Using data from the American Society of Clinical Oncology International Affairs Committee’s Web survey of selected oncologists with research experience from 25 countries, the authors explored the characteristics of and barriers to global clinical cancer research. They concluded that lack of funding, lack of time and competing priorities, and procedures from competent authorities might be the main global barriers to academic clinical cancer research.
Keywords: Cancer research, Global, Barrier
Abstract
Background.
There are concerns about growing barriers to cancer research. We explored the characteristics of and barriers to global clinical cancer research.
Methods.
The American Society of Clinical Oncology International Affairs Committee invited 300 selected oncologists with research experience from 25 countries to complete a Web-based survey. Fisher’s exact test was used to compare answers between participants from high-income countries (HICs) and low- and middle-income countries (LMICs). Barriers to clinical cancer research were ranked from 1 (most important) to 8 (least important). Mann-Whitney’s nonparametric test was used to compare the ranks describing the importance of investigated obstacles.
Results.
Eighty oncologists responded, 41 from HICs and 39 from LMICs. Most responders were medical oncologists (62%) at academic hospitals (90%). Researchers from HICs were more involved with academic and industry-driven research than were researchers from LMICs. Significantly higher proportions of those who considered their ability to conduct academic research and industry-driven research over the past 5 years more difficult were from HICs (73% vs. 27% and 70% vs. 30%, respectively). Concerning academic clinical cancer research, a lack of funding was ranked the most important (score: 3.16) barrier, without significant differences observed between HICs and LMICs. Lack of time or competing priorities and procedures from competent authorities were the second most important barriers to conducting academic clinical research in HICs and LMICs, respectively.
Conclusion.
Lack of funding, lack of time and competing priorities, and procedures from competent authorities might be the main global barriers to academic clinical cancer research.
Abstract
摘要
背景. 越来越多的癌症研究障碍引起了全球关注。本文探索了全球临床癌症研究的特征及其障碍。
方法. 美国临床肿瘤学会国际事务委员会从25个国家中遴选并邀请了300位具有研究经验的肿瘤科医生来完成一项网上调查。使用Fisher确切概率法来比较来自高收入国家(HIC)与低至中等收入国家(LMIC)参与者之间所提供的答案。临床癌症研究的各项障碍被分为1级(非常重要)到8级(最不重要)。通过Mann-Whitney非参数检验来比较纳入调查的障碍的重要性等级。
结果. 80位肿瘤科医生作出应答,其中41位来自HIC,39位来自LMIC。大多数应答者为就职于学术性医院(90%)的肿瘤内科医生(62%)。来自HIC的研究者在学术研究以及产业驱动的研究方面参与程度高于来自LMIC的研究者。有显著更高比例的HIC研究者认为,在过去的5年内开展学术研究以及产业驱动的研究 所面临的困难更大(与LMIC研究者相比,分别为73% vs. 27% 、70% vs. 30%)。就学术性临床癌症研究而言,缺乏资金被列为最重要(评分3.16)的障碍,HIC研究者与LMIC研究者之间未观察到显著差异。时间不够多或相互竞争的优先级以及监管当局的审批流程分别被HIC与LMIC列为第二重要的障碍。
结论. 缺乏资金和时间、相互竞争的优先级以及监管当局审批流程可能是学术性临床癌症研究所面临的全球性障碍。The Oncologist 2014;19:1-7
Implications for Practice:
To further improve cancer outcomes globally, barriers and hurdles to conducting research must be recognized and eliminated. Although barriers to clinical cancer research have been extensively studied to date in Western countries, limited information is available on barriers in the rest of the world. Results of our survey, which included oncologists from countries around the world, showed that lack of funding, lack of time and competing priorities, and regulatory procedures might be the most important barriers to academic cancer research. To get a clear picture, additional investigation into these barriers is needed.
