Abstract
Ca2+ flux through L-type CaV1.2 channels shapes the waveform of the ventricular action potential (AP) and is essential for excitation-contraction (EC) coupling. Timothy syndrome (TS) is a disease caused by a gain-of-function mutation in the CaV1.2 channel (CaV1.2-TS) that decreases inactivation of the channel, which increases Ca2+ influx, prolongs APs, and causes lethal arrhythmias. Although many details of the CaV1.2-TS channels are known, the cellular mechanisms by which they induce arrhythmogenic changes in intracellular Ca2+ remain unclear. We found that expression of CaV1.2-TS channels increased sarcolemmal Ca2+ “leak” in resting TS ventricular myocytes. This resulted in higher diastolic [Ca2+]i in TS ventricular myocytes compared to WT. Accordingly, TS myocytes had higher sarcoplasmic reticulum (SR) Ca2+ load and Ca2+ spark activity, larger amplitude [Ca2+]i transients, and augmented frequency of Ca2+ waves. The large SR Ca2+ release in TS myocytes had a profound effect on the kinetics of CaV1.2 current in these cells, increasing the rate of inactivation to a high, persistent level. This limited the amount of influx during EC coupling in TS myocytes. The relationship between the level of expression of CaV1.2-TS channels and the probability of Ca2+ wave occurrence was non-linear, suggesting that even low levels of these channels were sufficient to induce maximal changes in [Ca2+]i. Depolarization of WT cardiomyocytes with a TS AP waveform increased, but did not equalize, [Ca2+]i compared to depolarization of TS myocytes with the same waveform. We propose that CaV1.2-TS channels increase [Ca2+] in the cytosol and the SR, creating a Ca2+overloaded state that increases the probability of arrhythmogenic spontaneous SR Ca2+ release.
Keywords: CaV1.2, Timothy syndrome, ventricular myocyte, excitation-contraction coupling, calcium wave
1. Introduction
The function of the heart is to pump blood. To achieve this function, the heart chambers must contract in a specific, electrically coordinated sequence. The cardiac electrical cycle starts with the firing of an action potential (AP) by sinoatrial node cells. This AP propagates via gap junctions to neighboring atrial myocytes and the atrio-ventricular node, eventually reaching the ventricles. Excitation-contraction (EC) coupling is the coordinated process by which this AP triggers cell contraction.
During the AP, depolarization of the sarcolemma briefly opens L-type CaV1.2 channels, thus allowing a small amount of Ca2+ to enter the cytosol [1–4]. The increased [Ca2+]i in the cytosol activates ryanodine-sensitive Ca2+ channels in the sarcoplasmic reticulum (SR) via the mechanism called “Ca2+-induced Ca2+ release” (CICR) [5]. This results in the production of a “Ca2+ spark”[6]. Synchronous activation of thousands of Ca2+ sparks throughout the myocytes causes a cell-wide increase in [Ca2+]i that triggers contraction. Closure of CaV1.2 channels, due to inactivation and membrane repolarization, terminates both Ca2+ influx and release. Re-sequestration of Ca2+ into the SR by the Ca2+ ATPase and Ca2+ extrusion by the sarcolemmal Na+/Ca2+ exchanger (NCX) restores [Ca2+]i to diastolic levels.
This process of Ca2+ release and recovery is critical for the physiological heartbeat. Disruption of the cycle via prolongation of the AP can lead to an inability of the cell to return to diastolic levels before the next systole, thus creating spontaneous Ca2+ release and fatal arrhythmia. Long QT syndrome is one set of disorders in which ventricular repolarization is prolonged. Timothy syndrome (TS), also known as long QT syndrome 8, is a rare childhood disorder caused by a single amino acid substitution (G406R) in exon 8 of CaV1.2, creating a mutant channel (CaV1.2-TS) [7]. It is an autosomal dominant, multisystem disorder, leading to congenital heart disease, syndactyly, immunodeficiency, cognitive abnormalities, and autistic spectrum defects. TS patients commonly suffer sudden cardiac death as a result of lethal cardiac arrhythmias characterized by a long QT interval [7].
In the sarcolemma, CaV1.2 channels can exist in 3 gating modes: 0, 1, and 2. CaV1.2 channels in mode 0 are closed [8]. In mode 1, CaV1.2 channels have a low open probability (Po) and undergo brief openings (<1 ms). In contrast, CaV1.2 channels in mode 2 have a high Po and relatively long open times (>10 ms). CaV1.2 channels in this mode of operation allow relatively large amounts of Ca2+ to enter the cell [9, 10]. CaV1.2-TS channels exhibit increased “mode 2” gating, and undergo a slower voltage-dependent inactivation (VDI). Together, these properties contribute to a longer mean open time of these channels compared to WT channels [11, 12].
