Abstract
Interventional radiology-guided percutaneous drainage of liver abscesses with concomitant use of antibiotics has been the conventional approach for the treatment of liver abscesses. Hepatic abscesses refractory or not amenable to percutaneous drainage have been treated with surgical drainage, either via laparoscopic or open laparotomy techniques. The aim of this review was to evaluate the technical feasibility and efficacy of endoscopic ultrasound (EUS)-guided drainage of liver abscesses. A literature review was performed to identify the studies describing the technique. In this review article we have summarized case series or reports describing EUS-guided liver abscess drainage. The indications, techniques, endoprostheses, limitations and complications reported are discussed. A total of seven cases have been described so far in the literature which included patients with failed conventional treatment modalities. The EUS-guided drainage technique involves puncturing the abscess using endosonography to gain access, passing a guidewire followed by tract dilation and placement of an endoprosthesis for drainage. Studies have reported 100% technical and clinical success rates in selected cases. No complications were reported. EUS-guided drainage of liver abscesses can be a safe and effective alternative approach in the management of liver abscesses in selected patients.
Keywords: drainage, endoscopic ultrasound, liver abscess, management
Introduction
Liver abscesses are collections of infected or necrotic material in the liver parenchyma. The most common source of liver abscesses is ascending infection through the biliary tree and rarely extension of intra-abdominal processes and hematogenous spread during bacteremia. The etiology may be pyogenic, amebic, tubercular or rarely fungal [Bednarek et al. 2011; Slaughter, 2013]. Liver abscesses can be fatal if untreated. The traditional approach of management is antimicrobial therapy plus percutaneous or surgical drainage of abscesses. Surgical drainage is associated with a high mortality and morbidity rate of up to 25–30% [Zerem and Susic, 2012]. Surgery is usually indicated when there is evidence of multiple abscesses, failure of drainage by the percutaneous approach or perforated abscesses causing acute abdomen [Ferreira et al. 2011]. Currently, percutaneous drainage (PCD) is the first-line approach for the management of liver abscesses with success rates of 85–95% [Bertel et al. 1986; Gerzof et al. 1985; Onder et al. 2011; Rajak et al. 1998; Wuerz et al. 2012]. Advantages of this approach are minimal invasiveness, short hospital stays, low procedure-related morbidity and mortality, and low cost compared with surgery [Vogl and Estifan, 2001]. Limitations and complications include intraperitoneal bleeding, needle tract infection, hepatovenous fistula, sepsis, injury to surrounding vascular structures, and patient discomfort due to external drainage [Chung et al. 2003]. Dull and colleagues have reported endoscopic retrograde cholangiopancreatography-guided drainage of hepatic abscesses as an alternative [Dull et al. 2000]. The procedure is technically challenging and only suitable where there is a possibility of communication of the abscess with the biliary tree [Dull et al. 2000]. There is a need for alternative approaches of liver abscess drainage as all abscesses are not amenable to minimally invasive techniques, which lead to surgery in a significant proportion of patients. Ongoing advances in endoscopic ultrasound (EUS) techniques and accessories have led to the development of EUS-guided intraluminal drainage of collections at sites such as pancreas, gallbladder, and pelvis. Few authors have recently described successful transgastric or transduodenal drainage of liver abscesses. In the present review the indications, techniques, accessories, endoprostheses, limitations, and complications reported with EUS-guided liver abscess drainage are described.
Materials and methods
An extensive English language literature search was conducted using Pubmed, Medline, and Google to identify the peer-reviewed original and review articles using the keywords ‘endoscopic ultrasound’, ‘liver abscess’, and ‘endoluminal drainage’. Only human articles were selected. The references of pertinent studies were manually searched to identify additional relevant studies. The indications, procedural details, technical and clinical success rates, complications, and limitations were considered as part of inclusion criteria. Search results yielded mostly small sample sized retrospective studies including case reports and case series. Search results yielding mostly retrospective studies with small numbers of patients and a variety of devices used for drainage limited statistical analysis in the form of meta-analysis.
