Surgery plays a key role in the management of Zollinger-Ellison syndrome; however, extent of resection, timing of intervention, performance of prophylactic adjunctive procedures, and reoperation for recurrent disease are topics of controversy. Historical considerations as well as evidence-based recommendations are summarized in this review.
Keywords: Gastrinoma, Peptic ulcer, Multiple endocrine neoplasia type 1, Gastric acid
Learning Objectives
Compare the approaches to management of sporadic and MEN-1 associated Zollinger-Ellison syndrome variants.
Discuss the controversies in surgical and medical management of Zollinger-Ellison syndrome.
Abstract
Zollinger-Ellison syndrome (ZES) is an endocrinopathy characterized by gastrin-secreting tumors, responsible for causing the formation of multiple, refractory, and recurrent peptic ulcers in the distal duodenum and proximal jejunum. Two main variants have been described, sporadic and those found in association with parathyroid and pituitary tumors, a genetic disorder known as multiple endocrine neoplasia-1 (MEN-1). Biochemical serum evaluation for elevated gastrin, followed by radiological or nuclear localization of the primary lesion, is mandated for establishing diagnosis. The mainstays of treatment include management of hypersecretory state with medical suppression of gastric acid production and surgical resection of primary tumor for the prevention of malignant transformation and metastatic complications. Medical therapy with proton pump inhibitors has virtually eliminated the need for acid-reducing surgical procedures. Surgical approach to sporadic and MEN-1-associated ZES varies based on our understanding of the natural history of the condition and the probability of cure; however, resection to a negative microscopic margin is indicated in both cases. Postoperative surveillance involves measurement of gastrin level, followed by imaging if elevation is detected. Re-excision of recurrent or resection of metastatic disease is a subject of controversy; however, at the present time aggressive cytoreductive approach is favored.
Implications for Practice:
Surgery plays a key role in the treatment of Zollinger-Ellison syndrome, an endocrinopathy characterized by gastrin-secreting tumors responsible for causing multiple recurrent and often refractory ulcers in the GI tract. Although medical therapy with proton pump inhibitors has virtually eliminated the need for acid-reducing surgical procedures in patients with this condition, resection of the primary lesion is still indicated in most cases. However, the extent of resection, timing of intervention, and reoperation for recurrent disease are topics of controversy. The historical considerations as well as evidence-based recommendations for management are summarized in this article.
Introduction
In April 1956, at the annual meeting of the American Surgical Association in Philadelphia, Dr. Robert M. Zollinger and Dr. Edwin H. Ellison described two cases of a condition whereby patients developed severe, recurrent, multifocal ulcerative lesions of the proximal gastrointestinal tract. These lesions appeared to be refractory to any attempt at surgical resection and were curiously associated with tumors located in the adjacent pancreas. The manuscript, titled “Primary Peptic Ulcerations of the Jejunum Associated with Islet Cell Tumor of the Pancreas,” proposed a diagnostic triad for the clinical syndrome they had identified. This included mucosal ulcerations in unusual locations such as distal duodenum or proximal jejunum, gastric acid hypersecretion, and presence of non-β-cell pancreatic tumors [1]. Based on clinical observations, Zollinger and Ellison hypothesized a presence of a previously unknown pancreatic cell tumor capable of secreting a humoral factor effecting excitability of gastric acid-producing cells. Although the elaboration of the epidemiology, pathogenesis, clinical manifestations, and physiological sequelae of this entity was to follow in the upcoming decades, this was the first formal discussion at a scientific forum of what eventually became known as Zollinger-Ellison syndrome (ZES).
In the 1960s, gastrin was discovered as the key hormone in the pathogenesis of the gastric hypersecretion [2]. With further advances in biochemical detection techniques, as well as improved understanding of the gastrointestinal hormone interactions, specifically the identification of the role of secretin stimulation on the serum gastrin levels [3], the diagnosis of ZES could now be conclusively established. It was observed that some cases of ZES were decidedly sporadic, whereas others occurred in constellation with other clinical features that comprised what we now understand to be a genetic syndrome that later became known as MEN-1 [4]. Advances in radiological imaging, angiography, and endoscopic techniques allowed for more precise tumor localization. Finally, understanding the natural history of the tumors responsible for ZES has allowed us to make evidence-based recommendations about their ultimate management.
It is the purpose of the current review to present a historically based overview of the diagnosis and treatment of Zollinger-Ellison syndrome and to discuss some of the controversies that exist today with regard to its management.
Epidemiology and Pathogenesis
The early estimates of ZES incidence were most likely falsely low given large degree of overlap with peptic ulcer disease [5]. Whereas the true incidence remains unknown, there is general consensus that approximately 0.1–3 persons per million develop gastrinoma each year in most geographical areas [6, 7]. This rate has largely remained constant since ZES was originally described. Females have a slightly greater preponderance for developing the disease, and cases have been reported in both the very young and the very old, although gastrinoma is most frequently diagnosed between the ages of 20 and 50 [5, 7–9].
Approximately 80% of the time, the primary causative lesion is thought to arise sporadically; in the remainder of recorded cases, this entity exists as part of MEN-1, an autosomal dominant disorder characterized by tumors of the pituitary, the parathyroid, and the pancreas [4].
Gastrinomas are derived from the enteroendocrine cells that arise from the embryologic endoderm, and form tumors mainly in the pancreas, but also in the proximal small intestine. Because of their origin, these are generally classified under the larger umbrella term of neuroendocrine tumors (NETs) [10]. The World Health Organization classifies NETs into two broad categories, well differentiated and poorly differentiated, and most gastrinomas are considered well-differentiated NETs on the basis of histopathological analysis [11]. It has been observed that most gastrinomas arise in the duodenum, with tumors located in the pancreas carrying greater malignant potential [12].
