Abstract
Fusogenic membrane glycoproteins (FMGs) may enhance the cytotoxicity of conditionally replicative adenoviruses. However, expression at early stages of infection impairs virus replication. We have inserted the hyperfusogenic form of the gibbon ape leukemia virus (GALV) envelope glycoprotein as a new splice unit of the major late promoter (MLP) to generate a replication-competent adenovirus expressing this protein. At high multiplicity of infection (MOI), this virus replicated efficiently forming clumps of fused cells and showing a faster release. In contrast, at low MOI, infected cells formed syncytia where only one nucleus contained virus DNA, decreasing total virus production but increasing cytotoxicity.
Keywords: replicative adenovirus, syncytia, fusogenic membrane glycoproteins
Conditional replicative adenoviruses hold great promise for treating malignant solid tumors, because of their ability to replicate selectively within the tumor and kill neighboring cancer cells upon tumor cell lysis and secondary infection.1 However, the oncolytic activity of these viruses needs to be improved substantially for effective cancer treatment.2,3 One possible method to enhance the efficacy of replicating adenovirus is arming the viruses with therapeutic transgenes.4–7 In this respect, it has been previously shown that expression of FMGs efficiently kills tumoral cells by inducing cell–cell fusion and massive syncytia formation.8–11 Various FMGs have been evaluated as a means to increase the potency of oncolytic viruses.4,6,12–16 Expression of the HIV gp120 FMG by a replicative adenovirus enhanced viral release and facilitated dispersion of virus particles through a culture of tumor cells in vitro.4 In addition, a more recent report demonstrated that coinjection of plasmid DNA encoding the hyperfusogenic form of the GALV envelope glycoprotein significantly enhanced the in vivo efficacy of the adenovirus therapy.6 However, despite the interest on generating a replicative adenovirus expressing the GALV glycoprotein, previous attempts to produce adenoviral vectors encoding the GALV fusogenic protein have proven difficult, possibly due to premature cell fusion precluding virus replication. To overcome this problem the use of the modified human HSP70b promoter, a hyperthermia responsive promoter,17 or the use of metalloproteinase-cleavable linkers to target the cytotoxicity of GALV-expressing adenoviral vectors against gliomas18 has been reported. Here, we address this problem expressing GALV under the MLP. Further, GALV expression under the control of the MLP in a conditionally replicative adenovirus is desirable to prevent GALV expression in normal cells and uncontrolled induction of syncytia formation, because GALV receptor Pit-1 is ubiquitously expressed in human cells. In this paper, we describe a new replication-competent adenovirus that expresses the GALV glycoprotein in the late stage of virus replication. We have used this virus to evaluate how fusogenesis affects adenovirus production and cytotoxicity for future application in conditionally replicative adenoviruses.
Generation and characterization of AdwtRGD-GALV, a replicative adenovirus expressing the GALV FMG
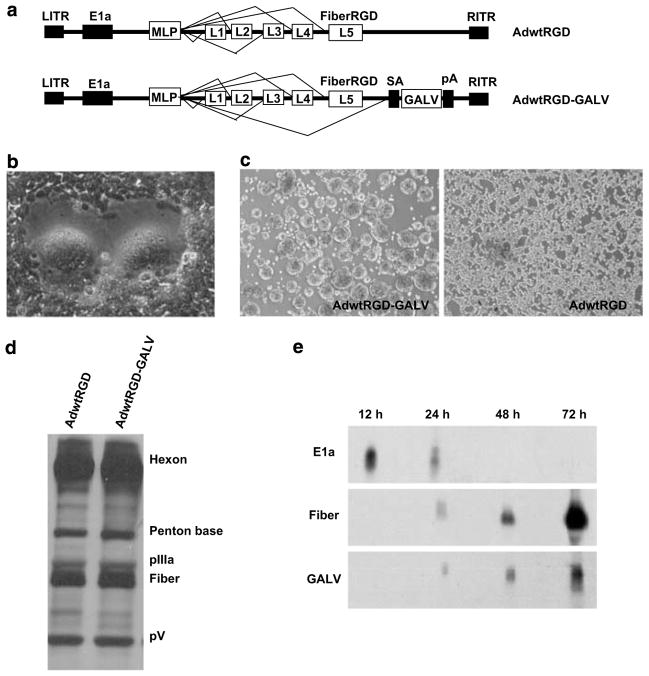
