This study sought to determine whether the addition of a vascular endothelial growth factor signaling inhibitor (cediranib) to conventional chemoradiation had an impact on the frequency of pseudoprogression. The results show that administration of a VEGF inhibitor during and after chemoradiation modifies the expression of pseudoprogression by imaging.
Keywords: Pseudoprogression, Vascular endothelial growth factor, Cediranib, Glioblastoma
Abstract
Background.
Chemoradiation (CRT) can significantly modify the radiographic appearance of malignant gliomas, especially within the immediate post-CRT period. Pseudoprogression (PsP) is an increasingly recognized phenomenon in this setting, and is thought to be secondary to increased permeability as a byproduct of the complex process of radiation-induced tissue injury, possibly enhanced by temozolomide. We sought to determine whether the addition of a vascular endothelial growth factor (VEGF) signaling inhibitor (cediranib) to conventional CRT had an impact on the frequency of PsP, by comparing two groups of patients with newly diagnosed glioblastoma before, during, and after CRT.
Methods.
All patients underwent serial magnetic resonance imaging as part of institutional review board-approved clinical studies. Eleven patients in the control group received only chemoradiation, whereas 29 patients in the study group received chemoradiation and cediranib until disease progression or toxicity. Response assessment was defined according to Response Assessment in Neuro-Oncology criteria, and patients with enlarging lesions were classified into true tumor progressions (TTP) or PsP, based on serial radiographic follow-up.
Results.
Two patients in the study group (7%) showed signs of apparent early tumor progression, and both were subsequently classified as TTP. Six patients in the control group (54%) showed signs of apparent early tumor progression, and three were subsequently classified as TTP and three as PsP. The frequency of PsP was significantly higher in the control group.
Conclusion.
Administration of a VEGF inhibitor during and after CRT modifies the expression of PsP by imaging.
Implications for Practice:
Pseudoprogression is an unsolved clinical dilemma during postchemoradiation surveillance for malignant gliomas. It has received substantial attention in the era of temozolomide-based regimens, mimicking early disease progression on imaging studies and challenging patient management and interpretation of clinical trials. This study suggests that the incidence of pseudoprogression in patients receiving antiangiogenic therapy is low and that enlarging lesions in this patient population are more likely to represent true tumor progression than transient post-treatment effects.
Introduction
Glioblastomas (GBMs) have proven to be one of the most challenging neoplasms in modern clinical oncology. They are associated with devastating neurological sequelae and dismal prognosis, and because of their inherent genetic, cellular, and histologic complexity, this neoplasm is resistant to most treatment modalities. Almost all patients will present with recurrent disease within 12 months [1], and few survive past 5 years. Despite the advancements in anatomical and functional imaging technology, there are still significant limitations in imaging characterization of GBM, including determination of tumor boundaries, characterization of tumoral versus pathological nontumoral tissue in the brain, and differentiation of therapy-induced changes from recurrent tumor.
Pseudoprogression (PsP) is a transient phenomenon that has recently gained substantial attention. PsP mimics true tumor progression (TTP) and is a considerable challenge for patient management and interpretation of clinical trials [2]. On magnetic resonance imaging (MRI), PsP appears as increasing contrast enhancement and vasogenic edema at the tumor site or resection margins, most commonly in the first 3 months following chemoradiation (CRT). PsP is thought to be induced by a treatment-related local tissue reaction accompanied by a robust inflammatory process that manifests as edema and transient increase in permeability of the blood-brain barrier [3–5]. It is not possible to distinguish between PsP and early TTP clinically or radiographically, and the PsP diagnosis is usually made by histological evaluation or on follow-up imaging studies when enhancing lesions subsequently regress or become stable without changes in treatment. PsP is relatively common in the early post-CRT follow-up of patients with GBM [5]. Population-based studies have reported frequencies of PsP ranging from 28% to 50% of early radiographically progressing GBMs [4–11].