Introduction
Clinical trials have an unquestioned role in improving treatment for cancer patients. Major improvements in survival rates observed in various malignancies were made possible through intensive clinical and translational research conducted in the past few decades [1, 2]. Clinical trials allow access to effective treatments and improved individual-patient care for all participating patients, including diagnostic procedures and supportive cancer care. Participation in clinical research enables the quick and smooth introduction of new, effective treatment strategies in everyday clinical practice. Survival of cancer patients treated at institutions involved with cooperative group clinical trials may be superior to survival of patients treated at institutions not involved with cooperative trials [3], although results of a recently published meta-analysis of randomized and nonrandomized clinical trials challenged this observation [4].
Because of the fragmentation of particular cancers to molecular subtypes and the challenges in accruing sufficient numbers of patients who have these cancers, the need for international collaboration in cancer clinical research has grown. International collaboration enables faster enrollment of patients, and results of such international clinical trials are more generalizable. Although some improvements in global harmonization and support of international research have been made, many hurdles and barriers remain [5]. One of the major issues is harmonization of ethical, scientific, and regulatory demands for clinical research worldwide. In addition, financial support for academic clinical research remains challenging.
Lack of adequate funding and other barriers have resulted in the conduct of very few clinical trials in developing countries [6] and may result in unmet needs related to cancer control in the developing world. Globally recommended evidence-based treatments do not reflect ethnic (genetic), cultural, and resource differences between wealthy and developing countries. In addition, little research is conducted on those cancer types that primarily affect inhabitants of the developing countries, thus the ability to diagnose and treat patients with these cancer types may be inadequate [7, 8].
A key factor in the timely completion of a clinical investigation is patient accrual, which can be influenced by a number of factors. Only about 3% of cancer patients are participating in clinical trials in the United States [9]. In contrast, the National Cancer Research Network in the U.K. is one of the most spectacular examples because it has succeeded in increasing the accrual rate from 3.7% to more than 10% in a few years [10]. This was made possible in part by logistical and financial support provided by the National Cancer Research Network to all sites participating in approved trials. Another example is the pediatric oncology community, which accrues more than half of affected children to clinical trials [11]. There are still many hurdles and obstacles in clinical cancer research. Although U.S. oncologists commonly have positive attitudes toward clinical trials, there is a critical need for better infrastructure to support greater patient participation in clinical trials [12].
Oncologic clinical trials have changed over time; for example, phase III clinical trials have become larger and are more often sponsored by pharmaceutical companies [13]. It is not known what impact this evolution of clinical trials has on global clinical cancer research.
Many organizations, including the American Society of Clinical Oncology (ASCO), have raised concerns about growing barriers to clinical cancer research. Better understanding of clinicians’ attitudes toward clinical trials, especially those involved with research outside the U.S., is needed to address the problem of low and selective accrual and to strengthen international collaboration in cancer research [14]. In the present analysis, we explored characteristics and barriers to the global clinical cancer research, as perceived by oncologists.
Materials and Methods
Data Collection
We conducted an ASCO-sponsored Web-based survey. The survey consisted of short questions inquiring about participants’ research and barriers they might have encountered while conducting clinical trials (Table 1). The survey was sent to 300 non-U.S. oncologists from 25 countries across all continents, who had experience with clinical cancer research and thus who might have encountered barriers. Countries were selected on the basis of a high number of cancer clinical trials per population registered at ClinicalTrials.gov (Africa: Egypt, Saudi Arabia, Kenya, Nigeria, South Africa; Asia: China, India, Japan, Nepal, Singapore; Australia; Europe: France, Germany, Israel, Poland, Slovenia, Spain, Sweden; Middle/South America: Argentina, Brazil, Mexico, Panama, Peru, Uruguay; North America: Canada). Eligible oncologists were identified by the ASCO country liaisons, who were current or recent members of the ASCO International Affairs Committee. To increase the response rate and the credibility of our survey, the liaisons were asked to identify oncologists involved in clinical cancer research who could give us a reliable and trustworthy opinion on the research barriers in their countries. Each participant was categorized as being from either a high-income country (HIC) or a low- to middle-income country (LMIC), using country classifications provided by the World Bank [15]. Selection criteria and process were the same for oncologists from HICs and LMICs. Because there was no reason to believe that oncologists from HICs or LMICs were more or less likely to respond, we sent the survey to approximately equal number of oncologists from HICs and LMICs.