When dealing with replication-competent adenoviruses, it is desirable to express cytotoxic transgenes late in the viral life cycle to avoid early cell death.5 Therefore, we cloned the coding sequence of the hyperfusogenic form of the GALV glycoprotein in the AdwtRGD genome under the control of the MLP. The adenovirus IIIa splicing acceptor was placed upstream of the GALV cDNA, and a polyA site downstream. This cassette was placed directly downstream of the fiber gene, as an L6 unit (Figure 1a). This strategy has been used previously7,19 and cloning strategies are available upon request. Transfection of the AdwtRGD-GALV genome into 293 cells led to massive syncytia formation (Figure 1b) and virus generation. The new fusogenic adenovirus, AdwtRGD-GALV, and the control virus, AdwtRGD,20 were efficiently propagated in 293 cells at high MOI. Whereas AdwtRGD induced a typical cytopathic effect characterized by cell rounding, swelling and loss of cell–cell contact, cells infected at high MOI with AdwtRGD-GALV formed large spherical clumps of fused cells (Figure 1c). The transducing units per ml of each cell extract were determined by α-hexon staining-based method,21 and high-titer stocks were obtained for both viruses: 625 TU per cell for AdwtRGD-GALV and 486 TU per cell for AdwtRGD, indicating that GALV late expression was not impairing effective virus production. Because it was possible that the insertion of the GALV cassette downstream of the fiber gene reduced fiber expression, protein content of purified adenoviral vectors was confirmed by silver nitrate staining. Figure 1d shows that no differences in capsid composition were detected. To confirm the late expression of the GALV gene, A549 cells were infected at MOI of 10 with AdwtRGD-GALV. Total RNA from cell lysates was isolated at 12, 24, 48 and 72 h post infection, and was subjected to northern blot analysis with specific probes for E1a, Fiber and GALV RNAs. As shown in Figure 1e, E1a RNA was detected early in viral replication, whereas GALV RNA was expressed in the late phase, reproducing the pattern of fiber RNA.
Figure 1.
Generation and characterization of AdwtRGD-GALV. (a) Schematic representation of viruses used in this study. The AdwtRGD genome encodes a fiber protein with the RGD peptide inserted in the HI loop of the knob. To drive GALV expression in AdwtRGD-GALV from the MLP, an expression cassette consisting of a splice acceptor (SA) in front of the GALV cDNA and a polyA sequence (pA) is inserted downstream of the fiber in the AdwtRGD genome. The total length of the inserted GALV-splice cassette is 2053 pb. (b) Cell morphology upon transfection of AdwtRGD-GALV genome. 293 cells were transfected with linearized plasmid containing AdwtRGD-GALV genome. Extensive syncytia formation was observed 5 days after transfection. (c) Characterization of cytophatic effects induced by infection of 293 cells with high MOI of AdwtRGD-GALV and AdwtRGD. (d) Protein content of purified adenoviruses. Adenoviral vectors were purified by CsCl gradients and dialyzed in PBS/Cl/Mg (PBS++) with 5% glycerol, and the particle titer was determined by OD. About 5 × 1010 vp of purified virions were boiled and separated by SDS-polyacrylamide gel electrophoresis and then stained with silver nitrate. (e) Time course of E1a, Fiber and GALV RNA expression from AdwtRGD-GALV. A549 cells were infected with AdwtRGD-GALV at MOI of 10 and total RNA was isolated at indicated time points. Extracted RNA were subjected to northern blot analysis with specific probes for E1a, Fiber and GALV. GALV, gibbon ape leukemia virus; MLP, major late promoter; MOI, multiplicity of infection; OD, optical density; PBS, phosphate-buffered saline.
