Abstract
Background:
Anaphylactic events due to immunotherapy are probably not completely preventable. There is always an inherent risk surrounding the administration of an allergen to an individual who is sensitized to the substance administered.
Methods:
There are, however, effective measures to reduce the risk of these events, and to optimize the assurance of a good outcome in the face of such an event.
Results:
Of prime importance in preventing these episodes is the regular assessment of the patient's health status, especially in regard to asthma, and the careful attention to the prevention of dosing errors.
Conclusion:
Of equal importance, in regard to assuring a good outcome should such an event occur, are the rapid recognition of symptoms and the immediate injection of epinephrine, the drug of choice for the treatment of any episode of anaphylaxis.
Keywords: Anaphylaxis, anaphylaxis prevention, anaphylaxis treatment, epinephrine, immunotherapy, treatment
Because allergen immunotherapy introduces an allergen into an allergic individual, hypersensitivity reactions are probably unavoidable. There are, however, measures to minimize the risk and effective therapy to treat any such reactions. This is a review of procedures that have been suggested to minimize these risks and protocols designed to treat such reactions if they do occur.
It draws heavily on consensus statements and evidence-based guidelines. The three references used extensively are the most recent allergen immunotherapy parameter,1 the most recent update of the anaphylaxis parameter,2 and a consensus publication on systemic reactions to immunotherapy sponsored by the World Allergy Organization.3
The most recent immunotherapy practice parameter1 states, “Although there is a low risk of severe systemic reactions with appropriately administered allergen immunotherapy, life-threatening and fatal reactions do occur.”
Because such reactions are life-threatening, although they are extremely rare, it is imperative that actions be taken to minimize them and protocols designed to treat them rapidly and efficiently are in place.
INCIDENCE
Allergic disease exerts a significant toll on the health care system4 and allergen immunotherapy is an effective and cost-effective therapy in the treatment of allergic respiratory tract disease.5 With this therapy, however, as noted, anaphylactic reactions are probably inevitable. Unfortunately, the exact incidence of these events is unknown. In addition, although we have some data, the exact incidence of near fatal or fatal reactions is also imprecisely established. The reasons for this are numerous. For example, reaction rates differ with the dose and technique used, the allergen used, and the definition applied to define a reaction. For example, severe systemic reactions occur at markedly different rates depending on the frequency of administration of allergy injections. With conventional immunotherapy, the rates of severe systemic reactions are probably <1%, whereas with rush immunotherapy reported reaction rates have been in some instances >30%.6–9
In addition, as with any adverse reaction to a therapeutic agent, reporting rates are probably not completely trustworthy. Also, response rates to surveys designed to assess incidence are usually, low, <30%.10
Another difficulty innate to the determination of the incidence of such reactions is that data gathering techniques are limited for the most part to retrospective analyses or surveys taken of allergists practicing immunotherapy. In addition, there are reviews of such studies. For example, in the previously mentioned World Allergy Organization document,3 it was concluded that by analyzing reaction rates reported from studies between 1995 and 2010, the percentage of systemic reactions per injection with conventional immunotherapy protocols was ∼0.2%.
One example of survey collected data was published by Amin et al.10 in 2006. This survey was sent to members of the American Academy of Allergy, Asthma, and Immunology seeking information about reactions encountered in their practice. The desire was to evaluate the incidence of fatal and near fatal reactions. There were 646 respondents. Two hundred seventy-three reported near fatal reactions between 1990 and 2001. This gave an incidence of 23 per year, or 5.4 events per million injections. The authors performed the study because they noted that in previous evaluations, there were very few if any descriptions of serious or near fatal systemic reactions. In these previous studies, they noted that it was reported that 5–7% of patients receiving immunotherapy experienced reactions, but there was no mention of the number that were fatal or near fatal or a detailed description of these events.11–13
Before this survey of fatal and near fatal episodes there were other studies in North America that were performed to characterize and estimate the incidence of reactions to immunotherapy. Lockey et al.14 reported 24 fatal reactions that occurred between 1973 and 1984. They estimated that there was one fatal reaction per 2.8 million injections. Reid et al.15 recorded 15 immunotherapy-related deaths between 1985 and 1989. They estimated one fatality in every 2 million injections. Bernstein and colleagues performed a survey that documented 41 fatal reactions between 1990 and 2001.16 Their estimate was that there was one fatal reaction per every 2.5 million injections.