We generated transgenic mice expressing variable levels of CaV1.2-TS solely in cardiac myocytes (TS mice) [13]. TS mice have a long QT interval and increased arrhythmia frequency despite having similar resting and exercise heart rates [13]. Two studies have investigated the effects of CaV1.2-TS channels on [Ca2+]i in cultured inducible pluripotent stem cell-derived cardiomyocytes and rat ventricular myocytes [14, 15]. Although important, these studies focused on Ca2+ current changes, leaving some biophysical aspects of EC coupling unexplored, which are difficult to study in cultured adult or immature myocytes due to absence or loss of transverse tubules. Thus, the mechanisms by which CaV1.2-TS causes arrhythmogenic changes in [Ca2+]i remain unclear. In the present study, we found that even a low level of expression of CaV1.2-TS is sufficient to dramatically alter [Ca2+]i in ventricular myocytes. CaV1.2-TS channels increased resting [Ca2+]i, SR load, Ca2+ spark frequency and amplitude, AP-evoked [Ca2+]i transients, and probability of Ca2+ waves, providing evidence that [Ca2+] increases in both the cytosol and SR. We propose that CaV1.2-TS channels create a Ca2+ overloaded state that increases the probability of arrhythmogenic events due to spontaneous SR Ca2+ release.
2. Material and methods
2.1. Isolation of ventricular myocytes
Mice (TS and WT controls) were euthanized with a lethal dose of sodium pentobarbital administered intraperitoneally as approved by the University of Washington Institutional Animal Care and Use Committee. Ventricular myocytes were isolated using a Langendorff perfusion apparatus as previously described [16, 17]. The isolated ventricular myocytes were kept at room temperature (22–25 °C) in Tyrode’s solution containing (mM): 140 NaCl, 5 KCl, 10 HEPES, 10 glucose, 2 CaCl2, and 1 MgCl2; pH 7.4 with NaOH, and used 0.5–8 hours after isolation.
2.2 Additional experimental methods
Please see Appendix A for online data supplement with additional experimental methods.
2.3. Statistics
Data are presented as mean ± standard error of the mean (S.E.M.). Two-sample comparisons were made using a Student’s t test (* P < 0.05, ** 0.001 < P ≤ 0.01, *** P ≤ 0.001).
3. Results
3.1. Higher resting sarcolemmal Ca2+ leak and [Ca2+]i transients in TS compared to WT ventricular myocytes
We have three TS mouse lines. Real time PCR was used to quantify CaV1.2-TS transcript expression in these lines (Figure S1A). Data were normalized to β-actin mRNA levels because it was expressed to a similar extent in WT and TS lines. Using this analysis, we found increasing levels of CaV1.2-TS channels in our 3 TS lines with line 1 expressing the lowest and 3 the highest mRNA (n = 3 mice per group; P < 0.01) (Figure S1A). We also quantified relative protein expression of CaV1.2-TS compared to CaV1.2-WT in these lines and found that line 1 expression was 0.20 ± 0.04, line 2 was 0.40 ± 0.3, and line 3 expression was 1.54 ± 0.68 (n = 4 mice per group; P < 0.05) (Figure S1B). The relationship between CaV1.2-TS mRNA and protein was linear, suggesting that higher mRNA translated into higher CaV1.2-TS protein expression in ventricular myocytes (Figure S1C). Unless specified, TS line 1 was used in the experiments described below because the expression level of this channel is similar in humans with TS [12].
To examine [Ca2+]i in WT and TS myocytes, cells were loaded with the fluorescent Ca2+ indicators Asante Ca2+ Red (ACaR) or Fluo-4 AM and imaged using a confocal microscope. ACaR is a ratiometric Ca2+ indicator that can be excited with 488 nm light. The emission spectrum of the Ca2+-free and -bound forms of ACaR peak at 525 and 650 nm, respectively. The ratio ACaR fluorescence at 650 and 525 nm increases ≈35-fold when [Ca2+] goes from Ca2+-free to saturating levels. Like ACaR, the single wavelength indicator Fluo-4 was excited with 488 nm light. Fluo-4 emission was measured at 525 nm. Fluo-4 undergoes much larger changes in fluorescence than ACaR in response to changes in Ca2+, increasing ≈255-fold when [Ca2+] is elevated from Ca2+-free to saturating levels.
The relationship between the fluorescence intensities of ACaR or Fluo-4 and [Ca2+] is non-linear. Thus, ACaR and Fluo-4 fluorescence values were converted to concentration (nM) units as described by Grynkiewicz et al. [18] and Maravall et al. [19], respectively. The rationale for using these two indicators in the initial set of experiments was manifold. Although the ratiometric ACaR has a lower dynamic range than Fluo-4, it has the advantage that its calibration is not affected by dye concentration or photo-bleaching rates. Fluo-4’s large dynamic range makes it a better choice for the detection of small [Ca2+]i levels than ACaR in TS and WT myocytes. However, because Fluo-4 is a single wavelength indicator, calibrating it assumes no change in laser intensity, photomultiplier gain, pinhole size, and indicator concentration during the experiment. By comparing the [Ca2+]i values obtained using ACaR or Fluo-4, we tested the assumption that these parameters did not change during Fluo-4 experimentation, which would validate the calibration of this indicator.