The pathophysiology of ZES is related to the trophic action of gastrin on parietal cells of the gastric antrum and the resulting hypersecretory acid milleu [3]. An overwhelming majority of patients with this disease consequently develop peptic ulcers, often large and multiple, frequently in distal duodenum and even proximal jejunum (an uncommon location for ulcers resulting from Helicobacter pylori or the use of nonsteroidal anti-inflammatory drugs).
Detection and Diagnosis
Most patients experience severe refractory heartburn and epigastric pain, often accompanied by profound diarrhea, which is a result of the combination of osmotic load of high gastric acid secretion as well as a malabsorptive component from inactivation of pancreatic digestive enzymes by the acid [13, 14]. In addition, high serum gastrin concentration may inhibit sodium and water reabsorption by the intestinal brush border, thus proving a secretory component as well. Severe diarrhea may be a sole presentation of ZES in as many as 20% of the patients [15]. Other common complaints at presentation include abdominal pain, weight loss, or history of renal colic/nephrolithiasis in patients with MEN-1.
Only a small proportion of patients with peptic ulcer disease (PUD) are ultimately diagnosed with ZES. A high index of suspicion should exist in cases of refractory gastric hyperacidemia, in cases of ulcers that recur in spite of maximal medical management, and in the presence of ulcers that are large, multiple, or located in uncommon areas (distal to the first portion of the duodenum). Biochemical analysis of the serum in patients with suspected ZES includes first measuring fasting gastrin levels, 72 hours off proton pump inhibitors (PPIs). A value greater than 1,000 pg/mL is diagnostic, and a value greater than 100 pg/mL is suggestive of this diagnosis, when identified in the presence of gastric acid. Measurement of gastric pH is required to exclude secondary hypergastrinemia (such as that associated with chronic achlorhydria) [5] (Table 1).
Table 1.
Differential diagnosis of hypergastrinemia
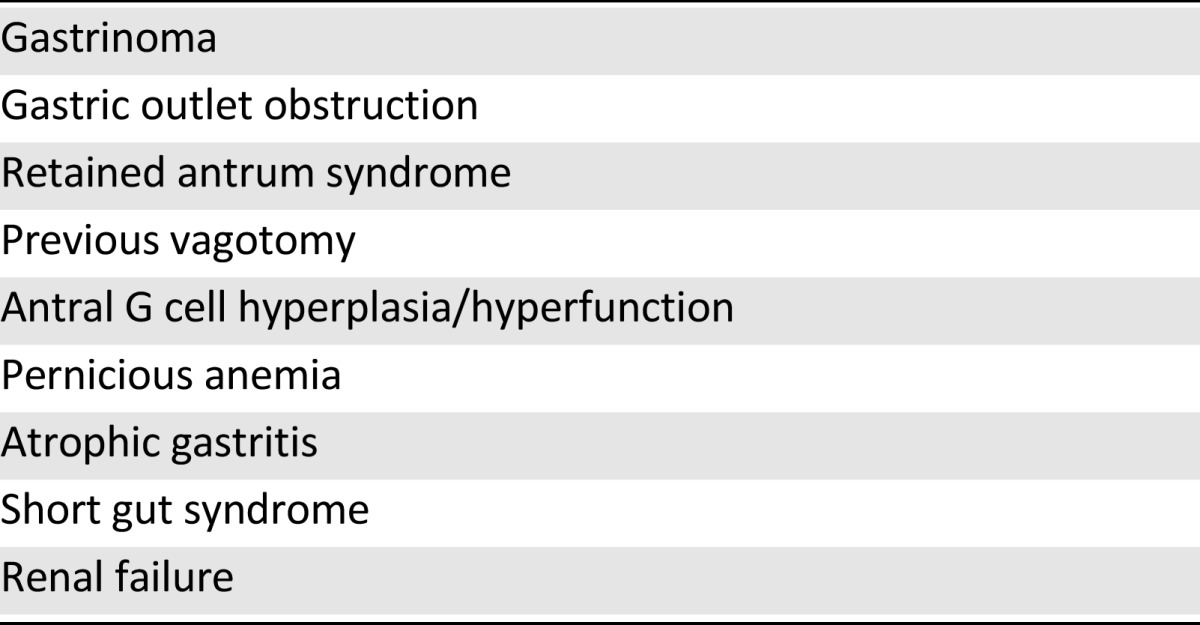
A high index of suspicion should exist in cases of refractory gastric hyperacidemia, in cases of ulcers that recur in spite of maximal medical management, and in the presence of ulcers that are large, multiple, or located in uncommon areas (distal to the first portion of the duodenum).
Falsely low gastrin levels, despite the presence of gastrinoma, have been reported; this occurs because the tumor secretes bioactive gastrin precursor molecules, only one subtype of which is detected by the commercially available assay [16]. Therefore, in situations in which gastrin level testing is nondiagnostic, or is low but the suspicion for a tumor remains, additional testing is recommended to identify and localize the lesion. The simplest, and most sensitive and reliable, confirmatory test in this setting is the provocative secretin test, which consists of intravenous administration of 2 μg/kg secretin. An increase of greater than 100 pg/mL in serum gastrin levels is considered positive, whereas a rise of 200 pmg/mL above baseline is virtually diagnostic [3, 10, 17]. This test is also the most sensitive indicator of recurrent or persistent disease in patients who have undergone attempted surgical resection. Positivity necessitates further radiological investigation for the presence of another lesion [18].
Any patient diagnosed with ZES should also be screened for MEN-1, which includes evaluating levels of prolactin, calcium, PTH, and pancreatic polypeptide. Interestingly, in patients with MEN-1, hyperparathyroidism is diagnosed after establishing ZES in approximately 50% of the patients [19].
