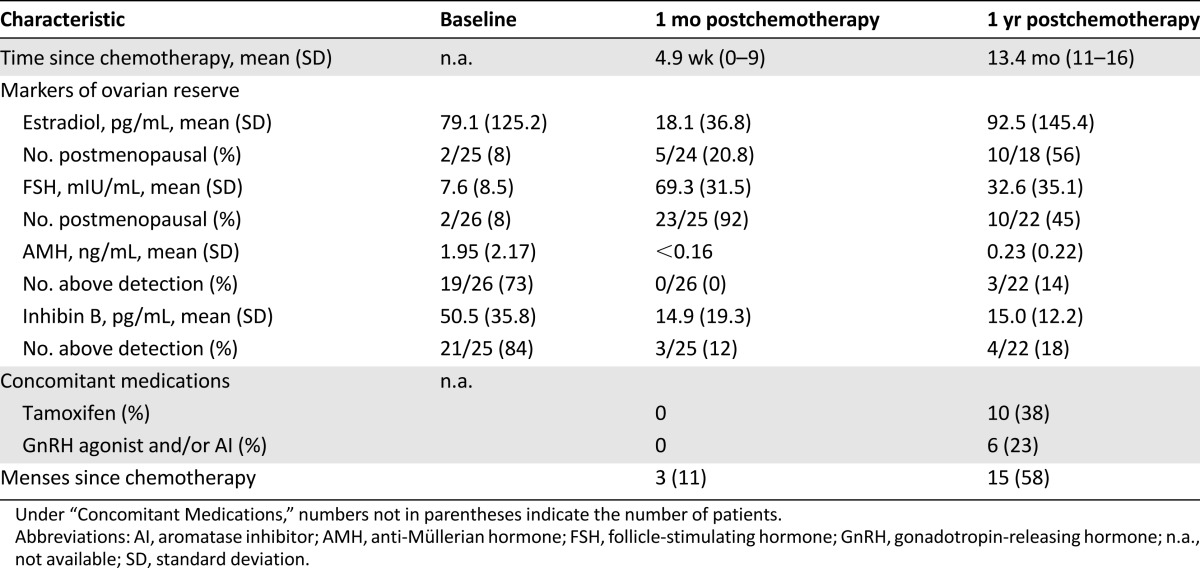
At the second study visit, which took place an average of 4.9 weeks (standard deviation [SD]: 2.1 weeks) following completion of chemotherapy, no subjects were taking adjuvant endocrine therapy (tamoxifen, luteinizing hormone-releasing hormone agonist, and/or AI). Average time since last menstrual period was 16.3 weeks (95% confidence interval [CI]: 7.7–24.9). One subject reported continued menstruation during chemotherapy. Two subjects, both of whom had serum estradiol concentrations >100 pg/mL, reported that menses had recurred since completing chemotherapy. Of the other 24 subjects with available serum estradiol concentrations, 4 had levels >10 pg/mL but had not experienced the return of menses.
At the final assessment an average of 13.6 months (SD: 1.1 months) following chemotherapy, 17 subjects were receiving treatment with tamoxifen and 6 subjects were not taking endocrine therapy. Four additional subjects resumed menstruation following chemotherapy and then started treatment with ovarian suppression or ablation plus AI therapy. One subject underwent bilateral salpingo-oophorectomy 4 months after completing chemotherapy, prior to resuming menses, and thus was not evaluable for the primary endpoint. Twenty of the 27 evaluable subjects (74%) had experienced recovery of ovarian function since completion of chemotherapy based on return of menses (n = 14) and/or serum estradiol concentration > 10 pg/mL (n = 10). Seven of 27 subjects (26%) had not had vaginal bleeding since completing chemotherapy, and 5 of these subjects were confirmed to have serum estradiol concentrations in the postmenopausal range.
