The inflammation hypothesis of depression, or more broadly, common mental disorders, proposes that chronic inflammation plays an important role in the pathophysiology of these conditions.1, 2 The hypothesis is supported by experiments of inflammatory stimuli, antidepressant trials and studies on depression-related genes and pathogen host defense,2, 3, 4, 5 but direct population-based evidence from long-term inflammation is scarce. Because of a lack of studies on the effects of chronically elevated inflammation, assessed over several years using repeat measurements, it has remained unclear whether the association between inflammation and common mental disorder is the consequence of acute or chronic inflammation.
This report is from the Whitehall II cohort study.6 In our analysis of up to 4630 adults without chronic disease, we used repeat measures of inflammatory markers and mental disorder. We measured the proinflammatory cytokine interleukin 6 (IL-6) in 1992, 1997 and 2003 and common mental disorder, based on the General Health Questionnaire (GHQ), in 1997, 2003 and 2008. The IL-6 distribution was categorized as: ⩽1.0 pg ml−1 (low), 1.1–2.0 pg ml−1 (intermediate) and >2.0 pg ml−1 (high). Details of study designs, methods and the included/excluded study populations are presented in Supplementary Material, Supplementary Figures S1-S4 and Supplementary Tables S1 and S2.
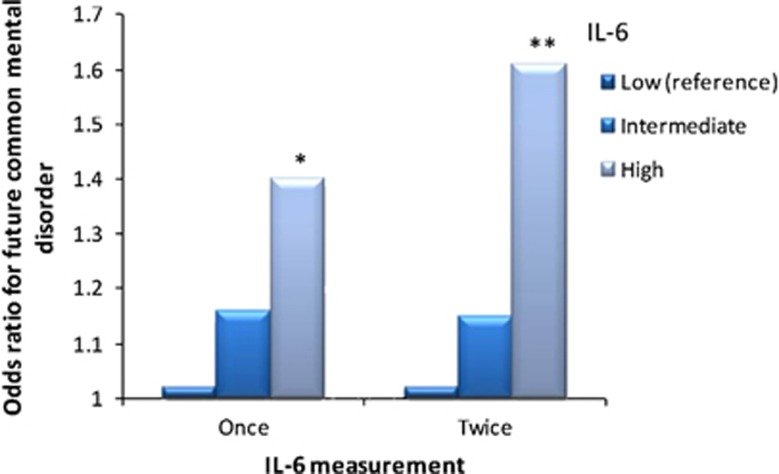
Cross-sectional analysis, using data from 1997, showed IL-6 not to be associated with common mental disorder (age- and sex-adjusted odds ratio for high versus low IL-6=1.04 (95% CI 0.85–1.27), P=0.69, Study Design A). Then we examined the cumulative 10-year risk of common mental disorder among the 2757 participants without the disorder at the first GHQ assessment in 1997. Compared to participants with low IL-6 in 1997, those with high IL-6 had a greater likelihood of common mental disorder in 2003 and/or 2008 (a total of 549 new cases) (age- and sex-adjusted odds ratio=1.40 (1.07–1.82), P=0.01)(Figure 1, Study Design B). Furthermore, participants with high IL-6 at both the 1992 and 1997 assessments had higher odds of a new-onset mental disorder in 2003 and/or 2008, 1.61 (1.14–2.28, P=0.007, Figure 1), with the odds ratio being even higher, 1.75 (1.19–2.57, P=0.004), among those who had high IL-6 in 1992, 1997 and 2003.
Figure 1.
Interleukin 6 (IL-6) and common mental disorder in the Whitehall II study of British civil servants. The figure shows that the increased 10-year risk of common mental disorder associated with high IL-6 levels is more marked when the assessment of IL-6 is based on repeat measurements (age- and sex-adjusted odds ratio=1.61 (95% CI: 1.14–2.28)) than a single measurement (age- and sex-adjusted odds ratio=1.40 (95% CI: 1.07–1.82)). *P<0.05, **P<0.01.