We recorded action potential (AP)-evoked [Ca2+]i transients in TS and WT myocytes. APs were evoked via field stimulation at a frequency of 1 Hz. Figure 1A–B shows representative AP-evoked [Ca2+]i transients in WT and TS cells under steady-state conditions. The amplitude of the global [Ca2+]i transient was larger in TS than in WT cells whether it was measured using ACaR (WT = 560 ± 46 nM, n = 8 vs. TS = 1003 ± 151 nM, n = 10; P < 0.05) or Fluo-4 (WT = 600 ± 40 nM, n = 49 vs. TS = 1000 ± 62 nM, n = 56; P < 0.001). Diastolic [Ca2+]i — defined as the [Ca2+]i measured at the end of the 1 s interval between APs — was also higher in TS than in WT cells in ACaR (WT = 152 ± 17 nM, n = 8 vs. TS = 237 ± 15 nM, n = 10; P < 0.01) and Fluo-4-loaded cells (WT = 158 ± 8 nM, n = 9 vs. TS = 215 ± 7 nM, n = 10; P < 0.001). [Ca2+]i in non-stimulated, resting myocytes (measured at least 2 seconds after the cessation of stimulation) was also higher in TS (ACaR = 222 ± 13 nM, n = 10 vs. Fluo-4 = 215 ± 15 nM, n = 10) than in WT myocytes (ACaR = 144 ± 12 nM, n = 8 vs. Fluo-4 = 150 ± 6 nM, n = 7; ACaR P < 0.001, Fluo-4 P < 0.01) (Figure 1C).
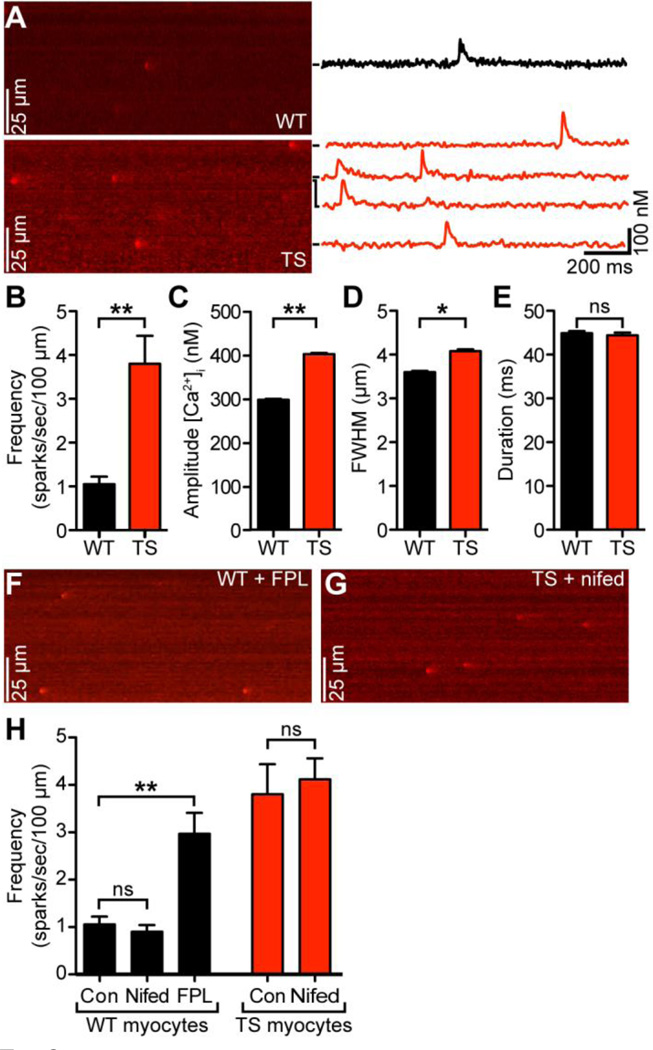
Figure 1. Higher diastolic, systolic, and resting [Ca2+]i and larger sarcolemmal leak in TS cells.
A–B, representative [Ca2+]i (nM) traces of WT (black) and TS (red) cells in diastole, systole, and at rest measured using the indicators ACaR (A) and Fluo-4 (B). C, bar plots of the mean ± S.E.M. of the diastolic, systolic, and resting [Ca2+]i (nM) levels of WT and TS cells measured using each indicator. D, representative time courses of WT (black) and TS (red) cell sarcolemmal [Ca2+]i (nM) leak (upon application of thapsigargin and removal of Na+ and Ca2+ from bath) and recovery (upon restoration of 2 mM Ca2+ to bath). Grey and pink traces indicate same experiment upon application of nifedipine.
We tested the hypothesis that higher “Ca2+ leak” through sarcolemmal CaV1.2 channels contributes to higher resting [Ca2+]i in TS than in WT myocytes (Figure 1D). To test this hypothesis, we recorded [Ca2+]i in resting TS and WT myocytes before and after the application of a 0 Na+/0 Ca2+ external solution in the presence or absence of the CaV1.2 channel blocker nifedipine (10 µM). All solutions in these experiments contained the SERCA pump inhibitor thapsigargin (1 µM) to eliminate SR Ca2+ release. As shown in the representative traces in Figure 1D, application of the 0 Na+/0 Ca2+ external solution decreased [Ca2+]i in TS and WT myocytes, presumably due to the termination of Ca2+ leak via Na+/Ca2+ exchanger and Ca2+-permeable channels and extrusion via the sarcolemmal Ca2+ pump. In the WT myocyte, return to the Ca2+ containing extracellular solution increased resting [Ca2+]i to control levels (150 ± 4 nM, n =6) even in the presence of nifedipine (153 ± 4 nM, n = 6, P > 0.05). This suggests that Ca2+ flux via CaV1.2 channels does not contribute to resting [Ca2+]i in WT myocytes. However, in TS myocytes, [Ca2+]i increased only to 160 ± 4 nM upon return to control conditions in the presence of nifedipine compared to 207 ± 13 nM without nifedipine (P < 0.05).