Finally, an endoscopic gastroduodenoscopy should be performed to rule out secondary complications in patients with long-standing acid hypersecretion. Nearly all patients will develop ulcers at some point in the course of their disease, and more than half will exhibit endoscopic evidence of mucosal injury, such as erosive inflammation, stricturization, and perforation; these esophageal complications can develop even in the presence of antisecretory medications [20].
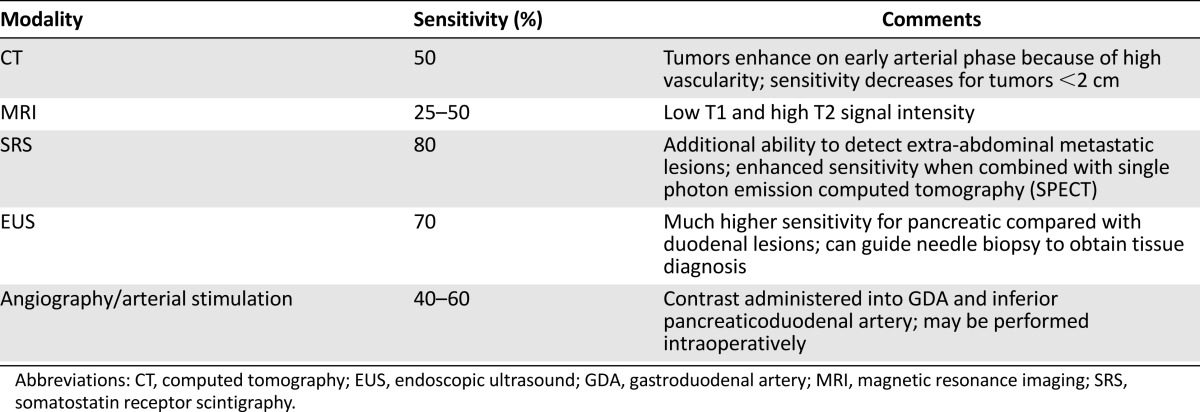
Radiologic imaging is recommended before undergoing any surgical exploration to localize the lesion and identify or rule out metastatic disease [21, 22]. Computer tomography (CT) and magnetic resonance imaging (MRI) both have good diagnostic accuracy for detecting lesions >3 cm; however, both frequently miss tumors smaller than 2 cm [22–25]. Somatostatin receptor scintigraphy has a higher sensitivity than conventional imaging (i.e., CT or MRI) and higher specificity than all combined for detection of extrahepatic gastrinoma, and is the imaging study of choice for identifying primary tumors and metastatic lesions in ZES [26, 27]. In recent years, endoscopic ultrasound has also become an important tool for localization of endocrine tumors located in the pancreas, as it allows good visualization of subcentimeter tumors (Table 2) [28–30].
Table 2.
Diagnostic accuracy of imaging for localization of gastrinoma
In select situations in which biochemical and radiological investigative testing fails to identify a discrete lesion in a patient with suspected ZES, a selective arterial secretin test can be used to ascertain the presence of a tumor. Using this method, a selective cannulation of splenic, hepatic, gastroduodenal artery and superior mesenteric artery is performed, and small amount of secretin is infused locally; serial gastrin measurements are then taken from the right hepatic vein, thus establishing the relative site of the lesion [31].
Even with all the sophisticated diagnostic testing available today, a primary lesion has not been identified in approximately 30% of the patients with ZES [32, 33]; in this situation, a surgical exploration with extensive and thorough examination of the pancreas and duodenum is warranted. The technique and rationale for this will be discussed later in this review.
Medical Management
In the era before the discovery and widespread introduction of histamine-2 receptor blockers and PPIs, acid-reducing surgical procedures were the mainstay of treatment for patients with ZES. The relative morbidity of surgical interventions, which often involved total gastrectomy, was thought to be far outweighed by the potential complications of chronic hypersecretory state. Today, as PPIs are highly effective antisecretory agents that are well tolerated and have few long-term negative side effects even with chronic use at high doses, these medications have become the first-line therapy for patients with hypergastrinemia [34, 35]. PPIs bind H+K+ATPase at the luminal aspect of the gastric parietal cell, thus interfering with both basal and stimulated gastric acid secretion. Most patients require doses that are slightly higher than those necessary for patients with idiopathic PUD; however, many can achieve acceptable outcomes with daily dosing because of the long duration of action of these drugs. A small percentage needs twice to multiple times the dose to maintain subphysiologic gastric acid levels [34]. Interestingly, mucosal healing is not correlated with symptom relief, and so endoscopic surveillance at regular intervals is recommended. There has been some concern about chronic hypergastrinemia and achlorhydria associated with long-term PPI use—several investigators have raised concerns about the development of gastric carcinoid tumors in this setting [36]. However, this has not been borne out in human clinical trials, and PPIs remain the recommended first-line agents for control of acid secretion in patients with ZES [34].
Surgical Management
With the advent of inexpensive, easy to administer, and well-tolerated pharmacologic options, acid-reducing surgical procedures largely fell by the wayside, instead replaced by the highly effective medical therapy. The role of surgical management of ZES has instead shifted to eradication of primary tumor and control/prevention of metastatic spread [21, 22].
Surgical approach to gastrinoma differs in sporadic ZES and ZES associated with MEN-1. As already mentioned, even although many gastrinomas are well-differentiated, over half do carry malignant potential and mortality results from metastatic disease (Table 3) [27, 37–42]. It has been observed that even though for the most part gastrinomas are slow-growing tumors, and their metastatic propensity is low if the lesion is less than 2 cm in size, presence of metastatic disease dramatically worsens prognosis and decreases survival. Because of this, a case has been made for early surgical exploration and excision of primary lesions in patients with ZES to prevent distant spread. Unfortunately, complete surgical resection is possible in less than half of patients with sporadic ZES and not at all in patients who also have MEN-1.
