Abstract
Reducing the nicotine content in tobacco products is being considered by the FDA as a policy to reduce the addictiveness of tobacco products. Understanding individual differences in response to nicotine reduction will be critical to developing safe and effective policy. Animal and human research demonstrating sex differences in the reinforcing effects of nicotine suggests that males and females may respond differently to nicotine-reduction policies. However, no studies have directly examined sex differences in the effects of nicotine unit-dose reduction on nicotine self-administration (NSA) in animals. The purpose of the present study was to examine this issue in a rodent self-administration model. Male and female rats were trained to self-administer nicotine (0.06 mg/kg) under an FR 3 schedule during daily 23 h sessions. Rats were then exposed to saline extinction and reacquisition of NSA, followed by weekly reductions in the unit dose (0.03 to 0.00025 mg/kg) until extinction levels of responding were achieved. Males and females were compared with respect to baseline levels of intake, resistance to extinction, degree of compensatory increases in responding during dose reduction, and the threshold reinforcing unit dose of nicotine. Exponential demand-curve analysis was also conducted to compare the sensitivity of males and females to increases in the unit price (FR/unit dose) of nicotine (i.e., elasticity of demand or reinforcing efficacy). Females exhibited significantly higher baseline intake and less compensation than males. However, there were no sex differences in the reinforcement threshold or elasticity of demand. Dose–response relationships were very well described by the exponential demand function (r2 values > 0.96 for individual subjects). These findings suggest that females may exhibit less compensatory smoking in response to nicotine reduction policies, even though their nicotine reinforcement threshold and elasticity of demand may not differ from males.
Keywords: Nicotine self-administration, Rat, Sex differences, Family Smoking Prevention and Tobacco, Control Act, Behavioral economics
1. Introduction
Progressive reduction of the nicotine content in tobacco products to render them non-addictive has been advocated by scientists and considered by policy makers for many years (Benowitz and Henningfield, 1994; Kessler, 1994; Henningfield et al., 2004; Hatsukami et al., 2010b). This approach has received increasing attention since the introduction of the Family Smoking Prevention and Tobacco Control Act (FSPTCA) to Congress in 2007 (http://www.govtrack.us/-congress/bills/110/hr1108). Passed in 2009, the FSPTCA provides the FDA regulatory authority over myriad aspects of tobacco products, including the performance standards of specific constituents in tobacco itself and tobacco smoke (www.gpo.gov/fdsys/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf). As such, the FDA now has the power to enforce a nicotine reduction policy as a population-wide strategy to reduce initiation of tobacco use in adolescents and promote cessation of use in current tobacco users.
Although, the public health benefits of a nicotine reduction policy could be vast (Tengs et al., 2005), there are significant knowledge gaps, conceptual issues, and ethical concerns that must be addressed to anticipate fully the feasibility and public health consequences of nicotine reduction (see Hatsukami et al., 2010b; Donny et al., 2012; Sofuoglu and LeSage, 2012; Benowitz and Henningfield, 2013 for review). For example, the threshold (i.e., lowest) nicotine content in tobacco products that engenders or maintains tobacco addiction is unknown. In addition, it is not clear to what extent a compensatory increase in smoking would be observed as cigarette nicotine content is reduced (Scherer, 1999), which is one of many potential adverse side effects of a nicotine reduction policy (Hatsukami et al., 2010b). Given that thousands of people die every week from tobacco-related disease (CDC, 2008), the FDA is in urgent need of research on these issues (see recent FOAs at http://www.fda.gov/tobaccoproducts/default.htm).
Understanding individual differences in response to nicotine reduction will be critical to anticipating the relative risk of addiction and adverse side effects (e.g., compensatory smoking) in subpopulations of smokers. Such knowledge will be vital to developing safe and effective policies, as well as clinical interventions to support those policies. For example, animal and human research suggests that there are sex differences in the addiction-related behavioral effects of nicotine. Female rats have been shown to acquire nicotine self-administration more rapidly (Donny et al., 2000; Lynch, 2009), work harder to self-administer nicotine (Donny et al., 2000; Lynch, 2009; Li et al., 2012), and exhibit greater cue-induced enhancement of nicotine self-administration (Chaudhri et al., 2005). In humans, women have shown less sensitivity to the discriminative stimulus effects of nicotine (Perkins et al., 1994), less change in subjective and reinforcing effects with changes in the nicotine content of a cigarette (Perkins et al., 1999), greater responsiveness to smoking-paired cues (Perkins, 1996), and less success in quitting smoking (for review, see Perkins, 2001). These findings suggest that males and females may respond differently to nicotine-reduction policies and may be at differential risk for side effects of such policies.
Animal research is vital to developing the science base to support FDA policy (for detailed discussion, see Donny et al., 2012). While there are a plethora of published studies in animals that have shown a dose–response relationship for nicotine’s reinforcing effects (i.e., self-administration, for review see Matta et al., 2007; Donny et al., 2012), very few have been designed in a way that models aspects of a nicotine reduction policy like that being considered by the FDA (e.g. Denoble and Mele, 2006; Smith et al., 2013). Specifically, doses have usually been manipulated between groups rather than within subjects; or in random order within subjects rather than in a strictly descending order as would be stipulated by a nicotine reduction policy. The range and number of doses has also typically been limited within a single study. Moreover, operationally defining and quantifying individual differences in the nicotine reinforcement threshold and the magnitude of compensation during repeated reductions in unit dose have not been a primary focus. Although some studies have demonstrated compensation in rats following within-subject reductions in unit dose (e.g., Corrigall and Coen, 1989; Shoaib et al., 1997; Denoble and Mele, 2006), few have specifically examined individual differences in compensation (Harris et al., 2009, 2011; Smith et al., 2013).
To our knowledge, only three studies have examined changes in NSA during progressive reductions in the unit dose (Shoaib et al., 1997; Denoble and Mele, 2006; Smith et al., 2013). None of these studies operationally defined and measured nicotine reinforcement thresholds for individual subjects, and it is not clear whether the dose range was wide enough to do so. In one study, a saline phase was not included in the repeated dose-reduction protocol to allow calculating a reinforcement threshold for maintenance of NSA in individuals (Smith et al., 2013). However, the lowest nicotine dose (0.001875 mg/kg) did not maintain infusion rates above those in a separate group of rats with access to saline, suggesting that the dose range in the progressive reduction protocol encompassed a reinforcement threshold for nicotine. Nonetheless, the nicotine solutions used in this study included a cocktail of several tobacco constituents known to alter the reinforcing effects of nicotine (Clemens et al., 2009), and the concentration of the cocktail remained constant over the course of nicotine dose reduction. While this provides vital face validity for modeling nicotine reduction policy in animals, it didn’t allow measurement of the nicotine reinforcement threshold per se and how non-nicotine constituents may have altered it.
The purpose of the present study was to examine sex differences in response to nicotine reduction using a rodent self-administration model. The study was specifically designed to model a nicotine reduction policy by arranging progressive decreases in the unit dose of nicotine to the point of extinction of self-administration in every subject. This approach is somewhat analogous to human studies that have examined progressive reduction of cigarette nicotine yield or content (e.g., Benowitz et al., 2007, 2009, 2012). However, in those studies, extinction of smoking behavior was not achieved in all subjects to allow measurement of individual reinforcement thresholds and sex differences were not examined. In the present study, the primary measures of interest, determined in individual rats, were the nicotine reinforcement threshold and magnitude of compensation.
Because changing the unit dose also changes the unit price of nicotine (response requirement/unit dose, Hursh, 1991), the present study was well suited to a behavioral economic analysis. This approach utilizes the concept of a demand curve; the function describing the consumption of a commodity (e.g., nicotine, y-axis) versus the unit price of that commodity (i.e., responses per unit dose, x-axis). Generally, a demand curve shows that as the price of a commodity increases, consumption decreases. The primary purpose of demand curve analysis is to characterize the “elasticity” of demand for a drug. Demand is inelastic if consumption declines slowly (i.e., proportionally less) as unit price increases, or elastic if consumption declines rapidly (i.e., proportionally greater) as unit price increases. Commodities for which demand is more inelastic are considered to have greater reinforcing efficacy or “essential value” (Hursh and Silberberg, 2008). In the present study, demand curve analysis provided a way to examine sex differences in the elasticity of demand for, or relative reinforcing efficacy of, nicotine in the context of a nicotine reduction model. Behavioral economics provides a conceptual and methodological framework commonly used in human laboratory, clinical, and epidemiological research (DeGrandpre et al., 1992; Emery et al., 2001; Tauras and Chaloupka, 2001; Bidwell et al., 2012; Mackillop et al., 2012a, 2012b), but seldom used in animal models of nicotine addiction (Diergaarde et al., 2011). As such, the use of a behavioral economic approach in the present study addresses an important knowledge gap in animal research and may facilitate the prediction of findings in human laboratory studies, clinical trials, and policy surveillance studies that utilize similar behavioral economic measures.