Table 1.
Response results

Statistical Analysis
Descriptive statistics were used to describe relevant characteristics of the participants. Barriers to clinical cancer research were ranked from 1 (most important) to 8 (least important). Fisher’s exact test was used to compare answers between participants of HICs and LMICs, and Mann-Whitney’s nonparametric test was used to compare the ranks describing the importance of investigated barriers. All tests were two-sided, and a p value of ≤.05 was considered statistically significant. No adjustment for multiple analyses was performed.
Results
Characteristics of Respondents
Of 300 oncologists, 27% (n = 80) responded; of these, 51% (n = 41) were from HICs and 49% (n = 39) were from LMICs. Overall, 62% of respondents (n = 50) were medical oncologists, 11% (n = 9) were surgical oncologists, 9% (n = 7) were radiation oncologists and 14% (n = 18) were of other specialties or did not specify their specialty. Moreover, 90% (n = 72) worked in academic hospitals and 6% (n = 5) worked in nonacademic hospitals; the rest (4%) did not specify their working environment. We did not observe any significant differences related to type of medical profession, working environment, or leadership position between respondents from the HICs and LMICs. It should be noted that the potential differences between respondents and nonrespondents could not be analyzed because the survey design guaranteed anonymity.
Characteristics of Clinical Research
Characteristics of the research are summarized in Table 1 and comparisons between LMICs and HICs are reported in Table 2. For 85% of oncologists (n = 68), phase I, II, or III clinical trials represented the majority of their clinical research. In comparison with oncologists from LMICs, oncologists from HICs were more often involved with phase I, II, or III clinical trials (p = .01). Forty-one percent of respondents (n = 33; with no differences between researchers from HICs and LMICs) reported that less than 50% of all clinical trials with which they have been involved in the past 5 years have been registered in a publicly accessible Internet format.
Table 2.
Comparison of characteristics of research between LMICs and HICs
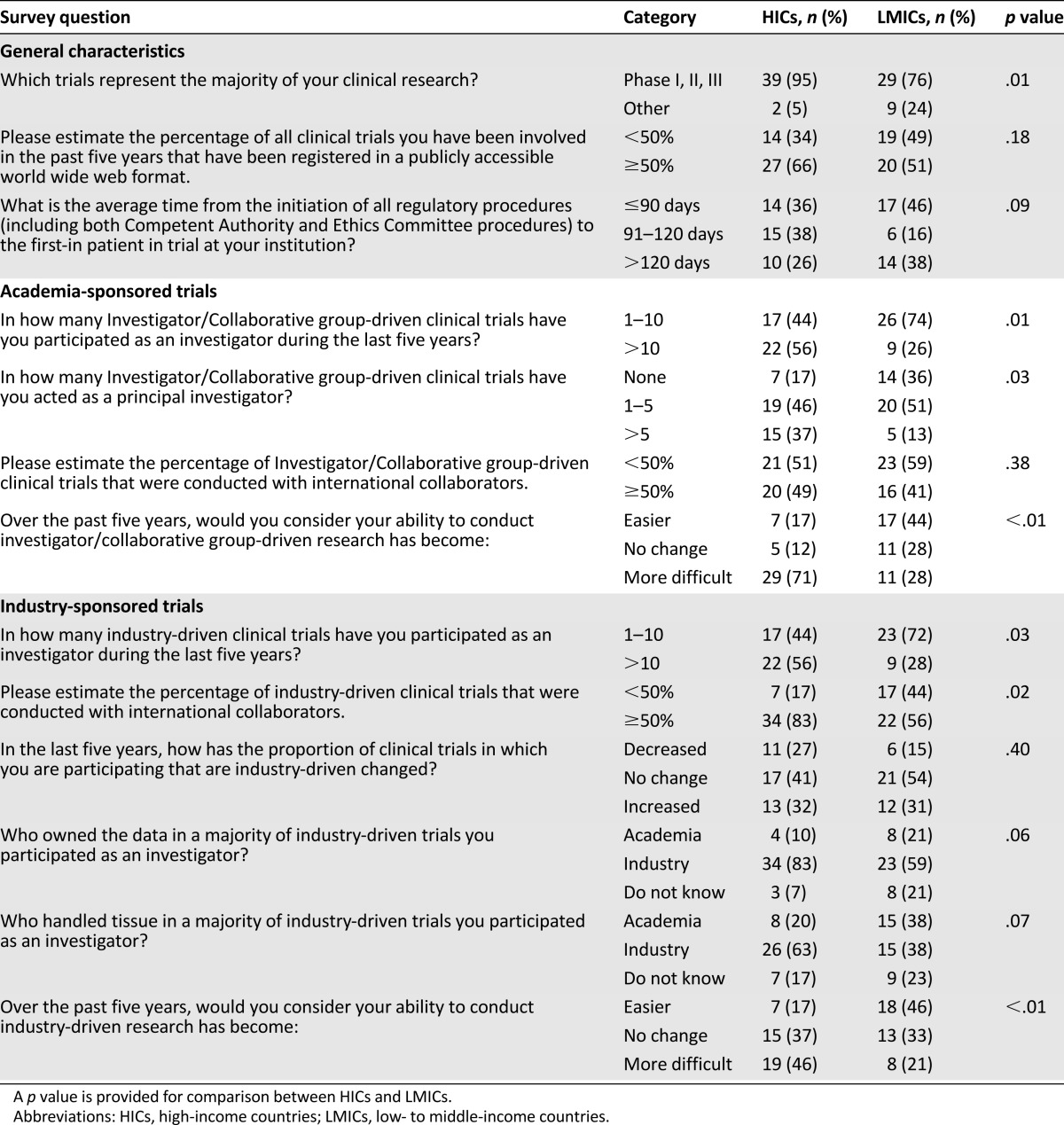