Change in Markers of Ovarian Reserve With Chemotherapy
Prior to chemotherapy, 19 of 26 (73%) and 21 of 25 (84%) evaluable subjects had serum AMH and inhibin B concentrations above the level of detection, including 3 of 7 (43%) and 6 of 7 (86%), respectively, in the oldest age cohort. Two of 26 subjects (8%) had serum FSH concentrations in the postmenopausal range, defined as >21 mIU/mL; both subjects were aged >40 years, and one reported irregular menses prior to enrollment.
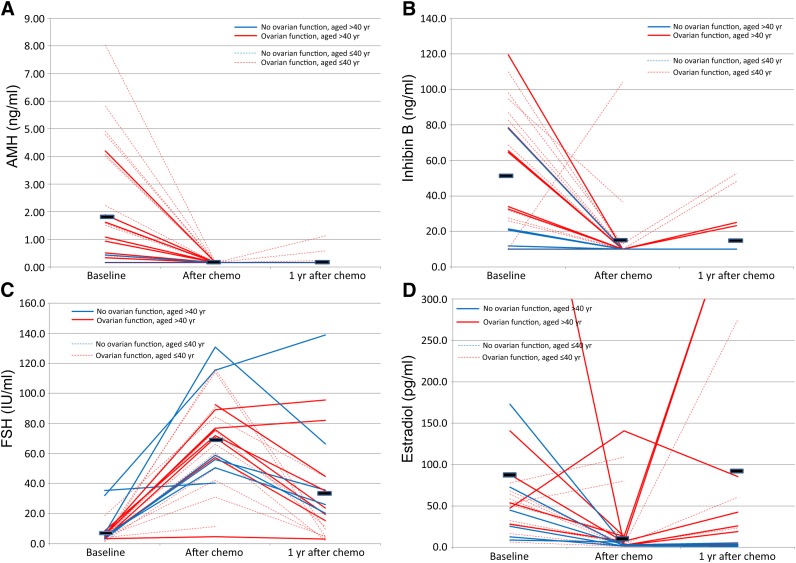
At the assessment 1 month after completion of chemotherapy, no subjects with available serum samples had AMH concentrations above the level of detection (Fig. 1A, Table 2). Similarly, only 3 of 25 (12%) had inhibin B concentrations above the level of detection (Fig. 1B, Table 2). Twenty-three of 25 subjects (92%) had FSH concentrations in the postmenopausal range (Fig. 1C, Table 2). The subject whose inhibin B concentration increased with chemotherapy did not have a concomitant decrease in FSH concentration.
Figure 1.
Change in serum concentrations of ovarian reserve markers with chemotherapy. Each line signifies an individual subject. Solid lines: subjects aged >40 years. Dotted lines: subjects aged ≤40 years. Blue lines: subjects with ovarian failure. Red lines: subjects with ovarian function recovery. Black bars: mean hormone concentration at each time point. (A): AMH. (B): Inhibin B. (C): FSH. (D): Estradiol.
Abbreviations: AMH, anti-Müllerian hormone; chemo, chemotherapy; FSH, follicle-stimulating hormone.
One year following completion of chemotherapy, 3 of 22 subjects (13%) had serum AMH levels above the level of detection. These three subjects were all under age 40 and had recovery of ovarian function (Fig. 1A, Table 2). Among 4 of 22 subjects (18%), one with a detectable AMH level also had a detectable inhibin B level and three others had detectable serum inhibin B but not AMH concentrations. Ten of 22 subjects (45%) had FSH concentrations in the postmenopausal range (Fig. 1C, Table 2). As can be seen in Figure 1C, all but one of the women aged >40 years had higher FSH concentrations following chemotherapy, regardless of whether they recovered ovarian function. All subjects with detectable AMH and half with detectable inhibin B levels had FSH concentrations below the postmenopausal range.