Anti-vascular endothelial growth factor (anti-VEGF) agents are known to induce prominent changes in vessel permeability by restoring the blood-brain barrier and alleviating edema [12, 13] and have recently been incorporated into clinical practice after the failure of CRT [13, 14]. More recently, experimental trials have explored the potential of anti-VEGF therapy in the treatment of newly diagnosed GBM [15, 16] as a means to exploit a “normalization window” for tumor vasculature, in which a more organized and efficient vascular supply could improve chemotherapeutic delivery and tumor oxygenation, resulting in improved efficacy of CRT [12, 17, 18]. Because this new strategy places the use of anti-VEGF agents in the same timeline as the development of PsP, we sought to determine the extent to which the antipermeability effect of VEGF blockade modifies the imaging appearance of GBM in the early treatment period, with a special focus on the incidence of PsP.
Materials and Methods
Study Population and Treatment Strategies
We evaluated and compared clinical and imaging data from two groups of patients with newly diagnosed GBM who were enrolled in two independent institutional review board-approved clinical trials performed at Massachusetts General Hospital and the Dana-Farber Cancer Institute. The first group (study) came from a phase II study of cediranib in combination with daily temozolomide and radiation in patients with newly diagnosed GBM. The second group (contemporary control) came from a parallel imaging trial designed to assess the effects of standard radiation and chemotherapy on MRI for newly diagnosed GBM. Additional trial details can be found online (ClinicalTrials.gov identifiers NCT00662506 and NCT00756106) and in recently published work from our group [19]. All patients participating in this study signed institutional review board-approved informed consent forms. Inclusion criteria for the two groups were similar and included patients aged 18 years or older with pathology-proven GBM after biopsy or resection who were eligible to receive standard postsurgical temozolomide (TMZ) and radiation. Evidence of residual contrast-enhancing tumor on postsurgical MRI (≥1 cm in at least one dimension) for evaluation of tumor response was necessary. Other inclusion criteria for both studies included a Karnofsky performance score ≥60 and Mini-Mental score >15, adequate organ and bone marrow function, no concomitant enzyme-inducing antiepileptic drugs, no prior anti-VEGF therapy, and no Gliadel wafers.
Both groups (study and control) received the same dose and schedule of CRT as described by Stupp el al. [1]. In addition, the study group received cediranib 30 mg per day orally, starting concomitantly with CRT and continuing without interruption until disease progression or toxicity. Enrolled patients did not receive any additional chemotherapeutic or investigational agent on both studies. Patient accrual occurred from February 2009 to February 2011 for the study group and from August 2008 to August 2010 for the control group. When available, O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status was obtained through chart review.
MRI
Serial imaging studies for both study and control groups were performed on similar 3.0T magnetic resonance scanners (Tim Trio; Siemens, Munich, Germany, http://www.medical.siemens.com). Patients had to be on a stable dose of steroids for 5 days before the MRI. Two baseline MRI scans were acquired before initiation of therapy as well as on day +1, weekly during CRT until day 50 and then monthly until disease progression. The imaging protocol used for this analysis included standard two- and three-dimensional anatomical pre- and postcontrast T1-weighted images (repetition time: 600 ms; echo time: 12 ms; 5-mm slice thickness; 1-mm interslice gap; 0.43-mm in-plane resolution; 23 slices and 512 × 512 matrix) and fluid-attenuated inversion recovery (FLAIR) images (repetition time: 10,000 ms; echo time: 70 ms; 5-mm slice thickness; 1-mm interslice gap; 0.43-mm in-plane resolution; 23 slices and 512 × 512 matrix). The postcontrast T1 images were acquired after the administration of 0.2 mmol/kg of gadolinium-diethylene-triamine penta-acetic acid. Scan-to-scan reproducibility was improved by usage of the “AutoAlign” method [20].