Virus replication
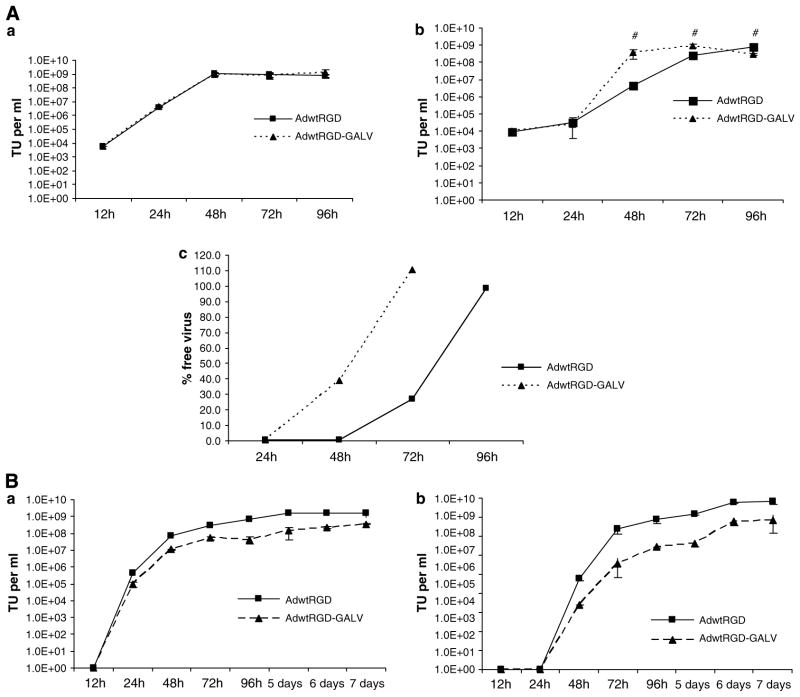
To determine whether the insertion of the GALV transgene in the adenovirus genome and syncytium formation is detrimental to virus replication, we compared the one-step growth curves of AdwtRGD-GALV and AdwtRGD. A549 cells were infected at MOI of 10 with AdwtRGD and AdwtRGD-GALV, and the amount of virus in the supernatant and cell extracts was determined by α-hexon staining at various time points after infection. As shown in Figure 2Aa, total virus produced of both viruses was very similar, reaching approximately the same titer at the same time points. Interestingly, the amount of AdwtRGD-GALV released into the medium at 48 h post infection was almost 2-log higher than that of AdwtRGD, indicating a greater adenoviral release in presence of syncytia at high MOI (Figure 2Ab). A representation of the ratio of virus released to total virus shows that at 48 h post infection almost 40% of the AdwtRGD-GALV produced is released to the supernatant in contrast to less than 0.5% of the AdwtRGD (Figure 2Ac). At 72 h after infection, whereas more than 70% of AdwtRGD remained cell associated, all AdwtRGD-GALV was released. Next, we performed a multistep growth curve by infecting A549 at MOI of 0.1. Supernatants and cell extracts were harvested at 24-h intervals up to day 7 after infection. Large syncytia formed around infected cells and AdwtRGD-GALV viral titers were significantly lower than that of the AdwtRGD at all time points (Figures 2Ba and 2Bb). Given a production impairment of AdwtRGD-GALV, one would expect a progressive divergence of titers with time. Initially, up to 72 and 96 h, there is such a clear divergence in the titers in supernatants and cell extracts, respectively. Later, the lack of divergence is not obvious and it may be explained by a saturating (plateau) level of AdwtRGD virus production. In summary, at high MOI, the fusogenic virus is produced as efficiently as AdwtRGD, but, at low MOI, the expression of the fusogenic protein decreases the amount of virus produced.
Figure 2.
Virus yield after infection with AdwtRGD-GALV and AdwtRGD. (A) Viral replication at high MOI. Confluent A549 cells were infected with AdwtRGD-GALV and AdwtRGD at MOI of 10. Four hours post infection, viral solution was removed and cell cultures where washed with PBS and incubated with 1 ml of fresh virus-free medium. Cell extracts (a) and supernatants (b) were harvested 12, 24, 48, 72 and 96 h after infection and titrated by α-hexon staining. Virus found at 12 h post infection correspond to the input virus. #P<0.05. (c) Graphic representation of the ratio of free virus released to the medium to total virus produced. (B) Viral replication at low MOI. Confluent A549 cells were infected with AdwtRGD-GALV and AdwtRGD at MOI of 0.1 as in (A). Cells extracts (a) and supernatants (b) were harvested at 24-h intervals up to day 7 after infection. Input virus at 12 h post infection was not detectable. All comparisons showed a significant advantage in replication for AdwtRGD. P<0.05. GALV, gibbon ape leukemia virus; MOI, multiplicity of infection.