Markers of Ovarian Reserve and Recovery of Ovarian Function
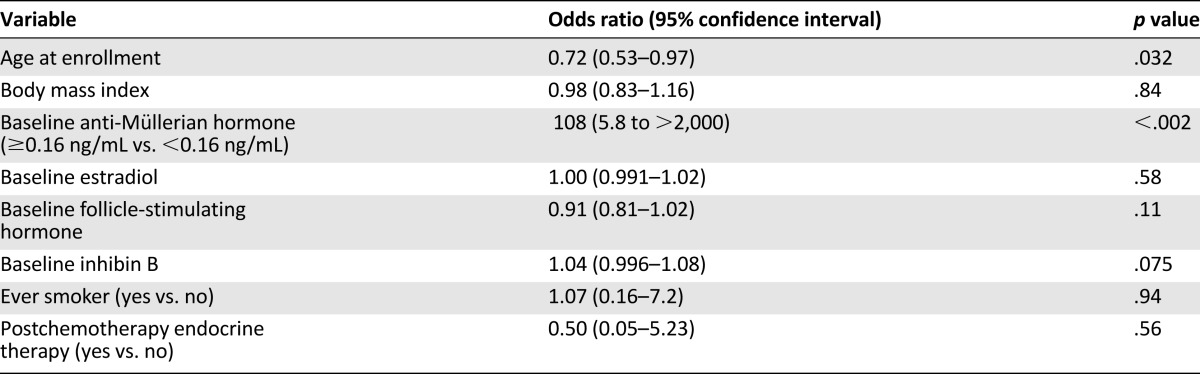
Clinical and laboratory factors were evaluated using univariate analysis to identify associations with recovery of ovarian function following chemotherapy. As shown in Table 3, only younger age at chemotherapy (odds ratio [OR]: 0.72 [95% CI: 0.53–0.97]; p = .032) and detectable baseline serum AMH concentration (OR: 108 [95% CI: 5.8 to >2,000]; p < .002) were statistically significantly associated with recovery of ovarian function. Of the seven subjects without recovery of ovarian function, six (85.7%) had undetectable serum AMH concentrations at baseline. In contrast, of the 19 subjects with serum samples available who had recovery of ovarian function, only 1 (5%) had an undetectable baseline AMH value. In addition, there was a trend toward an association between baseline serum inhibin B concentration and recovery of ovarian function, with an OR of 1.04 (95% CI: 0.996–1.08; p = .075). None of the factors remained significant in the multivariate analysis (data not shown).
Table 3.
Univariate analysis of the effect of potential covariates on recovery of ovarian function following chemotherapy
In an exploratory analysis, conditioned on subjects with detectable serum AMH concentrations at baseline, the likelihood of ovarian function recovery was 0.998 for subjects aged <35 years, 0.990 for subjects aged 35–39 years, and 0.95 for subjects aged 40–44 years. Too few subjects in the cohort aged 45–50 years had detectable baseline AMH concentrations to perform the analysis.
Discussion
In this study of young women with breast cancer who were initiating chemotherapy, we observed that older age and prechemotherapy serum AMH concentration below the level of assay detection were associated with increased likelihood of biochemically determined ovarian failure at 18 months following chemotherapy. The positive predictive value of a detectable baseline serum AMH concentration for prediction of recovery of ovarian function after chemotherapy was 94.7%, and the negative predictive value was 85.7%. These findings are consistent with previously published data on prechemotherapy AMH and biological underpinnings [11].
Our data are also consistent with previous reports that postchemotherapy AMH and inhibin B serum concentrations are lower in women treated with chemotherapy compared with age-matched healthy controls [19–21]. In a cohort of women with a median age of 43 years at the time of chemotherapy, serum concentrations of both hormones following chemotherapy were shown to be associated with chemotherapy-related amenorrhea [20]. Postchemotherapy serum AMH concentrations were also lower in a younger cohort of breast cancer survivors who continued menstruation following chemotherapy compared with healthy controls [19]. Finally, a recently reported trial of pre- and perimenopausal women who developed chemotherapy-induced ovarian failure and subsequently started treatment with anastrozole failed to identify an association between serum AMH concentration immediately prior to AI initiation and recovery of ovarian function [22]. Based on these data, postchemotherapy AMH assessment does not appear to be useful for endocrine therapy decision making.