Notably, 93% (n = 74) and 89% (n = 71) of respondents were involved with investigator- or collaborative group-driven (i.e., academic) and industry-driven clinical trials, respectively. A higher proportion of oncologists with more than 10 academic and industry-driven clinical trials were from HICs compared with LMICs (academic: 71% vs. 29%; p = .01; industry: 71% vs. 29%; p = .03). Oncologists from HICs more often served as principal investigators than did researchers from LMICs (p = .03). For academic research, we did not observe any significant difference in the international collaboration between oncologists from HICs and LMICs (p = .38). In contrast, for industry-driven trials, oncologists from HICs were more intensively involved with international collaboration than oncologists from LMICs (p = .02).
Overall, 71% (n = 57) and 51% (n = 41) of respondents reported that the industry owned the data and handled tissue, respectively, in a majority of industry-driven clinical trials; 14% and 20% of oncologists did not know who owned the data and handled tissue, respectively, in industry-driven trials in which they participated as an investigator. There was a trend of a higher proportion of oncologists from HICs who reported that industry owned the data and handled tissue in the majority of industry-driven clinical trials.
Barriers in Clinical Research
Oncologists from HICs responded that their ability to conduct academic research (71%, n = 29) and industry-driven research (46%, n = 19) has become more difficult over the past 5 years. A significantly higher proportion of those who considered their ability to conduct academic research over the past 5 years more difficult were from HICs (73% vs. 27%; p < .01). Similarly, a significantly higher proportion of those who considered their ability to conduct industry-driven research over the past 5 years more difficult were from HICs (70% vs. 30%; p < .01). Overall, 41% (n = 31), 28% (n = 21), and 31% (n = 24) of respondents estimated that the average time from the initiation of all regulatory procedures (including both “competent authority” and ethics committee procedures) to the first-in patient in trial at their institution was <90 days, 90–120 days, and >120 days, respectively; extreme times were most frequently reported by oncologists from LMICs.