Table 3.
Sporadic and MEN-1-associated ZES
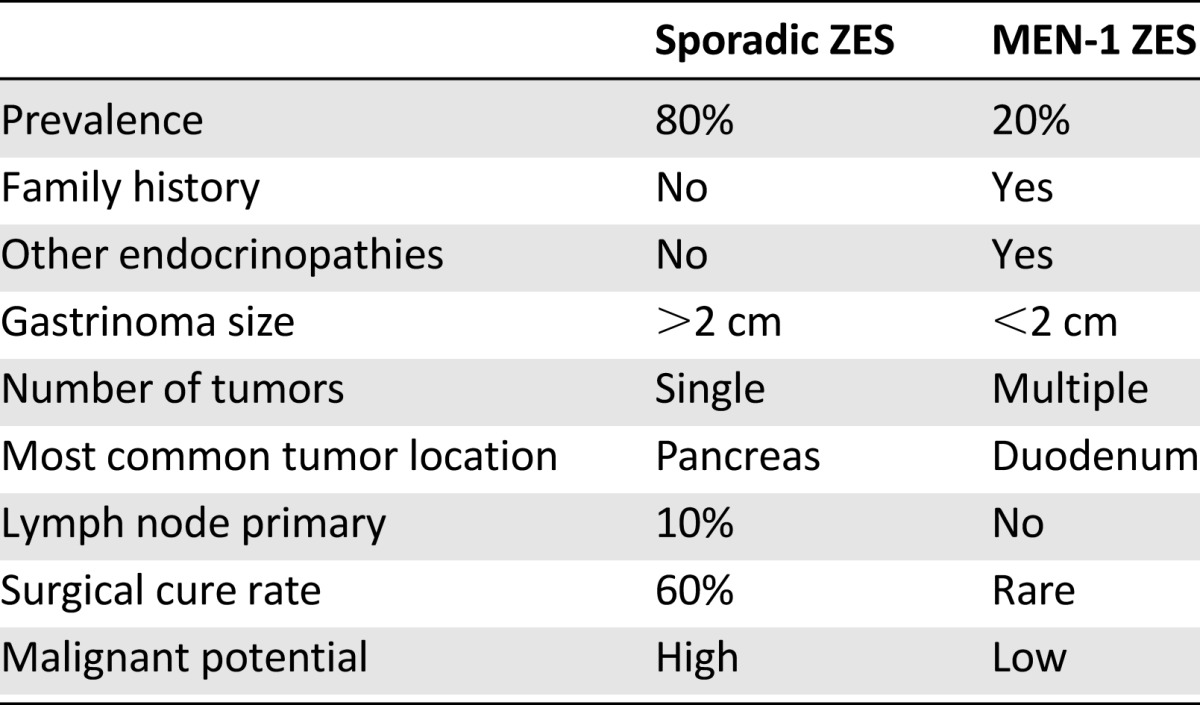
In sporadic ZES, as many as 40%–70% of patients already have lymph node metastases at surgery, and 20%–40% present with unresectable liver metastases [27, 43, 44]. In patients with MEN-1, gastrinomas are frequently small (and thus undetectable by localization imaging), multiple, and have a very high metastatic propensity. This makes it virtually impossible to achieve cure without an aggressive surgical resection such as a pancreaticoduodenectomy [27, 39, 41–48]. Despite this, surgical excision is advocated for patients with solitary gastrinomas who do not have other contraindications to surgery. Surgical excision is also advocated for patients whose metastatic disease is amenable to resection.
The general approach to surgical exploration of the region should first involve identification of any metastatic lesions in the liver and the peritoneum. This should be followed by exposure and mobilization of the entire duodenum and pancreas, allowing for the examination of the gastrinoma triangle. In the late 1980s, Dr. Bruce Stabile and Dr. Ed Passaro noted that more than two thirds of all primary gastrinoma lesions are located in this region, roughly defined by the confluence of the cystic and common bile duct superiorly, the second and third portions of the duodenum inferiorly, and the neck and body of the pancreas medially, both dorsally and ventrally (Fig. 1) [49, 50]. Mandating a thorough surgical exploration of this area resulted in nearly doubling the detection rate and removal of primary gastrinoma lesions.
Figure 1.
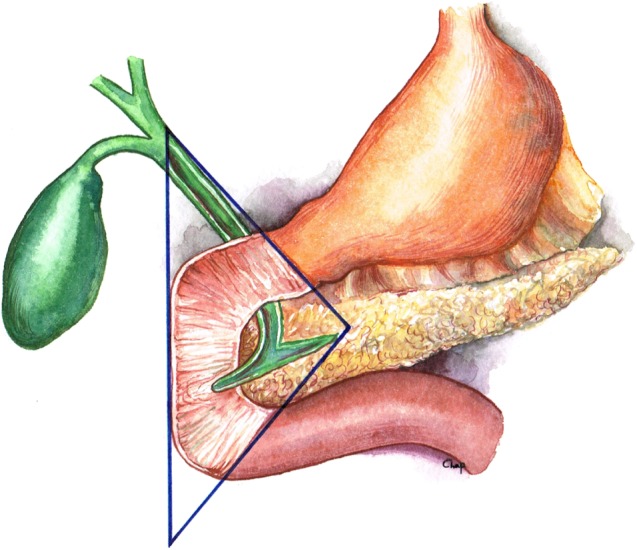
Gastrinoma triangle. Borders are defined by confluence of the cystic and common bile duct superiorly, the second and third portions of the duodenum inferiorly, and the neck and body of the pancreas medially, both dorsally and ventrally.