Identification of a measurable factor that could be used to predict postchemotherapy ovarian reserve would be important for management of younger women who are concerned about ovarian function recovery and future fertility. American Society of Clinical Oncology guidelines recommend that oncologists address the possibility of infertility with all patients of child-bearing potential who may desire future fertility [12]; however, in practice, fertility-preservation measures are time consuming and expensive and may delay initiation of therapy. If preservation of ovarian function and, more important, fertility could be better predicted with a circulating biomarker such as AMH, then embryo or oocyte preservation may not be necessary in a large number of women aged <40 years who have a detectable serum AMH concentration prior to initiation of standard breast cancer adjuvant chemotherapies.
More applicable to older pre- and perimenopausal women is the need to accurately determine ovarian function in order to make treatment decisions about adjuvant endocrine therapy. It is known that the risk of chemotherapy-induced ovarian failure increases with increasing age [4, 5]; however, the absolute age above which oncologists can be certain that a woman will not have residual ovarian function has not been established. There is concern that a proportion of women who are believed to have ovarian failure following chemotherapy may in fact recover ovarian function and thus should not be treated with AI monotherapy [14, 22]. It has been suggested that all women who are premenopausal prior to chemotherapy, even those in their late 40s and early 50s, should be treated with adjuvant tamoxifen therapy or, if they are going to receive an aromatase inhibitor, should have their ovaries removed or chemically suppressed [22]. For the latter group, these strategies are invasive and are associated with increased side effects. Consequently, prediction of permanent ovarian failure using information other than patient age is of interest. Although it is known that serum AMH concentration and age are correlated [8], the data from this study and others [11] suggest that prechemotherapy assessment of serum AMH concentrations, or possibly the combination of AMH and inhibin B, may provide important information about the likelihood of developing permanent ovarian failure with chemotherapy and could identify a patient population in which it is safe to treat with upfront AI monotherapy.
Strengths of our study include prospective, serial assessment of markers of ovarian reserve both before and after chemotherapy in subjects over a wide age range. In particular, serial monitoring of subjects permitted assessment of intrapatient change rather than relying on comparison of population averages. In addition, serum estradiol levels were assessed using an ultrasensitive assay designed for increased accuracy at very low estradiol levels, such as those found in postmenopausal women. This assessment enabled accurate biochemical assessment of ovarian function, as opposed to relying on patient self-report of menstrual function.
One limitation is the heterogeneity of administered chemotherapy regimens; however, the impact of this limitation is expected to be minimal because all but one subject were treated with a cyclophosphamide-containing chemotherapy regimen—the chemotherapy agent that appears to have the greatest impact on ovarian function—and all regimens contained similar total doses of the medication. An additional limitation is the use of adjuvant endocrine therapy by the majority of subjects at the final assessment. Ovarian function could still be assessed in those treated with tamoxifen using the estradiol assay, but those who underwent chemical ovarian suppression or bilateral oophorectomy were noninformative. Imaging methodologies such as transvaginal ultrasound were not performed in this clinical study. Recent research has suggested that imaging findings such as antral follicle count may provide additional information regarding ovarian function in chemotherapy-treated women [23]. Most important, the relatively small sample size limits the statistical power to detect associations between clinical or biochemical factors and recovery of ovarian function on multivariate analysis. Consequently, these data alone do not provide validated assay cutoffs for either recovery or permanent loss of ovarian function, and more work is needed in order to make measurement of baseline AMH a routine clinical practice.
Conclusion
These results suggest that in women with a serum AMH concentration of at least 0.16 ng/mL and who are aged <40 years, ovarian function recovery is likely. In contrast, in older pre- and perimenopausal women with breast cancer who undergo treatment with standard adjuvant chemotherapy regimens, low prechemotherapy serum AMH, and possibly inhibin B, concentrations may be predictive of permanent ovarian failure. Currently, data are insufficient to support routine assessment of these biomarkers prior to chemotherapy initiation. Prospective validation of this hypothesis is warranted because these findings could have significant implications for decision making about adjuvant endocrine therapy in this cohort of patients.