To further examine dose–response associations, we used the number of times a participant had IL-6>2 pg ml−1 as the exposure (Study Design B). Results show that those demonstrating high IL-6 on 0, 1, 2 and 3 occasions had odds ratios of 1.00 (reference), 1.18 (0.94–1.47), 1.38 (1.04–1.83) and 1.56 (1.10–2.21), respectively, for a 10-year risk of common mental disorder (total N=2757; Ptrend=0.002).
Multivariable adjusted results and the sensitivity analyses in Supplementary Table S3 (Study Design C) show that the association between the 5-year average level of IL-6 and subsequent 10-year risk of common mental disorder was little affected by adjustments for acute inflammation, obesity, smoking and drug treatments. The relationship between IL-6 and common mental disorder was evident in both men (odds ratio per doubling of IL-6=1.46 (1.19–1.78)) and women (1.34 (1.00–1.79)), and there was no statistical evidence of sex difference in this relationship (Psex interaction=0.67).
In the subgroup of participants without common mental disorder at the third inflammation measurement (2003, Study Design D), odds ratio of new common mental disorder in 2008 for high IL-6 in 1992, 1997 and 2003 was 1.42 (0.78–2.57) (details in Supplementary Material).
These findings support the hypothesis that persistently elevated levels of IL-6 contribute to the development of common mental disorder.1, 2 The fact that previous studies relied on a single measurement of IL-6 may partially explain the mixed findings:7 a one-off measure does not reliably capture the chronicity of inflammation.8 The present study has limitations: common mental disorder measured by a questionnaire is not the same as clinical diagnosis of depression or anxiety.9 Our data are from an occupational cohort where participants are likely to be healthier than the general population. Loss to follow-up accumulated over the extended follow-up; however, there was no evidence of major differences between the analytic sample and the sample at study recruitment.
Our findings have important clinical implications. If the observed association is causal, then targeting chronic inflammation with anti-inflammatory drugs could be useful in prevention of common mental disorder. Further studies are needed to clarify the underlying mechanisms, such as activation of the tryptophan-degrading enzyme, changes in indoleamine 2,3-dioxygenase, and abnormalities of the hypothalamic–pituitary–adrenal axis. Future investigations should also test the side effects, since anti-inflammatory strategies might increase the risk of infection and malignancy.10
Acknowledgments
KPE, on behalf of the Oxford Department of Psychiatry, receives funding for organization of local continued professional development events from Eisai, Lundbeck, Novartis, Boeringer-Ingelheim, and Pfizer.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. Nat Rev Neurosci. 2008. pp. 46–56. [DOI] [PMC free article] [PubMed]
- Raison CL, Miller AH. Mol Psychiatry. 2013. pp. 15–37. [DOI] [PMC free article] [PubMed]
- Dantzer R, Kelley KW. Brain Behav Immun. 2007. pp. 153–160. [DOI] [PMC free article] [PubMed]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Biol Psychiatry. 2009. pp. 407–414. [DOI] [PMC free article] [PubMed]
- Hannestad J, DellaGioia N, Bloch M. Neuropsychopharmacology. 2011. pp. 2452–2459. [DOI] [PMC free article] [PubMed]
- Kivimäki M, Lawlor DA, Singh-Manoux A, Batty GD, Ferrie JE, Shipley MJ, et al. BMJ. 2009. p. b3765. [DOI] [PMC free article] [PubMed]
- Howren MB, Lamkin DM, Suls J. Psychosom Med. 2009. pp. 171–186. [DOI] [PubMed]
- Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, et al. PLoS Med. 2008. p. e78. [DOI] [PMC free article] [PubMed]
- Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. Lancet. 2007. pp. 859–877. [DOI] [PubMed]
- Miller AH, Maletic V, Raison CL. Biol Psychiatry. 2009. pp. 732–741. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.