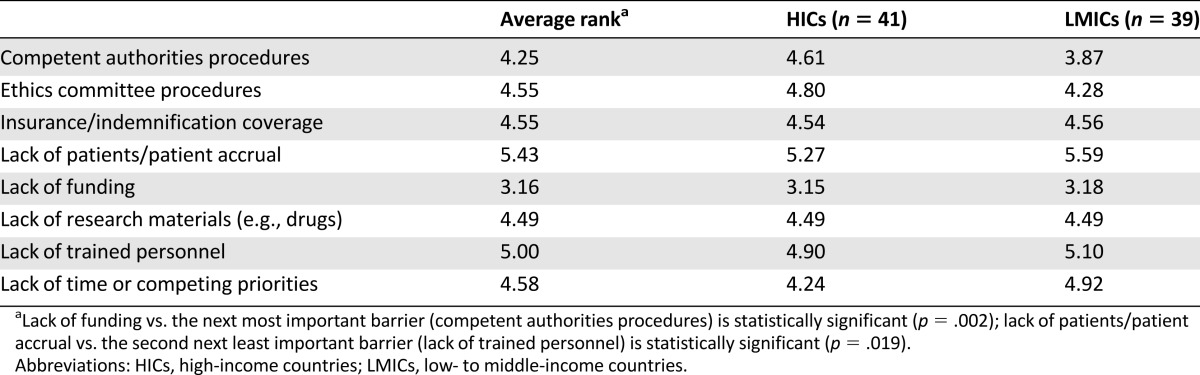
Table 3 summarizes obstacles to academic research. Lack of finances was reported as the most important obstacle (average rank: 3.16) and lack of patients as the least important obstacle (average rank: 5.43) to academic research. Competent authorities and ethics committees procedures and lack of time or competing priorities were the second most important barriers in academic clinical research in LMICs and HICs, respectively. Procedures involving competent authorities and ethics committees were a bigger obstacle for the researchers from LMICs compared with the researchers from HICs, but the difference did not reach statistical significance (average rank: 3.87 vs. 4.61; p = .15, and 4.28 vs. 4.80; p = .30, respectively). In contrast, a lack of time and competing priorities came out as a more important obstacle in HICs than in LMICs, but the difference was not statistically significant (average rank: 4.24 vs. 4.92; p = .20).
Table 3.
Obstacles for academic research, ranked from 1 (most important) to 8 (least important)
Discussion
Physician- and patient-related barriers to clinical cancer research have been extensively studied to date in Western countries [16–20]. In contrast, limited information is available on barriers to clinical cancer research in the rest of the world. In our Web-based survey, we aimed to analyze the characteristics of and barriers to global clinical cancer research, as perceived by oncologists, and to compare them between HICs and LMICs.
Not surprisingly, we found that oncologists from HICs are still more involved with academic and industry-driven research than are those from LMICs. In addition, oncologists from HICs acted more frequently as principal investigators for academic research. Oncologists from HICs also collaborated more intensively in international research, especially in industry-sponsored trials. This opposes our view that industry globalization stimulated international clinical research to a greater extent in less wealthy countries than in wealthy ones [21]; however, our results can be explained by the fact that investigators and sponsors from HICs who still have leading roles in cancer research are seeking a limited number of selected partners in the rest of the world. The majority of both academic and industry-sponsored trials are financed by funds originated in HICs. Hence, it is not surprising that the majority of the HIC-originating trials have investigators from HICs. In addition, investigators from LMICs may seldom be invited to participate in designing and drafting protocols for large international clinical trials. We believe that international collaboration in clinical research would be improved by increased participation of researchers from LMICs.
Researchers from HICs are still facing substantial barriers to the conduct of the academic and international cancer research. American experience shows that there is a critical need for better infrastructure to support participation in clinical trials [14]. European experience with the Clinical Trial Directive (EUCTD 2001/20/EC) teaches us how overmanagement and overregulation might negatively affect cancer research and how important it is to harmonize and not overregulate the field of clinical research [20, 22]. Because of the substantial increase in costs and administrative burdens after implementation of EUCTD 2001/20/EC, international collaboration in academia-driven clinical research decreased in Europe [23]. Currently, some procedures and initiatives are under way that might improve European collaboration in academia-driven research in the future.
