Abstract
Background.
Local and systemic recurrence are important sources of treatment failure following surgical resection of esophageal adenocarcinoma. We hypothesized that adding preoperative cetuximab and radiotherapy (cetux-RT) to perioperative chemotherapy would increase treatment efficacy with acceptable toxicity.
Methods.
In this prospective phase II trial, patients were treated with three cycles of epirubicin, cisplatin, and capecitabine (ECX), followed by cetux-RT. After surgery with curative intent, patients received three more cycles of ECX. Primary endpoints were efficacy, determined by histopathological complete response (pCR) rate, and safety, which was assessed with resectability rate.
Results.
Of the 12 patients enrolled in this trial, six received at least one dose of cetux-RT. In five patients, cetux-RT was not started because of adverse events (AEs) related to preoperative chemotherapy; one patient had progressive disease. Addition of cetux-RT was well tolerated and did not interfere with the resectability rate (100%). However, the pCR rate was 0, and 50% of patients experienced serious adverse events (SAEs) postoperatively.
Conclusion.
With 12 patients enrolled, the lack of initial signs of efficacy and a high incidence of postoperative SAEs prompted us to end this study prematurely. Perioperative ECX was associated with considerable toxicity, and further treatment intensification is problematic.
Abstract
摘要
背景. 局部与全身复发是食管腺癌手术切除后治疗失败的重要原因。我们假定术前西妥昔单抗联合放疗(cetux-RT)作为围手术期化疗方案能够提高疗效且毒性反应可接受。
方法. 在本项II期前瞻性试验中,给予患者表柔比星、顺铂以及卡培他滨(ECX)治疗3周期,继以cetux-RT。在进行了根治性手术之后,再给予患者ECX治疗3周期。主要终点为疗效以及安全性,前者根据组织病理学完全缓解(pCR)率来确定, 后者通过可切除率来评估。
结果. 本试验入组的12例患者中,6例接受了至少1剂次cetux-RT。5例患者因术前化疗相关的不良事件(AE)而未进行cetux-RT;1例患者出现疾病进展。Cetux-RT加入到原治疗方案后,患者的耐受情况良好,可切除率也未受到影响(100%)。然而,pCR率为0,50%的患者术后出现了严重AE(SAE)。
结论. 在12例入组患者中,未观察到初始疗效征象且术后SAE发生率很高,我们因而提前结束了研究。围手术期ECX引起严重的毒性反应,因此进一步强化治疗很难进行。The Oncologist2014; 19:1-2
Discussion
Long-term survival in patients with resectable esophageal adenocarcinoma remains poor, with a 5-year survival rate of only 20%–42% [1]. Perioperative ECX chemotherapy has improved survival rates but failed to deliver a significant proportion of pathologic complete responses. Since better locoregional control would probably improve survival rates, we added cetux-RT preoperatively (Fig. 1).
Figure 1.

Treatment schedule. ECX: epirubicin (day 1, 50 mg/m2), cisplatin (day 1, 60 mg/m2), capecitabine (days 1–21, 1,250 mg/m2); Cetux: cetuximab (day 1, 400 mg/m2); Cetux-RT: cetuximab 250 mg/m2 weekly, radiotherapy 45 Gy (25 × 1.8 Gy).
Abbreviations: Cetux, cetuximab; Cetux-RT, cetuximab plus radiotherapy; ECX: epirubicin, cisplatin, and capecitabine; wks, weeks.
We found that intensification of the preoperative treatment was poorly feasible, as 42% of patients discontinued treatment because of toxicity of preoperative ECX. Addition of cetux-RT was well tolerated (Table 1) and did not interfere with the resectability rate; however, the extension of the preoperative treatment led to a high postoperative complication rate. The combination of an extensive surgical procedure, the type of disease, a prolonged preoperative period, and high preoperative toxicity may have contributed to the postoperative toxicity of this regimen.
Table 1.
Adverse events related to cetux-RT
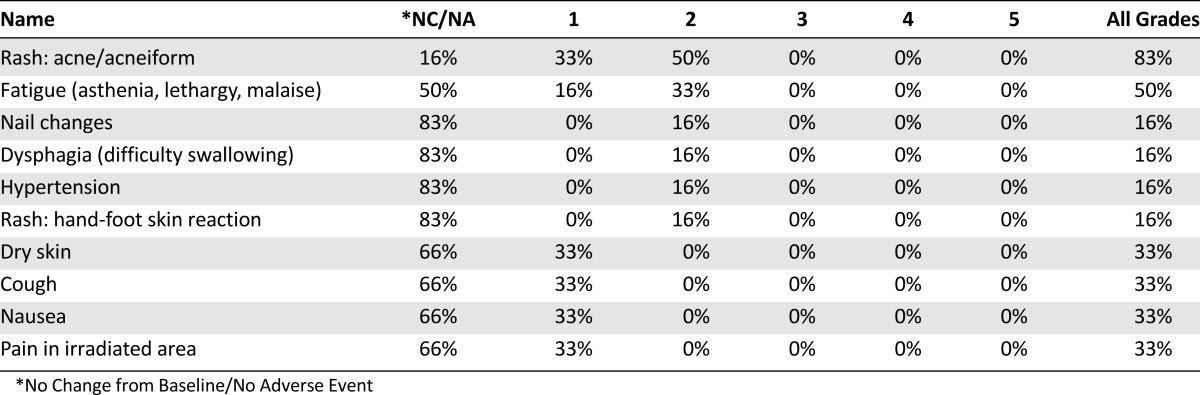
Although we did not complete full accrual, analysis of the six evaluable patients showed disappointing efficacy, as none of the resected tumors showed pCR. Previous studies with cetux-RT in patients with esophageal cancer did show an increase in pCR to 27%–33% [2–4]. However, cetux-RT efficacy appears to be limited to squamous cell carcinomas [4, 5].
Since there were no preliminary signs of efficacy, we feel that our study does not warrant further investigation of cetux-RT for resectable esophageal adenocarcinoma. Furthermore, since intensification of ECX will be problematic, alternative multimodality neoadjuvant schedules need to be identified.
Supplementary Material
Footnotes
ClinicalTrials.gov Identifier: NCT00827671
Sponsor(s): University Medical Center Utrecht and Merck KGaA
Principal Investigator: M.P. Lolkema
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