The pancreas should be examined in its entirety, including intraoperative ultrasound, whether the lesion of interest is palpable or not. Small tumors may be enucleated, with care taken not to disrupt the pancreatic duct. Larger tumors may necessitate more extensive resections, such as a distal or a proximal pancreatectomy. In select situations, a central pancreatectomy may be considered for tumors located in the body of the gland to maximally preserve endocrine function [51].
Examination of the duodenum is also an obligatory step of this procedure. A lateral duodenotomy must be made, and the duodenum can be evaluated using manual palpation, taking special care to assess the second and third portions. The duodenotomy can then be closed in a single layer longtitudinally without appreciably narrowing the lumen. The extent of exploration and resection in patients with MEN-1 is a subject of controversy, as these patients are almost never cured of their disease by removing the primary tumor.
For patients who have metastatic disease to the liver at presentation, surgical options do include resection of both the primary and the metastatic tumor, provided there is no extrahepatic disease and that sufficient size and function of the remnant liver can be ensured. Other therapies exist, such as administration of octreotide, hepatic artery embolization, chemo- and radiotherapy, and molecular targeted therapies, and all have been investigated, but no level 1 data exist regarding their efficacy [34, 52–55]. Additionally, patients with recurrent disease may be considered for re-excision, as some reports have demonstrated adequate disease-free survival at relatively long term follow-up with this strategy [56–58].
Outcomes and Surveillance
Surgical cure rates for ZES are a debated topic in current literature. Some groups have published complete surgical cures in sporadic gastrinoma; however, it should be noted that many of these studies have follow-up that is short and incomplete. Additionally, many of these define recurrence based on identification of radiologically detectable tumors rather than biochemical serum testing, even though the latter is a more sensitive and specific modality for detection of recurrent disease [44, 59, 60]. A more accurate estimate of approximately 40% at 10 years comes from several groups that present prospective data on patients with sporadic tumors who underwent resection [21, 42, 44, 61].
It is virtually impossible to achieve surgical cure in patients with MEN-1 ZES, with evidence of biochemical relapse present in more than 95% of patients within 3 years of surgery. However, resection of biologically active and radiologically/intraoperatively identifiable lesions remains the current recommendation because of improved survival thought to be secondary to prevention of distant metastatic spread.
It is virtually impossible to achieve surgical cure in patients with MEN-1 ZES, with evidence of biochemical relapse present in more than 95% of patients within 3 years of surgery. However, resection of biologically active and radiologically/intraoperatively identifiable lesions remains the current recommendation because of improved survival thought to be secondary to prevention of distant metastatic spread.
For all comers, data collected over the last 50 years suggest that, after a R0/R1 resection, most patients should enjoy a greater than 10-year survival, even if the disease recurs. Lymph node status and achieving eugastrinemia are not associated with a survival benefit; instead, the presence of metastatic disease is the marker most profoundly associated with poor long-term prognosis [37, 61, 62].
It is recommended that fasting gastrin level be checked annually and that subsequent imaging/localization investigations be performed based on the presence of symptoms and elevated gastrin levels in those who undergo successful surgical resection [63].
Controversies in Current Management
As laparoscopic techniques become more widely adopted in the surgical community, there has been increasing interest in using minimally invasive methods for the management of gastrinomas. Endoscopic resections of duodenal tumors, as well as laparoscopic enucleations or distal parenchymal resections of pancreatic lesions, have been described [64–67]. However, manual palpation still remains an essential component of surgical exploration for gastrinoma, as approximately 30% of the tumors are not detected with standard radiological localization techniques preoperatively. Specialized training and skill in advanced laparoscopic techniques are required of any surgeon undertaking resection of a lesion in this anatomic region. In addition, when dealing with larger tumors, it is sometimes difficult to assess the extent of disease and lymph node involvement, as well as to perform the required extent of resection, with minimally invasive approach only. Thus, role of laparoscopy remains limited in the definitive surgical management of gastrinoma.
The extent of surgical resection, in particular for patients with MEN-1, remains subject of debate as well. Tumors in MEN-1/ZES tend to be smaller, multifocal in location, and poorly defined with preoperative localization methods, compared with sporadic ZES. Because of this, there is a nearly universal recurrence of disease in this group of patients. Several small series have reported high cure rates with aggressive resection, favoring pancreaticoduodenectomy (Whipple procedure); however, this approach, although intuitively reasonable, suffers from a number of issues [41, 68–73]. First, no randomized controlled prospective data exist that support higher and more durable cure rates using Whipple procedures compared with less extensive resections. Second, in cases of local recurrence, reoperation is far more difficult and wrought with complications if a pancreaticoduodenectomy had already been performed. Third, if a metastatic lesion in the liver develops after the primary resection, the patient who has undergone a Whipple procedure would not be a candidate for local control therapies such as hepatic artery embolization due to concern for ascending infection. Thus, the recommendation to perform pancreaticoduodenectomy in patients with gastrinomas exists only for those with large lesions in head of pancreas not amenable to enucleation, bulky proximal disease, multiple duodenal lesions, and clinically involved regional lymph nodes.
The role of parietal cell vagotomy simultaneous with resection of the primary lesion is controversial. With use of PPIs as well as in the absence of primary gastrin-secreting tumor, acid-reducing surgical procedures have fallen out of favor. However, the long-term effects of chronic PPI therapy have not been well-elaborated, and the financial burden of approximately $3,300 per year is not inconsequential [44]. In response to this, several groups have continued to perform vagotomy at the time of primary tumor resection, and have observed that postoperatively, close to one third of the patients were able to stop antisecretory medications completely [74]. Therefore, selective vagotomy should be considered for patients undergoing surgical exploration for ZES.