This article is available for continuing medical education credit at CME.TheOncologist.com.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
For Further Reading: Di Paola R, Costantini C, Tecchio C et al. Anti-Müllerian Hormone and Antral Follicle Count Reveal a Late Impairment of Ovarian Reserve in Patients Undergoing Low-Gonadotoxic Regimens for Hematological Malignancies. The Oncologist December 2013;18:1307–1314.
Implications for Practice: The need for accurate and personalized counseling of women diagnosed with hematological malignancies and exposed to anticancer therapy in their reproductive age is the major clinical implication of this study. More specifically, the study implies that adequate fertility preservation methods should be discussed when therapies with a low toxicity to the reproductive system are used. Indeed, patients may experience impairment of their reproductive potential, even several years post-therapy, hampering their fertility wishes. Patients should undergo frequent evaluation of their fertility potential to detect eventual impairments in a timely manner.
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (Grant 5K07CA134747 to N.L.H.) and the University of Michigan Comprehensive Cancer Center (to N.L.H.). These findings have not been presented previously.
Author Contributions
Conception/Design: N. Lynn Henry, Mousumi Banerjee, Daniel F. Hayes
Financial support: N. Lynn Henry
Provision of study material or patients: N. Lynn Henry, Anne F. Schott, Daniel F. Hayes
Collection and/or assembly of data: N. Lynn Henry, Daniel McConnell
Data analysis and interpretation: N. Lynn Henry, Rong Xia, Anne F. Schott, Daniel McConnell, Mousumi Banerjee, Daniel F. Hayes
Manuscript writing: N. Lynn Henry, Rong Xia, Anne F. Schott, Mousumi Banerjee, Daniel F. Hayes
Final approval of manuscript: N. Lynn Henry, Rong Xia, Anne F. Schott, Daniel McConnell, Mousumi Banerjee, Daniel F. Hayes
Disclosures
Daniel F. Hayes: Veridex, Janssen (RF), Roche (H). The other authors indicated no financial relationships.
Section Editors: Dennis Chi: None; Peter Harper: Sanofi, Roche, Imclone, Pfizer, GlaxoSmithKline, Lilly, Genentech (C/A); Lilly, Novartis, Sanofi, Roche (H).
Reviewer “A”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 3.Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 5.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 6.Fanchin R, Schonäuer LM, Righini C, et al. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 7.van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: A novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 8.van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: A longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Pellicer A, Ardiles G, Neuspiller F, et al. Evaluation of the ovarian reserve in young low responders with normal basal levels of follicle-stimulating hormone using three-dimensional ultrasonography. Fertil Steril. 1998;70:671–675. doi: 10.1016/s0015-0282(98)00268-4. [DOI] [PubMed] [Google Scholar]
- 10.Anders C, Marcom PK, Peterson B, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26:286–295. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RA, Cameron DA. Pretreatment serum anti-müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 13.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith IE, Dowsett M, Yap Y-S, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: Caution and suggested guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 15.Burstein HJ, Mayer E, Patridge AH, et al. Inadvertent use of aromatase inhibitors in patients with breast cancer with residual ovarian function: Cases and lessons. Clin Breast Cancer. 2006;7:158–161. doi: 10.3816/cbc.2006.n.026. [DOI] [PubMed] [Google Scholar]
- 16.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 17.Santoro N, Crawford SL, Allsworth JE, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284:E521–E530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 18.Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu B, Douglas N, Ferin MJ, et al. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010;116:2099–2105. doi: 10.1002/cncr.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011–2016. doi: 10.1093/annonc/mdt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su HI, Chung K, Sammel MD, et al. Antral follicle count provides additive information to hormone measures for determining ovarian function in breast cancer survivors. Fertil Steril. 2011;95:1857–1859. doi: 10.1016/j.fertnstert.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.