We differentiated these records to determine the rate of change in resting [Ca2+]i during the switch from the 0 Na+/0 Ca2+ to the 2 mM external Ca2+ solution. The maximum d[Ca2+]i/dt was 0.0010 ± 0.0001 nM/ms and 0.004 ± 0.001 nM/ms in WT and TS myocytes (P < 0.05), respectively. By contrast, the maximum d[Ca2+]i/dt of the AP-evoked [Ca2+]i transients in the presence of 1 µM thapsigargin was 2.5 ± 0.1 nM/ms (n = 8 WT cells). Thus, while the maximum rate of Ca2+ influx at rest is faster in TS than in WT myocytes, it is about three orders of magnitude slower than the rate of Ca2+ influx into a ventricular myocyte during EC coupling.
Collectively, these data suggest that TS myocytes have a higher resting, diastolic, and systolic [Ca2+]i than WT myocytes. Although Ca2+ influx through CaV1.2 channels does not contribute to resting [Ca2+]i in WT cells, expression of CaV1.2-TS channels results in higher resting sarcolemmal leak and consequently higher [Ca2+]i in TS than in WT cells. Furthermore, our data indicate that ACaR and Fluo-4 report similar [Ca2+]i in ventricular myocytes, suggesting that laser intensity, photomultiplier gain, pinhole size, and Fluo-4 concentration did not change under our experimental conditions. Thus, calibration of Fluo-4 using the Maravall et al. [19] method is valid. Based on this, and the higher dynamic range of Fluo-4, this indicator was used in the experiments presented below.
3.2. TS myocytes have a higher frequency of Ca2+ sparks and waves than WT myocytes
We investigated the mechanisms underlying higher [Ca2+]i transients in TS myocytes. Experiments tested the hypothesis that RyR activity is higher in TS myocytes than in WT myocytes. A testable prediction of this hypothesis is that spontaneous Ca2+ spark activity is higher in TS myocytes than in WT myocytes.
Figure 2A shows representative confocal line-scan images of Ca2+ sparks in quiescent/resting WT and TS cells. Consistent with our hypothesis, we found that spontaneous Ca2+ spark frequency was ≈3.5-fold higher in TS (3.8 ± 0.6 sparks/100 µm/s, n = 11 cells) than in WT cells (1.1 ± 0.2 sparks/100 µm/s, n = 15 cells; P < 0.01) (Figure 2B). Ca2+ sparks in TS cells also had larger amplitude (401 ± 3 nM, n = 1602 vs. 297 ± 2 nM, n = 1782, P < 0.01) (Figure 2C) and full width at half-maximal amplitude (4.0 ± 0.1 ms, n = 1602 vs. 3.6 ± 0.1 ms, n = 1782; P < 0.05) (Figure 2D) compared to WT cells. Ca2+ spark duration, however, was similar in these cells (WT = 45 ± 1 ms, n = 1782 vs. TS = 44 ± 1 ms, n = 1602; P > 0.05) (Figure 2E).
Figure 2. Higher Ca2+ spark activity in TS cells.
A, representative confocal line-scan images from WT (above) and TS (below) cells. [Ca2+]i (nM) plotted to the right for each spark seen, marked by black bars. B–E, bar plots of the mean ± S.E.M. of the frequency (B), amplitude (C), full width at half-maximal amplitude (D), and duration (E) of Ca2+ sparks in WT and TS myocytes. F, representative confocal line-scan image of a WT cell upon long-term application of FPL 64176. G, representative confocal line-scan image of a TS cell upon short-term application of nifedipine. H, bar plots of the mean ± S.E.M. frequency of Ca2+ sparks in WT myocytes upon application of FPL 64176 and nifedipine and TS myocytes upon application of nifedipine.
If higher Ca2+ channel activity is sufficient to increase resting [Ca2+]i and thus induce higher Ca2+ spark activity in TS than in WT ventricular myocytes, exposing WT cells to a CaV1.2 channel opener should increase Ca2+ spark frequency in these cells to a level similar to that of TS cells. Figure 2F shows a representative line-scan image of a quiescent WT cell exposed to 1 µM FPL 64176, a drug known to increase the open probability of CaV1.2 channels. Consistent with our hypothesis, FPL 64176 increased resting [Ca2+]i (from 150 ± 16 to 209 ± 26 nM) and Ca2+ spark frequency in WT cells to a level similar to the one observed in TS cells (3.0 ± 0.4 sparks/100 µm/s, n = 10 cells; P < 0.05) (Figure 2H). Application of 1 µM FPL 64176 to TS cells invariably induced Ca2+ waves and cell death. It is important to note, that the effects of FPL 64176 on WT and TS myocytes were not rapid. Rather, the effects of FPL 64176 on Ca2+ spark (or waves) activity were seen approximately 3 minutes after exposure to the drug, during which time the cell was not stimulated. Similarly, short-term application (i.e., <20 s) of nifedipine (10 µM) did not affect Ca2+ spark frequency in WT and TS cells (P > 0.05) (Figure 2G–H). This suggests that spontaneous Ca2+ sparks are not directly activated or produced by the opening of CaV1.2 channels in TS or WT myocytes.