Response Assessment
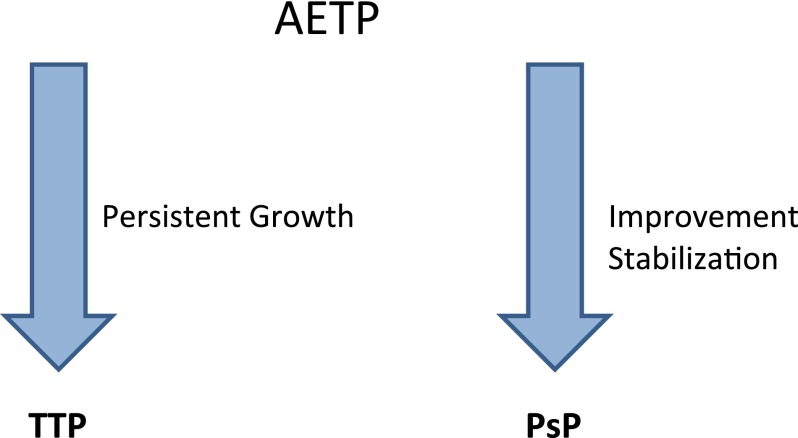
Central review of pre- and postcontrast T1-weighted and FLAIR images was performed by an independent radiologist who was blinded to clinical data and who obtained standard bidimensional measurements on T1-weighted postcontrast images. The reader was also blinded to the order of the imaging time points to avoid bias in tumor measurements from expected treatment effects. Clinical evaluations were performed by the treating oncologists. Tumor response was determined according to the updated Response Assessment in Neuro-Oncology (RANO) criteria for malignant gliomas [2], taking into account clinical performance, dose of steroids, dimensions of contrast-enhancing lesions, and extension of tumor-related FLAIR signal abnormalities. Patients who demonstrated signs of progression on imaging inside the radiation field during the first 12 weeks after completion of radiation were considered to have apparent early tumor progression (AETP) and (if clinically feasible) were maintained on the same treatment regimen and imaging surveillance. During follow-up, patients with lesions that maintained growth despite treatment were considered to have TTP. PsP was diagnosed in patients with stable or regressing lesions for a period of at least 6 months without changes in therapy (Fig. 1). Repeat biopsies or resections at the time of AETP were not deemed necessary by the treating neuro-oncologists for pathologic analysis in most cases, given the increasing recognition of PsP [5, 21–23].
Figure 1.
Classification of patients with AETP into TTP and PsP based on follow-up scans.
Abbreviations: AETP, apparent early tumor progression; PsP, pseudoprogression; TTP, true tumor progression.
Statistical Analysis
Percentages of observed frequencies of AETP, TTP, and PsP were calculated for the study and control groups, as were the percentages of patients with favorable response status at 3 months after completion of CRT (stable disease, partial response, and complete response). Differences between groups were assessed with Fisher’s exact test. Statistical significance was set at α = 0.05.
Results
Patient Characteristics
The study group enrolled 40 patients, and 14 patients were accrued to the control group. Patient characteristics are outlined in Table 1.
Table 1.
Patient characteristics
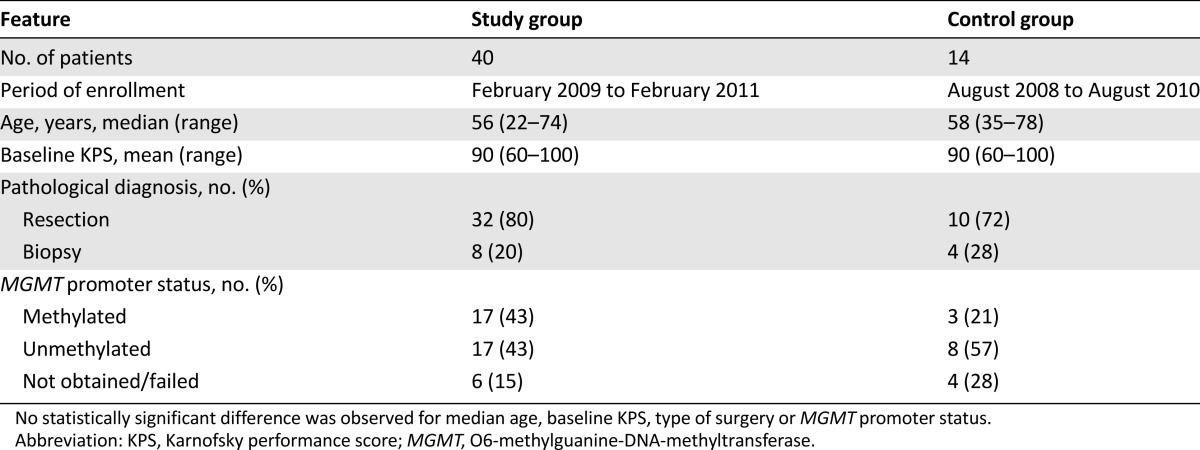
Eleven patients (27.5%) were removed from the study group before completion of a minimum of 3 months of follow-up after CRT exclusively because of drug toxicities and so were not included in the analysis. These toxicities included elevated liver enzymes, hematological toxicity, fatigue/anorexia, and hemorrhage. In the control group, three patients (21.4%) electively discontinued trial participation to enroll in experimental drug investigations immediately after CRT and were also excluded. In the study group, MGMT promoter methylation was detected in 17 patients (42.5%), was negative in 17 (42.5%), and was not obtained or failed in 6 (15%). In the control group, three patients (21%) had methylated MGMT promoters and eight patients (57%) had unmethylated promoters. For four patients (28%), methylation status could not be obtained or failed (Table 1).