Metastatectomy, re-excision of locally recurrent lesions, and cytoreductive resections in patients who present with multifocal metastatic disease have all been performed with adequate outcomes. Although no long-term prospective data exist, based on results of smaller scale retrospective studies, at present, it is believed that long-term benefits of aggressive surgical management sufficiently outweigh the potential risks of complications related to this approach, provided the patient is thought to be physiologically capable of tolerating the procedure [44, 71, 75, 76].
Conclusion
Zollinger-Ellison syndrome remains a challenging condition more than 50 years after its discovery. Diagnosis should be prompted by high index of suspicion based on clinical presentation, and confirmatory biochemical testing should be performed. Sporadic and MEN-1-associated variants should be distinguished. All patients without contraindication to surgery should undergo surgical exploration following radiological and nuclear localization studies. The optimal approach, open versus minimally invasive, remains the subject of debate; however, it is clear that gastrinoma triangle should be carefully and meticulously explored at the time of surgery, with inclusion of intraluminal duodenal evaluation. Extent of surgical resection, vis-à-vis prophylactic pancreaticoduodenectomy, is controversial; however, current recommendation remains to aim for R0/R1 outcome. Postoperative surveillance should center on biochemical serum testing, and cases of recurrence should be carefully considered for possible reoperation. Further studies with longer follow-up are necessary to conclusively describe the natural history of this condition, identify factors predictive of recurrence and survival, and make categorical recommendations regarding treatment.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
We thank Dr. Katya Chapchay for producing Figure 1.
Author Contributions
Conception/Design: Irene Epelboym, Haggi Mazeh
Provision of study material or patients: Irene Epelboym, Haggi Mazeh
Collection and/or assembly of data: Irene Epelboym, Haggi Mazeh
Data analysis and interpretation: Irene Epelboym, Haggi Mazeh
Manuscript writing: Irene Epelboym, Haggi Mazeh
Final approval of manuscript: Irene Epelboym, Haggi Mazeh
Disclosures
The authors indicated no financial relationships.
Section Editors: Herbert Chen: None; Stan Sidhu: None.
Reviewer “A”: None
Reviewer “B”: XOMA Pharmaceuticals (C/A)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709–723; discussion 724–728. [PMC free article] [PubMed] [Google Scholar]
- 2.Hou W, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2006;22:593–598. doi: 10.1097/01.mog.0000245538.43142.87. [DOI] [PubMed] [Google Scholar]
- 3.McGuigan JE, Wolfe MM. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology. 1980;79:1324–1331. [PubMed] [Google Scholar]
- 4.Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 5.Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine. 2006;85:295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson B, Oberg K, Skogseid B. Neuroendocrine pancreatic tumors: Clinical findings in a prospective study of 84 patients. Acta Oncol. 1989;28:373–377. doi: 10.3109/02841868909111209. [DOI] [PubMed] [Google Scholar]
- 7.Stage JG, Stadil F. The clinical diagnosis of the Zollinger-Ellison syndrome. Scand J Gastroenterol Suppl. 1979;53:79–91. [PubMed] [Google Scholar]
- 8.Sheppard BC, Norton JA, Doppman JL, et al. Management of islet cell tumors in patients with multiple endocrine neoplasia: A prospective study. Surgery. 1989;106:1108–1117; discussion 1117–1118. [PubMed] [Google Scholar]
- 9.Richardson CT, Peters MN, Feldman M, et al. Treatment of Zollinger-Ellison syndrome with exploratory laparotomy, proximal gastric vagotomy, and H2-receptor antagonists: A prospective study. Gastroenterology. 1985;89:357–367. doi: 10.1016/0016-5085(85)90337-3. [DOI] [PubMed] [Google Scholar]
- 10.Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg. 1994;31:77–156. doi: 10.1016/0011-3840(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 11.Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman TFCF, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2010. p. 13. [Google Scholar]
- 12.O’Toole D, Delle Fave G, Jensen RT. Gastric and duodenal neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:719–735. doi: 10.1016/j.bpg.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Deveney CW, Deveney KE. Zollinger-Ellison syndrome (gastrinoma): Current diagnosis and treatment. Surg Clin North Am. 1987;67:411–422. doi: 10.1016/s0039-6109(16)44192-7. [DOI] [PubMed] [Google Scholar]
- 14.Meko JB, Norton JA. Management of patients with Zollinger-Ellison syndrome. Annu Rev Med. 1995;46:395–411. doi: 10.1146/annurev.med.46.1.395. [DOI] [PubMed] [Google Scholar]
- 15.Jensen RT, Gardner JD. Zollinger-Ellison syndrome: clinical presentation, pathology, diagnosis and treatment. In A Dannenberg, D Zakim, eds. Peptic Ulcer and Other Acid-Related Diseases. New York NY: Academic Research Association, 1991:117–211. [Google Scholar]
- 16.Rehfeld JF, Bardram L, Hilsted L, et al. Pitfalls in diagnostic gastrin measurements. Clin Chem. 2012;58:831–836. doi: 10.1373/clinchem.2011.179929. [DOI] [PubMed] [Google Scholar]
- 17.Frucht H, Howard JM, Slaff JI, et al. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome: A prospective study. Ann Intern Med. 1989;111:713–722. doi: 10.7326/0003-4819-111-9-713. [DOI] [PubMed] [Google Scholar]