2. Methods and materials
2.1. Animals
Eight male and eight female Holtzman rats (Harlan, Indianapolis, IN) weighing 300–325 g and 225–250 g, respectively, at arrival were maintained under a restricted feeding regimen (18–20 g/day). Rats were fed daily during the 1-h interval between experimental sessions (see below). This strain was chosen to extend our previous studies that used the same strain to examine individual differences in compensatory NSA following unit dose reduction (Harris et al., 2008, 2009). Upon arrival, all rats were individually housed in a temperature- and humidity-controlled colony room with unlimited access to food and water under a reversed 12 h light/dark cycle (lights off at 11:00 h) for approximately one week. Rats were then moved to operant conditioning chambers and placed on food restriction in a separate room under the same light/dark cycle following recovery from catheter implantation for NSA (see below). Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
2.2. Apparatus
Each operant conditioning chamber (29 cm × 26 cm × 33 cm; Coulbourn Instruments, Allentown, PA) was made of aluminum and Plexiglas walls, an aluminum ceiling, and a stainless steel grid floor. Two response levers were located on the front wall 10 cm above the chamber floor on either side of an aperture for delivery of food pellets (not used in this study) located 2 cm above the floor. LED stimulus lights were located 2 cm above each response lever. Water was continuously available via a spout mounted on the back wall of the chamber. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) placed outside each cubicle delivered infusions through Tygon tubing connected to a fluid swivel mounted above the chamber, and from the swivel through a spring leash connected to a guide cannula mounted in a harness assembly on the back of the rat. MED-PC IV (Med Associates, St Albans, VT) software was used for operating the apparatus and recording data.
2.3. Drugs
Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline. The pH of the solution was adjusted to 7.4 with dilute NaOH, and heparin (30 units/ml) was added to help maintain catheter patency. Nicotine doses are expressed as the base.
2.4. Surgery
Each rat was implanted with a chronic indwelling catheter into the right jugular vein under droperidol (2 mg/kg)/fentanyl (0.04 mg/kg) anesthesia, described in detail elsewhere (LeSage et al., 2002; Harris et al., 2008). If this catheter became occluded prior to the Dose reduction protocol (see below) another catheter was implanted into the contralateral jugular or ipsilateral femoral vein. If catheter failure occurred during the dose reduction protocol, the rat was excluded from the study. The catheter was externalized between the scapulae and attached to a vascular-access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) that allowed connection to a fluid swivel via a tether for nicotine administration. Animals were allowed to recover for at least 4 days after surgery, during which time they received daily i.v. infusions of heparinized saline and ceftriaxone (5.25 mg) and s.c. injections of buprenorphine (0.1 mg/kg; first 2 days only) for analgesia. Infusions of methohexital (0.1 ml, 50 mg/ml, IV) were administered occasionally to determine catheter patency (production of ataxia) if malfunctions were suspected.
2.5. NSA training
The protocol timeline is illustrated in Fig. 1. Rats were trained to self-administer nicotine in daily 23 h sessions (11:00 am–10:00 am) according to similar protocols used for male and female rats in our laboratory (LeSage et al., 2002, 2007). This access schedule results in patterns of nicotine intake similar to those of smokers (see LeSage et al., 2002; Harris et al., 2009). Nicotine availability was signaled by illumination of the stimulus light above the active (right) response lever. Following completion of the response requirement, the stimulus light was extinguished and nicotine (0.06 mg/kg/inf) was infused in a volume of 100 μl/kg at a rate of 50 μl/s. This training dose was chosen because it lies on the descending limb of the “inverted U” NSA dose–response curve in both limited (e.g., 1 h) and unlimited access NSA models (e.g., Corrigall and Coen, 1989; Denoble and Mele, 2006), allowing measurement of compensatory increases in NSA following unit dose reduction and characterization of both ascending and descending limbs of the dose–response curve. Following a 7-s time-out, the stimulus light turned on and the next nicotine infusion was available. Responses on the other (inactive) lever were recorded but had no programmed consequences. The response requirement was initially a fixed ratio 1 (FR 1), and was gradually increased to FR 3 across several sessions. The criteria for acquisition were a minimum of 10 infusions per day under the FR 3 schedule and a ratio of active to inactive lever presses of at least 2:1 across 5 consecutive sessions. These acquisition criteria are typical for NSA under unlimited access conditions (e.g., Valentine et al., 1997; Brower et al., 2002; LeSage et al., 2003).
Fig. 1.

Illustration of the experimental protocol, showing each phase and associated unit dose available for self-administration.
2.6. Dose reduction protocol
When NSA was stable (no trend in infusion rates across at least 5 consecutive sessions and a coefficient of variation < 15%), extinction was arranged by substituting saline for nicotine for at least seven sessions until the number of infusions per day decreased to below 50% of baseline and no trend was evident for three consecutive sessions. This initial extinction period assured that rats were sensitive to changes in nicotine dose and set a target to define a non-reinforcing dose at the low-end of the dose range. This expedited completion of the protocol by avoiding unnecessary exposure to multiple non-reinforcing nicotine doses. At this point, the nicotine training dose was made available to allow reacquisition of NSA. Once NSA was reacquired and stable (same criteria as above), the unit dose reduction phase began. During the dose-reduction phase, the unit dose was reduced weekly until the number of infusions per day during the last 3 days at a given dose fell within range of the number of infusions during the last 3 days of the prior extinction phase. Therefore, the number of unit doses to which a rat was exposed differed between rats. Saline was then substituted for nicotine for one week to confirm that the last nicotine unit dose was functionally equivalent to saline (i.e., was not reinforcing). Then, the training dose was again made available to allow reacquisition of NSA to confirm catheter patency. One male and one female rat failed to reacquire NSA and all of their data were excluded from further analysis, resulting in a final sample size of seven for each group. Males and females did not significantly differ in the mean number of days to complete the entire protocol (155.1 ± 12.7 (5.5 months) in males and 179.9 ± 18.1 (6.4 months) in females, respectively). We did not include a separate group tested on the training dose throughout the protocol to control for changes (e.g., escalation) in NSA, because NSA remains stable under daily unlimited access conditions (Paterson and Markou, 2004; Kenny and Markou, 2006; O’Dell et al., 2007; Harris et al., 2008, 2009).
2.7. Data analysis
Mean lever presses on the active and inactive lever, number of infusions, and nicotine intake across the last three sessions at each unit dose and saline extinction were the primary dependent measures (Appendices A–D). Data from both the entire 23 h session and the first 2 h of the session were analyzed. The latter were used to examine whether recent food intake (i.e. satiation) might have influenced observed sex differences (see below). Unpaired t-tests were conducted to compare baseline measures at the 0.06 mg/kg unit dose between males and females. Because there was a significant sex difference in baseline NSA measures, data were also transformed to a percentage of baseline.
For the extinction phase, mean infusions were compared between males and females using a two-way mixed factor ANOVA to examine sex differences in resistance to extinction. For the dose-reduction phase, dose–response curves were compared between males and females via a Generalized Linear Mixed Model analysis using a random intercepts model with dose as a continuous variable (log scale) and cubic fitting as a model component, and post-hoc Bonferroni-corrected t-tests to compare males and females at individual unit doses. In addition, one-way Repeated Measures ANOVA (RMANOVA) followed by Dunnet’s post-hoc tests was used to examine within each sex the changes in dependent measures across unit dose. These ANOVAs did not include doses of 0.001 mg/kg and lower in males and 0.002 mg/kg and lower in females due to missing data for four males and two females at these doses. The missing data are due to these rats not receiving these doses because their responding had reduced to extinction levels at a higher dose.
A compensation index analogous to a measure of compensation used in smokers (Scherer, 1999) was calculated for each rat using the formula:
A compensation index (CI) of 0 indicates no compensation (total daily nicotine intake decreased proportionally to the reduction in unit dose), while a CI of 1.0 indicates full compensation (total daily nicotine intake was unchanged following dose reduction). Separate CIs were computed for each rat at each unit dose using the mean of the final 3 days of access to each unit dose. The CIs were compared between males and females at each unit dose via two-way mixed-factor ANOVA and post-hoc Bonferroni-corrected independent-samples t-tests.
Demand curve analysis was conducted according to the model proposed by Hursh and Silberberg (2008), using the exponential demand equation:
The dependent variable, Q, is the quantity consumed. The independent variable, C, is the cost of nicotine based on the unit price. The free parameters, Q0 and α, are estimated from the best-fit function and refer to the maximum level of consumption at zero price (i.e., level or “intensity” of demand) and the rate of change in consumption with increases in unit price, respectively. The range of the exponential function, k, is a constant specifying the range of consumption in log units. The k value is held constant across all data sets being compared (set to 2.5 in the present study) because changes in k impact the value of α. The exponential term, Q0 · C, represents the standardized price of a commodity, which corrects for variations in price due to different doses or potencies of the commodities being compared. It also serves to correct for differences in drug potency between subjects. Hursh and Silberberg (2008) posit that α is a measure of reinforcing strength or “essential value”, the degree to which a given commodity (e.g., drug) is capable of maintaining behavior under constraints of increasing price. The value of α is inversely related to reinforcing strength so that drugs that produce rapidly declining (elastic) demand curves have higher α values and lower reinforcing strength than demand curves with slower declining (inelastic) demand curves. Therefore, α served as the index of elasticity of demand for, or reinforcing efficacy of, nicotine in the present study. Other demand measures of interest included; Q0, the level of demand as described above; Omax, the maximal response output; and Pmax, the unit price (responses per unit dose) at which maximal response output occurred. Demand functions were generated using a template for GraphPad Prism software provided by the Institutes for Behavior Resources (Baltimore, MD) on their website (http://www.ibrinc.org/index.php?id=181). The demand measures for males and females were compared using independent-samples t-tests with Welch’s correction for unequal variances where appropriate.