FACTORS THAT MAY PREDISPOSE OR INCREASE THE SEVERITY OF SYSTEMIC REACTIONS DURING IMMUNOTHERAPY
Many factors have been identified that may enhance the risk of a systemic reaction during immunotherapy or make such a reaction more severe (Table 1). Very few of these, however, have been definitively established as a predisposing factor. Data collected regarding many such factors show conflicting results.
Table 1.
Factors that may increase the frequency or enhance the severity of a reaction during immunotherapy

ASTHMA
The presence of asthma may not increase the risk of a reaction, but asthma is a risk factor for a severe reaction, and if the asthma is unstable, it enhances this risk.17 In addition, it increases the risk of fatal reactions.10
CONCOMITANT MEDICATION
Although β-adrenergic blocking agents do not seem to affect the frequency of the occurrence of systemic reactions to immunotherapy, they are a risk factor for a more serious event and can complicate therapy.1,17 The issue of patients treated with immunotherapy and simultaneously receiving a β-adrenergic blocker is one that is commonly encountered and one that has generated intense interest as well as some controversy.18,19 This controversy has been generated in part because of the difficulties that are presented to the clinician when substitutions for β-blockers need to be made before the initiation of immunotherapy and because in some instances immunotherapy can be carefully performed even in patients who are receiving venom while on β-adrenergic blockers.20 In addition, it has been shown that β-adrenergic blockers may not increase the risk of anaphylactic events to radiocontrast material.21 However, there clearly are data that support the fact that β-adrenergic blockers may increase the risk of anaphylaxis after the administration of a known allergen, complicate its therapy, and worsen the severity of an event.14,22–46 Taken together, overall, it appears quite clear that β-adrenergic blocking agents can have an adverse effect on the outcome of an anaphylactic episode and perhaps can increase the predisposition toward these episodes. They may do so in several ways. When a patient is taking a β-adrenergic blocker, there is a diminished response to the β-adrenergic effects of epinephrine. This may make a patient less responsive to the endogenous compensatory response produced by the patient's own production of epinephrine as well as exogenously administered epinephrine given for therapy. In addition, it should be clarified that in a case of anaphylaxis, the relative contraindication extends not only to unselective β-adrenergic agents but also to relatively selective β-adrenergic blockers. This is because, in contrast to asthma, one is concerned not only with the β-adrenergic effect on smooth muscle in the lungs but also with the β-adrenergic effect on the cardiovascular system.3 Thus, it is desirable, in patients receiving immunotherapy, to, when possible, discontinue the use of β-adrenergic agents. Angiotensin-converting enzyme inhibitors clearly increase the risk of an anaphylactic event during immunotherapy to venoms, but no such risk has been noted, to date, regarding systemic reactions to inhalants.
PRECEDING LARGE LOCAL REACTIONS
Data regarding the occurrence of large local reactions are difficult to interpret in that results have been somewhat conflicting. Originally, studies failed to find that preceding large local reactions were a risk factor for a systemic event.46,48 At least, in these studies, there was no difference in the incidence of systemic reactions in a group of patients where dose adjustments were made based on the occurrence of large local reactions versus a group in which the large local reactions were not used to alter the immunotherapy dose. It was concluded that large local reactions were not accurate predictors of a subsequent systemic event. However, another investigation performed as a retrospective review designed to compare the frequency of preceding large local reactions in patients who had a systemic reaction versus a matched “control group” of subjects not experiencing a systemic reaction found that there was a significant increase in the frequency of large local reactions in patients who had experienced a systemic reaction.49
These data are difficult to interpret as far as their clinical significance; however, overall, it appears as if large local reactions are not adequate predictors of a future systemic event in that dosage adjustments based on local reactions fail to alter the frequency of systemic events. Nonetheless, individuals who have experienced a systemic event have a higher incidence of large local reactions than those who have never had a systemic reaction.