Response Assessment
Study Group
Of the final 29 patients, partial response was observed in 16 patients (55%) and stable disease was seen in 11 (38%) during the first 3 months of follow-up after CRT. No patients had complete responses. Two patients (7%) fulfilled criteria for AETP. Both patients were later confirmed as TTP, one by stereotactic biopsy at 4 months after completion of CRT and the other because of progressive disease outside of the radiation field (in the contralateral brain hemisphere) 3 months after completion of CRT. No patients fulfilled criteria for PsP (Table 2).
Table 2.
Pseudoprogression in study versus control groups
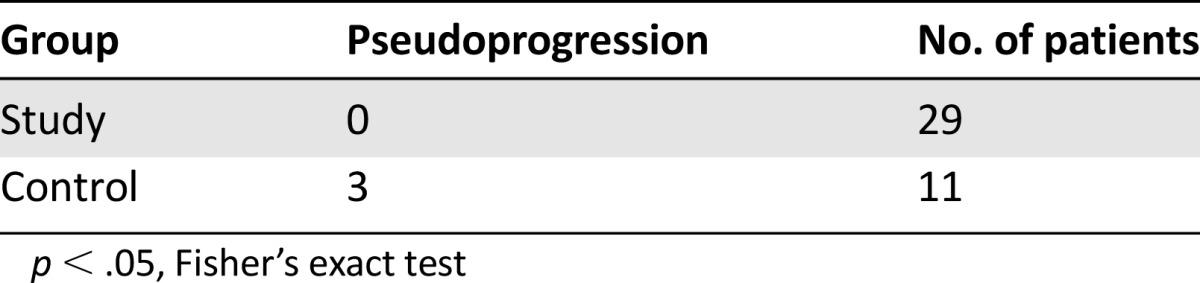
Control Group
Of the 11 patients included, one (9%) achieved partial response and four (36%) experienced stable disease. There were no complete responses. A total of six subjects (55%) demonstrated AETP and were kept on treatment with follow-up imaging. Three of these patients (50%) demonstrated persistent tumor growth despite continued treatment and were classified as TTP. Three patients (50%) had subsequent improvement or stabilization of enhancing lesions on MRI follow-up and were classified as PsP (Table 2). After initial lesion growth (AETP), patients diagnosed with PsP remained radiographically stable without changes in therapy for an average number of 209 days (range: 180–252 days). Patients on corticosteroids with TTP had persistent growth despite increased corticosteroid use, whereas those with PsP were able to taper steroids during follow-up. A significant higher frequency of PsP was observed in the control group compared with the study group (p < .05).
The frequency of early TTP cases was also higher in the control group but did not reach statistical significance (p = .08). The number of patients with partial responses was higher in the study group compared with controls (55% versus 9%), (p < .05) (Table 3). No correlation was observed between methylation status of MGMT promoter and development of PsP.
Table 3.
Partial response in study versus control groups

Examples of patients with AETP classified as PsP and TTP are demonstrated in Figures 2 and 3, respectively.