Using both the exponential demand model and the CI allowed analysis of elasticity of demand on two levels. On a more molar level, the exponential demand analysis measures the rate of change in consumption across all unit prices, providing an aggregate measure of elasticity of demand. On a more molecular level, the CI measures the change in consumption at individual prices, which is analogous to the concept of “local elasticity” (i.e., elasticity between two adjacent prices, Mackillop et al., 2012b). Analysis of local elasticity (i.e., the CI) can reveal marked sensitivity to specific changes in price even when an aggregate measure of demand (i.e. alpha) shows relatively low overall price sensitivity. Findings at each level of analysis can have important implications for designing public policy (see Mackillop et al., 2012b).
3. Results
Fig. 2 shows the mean number of infusions earned in males and females during saline extinction, expressed as both absolute values (left panel) and as a percentage of baseline (right panel). For absolute values, females exhibited significantly higher infusion rates compared to males, with a significant main effect of sex (F(1,12) = 9.465, p < 0.01) and session (F(8,96) = 33.91, p < 0.0001). However, the lack of a significant sex × session interaction indicated changes in NSA during extinction were parallel between males and females. Consequently, there was still a significant main effect of session (F(7,84) = 29.29, p < 0.0001), but no significant main effect of sex or sex × session interaction when data were expressed as a percentage of baseline. In addition, although females tended to require an average of 4.5 more sessions than males to meet extinction criteria (8.8 ± 1.1 SEM vs 13.3 ± 3.3 SEM days, respectively), this difference was not statistically significant. However, females showed significantly greater variability in this measure compared to males (F(6,6) = 9.16, p < 0.05).
Fig. 2.
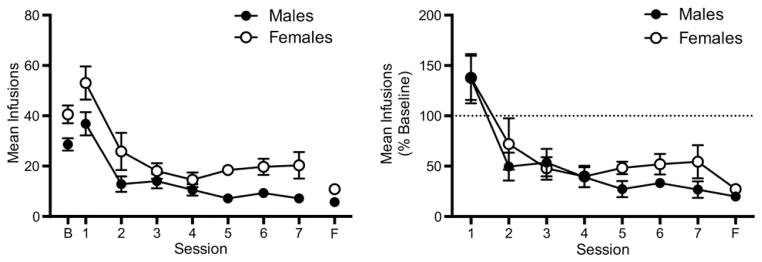
Mean (±SEM) number of infusions during saline extinction expressed as absolute values (left panel) and a percentage of the mean number of infusions earned during baseline (right panel). Each point is the mean of seven rats. Points above “B” represent the mean number of infusions during the last three sessions prior to extinction. Points above “F” represent values during the final extinction session at which extinction criteria were met.
Fig. 3 shows the mean dose–response curve for male and female rats during 23 h sessions, expressed as both absolute values (left panel) and as a percentage of baseline responding at the 0.06 mg/kg unit dose (right panel). Although baseline response rate at the 0.06 mg/kg dose was significantly higher in females compared to males (t(12) = 2.38, p < 0.05; Fig. 3, left panel), the overall dose response curves for absolute number of responses in males and females were not significantly different. Both curves exhibited an inverted U shape and a peak at the 0.01 mg/kg unit dose. The mean reinforcement threshold was also not significantly different between males (0.0032 mg/kg, range 0.004 to 0.0005 mg/kg) and females (0.0037, range 0.007 to 0.001 mg/kg). Although females consistently had somewhat higher mean rates of inactive lever pressing at unit doses above the reinforcement threshold, there were no statistically significant main effects for this measure. There were also no significant sex differences in the ratio of active/inactive lever presses (data not shown).
Fig. 3.
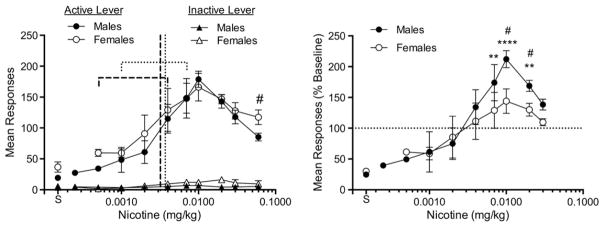
Mean (±SEM) number of responses on the active and inactive lever expressed as absolute values (left panel) and a percentage of baseline responding at the 0.06 mg/kg unit dose (right panel) during the course of progressive unit dose reduction. Each point is the mean of seven rats. Points above “S” represent the mean number of infusions during the last three sessions of saline extinction, prior to the dose reduction phase. Vertical and horizontal dotted lines represent the mean and range of the reinforcement threshold dose, respectively, in male rats. Vertical and horizontal dashed lines represent the same measures in female rats. Raw data are provided in Appendices A and D. Significantly different from males, #p < 0.05. Significantly different from baseline, **p < 0.01, ****p < 0.0001.
When data were transformed to a percentage of baseline (Fig. 3, right panel), there was a significant effect of sex (F(2,71.3) = 10.76, p < 0.0001) and significant linear (F(2,71.2) = 11.69, p < 0.0001), quadratic (F(2,71.3) = 10.15, p < 0.0001) and cubic (F(2,71.3) = 8.02, p < 0.001) sex × dose interactions. The peak and descending limb of the dose response curve was shifted upward in males compared to females, with males showing significant compensatory increases in NSA at the 0.02 to 0.007 mg/kg unit doses compared to baseline (q(36) = 3.86, p < 0.01; q(36) = 5.74, p < 0.001; q(36) = 3.55, p < 0.01, respectively). These increases were greater than those observed in females at the 0.02 and 0.01 mg/kg unit doses (t(12) = 2.79, p < 0.05 and t(12) = 2.81, p < 0.05, respectively). In fact, females as a group showed no significant increase in NSA from baseline at any dose. There were no sex differences at doses along the ascending limb of the unit dose–response curve (i.e., below 0.01 mg/kg).
Fig. 4 shows dose response curves for individual subjects. The curves for the majority of male rats were generally similar in shape to the group curve (Fig. 4, left panel), but the curves for females were somewhat skewed toward lower unit doses compared to the group curve. Although females as a group showed no significant increase in NSA, some females did exhibit substantial increases in NSA (Fig. 4, right pane) that were comparable in magnitude to males. However, such increases appeared slower to develop in females (see below). For instance, all but one male showed an increase in NSA at the 0.03 mg/kg unit dose, whereas several females showed little or no change at this unit dose. In addition, there appeared to be two distinct subgroups of females, those in which doses just below 0.01 mg/kg (primarily 0.004 to 0.007 mg/kg) sustained high rates of responding versus those in which responding had extinguished at those doses. Consequently, variability in the unit dose at which the peak of the dose–response curve occurred was greater in females compared to males (F(6,6) = 19.04, p < 0.01).
Fig. 4.
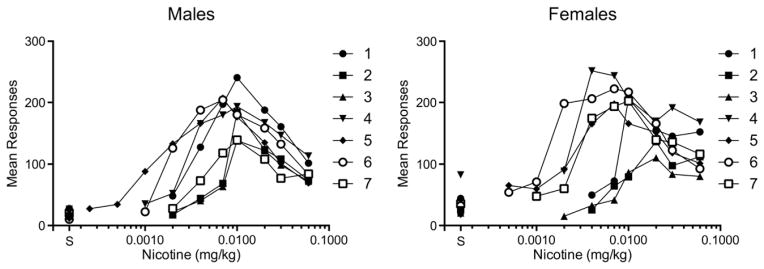
Mean number of responses on the active lever in each male (left panel) and female (right panel) rat during progressive unit dose reduction. Each point is the mean of the last three sessions at each unit dose. Raw data are provided in Appendix A. Points above “S” represent responding during the saline extinction phase prior to dose reduction.
Fig. 5 shows the compensation index at each unit dose. There was a main effect of sex (F(1,12) = 6.89, p < 0.05), dose (F(4,48) = 22.1, p < 0.001), and sex × dose interaction (F(4,48) = 6.34, p < 0.001). The CI was significantly lower in females compared to males at the 0.03 and 0.02 mg/kg doses (t(60) = 4.5, p < 0.001; t(60) = 2.9, p < 0.05, respectively). The mean CI at the 0.03 mg/kg unit dose was 0.39 ± 0.09 in males and 0.09 ± 0.06 in females. Most (5 out of 7) males showed maximal compensation at the 0.03 mg/kg unit dose, whereas the majority (5 out of 7) of females showed maximal compensation at lower unit doses (0.02 to 0.01 mg/kg), consistent with the data in Fig. 3 suggesting that compensation was slower to manifest in females.
Fig. 5.
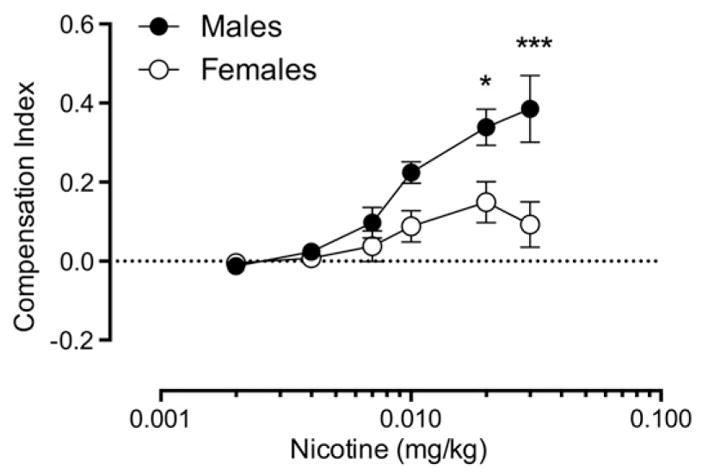
Mean (±SEM) compensation index (CI) in males and females at each unit dose. Data at doses below 0.002 mg/kg are not shown because less than half of the rats were exposed to those doses. The horizontal dotted line indicates the CI value at which no compensation is evident. Significantly different from females, *p < 0.05, ***p < 0.001.