ADMINISTRATION OF INJECTIONS DURING THE POLLEN SEASON
As with large local reactions, data are conflicting on whether or not immunotherapy injections given during the pollen season is a risk factor compared with injections administered outside the pollen season. Some studies have shown that there is no difference in the incidence of reactions when injections are given “in season” versus when they are given “out of season.”50,51
However, in the previously mentioned study by Amin and colleagues,10 the administration of injections during the pollen season was reported by 46% of respondents. In addition, it was hypothesized that “priming” during the season could be a predisposing factor in regard to near fatal reactions. Thus, as with local reactions, it is difficult to make a definitive statement on whether administration of injections during the pollen season is a risk factor, but data, to date, seem to imply that administration during the season may increase the risk of systemic reactions.
DOSING ERRORS
Dosing errors account for a significant number of anaphylactic reactions to immunotherapy. In the Amin et al.10 study they were the second most common factor reported to be associated with events, accounting for 26% of episodes.
FIRST INJECTION FROM A NEW VIAL
In two studies,15,16 the first injection from a new vial of extract was a risk factor for a systemic event. Because of this it has been suggested that the dose be lowered when a new vial is started.1 There is no accepted consensus as to the amount the dose should be lowered.
A HIGH LEVEL OF SENSITIVITY TO THE ALLERGEN ADMINISTERED
A high level of sensitivity to the allergen being administered has been found to be a risk factor for systemic events.1
A HISTORY OF A PREVIOUS SYSTEMIC REACTION TO ALLERGEN INJECTIONS
It is interesting to note that some patients experiencing a severe reaction on occasion report previous milder events occurring earlier in the course of immunotherapy.10
TIMING OF SYSTEMIC REACTIONS RELATED TO THE ADMINISTRATION OF THE INJECTION
It is clear that most systemic reactions occur within 30 minutes after an injection. In addition, almost all severe systemic reactions start within this period of time.1,3 However, fatal reactions can begin later than 30 minutes postinjection, and systemic reactions can occur in rare instances more than 2 hours after the shot is given.1,17 Of note, while speaking of timing, it is also necessary to recognize that, although rare, biphasic reactions to immunotherapy can occur. Thus, patients can be treated successfully, discharged from the office, and then experience a recurrence of symptoms.52,53 Based on an overall assessment of this information, a 30-minute waiting period for all patients receiving allergen immunotherapy has been suggested.1
Because anaphylactic episodes to immunotherapy can occur after patients have left the physician's office after a 30-minute wait,17 consideration has been given to supplying patients receiving allergen immunotherapy a prescription for an automatic epinephrine injector, and that the patient be required to have this injector with them on days when they receive their injections. To the author's knowledge, however, there is no consensus recommendation regarding this issue. Therefore, at least at this time, it appears that whether or not to issue epinephrine injectors to patients who are treated with immunotherapy remains at the discretion of the physician caring for the patient.
CLINICAL MANIFESTATIONS
The clinical manifestations of anaphylaxis during immunotherapy are similar to those occurring in anaphylactic reactions to any injected allergen. However, there are salient features of fatal and near fatal events that are of note10 (Table 2).
Table 2.
Unusual clinical manifestations of fatal and near fatal anaphylactic reactions due to the administration of immunotherapy

For example, although cutaneous features are the most common clinical manifestations in anaphylactic reactions taken as a whole,54 in near fatal and fatal immunotherapy reactions they do not predominate.10 Respiratory failure and hypotension or shock are the most frequently recorded events. Over 90% of patients with fatal reactions experience respiratory failure, and hypotension occurs in 88% of near fatal events and 81% of fatal reactions.