In our survey, we did not observe any difference in participation rate in academic and industry-driven trials because approximately 90% of the respondents from both HICs and LMICs were involved with both academic and industry-sponsored research. In contrast to our expectations, neither HIC nor LMIC oncologists reported a shift toward industry-driven trials in the past 5 years; however, it is not known how often academic studies were jointly sponsored by industry and academia or what academic researchers contributed to such trials. Increasing financial and logistical requirements of modern trials often exceed available resources of academic centers, and it seems that pharmaceutical companies may run international trials more easily than academic centers. We hope our data reflect a fruitful collaboration between industry and collaborative academic groups [24].
A substantial proportion of oncologists from HICs reported that their ability to conduct both academic and industry-driven research became more difficult in the past 5 years (71% and 46%, respectively) (Table 2). Lack of finances and lack of time or other competing priorities were the two most important barriers to their clinical cancer research. This finding is in line with ever-growing barriers and hurdles observed in some parts of the world. Rather than harmonizing and simplifying the regulatory environment, EUCTD 2001/20/EC has had the opposite effect, leading to increased costs with research and personnel costs and a reduction in the number of patients enrolled into academic clinical trials [23]. In addition, resources and time may be wasted with research that is destined to fail. Our study showed that a substantial proportion of oncologists from both HICs and LMICs (42% and 58%, respectively) reported that less than half of their trials were publicly registered, without a significant difference observed between HIC and LMIC responders. It is very unlikely that unregistered clinical cancer trials will have any substantial impact on clinical practice. To avoid publication bias and duplication of trials and to better plan future research, the International Committee of Medical Journal Editors mandated registration of all new clinical trials, with a penalty of denial of publication in participating journals should trials fail to be registered [25]. Furthermore, many phase II clinical trials (although they are publicly registered and may have encouraging results) do not lead to phase III clinical trials; therefore, fewer resources and time should be spent on phase II clinical trials that are not planned to lead to additional studies [26]. One of the reasons to do such phase II clinical trials among investigators is the pressure to undertake research and to publish. Time management should be addressed carefully at institutions where cancer research is conducted.
Results of the study by Somkin et al. [12] showed that to increase trial participation, there is a critical need for infrastructure to support trials, especially additional support staff and research nurses. Surprisingly, in our study, the lack of trained personnel has been ranked as the second least important barrier among oncologists from both HICs and LMICs.
In contrast to HIC oncologists, a substantial proportion of oncologists from LMICs reported that both academic and industry-driven research became easier for them to conduct (46% and 44%, respectively) (Table 2). Based on this response, one could speculate that, despite its lack, globalization in clinical research supports and facilitates the conduct of research in LMICs. Barriers still remain, with lack of funds and lengthy regulatory procedures (i.e., approval procedures by competent authorities and ethics committees) being the most important. Regulatory procedures were ranked as the second most important obstacle to clinical research in LMICs, with extremes in the time from the initiation of regulatory procedures to the first-in patient in the trial observed mainly in LMICs.
Based on our survey, a lack of funding is the main global barrier to clinical cancer research. There is a lack of data on cancer research investment in less wealthy countries, whereas cancer research funding surveys performed in the developed world point out huge disparities among different countries and continents [27]. Consequently, one may speculate that the financing of clinical research needs to be optimized in all countries and on all continents.
The analysis of our Web-based survey has limitations. Generalizability of our findings may be compromised by the methodology of our study and by the low response rate (27%). Alternative selection of oncologists and higher response rate might lead to different conclusions. Furthermore, low response rate might affect the credibility of the subanalysis comparing HICs and LMICs. Demographic data were obtained only for the respondents because the survey was anonymous; therefore, a comparison between respondents and nonrespondents could not be provided. The great majority of respondents were medical oncologists (62%) who were based at academic hospitals (90%), and we believe that our sample is representative of current clinical research practice. To obtain a clearer picture on the barriers, further research conducted among a broader population of oncologists is needed. In addition, there may be substantial differences in barriers to clinical cancer research not only between HICs and LMICs but also within the categories of HICs and LMICs. In-depth interviews with focus groups might add nuance and detail to the findings of our study; however, because of the low response rate, we were not able to do any further subanalyses.