Figs. 6 and 7 show group and individual subject demand curves, respectively. Table 1 shows the associated group and individual subject demand curve parameters. Nicotine consumption for both group and individual subject data was well described by the exponential demand function, with r2 values ranging from 0.96 to 1.0. None of the demand parameters were significantly different between males and females.
Fig. 6.
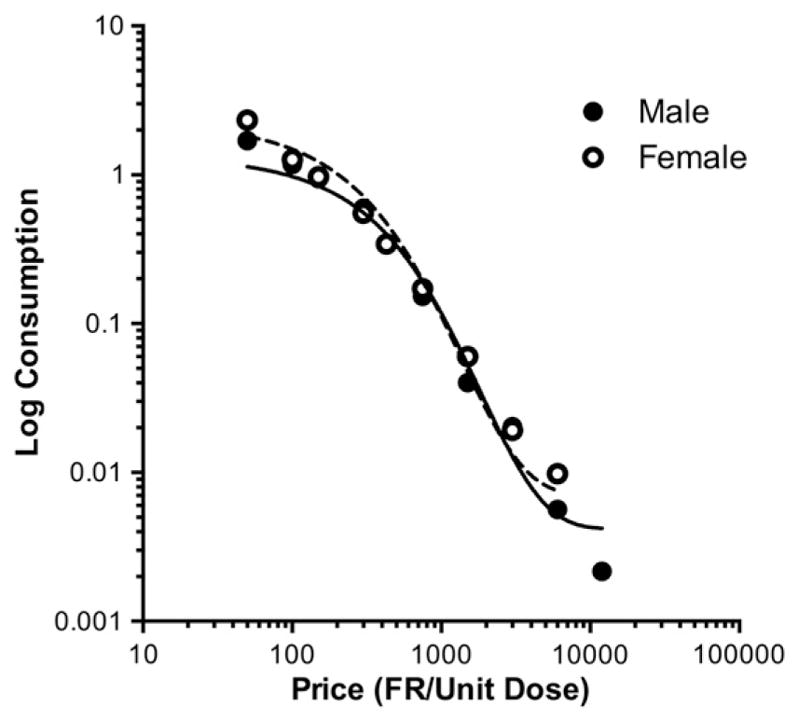
Group demand curves for male and female rats. Each point is the mean nicotine consumption (mg/kg/day) during the last three sessions at each unit price during progressive unit dose reduction, in log–log coordinates. Data points are fit with the predicted function according to the exponential demand equation. Raw data are provided in Appendix C.
Fig. 7.
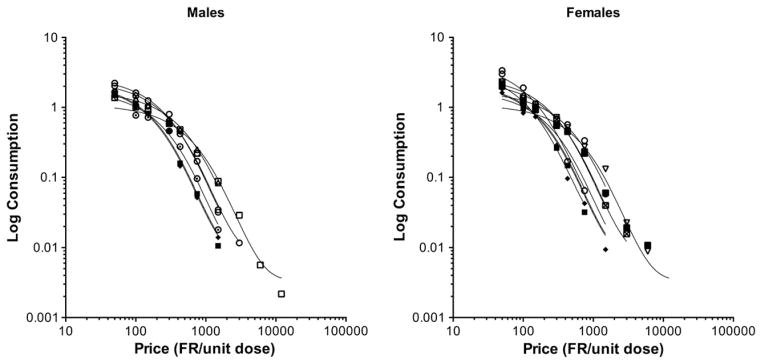
Demand curves for individual male and female rats. See Fig. 6 for further details.
Table 1.
Individual and group exponential demand curve parameters (k set to 2.5 log units globally).
| Subject | α | Q0 | R2 | Pmax | Omax |
|---|---|---|---|---|---|
| Males | |||||
| 1 | 3.30E-04 | 2.7 | 1.00 | 245.0 | 213.9 |
| 2 | 5.90E-04 | 2.3 | 0.97 | 160.8 | 119.7 |
| 3 | 6.20E-04 | 2.2 | 0.99 | 160.0 | 113.9 |
| 4 | 3.50E-04 | 2.4 | 0.99 | 259.8 | 201.7 |
| 5 | 3.40E-04 | 1.1 | 0.99 | 583.6 | 207.6 |
| 6 | 3.10E-04 | 1.7 | 0.99 | 414.1 | 227.7 |
| 7 | 5.60E-04 | 1.7 | 0.99 | 229.3 | 126.1 |
| Group | 4.43E-04 | 2.01 | 0.99 | 293.2 | 172.9 |
| SEM | 5.3E-005 | 0.21 | 0.003 | 58.1 | 19.0 |
| Females | |||||
| 1 | 4.20E-04 | 4.3 | 0.96 | 120.9 | 168.1 |
| 2 | 6.10E-04 | 3.5 | 0.98 | 102.2 | 115.7 |
| 3 | 7.90E-04 | 2.2 | 0.99 | 125.6 | 89.4 |
| 4 | 2.70E-04 | 2.9 | 0.96 | 278.7 | 261.5 |
| 5 | 3.40E-04 | 2.0 | 0.99 | 321.0 | 207.6 |
| 6 | 2.90E-04 | 1.6 | 0.99 | 470.4 | 243.4 |
| 7 | 3.60E-04 | 2.3 | 0.99 | 263.6 | 196.1 |
| Group | 4.40E-04 | 2.69 | 0.98 | 240.3 | 183.1 |
| SEM | 7.2E-005 | 0.36 | 0.005 | 50.7 | 24.0 |
Fig. 8 shows the mean infusions (% baseline, left panel), compensation indexes (middle panel), and demand curves (right panel) based on data from the first 2 h of the session. Similar to the data for the entire session (Fig. 3), there was a significant effect of sex (F(2,71.3) = 10.91, p < 0.0001) and significant linear (F(2,71.2) = 11.68, p < 0.0001), quadratic (F(2,71.3) = 10.92, p < 0.0001) and cubic (F(2,71.3) = 9.69, p < 0.001) sex × dose interactions. A significant increase in mean infusions was observed in males (F(6,36) = 12.36, p < 0.0001) but not females. Males showed a significant increase in infusions at the 0.02 and 0.01 mg/kg unit doses (q(36) = 3.7, p < 0.01 and q(36) = 4.8, p < 0.001, respectively), and the increase was greater than females at the 0.02 mg/kg unit dose (t(12) = 3.34, p < 0.01). The CI was also greater in males than females at the 0.02 mg/kg dose (t(60) = 3.12, p < 0.05). As with the overall session data, there was no sex difference in elasticity of demand during the first 2 h of the session.
Fig. 8.
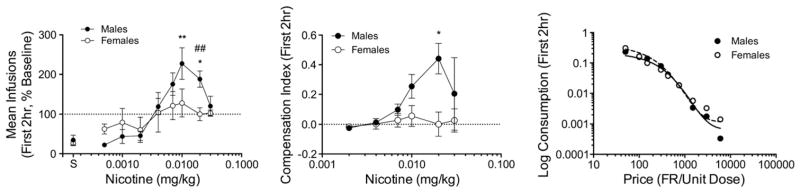
Data from the first 2 h of the session. The left panel shows the mean number of infusions at each unit dose. The middle panel shows the compensation index (CI) at each unit dose. The right panel shows exponential demand curves for consumption in the first 2 h of the session. See Figs. 3, 5, and 7, respectively, for further details.
4. Discussion
The primary findings of the present study were that, compared to males, females showed higher baseline rates of NSA, but significantly less compensation during the initial stages of progressive unit-dose reduction. In contrast, no sex differences were apparent in resistance to extinction, nicotine reinforcement threshold, or elasticity of demand for nicotine. To our knowledge, the present study is the first to explicitly model a nicotine reduction policy in animals for the purpose of measuring individual differences in compensation and the reinforcement threshold for nicotine per se (i.e., apart from other tobacco or smoke constituents). These findings are an important extension of the literature on sex differences in nicotine reinforcement and have important implications for future research on nicotine reduction policies being considered by the FDA (Hatsukami et al., 2010b; Donny et al., 2012; Sofuoglu and LeSage, 2012; Benowitz and Henningfield, 2013).
The higher rate of stable baseline NSA in females in the present study is consistent with several previous studies in rodents using i.v. administration under small FR or PR schedules or oral self-administration models (Donny et al., 2000; Klein et al., 2004; Chaudhri et al., 2005; Park et al., 2007; Lynch, 2009; Li et al., 2012), but is inconsistent with some other studies showing no sex difference or higher nicotine intake in males (Chen et al., 2007; Nesil et al., 2011; Feltenstein et al., 2012; Johnson et al., 2012; Li et al., 2012). It is unclear what accounts for these discrepancies, as numerous methodological factors vary across studies; including the unit dose, strain, age, session duration, and maintenance schedule. Much more research is needed to clarify the factors mediating sex differences in NSA in animal models (see below).