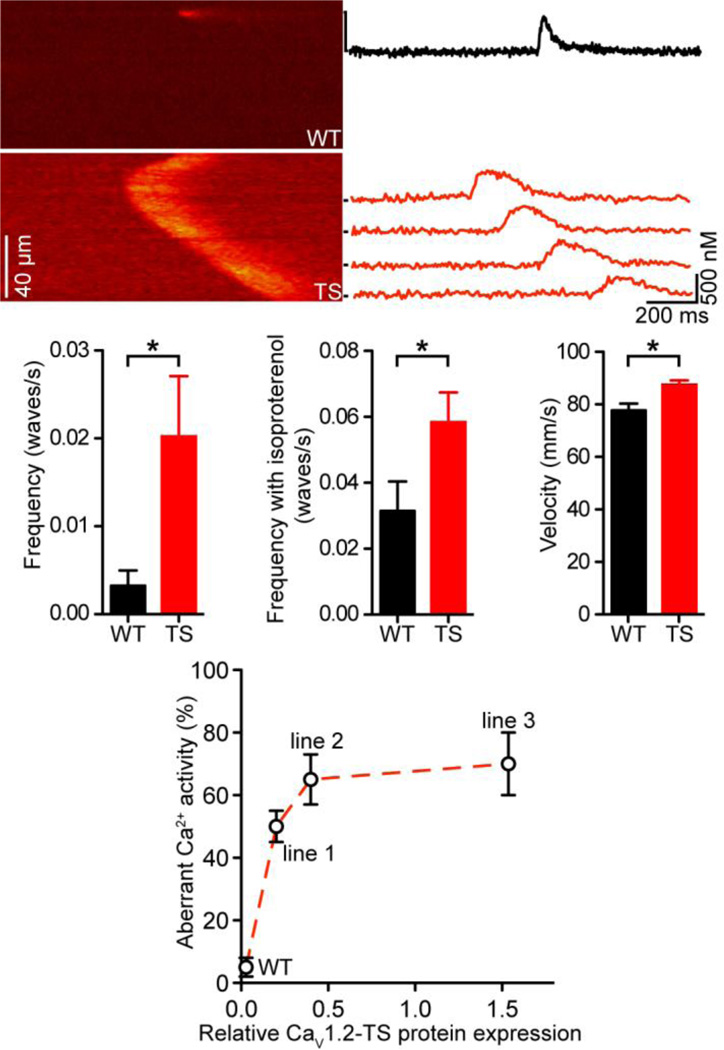
Having determined that TS myocytes have a higher propensity to undergo spontaneous SR Ca2+ release than WT myocytes, we performed a quantitative analysis of arrhythmogenic spontaneous Ca2+ waves in these cells. Figure 3A shows representative line-scan images of a large, non-propagated Ca2+ spark in a WT myocyte and a Ca2+ wave in a TS cell. Indeed, we found that while in WT cells Ca2+ waves are rare (1 wave per 308 ± 166 s, n = 25), the frequency of Ca2+ waves is dramatically higher in TS myocytes (1 wave per 49.4 ± 16.8 s, n = 37; P < 0.05) (Figure 3B). This relationship is maintained even upon application of 100 nM of the β adrenergic receptor agonist isoproterenol (WT, 1 wave per 31.7 ± 9.0 s, n = 17 vs. TS, 17.1 ± 2.7 s, n = 22; P < 0.05) (Figure 3C). Analysis of the Ca2+ waves suggested that they propagate at a faster rate in TS (87.4 ± 1.7 µm/s, n = 147) than in WT cells (77.7 ± 2.6 µm/s, n = 25; P < 0.05) (Figure 3D).
Figure 3. Higher Ca2+ wave activity in TS cells.
A, representative confocal line-scan images of a large spark in a WT cell (above) and a Ca2+ wave in a TS cell (below). [Ca2+]i (nM) plotted to the right for each line marked by black bars. B–D, bar plots of the mean ± S.E.M. of the frequency (B), frequency upon application of 100 nM isoproterenol (C), and velocity (D) of Ca2+ waves in WT and TS myocytes. E, plot of relative CaV1.2-TS expression (x-axis) vs. frequency of aberrant Ca2+ activity (%) (y-axis).
We extended this Ca2+ analysis to the other two CaV1.2-TS mouse lines to investigate the relationship between CaV1.2-TS expression and arrhythmogenic Ca2+ wave activity in ventricular myocytes. Interestingly, although only 5 ± 3% of the WT myocytes (n = 12) had spontaneous Ca2+ waves, 50 ± 3 (n = 16), 65 ± 8 (n = 19), and 70 ± 10% (n = 22) of the myocytes isolated from TS mice lines 1–3 had Ca2+ waves (Figure 3E). This suggests a nonlinear relationship between TS expression and aberrant Ca2+ wave activity in a cell.