Conclusion
Results of our survey-based analysis suggest that oncologists from HICs might be involved with clinical cancer research more intensively than those from LMICs. Lack of finances was identified as the most important barrier to clinical cancer research. In HICs, a lack of time or other competing priorities was the most important barrier, and regulatory procedures were the most important barrier in LMICs. Time management and regulatory issues should be critically addressed for more successful global clinical cancer research. Importantly, because of the methodology of our survey and the low response rate, our findings may not necessarily reflect the opinion of oncologists or all oncologists involved with research. To get a clearer picture, additional research on barriers to clinical cancer research in both HICs and LMICs needs to be done. To further improve cancer outcomes, international collaboration and the globalization of cancer research is indispensable to recognize and eliminate major barriers and hurdles.
Acknowledgment
This study was supported by the American Society of Clinical Oncology International Affairs Committee.
Author Contributions
Conception/Design: Bostjan Seruga, Eduardo L. Cazap, Natasha B. Leighl, Lucia Beatriz Delgado, Raghunadharao Digumarti, Mohamed M. Meshref, Hironobu Minami, Eliezer Robinson, Nise Hitomi Yamaguchi, Doug Pyle, Tanja Cufer
Provision of study material or patients: Doug Pyle
Collection and/or assembly of data: Bostjan Seruga
Data analysis and interpretation: Bostjan Seruga, Eduardo L. Cazap, Natasha B. Leighl, Aleksander Sadikov, Lucia Beatriz Delgado, Raghunadharao Digumarti, Mohamed M. Meshref, Hironobu Minami, Eliezer Robinson, Nise Hitomi Yamaguchi, Doug Pyle, Tanja Cufer
Manuscript writing: Bostjan Seruga, Eduardo L. Cazap, Natasha B. Leighl, Aleksander Sadikov, Lucia Beatriz Delgado, Raghunadharao Digumarti, Mohamed M. Meshref, Hironobu Minami, Eliezer Robinson, Nise Hitomi Yamaguchi, Doug Pyle, Tanja Cufer
Final approval of manuscript: Bostjan Seruga, Eduardo L. Cazap, Natasha B. Leighl, Aleksander Sadikov, Lucia Beatriz Delgado, Raghunadharao Digumarti, Mohamed M. Meshref, Hironobu Minami, Eliezer Robinson, Nise Hitomi Yamaguchi, Doug Pyle, Tanja Cufer
Disclosures
Eduardo L. Cazap: Union for International Cancer Control, Latin American and Caribbean Society of Medical Oncology (SLACOM), Breast Health Global Initiative (E), Bayer, Schering Pharma (C/A), Bayer, Bristol-Myers Squibb, Fresenius, Roche (H), Poniard Pharmaceuticals, Daiichi Sankyo Pharma, Breast Cancer Research Foundation (RF); Mohamed M. Meshref: Boehringer Ingelheim (E); Nise Hitomi Yamaguchi: lectures in meetings with proceeds donated for educational purposes (H); Tanja Cufer: Boehringer Ingelheim, Eli Lilly, Pfizer (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: Results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 2.Eurocare: Protocol EUROCARE-5. Available at http://www.eurocare.it/Eurocare5/DocumentsEU5/tabid/91/Default.aspx. Accessed March 23, 2013.
- 3.Du Bois A, Rochon J, Lamparter C, et al. Pattern of care and impact of participation in clinical studies on the outcome in ovarian cancer. Int J Gynecol Cancer. 2005;15:183–191. doi: 10.1111/j.1525-1438.2005.15202.x. [DOI] [PubMed] [Google Scholar]
- 4.Vist GE, Bryant D, Somerville L, et al. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database Syst Rev. 2008:MR000009. doi: 10.1002/14651858.MR000009.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimble EL, Abrams JS, Meyer RM, et al. Improving cancer outcomes through international collaboration in academic cancer treatment trials. J Clin Oncol. 2009;27:5109–5114. doi: 10.1200/JCO.2009.22.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado L. Clinical cancer research in Latin America. ASCO Daily News. 2010. p. 3C.