The inverted U-shape and position of the dose–response curves for NSA in the present study are consistent with numerous studies showing similar shaped functions across similar unit doses in either male or female rats. However, the resolution of the curves in the present study is novel in that the curves comprise a greater range and number of doses (10 doses over a 240-fold range) compared to previous studies of sex differences in NSA (3–4 doses over a 5 to 8-fold range). Studies that directly examined sex differences in NSA in adult rats primarily focused on responding at moderate to high unit doses on the descending limb that were reinforcing in most subjects (Donny et al., 2000; Chaudhri et al., 2005; Feltenstein et al., 2012). Two studies examined NSA in adolescent rats at only one dose (0.005 or 0.0075 mg/kg) that typically lies along the ascending limb of the curve (e.g., below 0.01 mg/kg), but that dose was still high enough to be reinforcing in some subjects (Chen et al., 2007; Lynch, 2009). In contrast, the present experiment examined several doses along the ascending limb of the curve, including a dose that was not reinforcing in any subject. Consequently, a relatively complete dose–response curve including both ascending and descending limbs was obtained in every subject, allowing measurement of each rat’s maximal level of compensation and nicotine reinforcement threshold. As such, the present study provides the most detailed account of sex differences in the dose–response relationship for maintenance of NSA to date.
The magnitude of compensation in male rats during the initial dose reduction to 0.03 mg/kg was comparable to our previous studies in which a single similar dose reduction was arranged (Harris et al., 2009, 2011). The present study extends these previous studies by arranging repeated reductions in unit dose, and shows that the magnitude of compensation is greatest at this initial stage of reduction in most males. In contrast, females showed less compensation overall than males and tended to show greater compensation at later stages of dose reduction. Importantly, the findings of a higher baseline rate and less compensation in females are consistent with our prior studies showing that higher baseline NSA rates are predictive of lower compensation in males (Harris et al., 2008, 2009, 2011). Thus, the present findings suggest further that the predictive utility of baseline nicotine intake for anticipating risk of compensation could be an important focus for human studies related to nicotine reduction policy.
The present study is unique in employing a within-subjects design to directly examine sex differences in NSA in a unit-dose reduction model. The lower degree of compensation observed in female rats is consistent with human studies showing that, compared to men, females are less sensitive to changes in nicotine dose (for review, see Perkins, 2009). In contrast, other studies in rats have shown either no sex difference or somewhat better regulation of nicotine intake in females (Chaudhri et al., 2005; Chen et al., 2007). This discrepancy may be attributable to differences in experimental design across studies. Compensation is typically examined in human studies by measuring changes in nicotine intake (or other biomarkers of smoking) following changes in nicotine dose in the same individual (Scherer, 1999). Therefore, the within-subject design in the present study may be better suited to detect and measure compensation than the between-subject designs more often used in prior studies. However, other factors may also be involved, such as our use of the Holtzman rat strain.
It is important to note that compensation in some females was similar to that in males, suggesting that there may be a subpopulation of females that are as sensitive to changes in nicotine dose as males. Larger studies are needed to better characterize this individual variability and identify factors that predict magnitude of compensation in females. As mentioned above, to the extent that baseline nicotine intake predicts compensation in females as it does in males (e.g., Harris et al., 2009), baseline nicotine intake might be a useful measure in clinical studies to identify women who are more sensitive to changes in cigarette nicotine content and at greater risk of compensatory smoking. Such work could have important clinical implications for use of nicotine replacement therapy (NRT) for smoking cessation or reduction in women. Although NRT appears to be less effective in women overall (Perkins and Scott, 2008), it may be more useful to a subgroup who is more sensitive to changes in cigarette nicotine content.
For the purpose of developing a nicotine reduction policy, the observed sex differences in baseline NSA and compensation are important in their own right, without any knowledge of the mechanisms underlying those differences (Donny et al., 2012). However, for the purpose of developing treatments to facilitate the success of policy, understanding the mechanisms could be useful and might involve a combination of several factors, including sex differences in a) nicotine pharmacokinetics (e.g., faster nicotine clearance or higher peak brain nicotine concentrations in females, Rosecrans, 1972; Klein et al., 2004; LeSage et al., 2007), b) sensitivity to the reinforcement enhancing effects of nicotine (i.e., greater enhancement of NSA by nicotine-paired environmental stimuli in females, Chaudhri et al., 2005), c) hormonal variables (e.g., estrous cycle effects in females, Lynch, 2009; but see Donny et al., 2000; Feltenstein et al., 2012), and d) pharmacodynamic responses to nicotine (e.g., reduced beta2-containing nicotinic acetylcholine receptor availability or upregulation in females, Koylu et al., 1997; Mochizuki et al., 1998; Cosgrove et al., 2012; but see Donny et al., 2000; Pehrson et al., 2008). Continued research on these mechanisms may identify sex-specific targets for behavioral or pharmacological treatments to promote policy-induced cessation or reduction of smoking.
Despite the presence of sex differences in degree of compensation, elasticity of demand and the reinforcement threshold were similar between males and females. This finding demonstrates that, females are less sensitive to initial reductions in unit dose (i.e. local elasticity is greater at lower prices), but that males and females are, overall, similarly sensitive to reductions in unit dose (i.e. aggregate elasticity is equal). This somewhat complex pattern of results may be attributed the different ways NSA is controlled by nicotine per se versus associated environmental stimuli and the resulting shape of the NSA dose–response curve. On one hand, greater sensitivity to nicotine’s primary reinforcing effects would serve to increase compensation, but reduce elasticity of demand and the reinforcement threshold. On the other hand, greater control by the discriminative and conditioned reinforcing effects of environmental stimuli would result in less compensation, and also reduce elasticity of demand and the reinforcement threshold. If NSA is more controlled by nicotine per se in males, but more controlled by environmental stimuli in females (see above), the net result could be similar aggregate elasticity of demand and reinforcement thresholds between males and females despite initial differences in compensation at lower unit prices (i.e. local elasticity). Parsing out sex differences in the relative contribution of nicotine per se versus associated environmental stimuli to elasticity of demand and nicotine reinforcement thresholds was beyond the scope of the present study, but certainly represents an important future direction.
Few studies have examined NSA at nicotine unit doses below 0.003 mg/kg, a dose that can still be reinforcing in some rats (e.g., Corrigall and Coen, 1989; Brower et al., 2002). In the NSA unit dose reduction model used by Smith et al. (2013) the unit dose was reduced to 0.001875 mg/kg. While this dose was quite likely below the reinforcement threshold for rats as a group when compared to a saline group, the lack of a saline phase within subjects and the presence of other tobacco constituents prevent confirming whether this was the case in individual rats. As such, it is unclear from these studies what the threshold reinforcing unit dose is for nicotine per se or what unit dose is ubiquitously not reinforcing in rats. The present study begins to address this issue by demonstrating that doses as low as 0.0005 to 0.002 mg/kg can continue to maintain NSA above saline responding in some rats, and that 0.00025 mg/kg was not reinforcing in any rat. In addition, sex was not a significant moderating factor. The present findings differ somewhat from Smith et al. (2013), in that NSA rates in the 0.004 to 0.001 mg/kg range maintained slightly higher mean rates of NSA. However, individual differences were similar between studies. For example, Smith et al. showed mean rates of NSA above or only slightly below baseline in some subjects at low doses near or below the mean reinforcement threshold. Certainly, it will be essential to confirm whether these findings apply to larger samples of rats; as well as other strains, ages, and species. The influence of key model parameters that often vary between studies will also be important to examine (e.g., session duration, infusion speed, cue conditions). Moreover, it will be critical to know whether the reinforcement threshold is moderated by individual differences in nicotine metabolism, history of nicotine intake, and concurrent drug intake (e.g., ethanol Lê et al., 2006, 2010).
It is important to note that at lower doses NSA may have been maintained more, if not solely, by presentation of the visual cues associated with nicotine delivery than nicotine itself. The higher rates of responding on the active lever compared to the inactive lever during saline suggest that NSA was, in part, controlled by either conditioned reinforcing effects of the cues, relatively weak primary reinforcing effects of the cues that were enhanced by nicotine exposure, or both. The present study wasn’t designed to directly address this issue. However, several studies using an unlimited-access model have shown that the specific cues used in the present study do not have any primary reinforcing effects in male and female rats (Fu et al., 2003; Parker et al., 2004; Chen et al., 2007; Wang et al., 2008; Yu and Sharp, 2010), suggesting that primary reinforcing effects of the cues and reinforcement enhancing effects of nicotine thereon were not playing a strong role in the present study. This contrasts with studies using a limited-access model and different visual cues that exhibit primary reinforcing effects that are enhanced by nicotine (Donny et al., 2000; Chaudhri et al., 2005; Palmatier et al., 2007), suggesting that reinforcement enhancing effects of nicotine may be dependent on access conditions, specific properties of visual cues, or both. These findings suggest that the higher rates of responding during saline were likely a function of conditioned, not primary, reinforcing effects of the cues.
The change in nicotine consumption over the course of gradual nicotine dose reduction (i.e., elasticity of demand) was well described by the exponential demand function for both group and individual subject data. This finding is consistent with the descriptive precision of exponential demand analysis observed in previous studies with nicotine, tobacco, and other drugs of abuse in both animals and humans (Christensen et al., 2008; Hursh and Silberberg, 2008; Mackillop et al., 2010, 2012b; Diergaarde et al., 2011). As such, the present study further supports Hursh’s (1991) seminal proposal that behavioral economic methods provide a means to model drug abuse policy in non-humans, and may be useful for further preclinical studies to model the potential effects of tobacco control policies (Donny et al., 2012; Hursh and Roma, 2013). None of the measures of demand for nicotine significantly differed between males and females, suggesting there was no sex difference in the reinforcing efficacy of nicotine per se. This finding is inconsistent with previous studies showing higher breaking points (i.e., reinforcing efficacy) for nicotine under PR schedules in female rats compared to males (Donny et al., 2000; Lynch and Sofuoglu, 2010; Li et al., 2012). As discussed above, this inconsistency may be attributable to differences in experimental design (between-versus within-subject manipulation of dose), access conditions (limited versus unlimited), and rat strain. Nonetheless, the lack of a sex difference in demand in the present study, despite differences in compensation, insinuates the hypothesis that overall consumer demand for cigarettes over the course of a nicotine reduction policy would be similar between male and female smokers, though males may show more compensation during initial nicotine reduction (i.e., less “local elasticity” Mackillop et al., 2012b). It will be important to test this hypothesis in comparable human studies.