Results
Four original articles published were considered appropriate to be included in the review article. Out of these, two were case reports from Germany [Seewald et al. 2005] and Singapore [Ang et al. 2009] and the other two were case series from Korea [Noh et al. 2010] and Japan [Itoi et al. 2011]. The total number of cases was seven. All cases have been summarized in Table 1.
Table 1.
Summary of reports describing endoscopic ultrasound-guided liver abscess drainage.
| Study, location | Indications | Location of Abscess | Size (cm) | Approach | Puncture needle | Tract dilator | Endoprosthesis for drainage | Follow up |
|---|---|---|---|---|---|---|---|---|
| Seewald et al. [2005], Germany | Failed ABX therapy (1 week) | Lateral segment of left lobe | 7 × 11 | Proximal TG | 22 G | 6F Teflon outer sheath | 7F Nasoabscess catheter with two side holes at distal end | 4 weeks, 3 months and 6 months |
| Ang et al. [2009], Singapore | Failed ABX and PCD (ruptured) | Left subhepatic space collection | 10.7 × 5.7 | TG | 19 G | 10F Soehendra biliary dilator and balloon dilation | 8F and 10F × 7cm double pigtail stents | 11 days |
| Noh et al. [2010], Korea | Failed ABX and PCD | Gastrohepatic space | 5.1 × 4 | TG | 19 G | 6F and 7F biliary dilator catheter | 7F double pigtail stent | Repeat CT on 10 days and 11 months, stent removal in 2 weeks |
| Noh et al. [2010], Korea | Failed ABX and PCD | Caudate lobe of liver | 4.5 × 6 | TG | 19 G | 6F and 7F biliary dilator catheter | 7F double pigtail stent | Repeat CT in 2 weeks and 9 months, stent removal in 3 months |
| Noh et al. [2010], Korea | Failed ABX and inaccessible to PCD | Caudate lobe of liver with portacaval extension | 5.5 × 4 | TD | 19 G | 6F and 7F biliary dilator catheter | Two 7F double pigtail stents with nasocystic tube | Repeat CT in 4 weeks and 3 months, nasocystic tube and stent removal in 5 days |
| Itoi et al. [2011], Japan | Failed ABX and PCD (TB) | Between pancreas and caudate lobe of liver | 7 (multiloculated) | TD | 19 G | 6 mm balloon dilatation | 7F straight stent and 5F nasocystic catheter | Repeat CT in 5 days and 6 months, nasocystic tube removal in 2 weeks. |
| Itoi et al. [2011], Japan | Failed ABX and PCD (TB) | Caudate lobe | NA | TG | 19 G | 6 mm balloon dilatation | 7F double pigtail stent and 5F nasocystic catheter | Repeat CT in 5 days and 6 months, nasocystic tube removal in 2 weeks. |
ABX, antibiotic; PCD, percutaneous drainage; TB, tubercular; NA, not available; TG, transgastric; TD, transduodenal; G, gauge.
Indications
Usual indications for EUS-guided liver abscess drainage were failure of antibiotic therapy or PCD approach [Ang et al. 2009; Itoi et al. 2011; Noh et al. 2010; Seewald et al. 2005]. Noh and colleagues reported three cases where septic shock with failure of antibiotics and abscess inaccessible to PCD were indications in one case each [Noh et al. 2010]. Ang and colleagues reported successful EUS-guided drainage of a ruptured liver abscess after failed antibiotics and PCD [Ang et al. 2009].