3.3. Higher SR Ca2+ load in TS than in WT ventricular myocytes
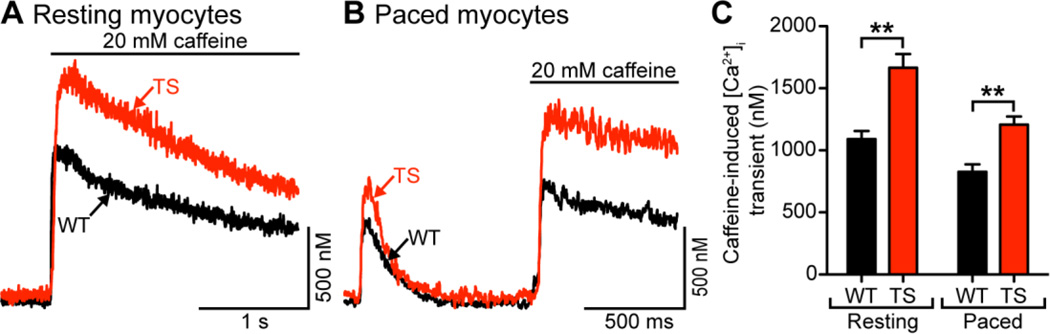
A potential mechanism for a higher frequency of spontaneous Ca2+ sparks, Ca2+ waves, and larger AP–evoked [Ca2+]i transients is an elevation in the SR Ca2+ load. Thus, we tested the hypothesis that SR Ca2+ content was higher in TS than in WT myocytes. We used a picospritzer to rapidly apply a Na+- and Ca2+-free saline solution containing 20 mM caffeine to both paced and quiescent/resting WT and TS cells. Figure 4A shows caffeine-induced [Ca2+]i transients from representative resting TS and WT myocytes. Under these experimental conditions, the amplitude of the caffeine induced [Ca2+]i transient was ≈1.5-fold higher in TS (1666 ± 109 nM, n = 11) compared to WT cells (1090 ± 66 nM, n = 17; P < 0.01) (Figure 4C), suggesting that TS myocytes have a higher SR Ca2+ load than WT myocytes.
Figure 4. Higher resting and paced SR load in TS cells.
A, representative SR Ca2+ release upon application of 20 mM caffeine in resting WT (black) and TS (red) cells. B, representative SR Ca2+ release upon application of 20 mM caffeine in WT (black) and TS (red) cells paced at 1 Hz. C, bar plot of the mean ± S.E.M. of SR Ca2+ release upon application of 20 mM caffeine in resting and paced WT and TS myocytes.
Figure 4B shows caffeine-induced [Ca2+]i transients from representative TS and WT myocytes which had been paced at 1 Hz until reaching steady-state. At this frequency, the amplitude of the [Ca2+]i transient of TS and WT myocytes reached steady state after 7 APs. On average, the amplitude of the caffeine-induced [Ca2+]i transient in paced cells was ≈1.4-fold higher in TS (1207 ± 66 nM, n = 60) compared to WT cells (828 ± 59 nM, n = 49; P < 0.01) (Figure 4C).
3.4. The large SR Ca2+ release during EC coupling in TS cells increases the rate of inactivation of ICa and hence limits Ca2+ influx during membrane depolarization into these cells
In the heart, the Ca2+ current (ICa) triggers SR Ca2+ release during EC coupling. Membrane depolarization opens CaV1.2 channels, but these channels inactivate due to Ca2+-dependent and voltage-dependent mechanisms [20]. SR Ca2+ release is a major contributor to Ca2+-dependent inactivation of ICa [21]. Although CaV1.2-TS currents inactivate at a slower rate than WT channels, the effects of SR Ca2+ release on these currents are unknown.
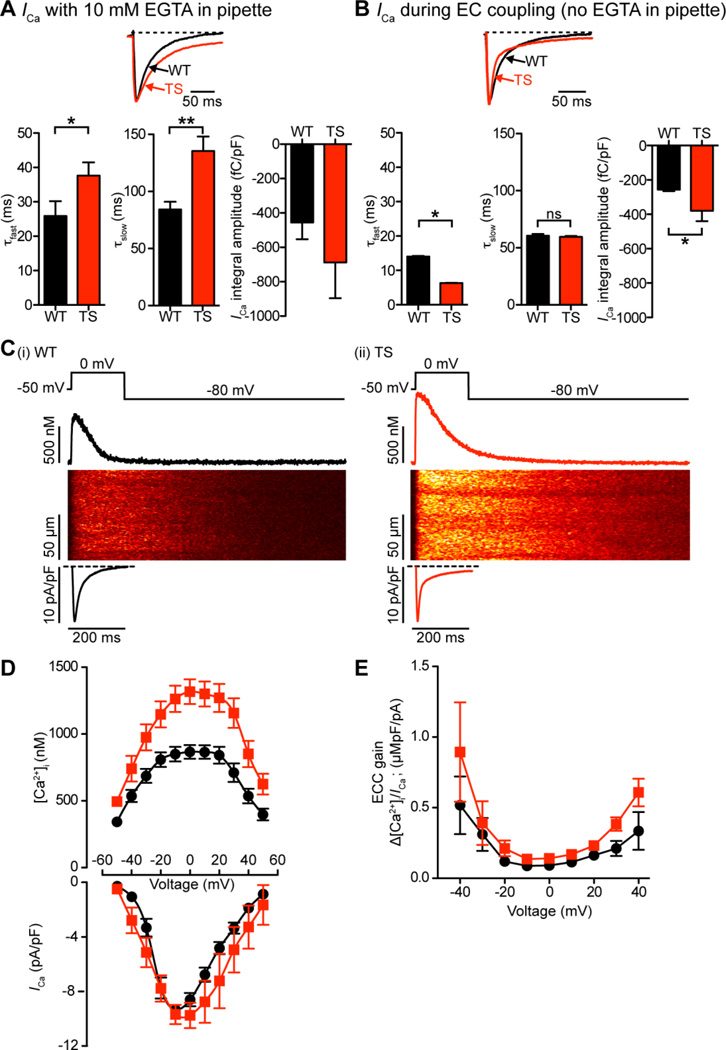
Figure 5A shows representative ICa records from TS and WT myocytes dialyzed with an intracellular solution containing 10 mM of the Ca2+ chelator EGTA to maintain low global [Ca2+]i and eliminate SR Ca2+ release. Peak ICa at 0 mV was −1154 ± 202 pA in WT (n = 5) and 1734 ± 541 pA in TS cells (n = 6). However, because TS cells (214 ± 25 pF) had a higher capacitance than WT cells (171 ± 25 pF), ICa density (i.e., pA/pF) was similar in these cells (WT = −6.9 ± 1.1 pA/pF vs. TS = −7.7 ± 1.8 pA/pF; P > 0.05). We fitted the decaying phase of these currents with the sum of two exponential functions. The time constants of the fast (τfast; WT = 26 ± 4 ms vs. TS = 38 ± 4 ms; P < 0.05) and slow component (τslow; WT = 84 ± 7 ms vs. TS = 136 ± 13 ms; P < 0.01) were larger in TS than in WT cells. Accordingly, the total charge associated with the ICa recorded from EGTA-dialyzed cells (determined by integrating these currents) was −688 ± 208 fC/pF in TS (n = 6) compared to −455 ± 98 fC/pF in WT cells (n = 5) (Figure 5A).