We are aware of only one other study that examined sex differences in extinction of NSA. Similar to the present study, Feltenstein et al. (2012) found a significant main effect of sex on absolute levels of active lever pressing in extinction, with females exhibiting higher rates compared to males. Moreover, there was no significant sex × session interaction, indicating a parallel change in response rates and no sex difference in resistance to extinction per se. Nonetheless, there were interesting trends in the present study that may warrant more intensive study. For example, there was a resurgence of responding after the initial extinction sessions in some females, as indicated by the upward trend in responding across extinction days 5 to 7 (Fig. 2), and females required somewhat longer to meet extinction criteria (4–5 days). Although these differences were not statistically significant, a larger study specifically powered to detect them would be of interest to help confirm whether or not there are modest but significant sex differences in resistance to extinction.
Unlike some other studies on sex differences in NSA using limited access models (Donny et al., 2000; Chaudhri et al., 2005; Li et al., 2012), the present study did not find higher rates of inactive lever pressing in females compared to males. The reason for this discrepancy may be due to differences in access conditions between studies, as another study using an unlimited-access model also showed no sex differences in inactive lever pressing (Chen et al., 2007). It’s possible that better lever discrimination develops in an unlimited access protocol, due to greater opportunity for learning (i.e., more reinforcers can be delivered per session to train the discrimination). Use of the Holtzman strain in the present study may also account for the discrepancy with other studies, as some studies showed this strain can exhibit lower rates inactive responding than Lewis rats (Brower et al., 2002; also see Shoaib et al., 1997).
There are a number of limitations to the present study. First, the limited number of days at which each dose was available may have resulted in thresholds that were lower than what would have been observed if NSA was allowed to stabilize at each unit dose. As such, the thresholds reported herein could be considered conservative estimates. We chose weekly exposure to each dose to try to balance expediting the protocol to avoid attrition and allowing a wide range of doses to be studied. Nonetheless, since the FDA will need to specify the duration that each nicotine standard is in effect under a nicotine reduction policy, it will be important to examine this variable in future studies. Second, the sample sizes in the present study were somewhat small. Larger studies will help to derive more accurate estimates of compensation, the nicotine reinforcement threshold, and elasticity of demand, and better characterize their individual variability. Third, only a single training dose was used. Though not directly measured in the present study, serum nicotine concentrations produced by the 0.06 mg/kg unit dose were likely significantly higher than those reported even in heavy smokers (LeSage et al., 2002; Hukkanen et al., 2005; Matta et al., 2007). This high dose was chosen because it facilitated the ability to study compensation. However, it is possible that sex differences in compensation would not have been observed if a lower clinically-relevant training dose was used. In addition, it is possible that the present protocol overestimates the general variation and degree of compensation that would be observed within each sex under more clinically-relevant circumstances. Fourth, only a single rate of unit dose reduction was used. It is possible that sex differences may manifest differently under different rates of dose reduction. In fact, the lack of sex differences in resistance to extinction in the present study, which could be considered the fastest rate of dose reduction, supports this notion. It will be critical to study this issue further. Although Benowitz and Henningfield (1994) initially suggested implementing a policy involving a gradual reduction of nicotine content in cigarettes, more research is needed to determine what the best rate of reduction would be to facilitate cessation and minimize side effects (Benowitz and Henningfield, 2013). Some studies in both humans and animals suggest that relatively rapid reduction of nicotine content in cigarettes might be as effective, if not more so, than slower reduction (Hatsukami et al., 2010a; Smith et al., 2013).
Finally, feeding conditions were not optimally tailored to each sex in the present study. In order to systematically extend previous studies (e.g., Donny et al., 2000), males and females were fed the same amount of food each day. However, because body weight was generally lower in females than males (mean = 298 (±8 SEM) g and 390 (±12 SEM) g, respectively) they received an average of 1.4% (±0.02 SEM) more food per kilogram of body weight and, therefore, might have been less food deprived than males. Since food deprivation can increase motivation to self-administer drugs of abuse (e.g., Comer et al., 1995; Rodefer and Carroll, 1997; also see Donny et al., 1998), females may have been somewhat less motivated than males in the present study and, therefore, less likely to compensate. However, this is not likely for several reasons. First, females normally consume more food per gram of body weight than males under ad lib conditions, which is likely due to their greater energy expenditure (e.g. thermogenesis Valle et al., 2005). As a result, when the same level of restriction is applied to both sexes (e.g. 75% of ad lib intake), females usually receive 1–1.5% more food per gram of body weight than males (Keenan et al., 1995, 1996, 1997; Hubert et al., 2000; Laaksonen et al., 2013). Therefore, the relative level of food restriction in the present study was likely similar between males and females. Second, differences in body weight were controlled for because unit doses were administered relative to body weight (mg/kg). Third, it has nonetheless been shown that reduced body weight and level of food restriction per se have less influence on NSA in rats than the acute state of food deprivation (i.e. hunger). Donny et al. (1998) showed that stable NSA in food-restricted rats fed 2 h before their session (pre-fed) was equivalent to that in ad lib fed rats, despite significant differences in body weight between groups. In contrast, NSA in food-restricted rats fed after their session was significantly higher than both the pre-fed and ad-lib rats, even though they were at a similar reduced body weight as pre-fed food-restricted rats. These findings demonstrate that the acute state of hunger exerts greater influence over NSA than the degree of food restriction or body weight per se. Our finding that sex differences in the first 2 h of the session were the same as for the entire session suggests that any potential differences in hunger between males and females was negligible.
In order to implement a nicotine reduction policy, the FDA must specify nicotine performance standards for tobacco products. Such standards need to be based on scientific understanding of the nicotine reinforcement threshold and the factors that moderate it. The purpose of animal studies is not to specify an actual threshold reinforcing nicotine dose for the FDA to consider in setting a performance standard (Donny et al., 2000), but to simply identify variables that moderate that dose. This information could, in turn, help direct the human research needed by the FDA to determine a nicotine performance standard. Despite its limitations, the present study is an important step toward addressing these issues. In summary, the unit dose reduction model was sufficiently sensitive to detect sex differences in compensation during gradual nicotine dose reduction, with females exhibiting significantly less compensation than males. The model also appears useful for measuring nicotine reinforcement thresholds in individual subjects, and suggests males and females have a similar threshold with substantial variability between subjects. Exponential demand curve analysis provided a precise description of the overall decrease in consumption with decreases in dose (i.e., increases in price), and suggests that elasticity of demand is similar in males and females. NSA dose reduction models may be useful for future animal studies to examine other factors that may modulate the nicotine reinforcement threshold, compensation, and elasticity of demand. Finally, our findings suggest that sex differences in compensation should be a focus in future human studies on nicotine reduction.
Appendix A.
Absolute individual and group active lever presses at each unit dose. Each value is the mean of three 23-h sessions
| Subject | Unit dose (mg/kg/infusion)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.03 | 0.02 | 0.01 | 0.007 | 0.004 | 0.002 | 0.001 | 0.0005 | 0.00025 | Saline | |
| Males | |||||||||||
| 1 | 101.7 | 160.7 | 187.7 | 240.7 | 197.0 | 127.7 | 48.0 | 27.7 | |||
| 2 | 74.0 | 108.3 | 123.7 | 184.3 | 68.7 | 44.0 | 17.3 | 15.7 | |||
| 3 | 72.0 | 98.7 | 122.0 | 138.3 | 63.3 | 40.3 | 22.3 | 22.7 | |||
| 4 | 113.3 | 146.7 | 167.0 | 193.3 | 179.7 | 165.3 | 52.0 | 35.0 | 26.7 | ||
| 5 | 69.3 | 101.3 | 135.0 | 176.3 | 207.0 | 166.0 | 133.0 | 88.0 | 34.3 | 27.3 | 11.7 |
| 6 | 83.0 | 132.7 | 158.3 | 180.0 | 204.0 | 187.7 | 126.0 | 22.3 | 10.0 | ||
| 7 | 84.0 | 77.0 | 108.0 | 139.0 | 118.0 | 73.0 | 27.7 | 20.3 | |||
| Group mean | 85.3 | 117.9 | 143.1 | 178.8 | 148.2 | 114.9 | 60.9 | 48.4 | 34.3 | 27.3 | 19.3 |
| SEM | 6.2 | 11.2 | 10.8 | 13.2 | 24.1 | 23.4 | 18.4 | 20.1 | NA | NA | 2.7 |
| Females | |||||||||||
| 1 | 152.0 | 145.0 | 155.0 | 206.0 | 72.7 | 49.7 | 44.0 | ||||
| 2 | 112.0 | 97.7 | 136.7 | 79.3 | 64.3 | 25.3 | 25.7 | ||||
| 3 | 80.0 | 83.7 | 110.3 | 86.7 | 41.7 | 32.7 | 15.0 | 20.3 | |||
| 4 | 168.0 | 191.3 | 169.7 | 205.3 | 243.7 | 251.7 | 88.7 | 82.7 | |||
| 5 | 101.3 | 118.3 | 151.3 | 165.7 | 197.7 | 165.0 | 91.3 | 59.7 | 65.3 | 18.0 | |
| 6 | 92.7 | 122.7 | 165.7 | 217.0 | 222.3 | 206.0 | 198.3 | 71.0 | 54.0 | 32.0 | |
| 7 | 116.3 | 136.0 | 139.3 | 202.3 | 193.7 | 174.3 | 60.0 | 47.7 | 37.3 | ||
| Group mean | 117.471 | 127.814 | 146.857 | 166.043 | 148.014 | 129.243 | 90.660 | 59.467 | 59.650 | 36.9 | |
| SEM | 12.008 | 13.240 | 7.653 | 22.291 | 32.076 | 34.726 | 30.212 | 6.727 | 5.650 | 8.4 | |
Appendix B.