Site of liver abscess
The left lobe of liver (segments two and three) and the caudate lobe usually lie in close proximity to the stomach or duodenum. These segments are likely to be accessible transluminally using EUS. Seewald and colleagues described drainage of liver abscesses located in the lateral segment of the left lobe of liver [Seewald et al. 2005]. Ang and colleagues reported drainage of collection in the subhepatic space after ruptured liver abscess [Ang et al. 2009]. Noh and colleagues reported three cases with two abscesses located in the caudate lobe of the liver, one with extension into the portal and inferior vena cava, and a third abscess located in the gastrohepatic space [Noh et al. 2010]. Itoi and colleagues described drainage of two different tuberculous abscesses in the same patient at different locations, one in the caudate lobe of liver and the other between the caudate lobe and the pancreas [Itoi et al. 2011].
Size of liver abscess
The size of abscesses ranged from 5 cm × 4 cm to 11 cm × 7 cm [Ang et al. 2009; Itoi et al. 2011; Noh et al. 2010; Seewald et al. 2005] (see Table 1).
Etiology
Of seven patients, five had a pyogenic abscess and two had a tubercular abscess [Ang et al. 2009; Itoi et al. 2011; Noh et al. 2010; Seewald et al. 2005].
Technique
Echoendoscope
Therapeutic linear array echoendoscopes with 2.8 mm diameter accessory channels that allow the passage of an endoprosthesis have been used by most endoscopists. Carbon dioxide (CO2) insufflation during endoscopy can potentially limit post-procedure abdominal discomfort and bowel remnant gas volume and is widely used at most centers performing EUS-guided drainage procedures.
Endoluminal puncture site
CT scan or alternative high-quality imaging prior to procedure is helpful in determining a guide map. A transgastric approach via the antral wall has been the preferred site reported for puncture because of proximity to the liver [Ang et al. 2009; Itoi et al. 2011; Noh et al. 2010; Seewald et al. 2005]. A transduodenal approach was successful for two cases with the abscess located in the caudate lobe of the liver [Itoi et al. 2011; Noh et al. 2010]. The decision regarding the puncture site should be individualized depending on the location of the abscess; where the abscess lies closest to the luminal wall with no intervening vascular structures assessed by color Doppler ultrasonography.
Puncture needle
Seewald and colleagues was the first reported case of using a 22-gauge needle with 6F Teflon outer sheath [Seewald et al. 2005] for initial puncture. In all other cases 19-gauge EUS fine-needle aspiration (FNA) needle was used [Ang et al. 2009; Itoi et al. 2011; Noh et al. 2010]. No preference in the puncture location on the abscess was reported by any author (Figure 1A).
Figure 1.
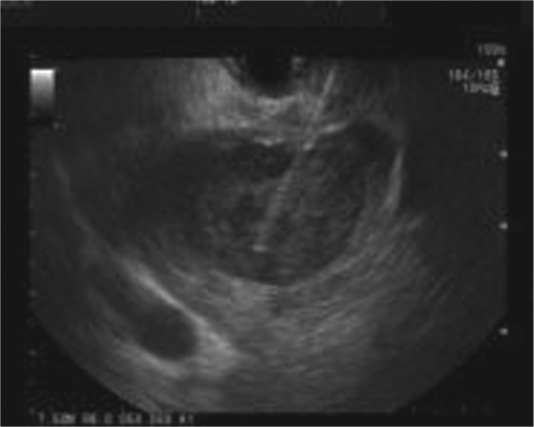
Endoscopic ultrasound image of a liver abscess with a puncture needle in the abscess cavity.
Tract dilator
After gaining access to the abscess cavity with a needle and confirming the position of the needle by fluoroscopy, a 0.035-inch guidewire is advanced to coil into the abscess cavity under EUS and fluoroscopy guidance (Figure 2). It is generally recommended to make at least two loops in the abscess cavity to make easy and safe passage of the catheter and to ensure stability of the guidewire. If initial puncture is made by a needle with outer Teflon sheath then no further tract dilation is needed [Seewald et al. 2005]. The endoscopist’s preference of either 6F or 10F biliary dilators or 6 mm biliary balloons either alone or sequentially has been used to achieve effective dilation with no difference in technical success rates (Figure 2).