Figure 5. Higher SR Ca2+ release during EC coupling in TS cells increases the rate of inactivation of ICa.
A–B, representative ICa records normalized to peak from TS and WT myocytes dialyzed with an intracellular solution containing (A) or lacking (B) 10 mM of the Ca2+ chelator EGTA. Currents were evoked by a voltage step from −50 mV to 0 mV in the presence of 2 mM external Ca2+. Below, bar plots of the mean ± S.E.M. of the inactivation components of ICa: τfast (ms), τslow (ms), and current integral (fC/pF) in WT and TS myocytes. C, representative ICa and confocal line-scan images from WT (i) and TS (ii) cells. ICa and [Ca2+]i transients were evoked by a 200 ms voltage step from −50 to 0 mV. Traces showing the time course of ICa and [Ca2+]i in these cells are shown below and above the line-scan images, respectively. D, voltage dependence of the amplitude of the [Ca2+]i transient on positive y-axis, current-voltage relationship of ICa on negative y-axis, for both WT (black) and TS (red) cells. E, voltage dependence of EC coupling (ECC) gain for WT (black) and TS (red).
Figure 5C shows line-scan images, ICa densities, and the time course of the spatially-averaged [Ca2+]i of TS and WT cells depolarized to the test potential of 0 mV. EGTA was absent from the patch pipette solution to ensure normal SR Ca2+ release during membrane depolarization. To achieve steady-state SR Ca2+ load, 10 pre-conditioning pulses (100 ms duration) from −80 to 0 mV (1 Hz) were applied just prior to the test pulse. Control experiments showed that TS myocytes (amplitude of the caffeine-induced transient = 1224 ± 74 nM) had a higher SR Ca2+ load than WT cells (amplitude of the caffeine-induced transient = 828 ± 59 nM) when depolarized with a similar waveform (P < 0.05). Test pulses began with a slow ramping depolarization (0.08 mV/ms) from −80 mV to −50 mV, where the cell was held for 50 ms to inactivate Na+ channels. In addition, to ensure no contaminating Na+ channel currents, 10 µM tetrodotoxin was added to the perfusion solution. Cells were then depolarized for 200 ms from this interim holding potential to voltages ranging from −40 to +60 mV.
Although, on average, peak ICa density was similar in WT and TS cells, the [Ca2+]i transients these currents evoked were larger in TS (n = 7) than in WT myocytes (n = 7) at all voltages examined (Figure 5D; P < 0.05). Consequently, EC coupling gain, defined here as the maximum change in [Ca2+]i divided by the peak ICa density at a given voltage, tended to be higher in TS than in WT cells over a broad range of voltages (Figure 5E).
Interestingly, with SR Ca2+ release enabled, ICa inactivated at a faster rate in TS than in WT cells. This was largely due to a faster first component in TS (τfast = 6 ± 1 ms, n = 6) than in WT cells (τfast = 14 ± 2 ms, n = 5; P < 0.05), as τslow was similar in these cells (WT = 61 ± 17 ms, n = 5 vs. TS = 60 ± 11 ms, n = 6; P > 0.05). However, because TS cells had a larger noninactivating component, total Ca2+ flux during the 200 ms pulse was ≈1.5-fold higher in TS cells (−389 ± 60 fC/pF, n = 3) than WT cells (−256 ± 8 fC/pF, n = 5; P < 0.05) (Figure 5B).
Taken together, the data in Figure 5 suggest that SR Ca2+ release and EC coupling gain are larger in TS than in WT myocytes. SR Ca2+ release has a profound impact on the kinetics of ICa in TS cells. In the absence of EC coupling ICa inactivates at a slower rate in TS than in WT myocytes. However, with SR Ca2+ release, ICa inactivates faster, but to a sustained current level in TS cells.