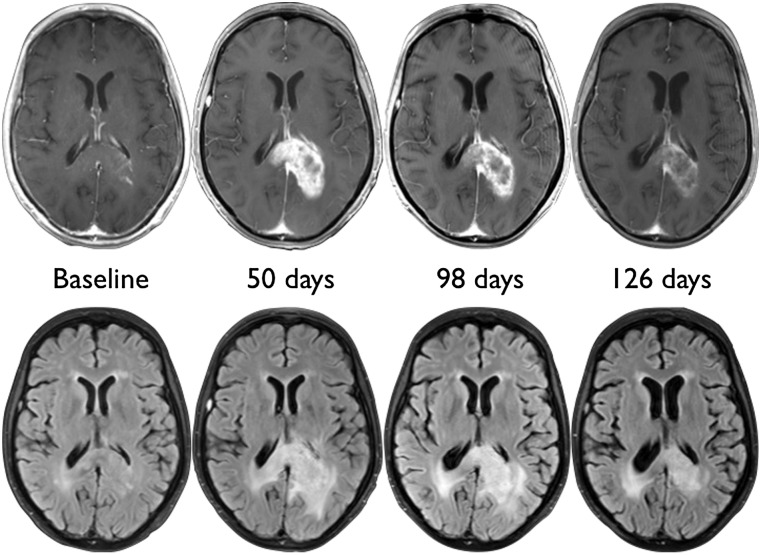
Figure 2.
Pseudoprogression in a 55-year-old glioblastoma patient from the control group. There is early increase in mass-like enhancement and edema on day 50 after initiation of chemoradiation, with subsequent regression on days 98 and 126.
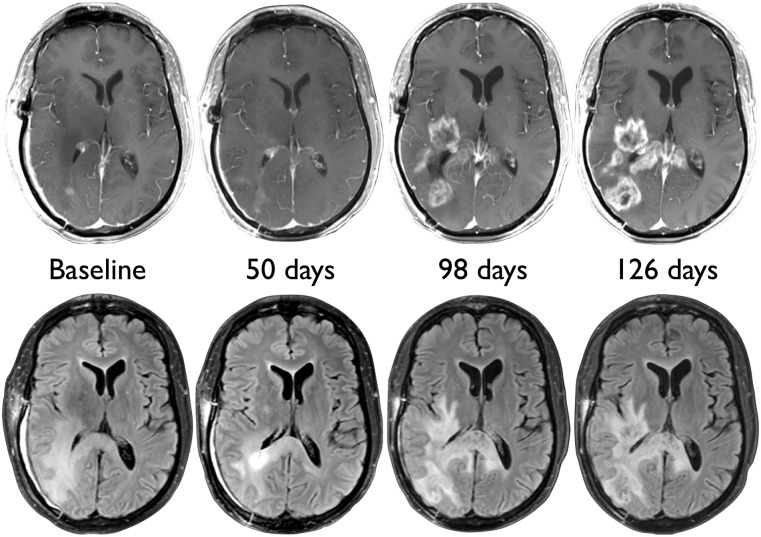
Figure 3.
True tumor progression in a 40-year-old glioblastoma patient from the control group. There is subtle increase in enhancement 50 days after initiation of chemoradiation, with pronounced worsening of enhancement, edema, and mass effect on days 98 and 126.
Discussion
The optimal role of anti-VEGF therapy for the treatment of GBMs remains subject to debate [24, 25], despite the accelerated approval for bevacizumab granted in May 2009 [26] as single-agent therapy for patients with progressive GBM. Although a clearly established standard of care still does not exist for recurrent GBMs, bevacizumab is being used for the majority of progressive tumors that have failed previous TMZ therapy [14, 27–30].
Despite some controversies regarding the antineoplastic properties of antiangiogenic drugs, the substantial antipermeability and antiedema effects of these agents have been demonstrated by MRI in investigational studies [12, 31, 32] and clinical practice. As such, anti-VEGF agents are sometimes used to counteract the adverse effects of radiation necrosis [33–36]. In this study, we aimed to test the hypothesis that anti-VEGF agents would also have a preventive effect on the development of early treatment-related inflammation (i.e., PsP), by using serial MRI to evaluate the incidence of PsP during and after conventional CRT. The opportunity to test such a hypothesis emerged from data prospectively collected from two contemporary cohorts of GBM patients undergoing CRT in experimental and imaging trials evaluating response of newly diagnosed GBMs to CRT with and without the addition of concomitant cediranib, a potent anti-VEGF small molecule that exerts its effects through inhibition of receptor tyrosine kinases [12, 37]. Our results demonstrated substantial radiographic response differences between the two groups, without a single case of PsP among the 29 patients that completed CRT and cediranib. In the control group, 3 cases of PsP with serial imaging were identified among the 11 patients evaluated—a 27% incidence, which is in agreement with previously published data from larger trials [4–11]. A case series from Chamberlain et al. [9] in 2007, for example, identified AETP in 51% of patients. Considering the patients that underwent reoperation, 47% had treatment-related necrosis, suggesting an incidence of roughly 25% in all patients at risk. Taal et al. [10] examined 85 patients with malignant gliomas and found very similar results (PsP corresponding to 50% of the early radiographically progressing subgroup and 25% of all patients). In 2008, a study of 208 GBM patients demonstrated a 30% incidence of PsP on the first MRI after CRT [4]. Based on these data, we expected to observe 7–10 cases of PsP (one-quarter to one-third of patients) in the study group receiving cediranib, but no cases were observed. The possibility that we may have missed cases of PsP in our cohort is very unlikely because our imaging follow-up was done at very short intervals as part of the trial protocol.