Figure 2.
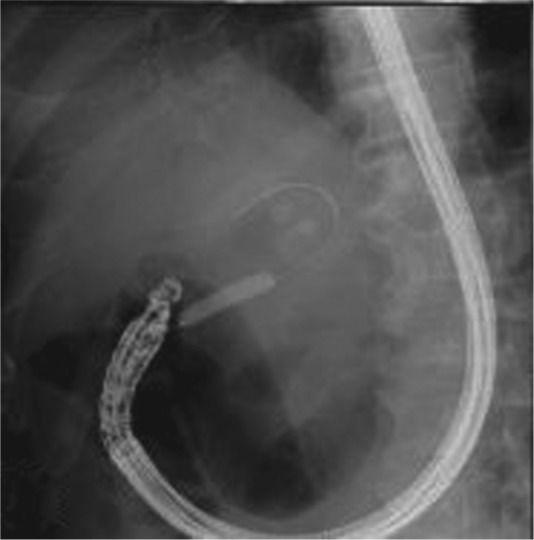
Fluoroscopic image showing 0.035-inch guidewire looped inside the abscess cavity with 8 mm biliary balloon dilators.
Endoprosthesis
After tract dilation, an endoprosthesis is placed into the abscess cavity for drainage. Most endoscopists have used 7F double pigtail plastic stents for drainage [Ang et al. 2009; Itoi et al. 2011; Noh et al. 2010; Seewald et al. 2005] (Figure 3). In most of the cases a single stent was used, while Ang and colleagues and Noh and coworkers used two pigtail stents possibly because of the larger abscesses involved [Ang et al. 2009; Noh et al. 2010]. Double pigtail stents are less likely to migrate and seem to be the preferred stents in most reports, while only one report (by Ito and colleagues) described the use of straight stents for drainage of multilocular tubercular abscess where the placement of pigtail stents might be challenging [Itoi et al. 2011]. Nasocystic catheters can provide a means for lavage and confirm continued drainage from abscesses by monitoring output (Figure 4). Clinical significance of using nasocystic drains is uncertain. Success rates have been similar in case reports with or without nasocystic drain [Ang et al. 2009; Noh et al. 2010; Seewald et al. 2005].
Figure 3.
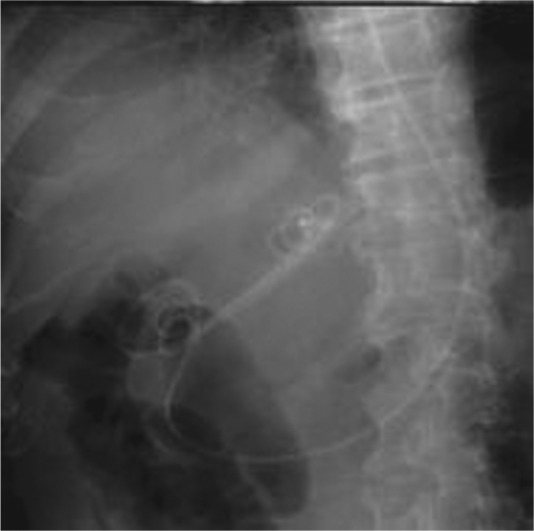
Fluoroscopic image showing double pigtail stents and nasocystic catheter in place.
Figure 4.