Cutaneous signs appeared in 70% of near fatal reactions and in only 29% of those that were fatal. This may be because of the fact that the hypotensive state of these patients prevents blood flow from reaching the skin.54
A striking finding was that when patients exhibited a history of poorly controlled or labile asthma, there was a prominently increased risk of fatal events, and most of these who did have reactions experienced fatal rather than near fatal episodes.10
PREVENTION
In light of these findings, there have been several suggestions to reduce the incidence and severity of systemic reactions due to immunotherapy1,3,10 (Table 3). Because asthma is clearly one of the most important risk factors, it has been suggested that patients not receive allergy injections when their asthma is unstable or when their peak expiratory flow is “considered low for that patient” or “is substantially reduced compared with the patient's baseline value.”1 It has also been suggested that the absolute value of the forced expiratory volume at 1 second be used as a measure to exclude patients from receiving immunotherapy. In this regard, it has been proposed that an forced expiratory volume at 1 second below 70% of their predicted value should eliminate an asthmatic patient for consideration of the institution of aeroallergen immunotherapy.17
Table 3.
Actions designed to diminish the risk of an anaphylactic event during immunotherapy
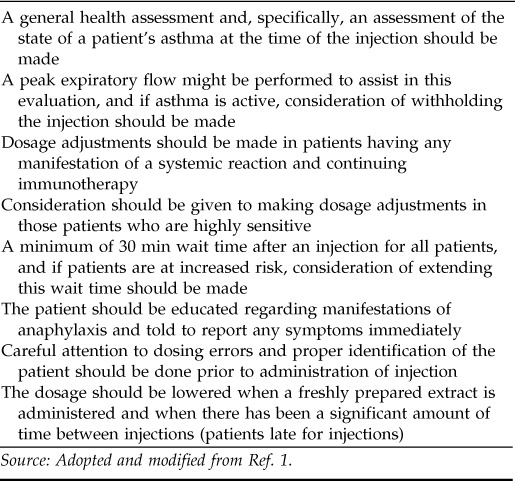
Source: Adopted and modified from Ref. 1.
Patients who have experienced systemic reactions should have dosage adjustments made. The amount the dose should be adjusted is dependent on the physician's judgment in regard to that particular patient. Obviously, this decision can be based on the severity of the event in question. In some instances, it may be decided that immunotherapy should be discontinued.
The degree of allergen sensitivity has been considered a risk factor for anaphylactic events occurring during immunotherapy.3 Thus, consideration of dose adjustments can be made in patients who show a high degree of sensitivity as manifested by skin test reactivity.
Any risk factor would lead the physician administering immunotherapy to consider a wait of >30 minutes. This includes a previous reaction, a high degree of skin test sensitivity, a patient with asthma, etc.
It is important that the patient recognize the early manifestations of an anaphylactic episode and be told to report any manifestation immediately. Episodes can begin insidiously, and patients may ignore the early clinical expressions of a harbinger of a more severe reaction. Thus, any patient receiving immunotherapy should be acquainted with all of the manifestations of anaphylaxis and be told to report any of these manifestations, as noted, promptly.
As mentioned earlier, dosing areas are one of the most common causes of anaphylaxis to immunotherapy injections. Therefore, measures should be in place to minimize the chance of error. Efforts should be made to enhance the distinction between different dilutions of extract. Color coding systems can be used to accomplish this. The person administering the injection should clearly identify the patient by name and assure that the vial from which the injection is drawn is for that patient. Careful record keeping as to dates and doses for each injection should be used. The patient's medication regimen should be frequently monitored to see if there have been changes in medication (e.g., the addition of a β-blocker), which might signify an increased risk for a reaction. In addition, as noted previously, consideration should be given to lowering the dose when a freshly prepared extract is administered, and a schedule for reduction of dosing should be available to delineate dose reductions due to an inordinate lapse of time between injections.