- 18.Gibril F, Jensen RT. Diagnostic uses of radiolabelled somatostatin receptor analogues in gastroenteropancreatic endocrine tumours. Dig Liver Dis. 2004;36(suppl 1):S106–S120. doi: 10.1016/j.dld.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Mignon M, Ruszniewski P, Podevin P, et al. Current approach to the management of gastrinoma and insulinoma in adults with multiple endocrine neoplasia type I. World J Surg. 1993;17:489–497. doi: 10.1007/BF01655108. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best Pract Res Clin Gastroenterol. 2012;26:737–753. doi: 10.1016/j.bpg.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 22.Norton JA, Fraker DL, Alexander HR, et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410–419. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gualdi GF, Casciani E, Polettini E. Imaging of neuroendocrine tumors [in Italian] Clin Ter. 2001;152:107–121. [PubMed] [Google Scholar]
- 24.Gibril F, Jensen RT. Comparative analysis of diagnostic techniques for localization of gastrointestinal neuroendocrine tumors. Yale J Biol Med. 1997;70:509–522. [PMC free article] [PubMed] [Google Scholar]
- 25.Krudy AG, Doppman JL, Jensen RT, et al. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography, and venous sampling. AJR Am J Roentgenol. 1984;143:585–589. doi: 10.2214/ajr.143.3.585. [DOI] [PubMed] [Google Scholar]
- 26.Alexander HR, Fraker DL, Norton JA, et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998;228:228–238. doi: 10.1097/00000658-199808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1) Surg Oncol. 2003;12:145–151. doi: 10.1016/s0960-7404(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 28.Klose KJ, Heverhagen JT. Localisation and staging of gastrin producing tumours using cross-sectional imaging modalities. Wien Klin Wochenschr. 2007;119:588–592. doi: 10.1007/s00508-007-0886-0. [DOI] [PubMed] [Google Scholar]
- 29.Gibril F, Reynolds JC, Doppman JL, et al. Somatostatin receptor scintigraphy: Its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: A prospective study. Ann Intern Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ruszniewski P, Amouyal P, Amouyal G, et al. Localization of gastrinomas by endoscopic ultrasonography in patients with Zollinger-Ellison syndrome. Surgery. 1995;117:629–635. doi: 10.1016/s0039-6060(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 31.Maton PN, Miller DL, Doppman JL, et al. Role of selective angiography in the management of patients with Zollinger-Ellison syndrome. Gastroenterology. 1987;92:913–918. doi: 10.1016/0016-5085(87)90964-4. [DOI] [PubMed] [Google Scholar]
- 32.Thom AK, Norton JA, Doppman JL, et al. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992;112:1002–1008; discussion 1008–1009. [PubMed] [Google Scholar]
- 33.Gibril F, Doppman JL, Chang R, et al. Metastatic gastrinomas: Localization with selective arterial injection of secretin. Radiology. 1996;198:77–84. doi: 10.1148/radiology.198.1.8539410. [DOI] [PubMed] [Google Scholar]
- 34.Ito T, Igarashi H, Uehara H, et al. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2013;14:307–321. doi: 10.1517/14656566.2013.767332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banasch M, Schmitz F. Diagnosis and treatment of gastrinoma in the era of proton pump inhibitors. Wien Klin Wochenschr. 2007;119:573–578. doi: 10.1007/s00508-007-0884-2. [DOI] [PubMed] [Google Scholar]
- 36.Jensen RT. Consequences of long-term proton pump blockade: Insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4–19. doi: 10.1111/j.1742-7843.2006.pto_378.x. [DOI] [PubMed] [Google Scholar]
- 37.Norton JA. Surgical treatment and prognosis of gastrinoma. Best Pract Res Clin Gastroenterol. 2005;19:799–805. doi: 10.1016/j.bpg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: A comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46:13–106. doi: 10.1067/j.cpsurg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic) Neuroendocrinology. 2006;84:173–182. doi: 10.1159/000098009. [DOI] [PubMed] [Google Scholar]
- 40.Gibril F, Jensen RT. Advances in evaluation and management of gastrinoma in patients with Zollinger-Ellison syndrome. Curr Gastroenterol Rep. 2005;7:114–121. doi: 10.1007/s11894-005-0049-2. [DOI] [PubMed] [Google Scholar]
- 41.Norton JA, Fang TD, Jensen RT. Surgery for gastrinoma and insulinoma in multiple endocrine neoplasia type 1. J Natl Compr Canc Netw. 2006;4:148–153. doi: 10.6004/jnccn.2006.0015. [DOI] [PubMed] [Google Scholar]
- 42.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495–505; discussion 505–506. doi: 10.1097/00000658-200110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacFarlane MP, Fraker DL, Alexander HR, et al. Prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia type 1. Surgery. 1995;118:973–979; discussion 979–980. doi: 10.1016/s0039-6060(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 44.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: Advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807–1843. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 49.Sawicki MP, Howard TJ, Dalton M, et al. The dichotomous distribution of gastrinomas. Arch Surg. 1990;125:1584–1587. doi: 10.1001/archsurg.1990.01410240066014. [DOI] [PubMed] [Google Scholar]
- 50.Stabile BE, Morrow DJ, Passaro E., Jr The gastrinoma triangle: Operative implications. Am J Surg. 1984;147:25–31. doi: 10.1016/0002-9610(84)90029-1. [DOI] [PubMed] [Google Scholar]