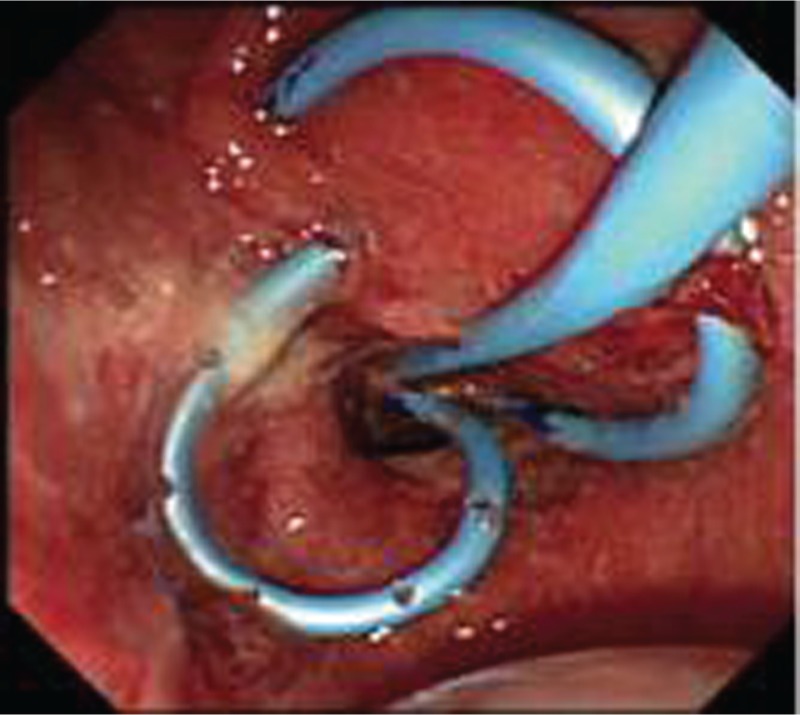
Endoscopic view of two double pigtail stents and nasocystic tube.
(Reproduced from Noh et al. [2010] with permission from Elsevier.)
Technical and clinical success rate
The combined technical and clinical success rate was 100% with improvement in the patient’s clinical condition and laboratory parameters. The success rates are unlikely to represent true clinical success rates in practice as unsuccessful cases are unlikely to be reported.
Complications
There were no procedural-related complications or failure reported.
Follow up
Most authors reported the use of CT scan with contrast for a follow up of 5 days to 4 weeks after EUS-guided drainage. Monitoring clinical signs and symptoms such as fever, abdominal pain, or high white blood cell count (WBC) should increase suspicions for failed drainage or re-accumulation of abscess. Repeat CT scans might be useful to guide timing of endoprosthesis removal. A delayed CT scan at 6 months might be helpful to document resolution of abscess.
Summary and future directions
EUS-guided transluminal liver abscess drainage can be a safe and minimally invasive alternative approach to surgery in selected patients who fail PCD. With present techniques and accessories, drainage of abscesses in the left and caudate lobes of the liver has been shown to be successful. Preliminary reports appear promising and large multicentric prospective studies are needed in the future to adopt it as a standard primary or alternative therapy. Inevitably, when a new procedure is attempted, there is a publication bias; only cases that are technically and clinically successful are published. With further experience and the development of more sophisticated accessories, the arena of EUS-guided drainage is likely to expand to other areas of the liver and other abdominopelvic organs. The technique has a potential in assisting in diagnosis by aspiration or biopsy of hepatic lesions as well as directed injection or placement of endoprosthesis for delivery of therapeutic agents into hepatic lesions.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: None of the authors have any conflicts of interest or financial relationships with the company that produces or distributes the device described in the review article.
Contributor Information
Shashideep Singhal, Division of Digestive and Liver Diseases, Columbia University Medical Center, 5141 Broadway, New York, NY 10034, USA.
Kinesh Changela, Division of Gastroenterology, Department of Medicine, The Brooklyn Hospital Center, Brooklyn, New York, USA.
Devin Lane, Division of Gastroenterology, Department of Medicine, The Brooklyn Hospital Center, Brooklyn, New York, USA.
Sury Anand, Division of Gastroenterology, Department of Medicine, The Brooklyn Hospital Center, Brooklyn, New York, USA.
Sushil Duddempudi, Division of Gastroenterology, Department of Medicine, The Brooklyn Hospital Center, Brooklyn, New York, USA.