Viral replication in nuclei within syncytia
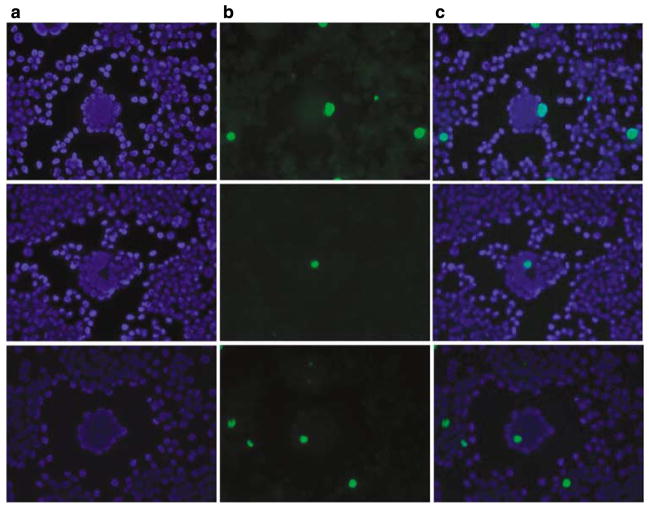
The low virus production in the presence of syncytia that arise at low MOI is contrasted with previous suggestions indicating that adenoviruses spread between the nucleus of a syncytium, thereby enhancing replication and increasing the amount of virus released.4,6 However, a more recent report indicated that adenovirus DNA does not normally undergo internuclear spreading between nuclei within a syncytium, suggesting that efficient adenovirus replication cannot occur without extracellular particle spread.22 As a result, most nuclei within a syncytium would die without producing new viral particles, limiting the total virus production. To test this, human melanoma cells, SKMel28, were infected at low MOI with AdwtRGD-GALV, and viral DNA was detected by fluorescent in situ hybridization 72 h after infection using AdwtRGD-GALV viral DNA stained by nick-translation as a probe.23 Nuclei were detected by DAPI (4′,6′-diamidino-2-phenylindole) staining. As shown in Figure 3, the presence of viral DNA was only detected in one nucleus within a syncytium, in almost all the syncytia evaluated, indicating that fusogenic adenoviruses cannot efficiently spread between nuclei within a syncytium. This lack of viral DNA spreading among the nuclei within the syncytia would explain the lower virus yields when infecting at low MOI, and suggest that syncytia formation does not directly interfere with adenovirus replication.
Figure 3.
Analysis of viral DNA ability to spread between nuclei within a syncytium. Confluent SKMel28 cells were infected at MOI of 0.5 with AdwtRGD-GALV. Three days after infection, cells were fixed and viral DNA was detected by fluorescent in situ hybridization. The total DNA was stained with DAPI (a) and the viral DNA was detected with a viral DNA probe stained by nick-translation (b). (c) DAPI and viral DNA patterns overlapping are shown. DAPI, 4′,6′-diamidino-2-phenylindole; GALV, gibbon ape leukemia virus; MOI, multiplicity of infection.