- 51.DiNorcia J, Ahmed L, Lee MK, et al. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery. 2010;148:1247–1254; discussion 1254–1256. doi: 10.1016/j.surg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Grozinsky-Glasberg S, Barak D, Fraenkel M, et al. Peptide receptor radioligand therapy is an effective treatment for the long-term stabilization of malignant gastrinomas. Cancer. 2011;117:1377–1385. doi: 10.1002/cncr.25646. [DOI] [PubMed] [Google Scholar]
- 53.Shojamanesh H, Gibril F, Louie A, et al. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer. 2002;94:331–343. doi: 10.1002/cncr.10195. [DOI] [PubMed] [Google Scholar]
- 54.Gut P, Fischbach J, Kamiński G, et al. Contemporary methods of therapy and follow-up of neuroendocrine tumours of the gastrointestinal tract and the pancreas. Contemp Oncol (Pozn) 2012;16:371–375. doi: 10.5114/wo.2012.31764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clouse ME, Perry L, Stuart K, et al. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Digestion. 1994;55(suppl 3):92–97. doi: 10.1159/000201208. [DOI] [PubMed] [Google Scholar]
- 56.Jaskowiak NT, Fraker DL, Alexander HR, et al. Is reoperation for gastrinoma excision indicated in Zollinger-Ellison syndrome? Surgery. 1996;120:1055–1062; discussion 1062–1063. doi: 10.1016/s0039-6060(96)80055-9. [DOI] [PubMed] [Google Scholar]
- 57.Grobmyer SR, Vogel SB, McGuigan JE, et al. Reoperative surgery in sporadic Zollinger-Ellison syndrome: Longterm results. J Am Coll Surg. 2009;208:718–722; discussion 722–724. doi: 10.1016/j.jamcollsurg.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Jensen RT. Carcinoid and pancreatic endocrine tumors: Recent advances in molecular pathogenesis, localization, and treatment. Curr Opin Oncol. 2000;12:368–377. doi: 10.1097/00001622-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1991;15:151–159. doi: 10.1007/BF01658992. [DOI] [PubMed] [Google Scholar]
- 60.Fishbeyn VA, Norton JA, Benya RV, et al. Assessment and prediction of long-term cure in patients with the Zollinger-Ellison syndrome: The best approach. Ann Intern Med. 1993;119:199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mortellaro VE, Hochwald SN, McGuigan JE, et al. Long-term results of a selective surgical approach to management of Zollinger-Ellison syndrome in patients with MEN-1. Am Surg. 2009;75:730–733. [PubMed] [Google Scholar]
- 62.Ellison EC, Sparks J, Verducci JS, et al. 50-year appraisal of gastrinoma: Recommendations for staging and treatment. J Am Coll Surg. 2006;202:897–905. doi: 10.1016/j.jamcollsurg.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 63. NCCN Clinical Practice Guidelines. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#neuroendocrine. Accessed September 10, 2013. [Google Scholar]
- 64.DiNorcia J, Lee MK, Reavey PL, et al. One hundred thirty resections for pancreatic neuroendocrine tumor: Evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg. 2010;14:1536–1546. doi: 10.1007/s11605-010-1319-3. [DOI] [PubMed] [Google Scholar]
- 65.Atalar K, Warren OJ, Jacyna M, et al. Laparoscopic resection for primary lymph node gastrinoma. Pancreas. 2013;42:723–725. doi: 10.1097/MPA.0b013e31826dcd52. [DOI] [PubMed] [Google Scholar]
- 66.Min BH, Kim ER, Lee JH, et al. Management strategy for small duodenal carcinoid tumors: Does conservative management with close follow-up represent an alternative to endoscopic treatment? Digestion. 2013;87:247–253. doi: 10.1159/000349958. [DOI] [PubMed] [Google Scholar]
- 67.Blanc P, Porcheron J, Pages A, et al. Laparoscopic excision of a duodenal neuroendocrine tumor [in French] Ann Chir. 2000;125:176–178. doi: 10.1016/s0001-4001(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 68.Lopez CL, Waldmann J, Fendrich V, et al. Long-term results of surgery for pancreatic neuroendocrine neoplasms in patients with MEN1. Langenbecks Arch Surg. 2011;396:1187–1196. doi: 10.1007/s00423-011-0828-1. [DOI] [PubMed] [Google Scholar]
- 69.Tonelli F, Fratini G, Nesi G, et al. Pancreatectomy in multiple endocrine neoplasia type 1-related gastrinomas and pancreatic endocrine neoplasias. Ann Surg. 2006;244:61–70. doi: 10.1097/01.sla.0000218073.77254.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giudici F, Nesi G, Brandi ML, et al. Surgical management of insulinomas in multiple endocrine neoplasia type 1. Pancreas. 2012;41:547–553. doi: 10.1097/MPA.0b013e3182374e08. [DOI] [PubMed] [Google Scholar]
- 71.Cherner JA, Sawyers JL. Benefit of resection of metastatic gastrinoma in multiple endocrine neoplasia type I. Gastroenterology. 1992;102:1049–1053. doi: 10.1016/0016-5085(92)90196-6. [DOI] [PubMed] [Google Scholar]
- 72.Partensky C, Berger F, Owono P, et al. Cephalic duodenopancreatectomy for endocrine tumor of the ampulla of Vater and of the minor papilla [in French] Gastroenterol Clin Biol. 1999;23:832–836. [PubMed] [Google Scholar]
- 73.Sarmiento JM, Farnell MB, Que FG, et al. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: Long-term survival analysis. World J Surg. 2002;26:1267–1271. doi: 10.1007/s00268-002-6714-9. [DOI] [PubMed] [Google Scholar]
- 74.Pisegna JR, Norton JA, Slimak GG, et al. Effects of curative gastrinoma resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology. 1992;102:767–778. doi: 10.1016/0016-5085(92)90157-t. [DOI] [PubMed] [Google Scholar]
- 75.Pachera S, Yokoyama Y, Nishio H, et al. A rare surgical case of multiple liver resections for recurrent liver metastases from pancreatic gastrinoma: Liver and vena cava resection. J Hepatobiliary Pancreat Surg. 2009;16:692–698. doi: 10.1007/s00534-009-0055-0. [DOI] [PubMed] [Google Scholar]
- 76.Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]