Absolute individual and group number of nicotine infusions at each unit dose. Each value is the mean of three 23-h sessions
| Subject | Unit dose (mg/kg/infusion)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.03 | 0.02 | 0.01 | 0.007 | 0.004 | 0.002 | 0.001 | 0.0005 | 0.00025 | Saline | |
| Males | |||||||||||
| 1 | 33.7 | 53.7 | 62.3 | 80.0 | 65.3 | 42.3 | 16.0 | 9.0 | |||
| 2 | 24.7 | 36.0 | 41.0 | 61.3 | 22.7 | 14.7 | 5.3 | 5.0 | |||
| 3 | 24.0 | 32.7 | 40.7 | 46.0 | 20.7 | 13.0 | 7.0 | 7.3 | |||
| 4 | 37.0 | 48.7 | 52.0 | 64.3 | 59.7 | 55.0 | 17.3 | 11.7 | 7.3 | ||
| 5 | 23.0 | 33.7 | 45.0 | 58.7 | 68.7 | 55.0 | 44.0 | 29.0 | 11.3 | 8.7 | 6.0 |
| 6 | 28.0 | 44.0 | 52.3 | 60.0 | 68.0 | 62.3 | 41.7 | 2.0 | |||
| 7 | 27.6 | 25.7 | 36.0 | 46.0 | 39.3 | 24.0 | 9.0 | 10.0 | |||
| Group mean | 28.3 | 39.2 | 47.0 | 59.5 | 49.2 | 38.0 | 20.0 | 20.4 | 11.3 | 8.7 | 6.7 |
| SEM | 2.0 | 3.7 | 3.4 | 4.4 | 8.0 | 7.8 | 6.1 | 8.7 | NA | NA | 1.0 |
| Females | |||||||||||
| 1 | 50.0 | 48.0 | 51.3 | 68.3 | 24.0 | 16.3 | 14.3 | ||||
| 2 | 37.0 | 32.3 | 45.0 | 26.3 | 21.0 | 8.0 | 8.3 | ||||
| 3 | 27.0 | 27.7 | 36.7 | 28.7 | 13.7 | 10.7 | 4.7 | 6.3 | |||
| 4 | 56.0 | 63.3 | 56.3 | 68.3 | 81.0 | 83.7 | 29.3 | 27.3 | |||
| 5 | 33.3 | 39.0 | 50.0 | 55.0 | 65.3 | 54.7 | 30.3 | 19.3 | 21.6 | 5.7 | |
| 6 | 30.7 | 40.7 | 54.7 | 72.0 | 73.7 | 68.3 | 65.7 | 22.7 | 17.7 | 10.7 | |
| 7 | 38.7 | 45.0 | 46.3 | 67.0 | 64.3 | 58.0 | 20.0 | 15.7 | 10.8 | ||
| Group mean | 38.957 | 42.286 | 48.614 | 55.086 | 49.000 | 42.814 | 30.000 | 19.233 | 19.650 | 11.9 | |
| SEM | 3.962 | 4.384 | 2.515 | 7.404 | 10.678 | 11.588 | 10.038 | 2.021 | 1.950 | 2.8 | |
Appendix C.
Absolute individual and group nicotine consumption (mg/kg/23 h) at each unit price. Each value is the mean of three 23-h sessions
| Males | 50 | 100 | 150 | 300 | 429 | 750 | 1500 | 3000 | 6000 | 12,000 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.022 | 1.611 | 1.246 | 0.800 | 0.457 | 0.169 | 0.032 | |||
| 2 | 1.482 | 1.080 | 0.820 | 0.613 | 0.159 | 0.059 | 0.011 | |||
| 3 | 1.440 | 0.981 | 0.814 | 0.460 | 0.145 | 0.052 | 0.014 | |||
| 4 | 2.220 | 1.461 | 1.040 | 0.643 | 0.418 | 0.220 | 0.035 | 0.012 | ||
| 5 | 1.380 | 1.011 | 0.900 | 0.587 | 0.481 | 0.220 | 0.088 | 0.029 | 0.006 | 0.002 |
| 6 | 1.680 | 1.320 | 1.046 | 0.600 | 0.476 | 0.249 | 0.083 | |||
| 7 | 1.656 | 0.771 | 0.720 | 0.460 | 0.275 | 0.096 | 0.018 | |||
| Group mean | 1.697 | 1.176 | 0.941 | 0.595 | 0.344 | 0.152 | 0.040 | 0.020 | 0.006 | 0.002 |
| SEM | 0.119 | 0.112 | 0.068 | 0.044 | 0.056 | 0.031 | 0.012 | 0.009 | NA | NA |
| Females | ||||||||||
| 1 | 3.000 | 1.440 | 1.026 | 0.683 | 0.168 | 0.065 | ||||
| 2 | 2.220 | 0.969 | 0.900 | 0.263 | 0.147 | 0.032 | ||||
| 3 | 1.620 | 0.831 | 0.734 | 0.287 | 0.096 | 0.043 | 0.009 | |||
| 4 | 3.360 | 1.899 | 1.126 | 0.683 | 0.567 | 0.335 | 0.059 | |||
| 5 | 1.998 | 1.170 | 1.000 | 0.550 | 0.457 | 0.219 | 0.061 | 0.019 | 0.011 | |
| 6 | 1.842 | 1.221 | 1.094 | 0.720 | 0.516 | 0.273 | 0.131 | 0.023 | 0.009 | |
| 7 | 2.322 | 1.350 | 0.926 | 0.670 | 0.450 | 0.232 | 0.040 | 0.016 | ||
| Group mean | 2.337 | 1.269 | 0.972 | 0.551 | 0.343 | 0.171 | 0.085 | 0.041 | 0.031 | |
| SEM | 0.238 | 0.132 | 0.050 | 0.074 | 0.075 | 0.046 | 0.030 | 0.022 | 0.022 | |
Appendix D.
Absolute individual and group inactive lever presses at each unit dose. Each value is the mean of three sessions
| Subject | Unit dose (mg/kg/infusion)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.03 | 0.02 | 0.01 | 0.007 | 0.004 | 0.002 | 0.001 | 0.0005 | 0.00025 | Saline | |
| Males | |||||||||||
| 1 | 0.3 | 2.7 | 1.0 | 4.3 | 10.7 | 5.7 | 4.0 | 2.7 | |||
| 2 | 1.3 | 4.3 | 2.7 | 2.0 | 0.3 | 1.0 | 1.0 | 0.7 | |||
| 3 | 7.0 | 4.0 | 8.0 | 5.0 | 1.7 | 1.3 | 2.7 | 2.3 | |||
| 4 | 13.3 | 5.7 | 5.7 | 4.3 | 3.7 | 2.7 | 0.3 | 0.3 | 2.3 | ||
| 5 | 0.0 | 3.0 | 1.7 | 6.7 | 14.0 | 14.7 | 14.3 | 7.3 | 4.3 | 4.7 | 3.7 |
| 6 | 11.3 | 9.0 | 5.7 | 9.3 | 8.3 | 5.3 | 3.7 | 2.7 | 5.7 | ||
| 7 | 6.0 | 2.7 | 6.7 | 15.3 | 9.0 | 6.7 | 2.0 | 6.0 | |||
| Group mean | 5.6 | 4.5 | 4.5 | 6.7 | 6.8 | 5.3 | 4.0 | 3.4 | 4.3 | 4.7 | 3.3 |
| SEM | 2.0 | 0.9 | 1.0 | 1.7 | 1.9 | 1.8 | 1.8 | 2.1 | NA | NA | 0.7 |
| Females | |||||||||||
| 1 | 40.0 | 38.0 | 34.0 | 22.7 | 7.3 | 7.0 | 6.7 | ||||
| 2 | 0.7 | 2.3 | 1.3 | 1.0 | 1.3 | 0.3 | 0.0 | ||||
| 3 | 8.0 | 7.3 | 24.0 | 16.0 | 32.0 | 31.7 | 12.3 | 10.7 | |||
| 4 | 10.0 | 20.7 | 22.3 | 16.0 | 25.0 | 19.3 | 7.3 | 8.7 | |||
| 5 | 0.0 | 0.0 | 18.0 | 12.7 | 4.0 | 1.0 | 0.0 | 0.0 | 0.7 | 1.0 | |
| 6 | 3.0 | 1.0 | 5.0 | 6.3 | 8.0 | 4.7 | 6.7 | 2.0 | 0.7 | 3.3 | |
| 7 | 4.3 | 9.0 | 9.3 | 9.3 | 7.0 | 3.0 | 0.7 | 1.7 | 4.7 | ||
| Group mean | 9.429 | 11.186 | 16.271 | 12.000 | 12.086 | 9.571 | 5.400 | 1.233 | 0.700 | 5.0 | |
| SEM | 5.277 | 5.204 | 4.400 | 2.707 | 4.393 | 4.411 | 2.282 | 0.623 | 0.000 | 1.5 | |
Acknowledgments
The authors would like to thank Drs. Steven Hursh and Pete Roma from the Institutes for Behavior Resources (Baltimore, MD) and Johns Hopkins University School of Medicine for providing the software for demand curve analysis and assistance with conducting the analysis, and Dr. Andrew Harris for his helpful comments on earlier drafts of the manuscript. The authors also thank Mylissa Staley and Luke Kane for technical assistance. Yan Zhang is now at Medtronic, Minneapolis, MN. Funding by NIDA grant R01DA026444 (LeSage, PI). NIDA had no role in the design or conduct of the study, and in the preparation of the manuscript.