As expected, the number of patients with objective radiographic responses in the early post-CRT period was also significantly different between the two groups, reflecting the major improvements observed in radiographic response when anti-VEGF therapy was used both in the newly diagnosed [15, 16, 38] and recurrent [29, 31, 32, 39, 40] settings. However, whether anti-VEGF agents have a true antitumor effect remains the subject of debate [24, 25]. Cediranib, for example, has been previously studied as monotherapy and in combination with lomustine in a randomized, multicenter, phase III study in patients with recurrent GBM, without significant survival benefits [41]. Recent data by our group, however, suggest that the combination of antiangiogenic therapy and CRT may indeed benefit some patients. Survival benefits were observed in a subset of GBM patients with improved tumor perfusion while receiving cediranib mono- and combination therapy [19, 42]. These changes were not seen in patients treated exclusively with CRT [19], suggesting that improved tissue perfusion resulting from a transient effect of “vascular normalization” may increase delivery of chemotherapeutic agents and enhance tumor oxygenation, potentially sensitizing tumor cells to the effect of CRT. Whether concurrent anti-VEGF therapy impedes or enhances delivery of chemotherapy to tumors appears to be a function of the dose of anti-VEGF therapy and the time when the drug uptake is measured [24, 25, 43].
In a prior study reporting safety data on the addition of bevacizumab to standard TMZ-based CRT, Vredenburgh et al. commented on the elimination of PsP in a cohort of patients with newly diagnosed GBM that received bevacizumab in addition to CRT [44]. In their retrospective nonblinded analysis, the investigators noted seven patients with “probable pseudoprogression” suspected on clinical grounds, with four patients demonstrating increasing enhancement according to McDonald criteria and three patients with increased FLAIR abnormalities only (which are nonspecific and could be related to postradiation white matter changes or nonenhancing progression). Although the observed trend in that study is comparable to ours (elimination of PsP), the lack of a clear definition and qualitative description limits a direct comparison between the two studies.
MGMT gene promoter methylation has been associated with increased incidence of PsP in previous studies [4]. In addition, patients with methylated MGMT within these cohorts had a significantly improved overall prognosis, a survival benefit that has been confirmed in subsequent studies [1, 22, 45–47]. Based on these findings, investigators have hypothesized that PsP may be the imaging manifestation of increased CRT efficacy on the residual tumor burden, resulting in improved survival [4]. Although assessment of MGMT status was performed in most patients in both our study and control groups, the overall small number of PsP cases precludes meaningful analysis.
Demonstrating that PsP tends to be eliminated has implications for patients receiving upfront anti-VEGF therapy, a therapeutic strategy that is currently being evaluated in prospective randomized trials (AVAglio, BO21990, NCT00943826). The first implication relates to treatment tolerance. Suppressing the inflammatory process, edema and mass effect that accompanies CRT may improve patient tolerability to adjuvant therapy and may have a steroid-sparing effect. For selected patients who have poor performance scores or large residual or inoperable tumors and who may not be candidates for adjuvant therapy because of concern about treatment-induced swelling, addition of an anti-VEGF agent could be a rational strategy to assess in future experimental trials. It remains unclear whether prophylactic anti-VEGF therapy would be more beneficial than starting an anti-VEGF agent when and if the patient becomes symptomatic, particularly given that patients who will experience PsP cannot yet be identified before initiation of CRT. In addition, it should be emphasized that PsP is sometimes asymptomatic or minimally and transiently symptomatic. In such cases, no treatment is usually necessary, and patients can be followed radiographically until the imaging abnormalities resolve.