- 7.Steinhausen K, Ren J, Cazap E, et al. Global core competencies for clinical trials. Lancet. 2012;380:728. doi: 10.1016/S0140-6736(12)61403-2. [DOI] [PubMed] [Google Scholar]
- 8.Seruga B, Hertz PC, Le LW, et al. Global drug development in cancer: A cross-sectional study of clinical trial registries. Ann Oncol. 2010;21:895–900. doi: 10.1093/annonc/mdp403. [DOI] [PubMed] [Google Scholar]
- 9.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 10. 3 Year Progress Report 2001–2004. London, U.K.: National Cancer Research Institute, 2004.
- 11.Bleyer WA. The U.S. pediatric cancer clinical trials programmes: International implications and the way forward. Eur J Cancer. 1997;33:1439–1447. doi: 10.1016/s0959-8049(97)00249-9. [DOI] [PubMed] [Google Scholar]
- 12.Somkin CP, Altschuler A, Ackerson L, et al. Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care. 2005;11:413–421. [PubMed] [Google Scholar]
- 13.Booth CM, Cescon DW, Wang L, et al. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26:5458–5464. doi: 10.1200/JCO.2008.16.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castel P, Négrier S, Boissel JP, Plateforme d’Aide à la Recherche Clinique en Cancérologie de la région Rhône-Alpes Why don’t cancer patients enter clinical trials? A review. Eur J Cancer. 2006;42:1744–1748. doi: 10.1016/j.ejca.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 15.World Bank. How we classify countries. Available at http://data.worldbank.org/about/country-classifications. Accessed October 2011.
- 16.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs SR, Weiner BJ, Minasian LM, et al. Achieving high cancer control trial enrollment in the community setting: An analysis of the Community Clinical Oncology Program. Contemp Clin Trials. 2013;34:320–325. doi: 10.1016/j.cct.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanarek NF, Kanarek MS, Olatoye D, et al. Removing barriers to participation in clinical trials, a conceptual framework and retrospective chart review study. Trials. 2012;13:237. doi: 10.1186/1745-6215-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton MK. Physician characteristics associated with clinical trial enrollment. CA Cancer J Clin. 2011;61:207–208. doi: 10.3322/caac.20123. [DOI] [PubMed] [Google Scholar]
- 20.Hearn J, Sullivan R. The impact of the ‘Clinical Trials’ directive on the cost and conduct of non-commercial cancer trials in the UK. Eur J Cancer. 2007;43:8–13. doi: 10.1016/j.ejca.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Glickman SW, McHutchison JG, Peterson ED, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–823. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan R. The good, the bad, and the ugly: Effect of regulations on cancer research. Lancet Oncol. 2008;9:2–3. doi: 10.1016/S1470-2045(07)70388-1. [DOI] [PubMed] [Google Scholar]
- 23.Conference on the Impact on Clinical Research of European Legislation – ICREL: Results & discussion. Available at http://pt.wkhealth.com/pt/pt-core/template-adis/jpm/media/ICREL.pdf. Accessed April 7, 2013.
- 24.Piccart M, Goldhirsch A, Wood W, et al. Keeping faith with trial volunteers. Nature. 2007;446:137–138. doi: 10.1038/446137a. [DOI] [PubMed] [Google Scholar]
- 25.De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351:1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- 26.Berthold DR, Gulamhusein A, Jackson JI, et al. The transition from phase II to phase III studies. J Clin Oncol. 2009;27:1150–1151. doi: 10.1200/JCO.2008.21.1938. [DOI] [PubMed] [Google Scholar]
- 27.Eckhouse S, Sullivan R. A survey of public funding of cancer research in the European Union. PLoS Med. 2006;3:e267. doi: 10.1371/journal.pmed.0030267. [DOI] [PMC free article] [PubMed] [Google Scholar]