References
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(Suppl 1):i14–7. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Progressive commercial cigarette yield reduction: biochemical exposure and behavioral assessment. Cancer Epidemiol Biomarkers Prev. 2009;18(3):876–83. doi: 10.1158/1055-9965.EPI-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Mackillop J, Murphy JG, Tidey JW, Colby SM. Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addict Behav. 2012;37(11):1257–63. doi: 10.1016/j.addbeh.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930(1–2):12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses — United States, 2000–2004. Morb Mortal Wkly Rep. 2008;57(45):1226–8. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180(2):258–66. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–9. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Huntsberry ME, Riley AL. Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology (Berl) 2008;198(2):221–9. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- Clemens K, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;15:1–12. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Comer SD, Turner DM, Carroll ME. Effects of food deprivation on cocaine base smoking in rhesus monkeys. Psychopharmacology (Berl) 1995;119(2):127–32. doi: 10.1007/BF02246152. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99(4):473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, Mckee SA, Bois F, Seibyl JP, Mazure CM, et al. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69(4):418–27. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. III. A reanalysis of the nicotine regulation hypothesis. Psycho-pharmacology. 1992;108(1–2):1–10. doi: 10.1007/BF02245277. [DOI] [PubMed] [Google Scholar]
- Denoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamyl-amine, hexamethonium and naloxone. Psychopharmacology (Berl) 2006;184(3–4):266–72. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer ANM, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 2011;17(3):576–87. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136(1):83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, LeSage MG, Levin M, Buffalari DM, Joel D, et al. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14(11):1319–38. doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery S, Ake CF, Navarro AM, Kaplan RM. Simulated effect of tobacco tax variation on Latino health in California. Am J Prev Med. 2001;21(4):278–83. doi: 10.1016/s0749-3797(01)00368-3. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–6. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Kane VB, Sharp BM. Norepinephrine release in amygdala of rats during chronic nicotine self-administration: an in vivo microdialysis study. Neuropharma-cology. 2003;45(4):514–23. doi: 10.1016/s0028-3908(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16(1):86–97. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology (Berl) 2009;205(4):599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, LeSage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl) 2011;217(2):153–66. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010a;105(2):343–55. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, et al. Nicotine reduction revisited: science and future directions. Tob Control. 2010b;19(5):e1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz N, Connolly G, Davis R, Gray N, Myers M, et al. Reducing tobacco addiction through tobacco product regulation. Tob Control. 2004;13(2):132–5. doi: 10.1136/tc.2003.006890. BMJ Publishing Group Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert M-F, Laroque P, Gillet J-P, Keenan KP. The effects of diet, ad libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague–Dawley rats. Toxicol Sci. 2000;58(1):195–207. doi: 10.1093/toxsci/58.1.195. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56(2):377–93. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99(1):98–124. doi: 10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, et al. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology (Berl) 2012;222(2):269–76. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan KP, Soper KA, Hertzog PR, Gumprecht LA, Smith PF, Mattson BA, et al. Diet, overfeeding, and moderate dietary restriction in control Sprague–Dawley rats: II. Effects on age-related proliferative and degenerative lesions. Toxicol Pathol. 1995;23(3):287–302. doi: 10.1177/019262339502300306. [DOI] [PubMed] [Google Scholar]
- Keenan KP, Laroque P, Ballam GC, Soper KA, Dixit R, Mattson BA, et al. The effects of diet, ad libitum overfeeding, and moderate dietary restriction on the rodent bioassay: the uncontrolled variable in safety assessment. Toxicol Pathol. 1996;24(6):757–68. doi: 10.1177/019262339602400620. SAGE Publications. [DOI] [PubMed] [Google Scholar]
- Keenan KP, Ballam GC, Dixit R, Soper KA, Laroque P, Mattson BA, et al. The effects of diet, overfeeding and moderate dietary restriction on Sprague–Dawley rat survival, disease and toxicology. J Nutr. 1997;127(5 Suppl):851S–6S. doi: 10.1093/jn/127.5.851S. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–11. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kessler DA. Statement on nicotine-containing cigarettes. Tob Control. 1994;3(2):148. BMJ Group. [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose–response experiment. Pharmacol Biochem Behav. 2004;78(1):13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Koylu E, Demirgören S, London ED, Pöün S. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci. 1997;61(12):L185–90. doi: 10.1016/s0024-3205(97)00665-6. [DOI] [PubMed] [Google Scholar]
- Laaksonen KS, Nevalainen TO, Haasio K, Kasanen IHE, Nieminen PA, Voipio H-M. Food and water intake, growth, and adiposity of Sprague–Dawley rats with diet board for 24 months. Lab Anim. 2013;47(4):245–56. doi: 10.1177/0023677213488103. [DOI] [PubMed] [Google Scholar]
- Lê AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26(6):1872–9. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 2010;208(3):475–86. doi: 10.1007/s00213-009-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72(1–2):279–89. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170(3):278–86. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Burroughs D, Pentel PR. Effects of pregnancy on nicotine self-administration and nicotine pharmacokinetics in rats. Psychopharmacology (Berl) 2007;194(3):413–21. doi: 10.1007/s00213-007-0830-z. [DOI] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. [Jul 11];Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00473.x. http://dx.doi.org/10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–61. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, O’Hagen S, Lisman SA, Murphy JG, Ray LA, Tidey JW, et al. Behavioral economic analysis of cue-elicited craving for alcohol. Addiction. 2010;105(9):1599–607. doi: 10.1111/j.1360-0443.2010.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Amlung MT, Wier LM, David SP, Ray LA, Bickel WK, et al. The neuroeconomics of nicotine dependence: a preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. Psychiatry Res. 2012a;202(1):20–9. doi: 10.1016/j.pscychresns.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Few LR, Murphy JG, Wier LM, Acker J, Murphy C, et al. High-resolution behavioral economic analysis of cigarette demand to inform tax policy. Addiction. 2012b;107(12):2191–200. doi: 10.1111/j.1360-0443.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190(3):269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Villemagne VL, Scheffel U, Dannals RF, Finley P, Zhan Y, et al. Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse. 1998;30(1):116–8. doi: 10.1002/(SICI)1098-2396(199809)30:1<116::AID-SYN15>3.0.CO;2-2. Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pöün S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61(1–2):189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320(1):180–93. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 2007;195(2):235–43. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han S-H, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86(2):297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, et al. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the alpha6 subunit. Mol Pharmacol. 2004;65(3):611–22. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173(1–2):64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Philibin SD, Gross D, Robinson SE, Vann RE, Rosecrans JA, et al. The effects of acute and repeated nicotine doses on spontaneous activity in male and female Sprague Dawley rats: analysis of brain area epibatidine binding and cotinine levels. Pharmacol Biochem Behav. 2008;89(3):424–31. doi: 10.1016/j.pbb.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol. 1996;4:166–77. AMERICAN PSYCHOLOGICAL ASSOCIATION. [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr Symp Motiv. 2009;55:143–69. doi: 10.1007/978-0-387-78748-0_9. [DOI] [PubMed] [Google Scholar]
- Perkins K, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10(7):1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perkins KA, DiMarco A, Grobe JE, Scierka A, Stiller RL. Nicotine discrimination in male and female smokers. Psychopharmacology (Berl) 1994;116(4):407–13. doi: 10.1007/BF02247470. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. A comparison of progressive ratio schedules versus behavioral economic measures: effect of an alternative reinforcer on the reinforcing efficacy of phencyclidine. Psychopharmacology (Berl) 1997;132(1):95–103. doi: 10.1007/s002130050325. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11(6):863–70. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychophar-macology (Berl) 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129(1):35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15(11):1918–25. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, LeSage MG. The reinforcement threshold for nicotine as a target for tobacco control. Drug Alcohol Depend. 2012;125(1–2):1–7. doi: 10.1016/j.drugalcdep.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauras JA, Chaloupka FJ. The demand for nicotine replacement therapies. 2001 doi: 10.1080/1462220031000073306. [DOI] [PubMed] [Google Scholar]
- Tengs TO, Ahmad S, Savage JM, Moore R, Gage E. The AMA proposal to mandate nicotine reduction in cigarettes: a simulation of the population health impacts. Prev Med. 2005;40(2):170–80. doi: 10.1016/j.ypmed.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133(3):300–4. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab. 2005;289(1):E15–22. doi: 10.1152/ajpendo.00553.2004. Am Physiological Soc. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM. Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J Neurochem. 2008;106(2):943–56. doi: 10.1111/j.1471-4159.2008.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Sharp BM. Nicotine self-administration diminishes stress-induced norepinephrine secretion but augments adrenergic-responsiveness in the hypothalamic paraventricular nucleus and enhances adrenocorticotropic hormone and corticosterone release. J Neurochem. 2010;112(5):1327–37. doi: 10.1111/j.1471-4159.2009.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