SUGGESTED EQUIPMENT IN THE OFFICE FOR TREATMENT OF A SYSTEMIC REACTION
There have been a number of articles written that have mentioned what equipment should be available for the treatment of an in-office anaphylactic reaction.54 Two recent documents1,2 list such equipment, and their suggestions are compared in Table 4.
Table 4.
Equipment suggested for treatment of an in-office anaphylactic event comparing two recent parameters published by the joint task force

In addition to the equipment noted in Table 4, any facility in which allergy injections are administered should have certain procedures in place to facilitate a rapid response to an event (Table 5).3
Table 5.
Practices and procedures to be in place for the management of an anaphylactic event

Source: Adopted from Ref. 3.
MANAGEMENT OF ANAPHYLAXIS
The management of an anaphylactic event occurring to immunotherapy is identical to the management of an episode due to exposure to any other injected allergen. Epinephrine is the drug of choice and should be given at the first sign of an anaphylactic episode.1–3 A delay in the administration of epinephrine has been found to be a risk factor for poor outcomes and, in some studies, for a biphasic reaction.55
Epinephrine can be administered every 5–10 minutes as necessary, and this can be liberalized based on clinical judgment. Intravenous administration can be considered if needed because of a poor response to intramuscular or subcutaneous injection, but it is preferably administered where cardiovascular monitoring is available.
Immediate assessment of vital signs and the airway should be performed, and the patient should be placed in a supine position with legs elevated. Oxygen should be started simultaneously with the initial evaluation. Patients should stay in this recumbent position until the cardiovascular system is stable. Fatalities have been associated with prematurely assuming the upright position.56
If there is a good and rapid response to these early measures consisting of oxygen, epinephrine, and positioning, the patient can be observed (the length of time must be individualized) and then discharged from the facility. It is suggested that they be supplied with a prescription for an automatic epinephrine injector at that time because symptoms can recur. They should be given the phone number where the physician on call can be reached should symptoms reappear.
One could also consider, at the time of administration of epinephrine, calling for emergency services, but that is usually done if there is no quick and adequate response to the initial therapy. Of course, this decision is dependent on the severity of the symptoms at the time of the initial evaluation.
In addition, should the blood pressure remain low, i.v. fluids should be administered, for wheezing an inhaled bronchodilator given, and consideration should be given to the i.v. administration of an H1/H2-antihistamine and corticosteroids.
An algorithm outlining the treatment of an office event is shown in Fig. 1.
Figure 1.
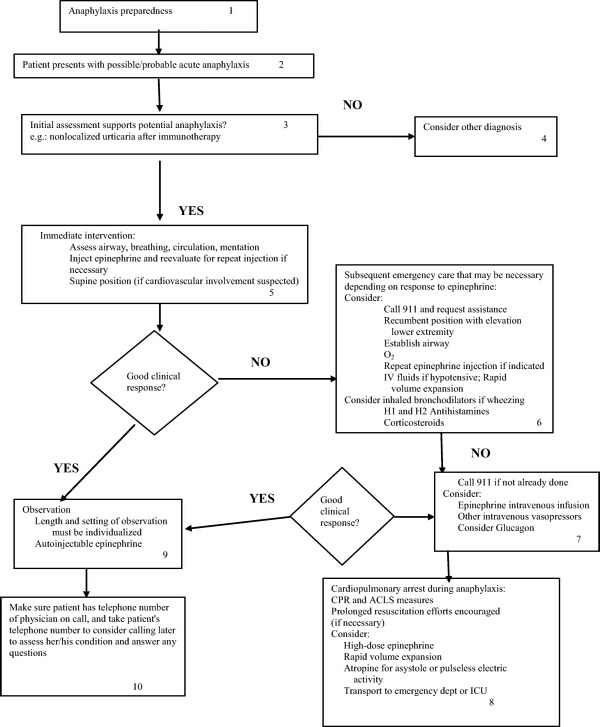
Algorithm for the treatment of an anaphylactic event in the outpatient setting (i.v.). (Adopted from Ref. 2.)