Comparison of tumor cell killing in vitro
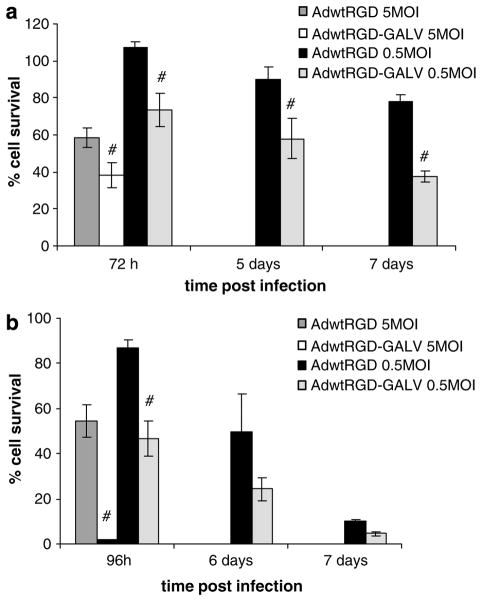
Despite the observed reduced virus yields, the cytotoxicity induced by a replication-competent adenovirus expressing a fusogenic protein, even at low MOI, could be enhanced compared with a non-fusogenic counterpart. To determine whether late GALV expression would enhance tumor cell killing, we infected SKMel28 cells with AdwtRGD and AdwtRGD-GALV at MOI of 5 or 0.5. Cells were harvested 72 h, 5 and 7 days after infection through trypsinization, and the number of viable cells was counted with a hemocytometer after Trypan blue staining. At MOI of 5, AdwtRGD-GALV killed more than 60% of tumor cells within 72 h, a time point where less than 45% of AdwtRGD-infected cells were dead. At MOI of 0.5, AdwtRGD-GALV reduced cell viability to less than 60% within 5 days, and at this same day most tumor cells infected with AdwtRGD were viable (Figure 4a). Differences at day 7 after infection were still significant. The degree of tumor cell killing appeared to correlate with the extent of syncytia formation. To test this further, A549 and pancreatic tumor cells (NP9) were infected in a similar manner. Both cells were killed more effectively by AdwtRGD-GALV compared with AdwtRGD. Interestingly, at MOI of 0.7, AdwtRGD-GALV killed all A549 cells within 96 h, a time point where more than 50% of the AdwtRGD-infected cells were still viable. At MOI of 0.05, AdwtRGD-GALV reduced cell viability to less than 50% within 96 h while at the same time most of tumor cells infected with AdwtRGD were viable (Figure 4b). These results indicate that the expression of GALV protein greatly enhances AdwtRGD cytotoxicity.
Figure 4.
Comparison of cell killing of AdwtRGD-GALV and AdwtRGD in tumoral cells. (a) Confluent SKMel28 were infected with MOIs of 5 and 0.5 of AdwtRGD-GALV and AdwtRGD, respectively, or remained uninfected. (b) Confluent A549 were infected with MOIs of 0.7 and 0.05 of AdwtRGD-GALV and AdwtRGD, respectively, or remained uninfected. At indicated time points, cells were collected, stained with Trypan blue and counted. The percentage of cell viability was determined by dividing the number of viable cells from the infected well by the number of cells from an uninfected well. Data are expressed as means±standard errors of triplicates experiments. #P<0.02. GALV, gibbon ape leukemia virus; MOI, multiplicity of infection.
Collectively, our data demonstrate that expression of GALV under the MLP allows the generation of viable replication-competent adenoviruses. These fusogenic viruses are characterized by an enhanced cell killing despite detrimental effects on virus production from syncytia. These results warrant the construction of tumor-selective oncolytic adenoviruses using this strategy and have implications when considering parameters such as virus yield or cytotoxicity with those viruses.
Acknowledgments
We thank Margarita Nadal, Eduard Serra and Jian Qiao for technical assistance. S Guedan was supported by a predoctoral fellowship (FI) granted by the Generalitat de Catalunya. AG was supported by a graduate student fellowship from Oncolytics Biotech. This work was supported by a grant from the Spanish Ministry of Education and Science, BIO2005-08682-C03-02/01 and received partial support from the Generalitat de Catalunya SGR0500008 and 200556R00066, and the Theradpox contract LSHB-CT-2005-018700 from the European Commission. RV is supported by the NIH Grant RO1CA085931.