References
- Ang T., Seewald S., Teo E., Fock K., Soehendra N. (2009) EUS-guided drainage of ruptured liver abscess. Endoscopy 41(Suppl. 2): E21-E22 [DOI] [PubMed] [Google Scholar]
- Bednarek M., Budzynski P., Drozdz W., Kedzierska J. (2011) [Use of the percutaneous drainage in the treatment of patients with hepatic abscesses]. Przegl Lek 68: 303-306 [PubMed] [Google Scholar]
- Bertel C., van Heerden J., Sheedy P., II (1986) Treatment of pyogenic hepatic abscesses. Surgical vs percutaneous drainage. Arch Surg 121: 554-558 [DOI] [PubMed] [Google Scholar]
- Chung Y., Tay K., Stan B., Htoo A., Thng C., Chow P., et al. (2003) Percutaneous drainage of liver abscess complicated by hepato-venous fistula. Singapore Med J 44: 299-301 [PubMed] [Google Scholar]
- Dull J., Topa L., Balgha V., Pap A. (2000) Non-surgical treatment of biliary liver abscesses: efficacy of endoscopic drainage and local antibiotic lavage with nasobiliary catheter. Gastrointest Endosc 51: 55-59 [DOI] [PubMed] [Google Scholar]
- Ferreira J., Abreu M., Rodrigues P., Carvalho L., Correia J. (2011) [Methicillin resistant Staphylococcus aureus and liver abscess: a retrospective analysis of 117 patients]. Acta Med Port 24(Suppl. 2): 399-406 [PubMed] [Google Scholar]
- Gerzof S., Johnson W., Robbins A., Nabseth D. (1985) Intrahepatic pyogenic abscesses: treatment by percutaneous drainage. Am J Surg 149: 487-494 [DOI] [PubMed] [Google Scholar]
- Itoi T., Ang T., Seewald S., Tsuji S., Kurihara T., Tanaka R., et al. (2011) Endoscopic ultrasonography-guided drainage for tuberculous liver abscess drainage. Dig Endosc 23(Suppl. 1): 158-161 [DOI] [PubMed] [Google Scholar]
- Noh S., Park do H., Kim Y., Chun Y., Lee H., Lee S., et al. (2010) EUS-guided drainage of hepatic abscesses not accessible to percutaneous drainage (with videos). Gastrointest Endosc 71: 1314-1319 [DOI] [PubMed] [Google Scholar]
- Onder A., Kapan M., Boyuk A., Gumus M., Tekbas G., Girgin S., et al. (2011) Surgical management of pyogenic liver abscess. Eur Rev Med Pharmacol Sci 15: 1182-1186 [PubMed] [Google Scholar]
- Rajak C., Gupta S., Jain S., Chawla Y., Gulati M., Suri S. (1998) Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR Am J Roentgenol 170: 1035-1039 [DOI] [PubMed] [Google Scholar]
- Seewald S., Imazu H., Omar S., Groth S., Seitz U., Brand B., et al. (2005) EUS-guided drainage of hepatic abscess. Gastrointest Endosc 61: 495-498 [DOI] [PubMed] [Google Scholar]
- Slaughter M. (2013) Use of percutaneous drainage for treatment of pyogenic liver abscess. JAAPA 26: 43-46 [DOI] [PubMed] [Google Scholar]
- Vogl T., Estifan F. (2001) [Pyogenic liver abscess: interventional versus surgical therapy: technique, results and indications]. Rofo 173: 663-667 [DOI] [PubMed] [Google Scholar]
- Wuerz T., Kane J., Boggild A., Krajden S., Keystone J., Fuksa M., et al. (2012) A review of amoebic liver abscess for clinicians in a nonendemic setting. Can J Gastroenterol 26: 729-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerem E., Susic A. (2012) Multiple pyogenic liver abscesses formed after appendectomy: the role of percutaneous drainage in a critically ill patient. Acta Med Acad 41: 210-213 [DOI] [PubMed] [Google Scholar]