In conclusion, anaphylactic episodes due to allergen immunotherapy probably are unavoidable, but there are strategies available to minimize the frequency of their occurrence and to enhance the outcome of these events. Of primary importance is a level of awareness and the institution of treatment immediately should any manifestation of an anaphylactic event occur.
Footnotes
Presented at the North American Rhinology & Allergy Conference, Puerto Rico, February 4, 2011
The author has no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: A practice parameter. J Allergy Clin Immunol 127:S1–S55, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Lieberman P, Nicklas R, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 Update. J Allergy Clin Immunol 126:477–480, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Cox L, Larenas-Linnemann D, Lockey R. Speaking the same language: The World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol 125:569–574, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Blaiss MS. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc 31:375–380, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Alzakar RH, Alsamarai AM. Efficacy of immunotherapy for treatment of allergic asthma in children. Allergy Asthma Proc 31:324–330, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Larenas-Linnemann D. Subcutaneous and sublingual immunotherapy in children: Complete update on controversies, dosing, and efficacy. Curr Allergy Asthma Rep 8:465–474, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Bernstein DI, Epstein T, Murphy-Berendts K, Liss GM. Surveillance of systemic reactions to subcutaneous immunotherapy injections: Year 1 outcomes of the ACAAI and AAAAI collaborative study. Ann Allergy Asthma Immunol 104:530–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Windom H, Lockey R. An update on the safety of specific immunotherapy. Curr Opin Allergy Clin Immunol 8:571–576, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Portnoy J, Bagstad K, Kanarek H, et al. Premedication reduces the incidence of systemic reactions during inhalant rush immunotherapy with mixtures of allergenic extracts. Ann Allergy 73:409–418, 1994. [PubMed] [Google Scholar]
- 10. Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol 117:169–175, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Ragusa FV, Passalacqua G, Gambardella R, et al. nonfatal systemic reactions to subcutaneous immunotherapy: A 10-year experience. J Investig Allergol Clin Immunol 7:151–154, 1997. [PubMed] [Google Scholar]
- 12. Greenberg MA, Kaufman CR, Gonzalez GE, et al. Late and immediate systemic-allergic reactions to inhalant allergen immunotherapy. J Allergy Clin Immunol 77:865–870, 1986. [DOI] [PubMed] [Google Scholar]
- 13. Ragusa VF, Massolo A. Non-fatal systemic reactions to subcutaneous immunotherapy: A 20-year experience comparison of two 10-year periods. Allergy Immunol (Paris) 36:52–55, 2004. [PubMed] [Google Scholar]
- 14. Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol 79:660–677, 1987. [DOI] [PubMed] [Google Scholar]
- 15. Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol 92:6–15, 1993. [DOI] [PubMed] [Google Scholar]
- 16. Bernstein DI, Wanner M, Borish L, Liss GM. Twelve-year survey of fatal reactions to allergen injections and skin testing. J Allergy Clin Immunol 113:1129–1136, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Nelson HS. (Ed). Allergen Immunotherapy. In: Immunotherapy for Inhalant Allergens. Philadelphia, PA: Mosby, an affiliate of Elsevier, Inc., 1657–1678, 2009. [Google Scholar]