References
- 1.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 2.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 3.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neurooncol. 2003;65:237–246. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Haviv YS, Derdeyn CA, Lam J, Coolidge C, Hunter E, et al. Human immunodeficiency virus type 1-mediated syncytium formation is compatible with adenovirus replication and facilitates efficient dispersion of viral gene products and de novo-synthesized virus particles. Hum Gene Ther. 2001;12:2155–2165. doi: 10.1089/10430340152710504. [DOI] [PubMed] [Google Scholar]
- 5.Sauthoff H, Pipiya T, Heitner S, Chen S, Norman RG, Rom WN, et al. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum Gene Ther. 2002;13:1859–1871. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Jevremovic D, Suzuki K, Kottke T, Thompson J, Emery S, et al. Intratumoral expression of a fusogenic membrane glycoprotein enhances the efficacy of replicating adenovirus therapy. Gene Therapy. 2003;10:1663–1671. doi: 10.1038/sj.gt.3302064. [DOI] [PubMed] [Google Scholar]
- 7.Cascante A, Abate-Daga D, Garcia-Rodriguez L, Gonzalez JR, Alemany R, Fillat C. GCV modulates the antitumoural efficacy of a replicative adenovirus expressing the Tat8-TK as a late gene in a pancreatic tumour model. Gene Therapy. 2007;14:1471–1480. doi: 10.1038/sj.gt.3303008. [DOI] [PubMed] [Google Scholar]
- 8.Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset FL, et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- 9.Fielding AK, Chapel-Fernandes S, Chadwick MP, Bullough FJ, Cosset FL, Russell SJ. A hyperfusogenic gibbon ape leukemia envelope glycoprotein: targeting of a cytotoxic gene by ligand display. Hum Gene Ther. 2000;11:817–826. doi: 10.1089/10430340050015437. [DOI] [PubMed] [Google Scholar]
- 10.Bateman AR, Harrington KJ, Kottke T, Ahmed A, Melcher AA, Gough MJ, et al. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 2002;62:6566–6578. [PubMed] [Google Scholar]
- 11.Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell–tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- 12.Fu X, Tao L, Jin A, Vile R, Brenner MK, Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol Ther. 2003;7:748–754. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 13.Nakamori M, Fu X, Meng F, Jin A, Tao L, Bast RC, Jr, et al. Effective therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane fusion mechanisms. Clin Cancer Res. 2003;9:2727–2733. [PubMed] [Google Scholar]
- 14.Ebert O, Shinozaki K, Kournioti C, Park MS, Garcia-Sastre A, Woo SL. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 2004;64:3265–3270. doi: 10.1158/0008-5472.can-03-3753. [DOI] [PubMed] [Google Scholar]
- 15.Simpson GR, Han Z, Liu B, Wang Y, Campbell G, Coffin RS. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 2006;66:4835–4842. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Trevino A, Castel S, Lopez-Iglesias C, Cortadellas N, Comas-Riu J, Mercade E. Effects of adenovirus-mediated SV5 fusogenic glycoprotein expression on tumor cells. J Gene Med. 2003;5:483–492. doi: 10.1002/jgm.371. [DOI] [PubMed] [Google Scholar]
- 17.Brade AM, Szmitko P, Ngo D, Liu FF, Klamut HJ. Heat-directed tumor cell fusion. Hum Gene Ther. 2003;14:447–461. doi: 10.1089/104303403321467216. [DOI] [PubMed] [Google Scholar]
- 18.Allen C, McDonald C, Giannini C, Peng KW, Rosales G, Russell SJ, et al. Adenoviral vectors expressing fusogenic membrane glycoproteins activated via matrix metalloproteinase cleavable linkers have significant antitumor potential in the gene therapy of gliomas. J Gene Med. 2004;6:1216–1227. doi: 10.1002/jgm.616. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Castro J, Martinez-Palacio J, Lillo R, Garcia-Sanchez F, Alemany R, Madero L, et al. Tumor cells as cellular vehicles to deliver gene therapies to metastatic tumors. Cancer Gene Ther. 2005;12:341–349. doi: 10.1038/sj.cgt.7700801. [DOI] [PubMed] [Google Scholar]
- 20.Cascallo M, Alonso MM, Rojas JJ, Perez-Gimenez A, Fueyo J, Alemany R. Systemic toxicity–efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 21.Majem M, Cascallo M, Bayo-Puxan N, Mesia R, Germa JR, Alemany R. Control of E1A under an E2F-1 promoter insulated with the myotonic dystrophy locus insulator reduces the toxicity of oncolytic adenovirus Ad-Delta24RGD. Cancer Gene Ther. 2006;13:696–705. doi: 10.1038/sj.cgt.7700940. [DOI] [PubMed] [Google Scholar]
- 22.Horn GP, Vongpunsawad S, Kornmann E, Fritz B, Dittmer DP, Cattaneo R, et al. Enhanced cytotoxicity without internuclear spread of adenovirus upon cell fusion by measles virus glycoproteins. J Virol. 2005;79:1911–1917. doi: 10.1128/JVI.79.3.1911-1917.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadal M, Pera G, Pujadas J, Abril J, Gonzalez L, Aguilo F, et al. Aneuploidy of chromosome Y in prostate tumors and seminal vesicles: a possible sign of aging rather than an indicator of carcinogenesis? Mol Carcinog. 2007;46:543–552. doi: 10.1002/mc.20301. [DOI] [PubMed] [Google Scholar]