Our findings also have implications for evaluation of response assessment for these patients. In the context of upfront adjuvant anti-VEGF therapy, enlarging contrast-enhancing lesions on MRI during or within the first 3 months after CRT are much more likely to represent TTP than PsP. Shorter-interval follow-up or tissue sampling may be warranted in these patients to confirm early tumor progression.
Suppression of PsP has raised concerns in the literature [24], because this inflammatory phenomenon has been associated with a survival benefit and may reflect increased tumor sensitivity to CRT. Some authors [24] have postulated that anti-VEGF agents, like bevacizumab, may decrease the correlation between MGMT status and PsP [4]. Although theoretically possible, this has not been observed in the few published phase II trials available using anti-VEGF drugs, in which MGMT-methylated patients maintained a survival benefit in comparison with patients without methylated promoters [15, 25]. Another possible concern regarding the antipermeability effect of anti-VEGF therapy would be to potentially mask and delay the detection of real tumor progression. To what extent this phenomenon truly occurs and affects patient outcomes remains to be determined.
Our study has some limitations that deserve mention. The most significant is the relatively small number of patients. Although 40 patients were accrued in the study group as part of the experimental trial, 11 patients (27.5%) could not be included in the final analysis because of study withdrawals secondary to drug toxicities. It is worth mentioning that although these patients were not included in the analysis because the minimal observation period was not reached (3 months after CRT), none demonstrated signs of progression or PsP while in the trial. Had we been able to include these patients in our analysis, the measured effect would potentially be even more significant. Patient sample size was a more significant issue in the control group mainly because of accrual limitations encountered in this imaging trial, which did not offer experimental drug interventions and thus had difficulties enrolling subjects. Even with a small patient sample, we were able to demonstrate a substantial difference between the two groups, a fact that strengthens the validity of these findings. Moreover, the incidence of PsP observed in the control group (27%) is in agreement with pooled data from larger studies [4, 5, 9, 11], although many factors such as different study designs and variable criteria for PsP may influence reported rates of PsP in the literature.
Conclusion
Our results suggest that anti-VEGF therapy for newly diagnosed GBM patients prevents the development of PsP on serial MRI during and after TMZ-based CRT. Knowing what to expect in the early postradiation period for these patients should raise awareness that enlarging contrast-enhancing lesions in this context are more likely to represent TTP. Future studies analyzing data from large phase III trials should be available in the near future to confirm these observations.
Acknowledgments
This work was conducted with support from the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers); National Institutes of Health R01CA129371 and K24CA125440A (T.T.B.), N01CM-2008-00060C and 5R01NS060918 (A.G.S., M.C.P), 1U01CA154601 (B.R.R.), P01-CA080124 (R.K.J), 8UL1TR000170-05 and Merck (A.G.S., E.R.G.); and Norwegian Research Council grant 191088/F20 and South-Eastern Norway Regional Health Authority Grant 2013069 (K.E.E).