- 18. Lieberman P, Kemp S, Oppenheimer J, et al. Letter to the editor. J Allergy Clin Immunol 116:3933–3936, 2005. [Google Scholar]
- 19. Miller MM, Miller MM. Beta-blockers and anaphylaxis: Are the risks overstated? J Allergy Clin Immunol 116:931–933, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Muller UR, Haeberli G. Use of beta-blockers during immunotherapy for hymenoptera venom allergy. J Allergy Clin Immunol 115:606–610, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Greenberger PA, Meyers SN, Kramer BL. Effects of beta-adrenergic and calcium channel antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 80:698–672, 1987. [DOI] [PubMed] [Google Scholar]
- 22. Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reactions from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 115:270–276, 1991. [DOI] [PubMed] [Google Scholar]
- 23. Lang DM, Alpern MB, Visintainer PF, Smith ST. Elevated risk of anaphylactoid reactions from radiographic contrast media is associated with both beta-blocker exposure and cardiovascular disorders. Arch Intern Med 153:2033–2040, 1993. [PubMed] [Google Scholar]
- 24. Hepner MJ, Ownby DR, Anderson JA, et al. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 86:407–411, 1990. [DOI] [PubMed] [Google Scholar]
- 25. Alarn MM, Alvarez del Real G, Hsieh FH. Cardiopulmonary resuscitation (CPR) in patients with acute anaphylaxis taking beta-blockers. J Allergy Clin Immunol 115:S38, 2005. (Abs). [Google Scholar]
- 26. Jacobs RL, Rake GW, Fournie DC, et al. Potentiated anaphylaxis in patients with drug-induced beta-adrenergic blockade. J Allergy Clin Immunol 68:125–127, 1981. [DOI] [PubMed] [Google Scholar]
- 27. Newman BR, Schultz LK. Epinephrine-resistant anaphylaxis in a patient taking propranolol hydrochloride. Ann Allergy 47:35–37, 1981. [PubMed] [Google Scholar]
- 28. Awai LW, Makori YA. Insect sting anaphylaxis and beta-adrenergic blockade: A relative contraindication. Ann Allergy 53:43–49, 1984. [PubMed] [Google Scholar]
- 29. Laxenaire MC, Torrens J, Moneret-Vautrin DA. Fatal anaphylactic shock in a patient treated with beta-blockers (French). Ann Fr Anesth Reanim 3:453–455, 1984. [DOI] [PubMed] [Google Scholar]
- 30. Benitah E, Nataf P, Herman D. Anaphylactic complications in patients treated with beta-blockers: Apropos of 14 cases (French). Therapie 41:139–142, 1986. [PubMed] [Google Scholar]
- 31. Comaille G, Leynadier F, Modiano D, Dry J. Severity of anaphylactic shock in patients treated with beta-blockers (French). Presse Med 14:790–791, 1985. [PubMed] [Google Scholar]
- 32. Toogood JH. Beta-blocker therapy and the risk of anaphylaxis. Can Med Assoc J 136:929–932, 1987. [PMC free article] [PubMed] [Google Scholar]
- 33. Berkelman RI, Finton RJ, Elsea WR. Beta-adrenergic antagonists and fatal anaphylactic reactions to oral penicillin (letter). Ann Intern Med 104:134, 1986. [DOI] [PubMed] [Google Scholar]
- 34. Capellier G, Boillot A, Cordier A. Anaphylactic shock in patients treated with beta-blockades (French). Presse Med 18:181, 1989. [PubMed] [Google Scholar]
- 35. Stark BJ, Sullivan TJ. Biphasic and protracted anaphylaxis. J Allergy Clin Immunol 78:76–83, 1986. [DOI] [PubMed] [Google Scholar]
- 36. Hamilton G. Severe adverse reactions to urography in patients taking beta-adrenergic blocking agents. Can Med Assoc J 133:122–126, 1985. [PMC free article] [PubMed] [Google Scholar]
- 37. Zaloga GP, DeLacey W, Holmboe B, Chernow B. Glucagon reversal of hypotension in a case of anaphylactic shock. Ann Intern Med 105:65–66, 1986. [DOI] [PubMed] [Google Scholar]
- 38. Kim Y. Interaction between beta-blockers and epinephrine on hemodynamics of spontaneously hypertensive rats. Res Commun Chem Pathol Pharmacol 80:3–19, 1993. [PubMed] [Google Scholar]
- 39. Matsummura Y, Tan EN, Vaughn JH. Hypersensitivity to histamine and systemic anaphylaxis in mice with pharmacologic beta-adrenergic blockade: Protection by nucleotides. J Allergy Clin Immunol 58:387–394, 1976. [DOI] [PubMed] [Google Scholar]
- 40. Forfang K, Simonsen S. Effects of atenolol and pindolol on the hypokalemia and cardiovascular responses to adrenaline. Eur J Clin Pharmacol 37:23–26, 1989. [DOI] [PubMed] [Google Scholar]
- 41. Raptis S, Rosenthal J, Wetzel D, Moulopoulos S. Effects of cardioselective and non-cardioselective beta-blockade on adrenaline-induced metabolic and cardiovascular response in man. Eur J Clin Pharmacol 20:17–22, 1981. [DOI] [PubMed] [Google Scholar]
- 42. Madowitz JS, Schweiger MJ. Severe anaphylactoid reaction to radiographic contrast media. J Am Med Assoc 241:2813–2815, 1979. [PubMed] [Google Scholar]
- 43. Brummett RE. Warning to otolaryngologists using local anesthetics containing epinephrine: Potential serious reaction occurring in patients treated with beta-adrenergic receptor blockers. Arch Otolaryngol 110:561, 1984. [DOI] [PubMed] [Google Scholar]
- 44. Anonymous. Physician's Desk Reference. Montvale, NJ: Thomson PDR, 3337, 2005. [Google Scholar]
- 45. Bousquet J, Lockey RF, Malling HJ, et al. WHO position paper: Allergen immunotherapy: Therapeutic vaccines for allergic diseases. Allergy 53(suppl 44):1–42, 1998. [DOI] [PubMed] [Google Scholar]
- 46. Lang DM. Anaphylactoid and anaphylactic reactions, hazards of betablockers. Drug Saf J 12:299–304, 1996. [DOI] [PubMed] [Google Scholar]
- 47. Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol 106:840–843, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol 92:225–227, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Roy SR, Sigmon JR, Olivier J, et al. Increased frequency of large local reactions among systemic reactors during subcutaneous allergen immunotherapy. Ann Allergy Asthma Immunol 99:82–86, 2007. [DOI] [PubMed] [Google Scholar]
- 50. Lin MS, Tanner E, Lynn J, et al. Nonfatal systemic allergic reactions induced by skin testing and immunotherapy. Ann Allergy 71:557–562, 1993. [PubMed] [Google Scholar]
- 51. Tikelman DG, Cole WQ, Tunno J. Immunotherapy: A one year prospective study to evaluate risk factors of systemic reactions. J Allergy Clin Immunol 95:8–14, 1995. [DOI] [PubMed] [Google Scholar]
- 52. Confino-Cohen R, Goldberg A. Allergen immunotherapy-induced biphasic systemic reactions: incidence, characteristics, and outcome: a prospective study. Ann Allergy Asthma Immunol 104:73–78, 2010. [DOI] [PubMed] [Google Scholar]
- 53. Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. J Allergy Clin Immunol 123:493–498, 2009. [DOI] [PubMed] [Google Scholar]
- 54. Lieberman P. Anaphylaxis. In Middleton's Allergy: Principles and Practice. Atkinson F, Bochner B, Busse W, Holgate S, Lemanske R, Simons FER. (Eds). Philadelphia, PA: Mosby, an affiliate of Elsevier, Inc., 1027–1051, 2009. [Google Scholar]
- 55. Lieberman P. Biphasic anaphylactic reactions. Ann Allergy Asthma Immunol 95:217–228, 2005. [DOI] [PubMed] [Google Scholar]
- 56. Pumphrey R. Fatal posture in anaphylactic shock. J Allergy Clin Immunol 112:451–452, 2003. [DOI] [PubMed] [Google Scholar]