Author Contributions
Conception/Design: Marco C. Pinho, Rakesh K. Jain, A. Gregory Sorensen, Tracy T. Batchelor, Elizabeth R. Gerstner
Provision of study material or patients: Patrick Y. Wen, A. Gregory Sorensen, Tracy T. Batchelor, Elizabeth R. Gerstner
Collection and/or assembly of data: Marco C. Pinho, Pavlina Polaskova, Dominique Jennings, Kyrre E. Emblem, Bruce R. Rosen, A. Gregory Sorensen
Data analysis and interpretation: Marco C. Pinho, Pavlina Polaskova, Jayashree Kalpathy-Cramer, Dominique Jennings, Kyrre E. Emblem, Bruce R. Rosen, Elizabeth R. Gerstner
Manuscript writing: Marco C. Pinho, Kyrre E. Emblem, Rakesh K. Jain, Patrick Y. Wen, Tracy T. Batchelor, Elizabeth R. Gerstner
Final approval of manuscript: Marco C. Pinho, Pavlina Polaskova, Jayashree Kalpathy-Cramer, Kyrre E. Emblem, Rakesh K. Jain, Bruce R. Rosen, Tracy T. Batchelor, Elizabeth R. Gerstner
Disclosures
Kyrre E. Emblem: NordicNeuroLab AS (IP); Rakesh K. Jain: Noxxon Pharma, Zyngenia, WebMD, Enlight, SynDevRx (C/A), MedImmune, Roche (RF), Enlight, XTuit, SynDevRx (OI); Bruce R. Rosen: Siemens Healthcare (C/A); Patrick Y. Wen: Astra Zeneca (RF); A. Gregory Sorensen: Siemens Healthcare (E, OI); Tracy T. Batchelor: Roche, Merck (C/A), AstraZeneca, Millennium, Pfizer (RF), UpToDate, Research 2 Practice, Robert Michael Educational Institute LLC, Kyrin Pharmaceuticals, Oakstone Medical Publishing (H); Elizabeth R. Gerstner: Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 3.Gerstner ER, Batchelor TT. Imaging and response criteria in gliomas. Curr Opin Oncol. 2010;22:598–603. doi: 10.1097/CCO.0b013e32833de96e. [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 5.Fink J, Born D, Chamberlain MC. Pseudoprogression: Relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12:240–252. doi: 10.1007/s11864-011-0157-1. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg. 1979;50:624–628. doi: 10.3171/jns.1979.50.5.0624. [DOI] [PubMed] [Google Scholar]
- 7.de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 8.Levin VA, Crafts DC, Norman DM, et al. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 10.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 11.Roldán GB, Scott JN, McIntyre JB, et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci. 2009;36:617–622. doi: 10.1017/s0317167100008131. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5:610–620. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain MC. Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas. Cancer. 2010;116:3988–3999. doi: 10.1002/cncr.25256. [DOI] [PubMed] [Google Scholar]
- 15.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 19.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benner T, Wisco JJ, van der Kouwe AJ, et al. Comparison of manual and automatic section positioning of brain MR images. Radiology. 2006;239:246–254. doi: 10.1148/radiol.2391050221. [DOI] [PubMed] [Google Scholar]
- 21.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–638. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- 22.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9:241–246. doi: 10.1007/s11910-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 23.Jahangiri A, Aghi MK. Pseudoprogression and treatment effect. Neurosurg Clin N Am. 2012;23:277–287, viii–ix. doi: 10.1016/j.nec.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;76:87–93. doi: 10.1212/WNL.0b013e318204a3af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain MC. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;77 doi: 10.1212/WNL.0b013e3182247068. ; author reply 803–804. [DOI] [PubMed] [Google Scholar]
- 26.FDA approval for bevacizumab: Second-line treatment of glioblastoma. Available at http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab#Anchor-Glioblastoma. Accessed October 13, 2012.
- 27.Beal K, Abrey LE, Gutin PH. Antiangiogenic agents in the treatment of recurrent or newly diagnosed glioblastoma: Analysis of single-agent and combined modality approaches. Radiat Oncol. 2011;6:2. doi: 10.1186/1748-717X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: Impact on local control and patient survival. J Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 29.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 30.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 31.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 32.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Kawabata S, Kuroiwa T, et al. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: A report of 2 cases. J Neurooncol. 2011;102:471–475. doi: 10.1007/s11060-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matuschek C, Bölke E, Nawatny J, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. 2011;187:135–139. doi: 10.1007/s00066-010-2184-4. [DOI] [PubMed] [Google Scholar]
- 37.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 38.Narayana A, Gruber D, Kunnakkat S, et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg. 2012;116:341–345. doi: 10.3171/2011.9.JNS11656. [DOI] [PubMed] [Google Scholar]
- 39.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: A single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19:1636–1640. doi: 10.1016/j.jocn.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31:3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang S, Stylianopoulos T, Duda DG, et al. Letter to the editor - benefits of vascular normalization are dose and time dependent. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-1989. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vredenburgh JJ, Desjardins A, Kirkpatrick JP, et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;82:58–66. doi: 10.1016/j.ijrobp.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert MR, Wang M, Aldape KD, et al. RTOG 0525: A randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (DD) schedule in newly diagnosed glioblastoma (GBM) J Clin Oncol. 2011;29(suppl):2006a. [Google Scholar]
- 46.Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 47.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]