3.5. Differences in AP waveform amplify differences in Ca2+ influx between TS and WT myocytes
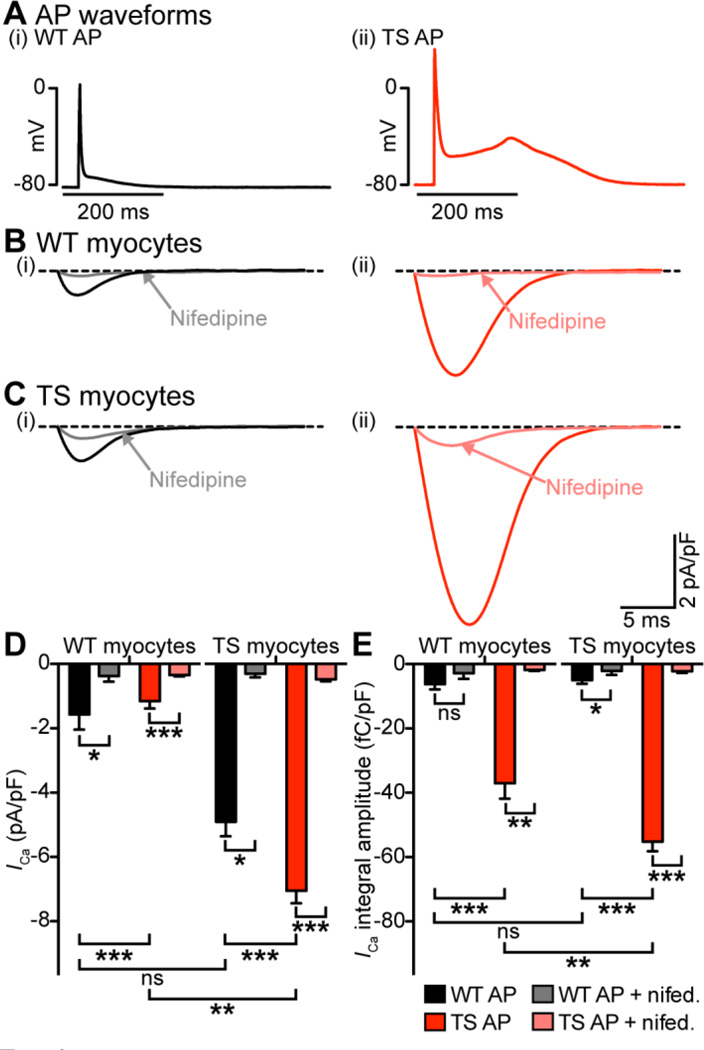
Although the use of square voltage pulses to evoke ICa is convenient for technical and analytical purposes, they do not provide information on Ca2+ influx during the physiological AP. We used the AP clamp technique to test the hypothesis that Ca2+ influx is larger in TS than in WT myocytes during the AP. Myocytes were depolarized with a previously recorded WT or TS AP (Figure 6A). Figure 6B–C shows averaged AP-evoked Ca2+ current traces from WT and TS myocytes in the absence or presence of 10 µM nifedipine. In WT cells, the TS AP evoked currents with a larger peak (−4.9 ± 0.5 pA/pF, n = 9) and integral (−37.0 ± 4.9 fC/pF, n = 9) than the WT AP (peak, −1.6 ± 0.5 pA/pF, n = 11; integral, −6.2 ± 1.7 fC/pF, n = 11; P < 0.001) (Figure 6D–E). Similarly, in TS cells, the TS AP evoked currents with a larger peak (−7.0 ± 0.4 pA/pF, n = 11) and integral (−55.2 ± 2.9 fC/pF, n = 11) than the WT AP (peak, −1.1 ± 0.2 pA/pF, n = 11; integral, −5.0 ± 1.2 fC/pF, n = 11; P < 0.001). Interestingly, applying the TS AP evoked a greater response from TS myocytes (−55.2 ± 2.9 fC/pF, n = 11) than WT myocytes (−37.0 ± 4.9 fC/pF, n = 9; P < 0.01) (Figure 6B–E). Strong reduction of these AP-evoked currents with 10 µM nifedipine suggests that these currents are produced by Ca2+ influx via CaV1.2 channels (Figure 6B–E). Importantly, these data suggest that both AP waveform and CaV1.2-TS gating properties contribute to the increase in ICa seen in TS.
Figure 6. Differences in AP waveform contributes to differences in L-type Ca2+ current between WT and TS myocytes.
A, representative WT (i) and TS (ii) APs. B–C Current traces from a representative WT myocyte (B) and a TS myocyte (C) stimulated with the WT AP (i) or TS AP (ii). Traces recorded in the presence of 10 µM nifedipine are labeled and indicated with arrows. D–E, bar plots of the mean ± S.E.M. of the amplitude of ICa (D) and the current integral (E) in WT and TS myocytes stimulated with the WT or TS APs with and without 10 µM nifedipine.
3.6. Differences in AP waveform contribute to differences in EC coupling between WT and TS cells
Next, we tested the hypothesis that differences in the AP waveform between WT and TS cells contribute to differences in [Ca2+]i between these cells. A testable prediction of this hypothesis is that if differences in [Ca2+]i between WT and TS cells were due exclusively to differences in AP waveform, then [Ca2+]i transients of WT cells stimulated with TS APs should resemble TS cells and vice versa. Thus, we recorded [Ca2+]i transients while using a AP clamp to again apply WT or TS AP waveforms.
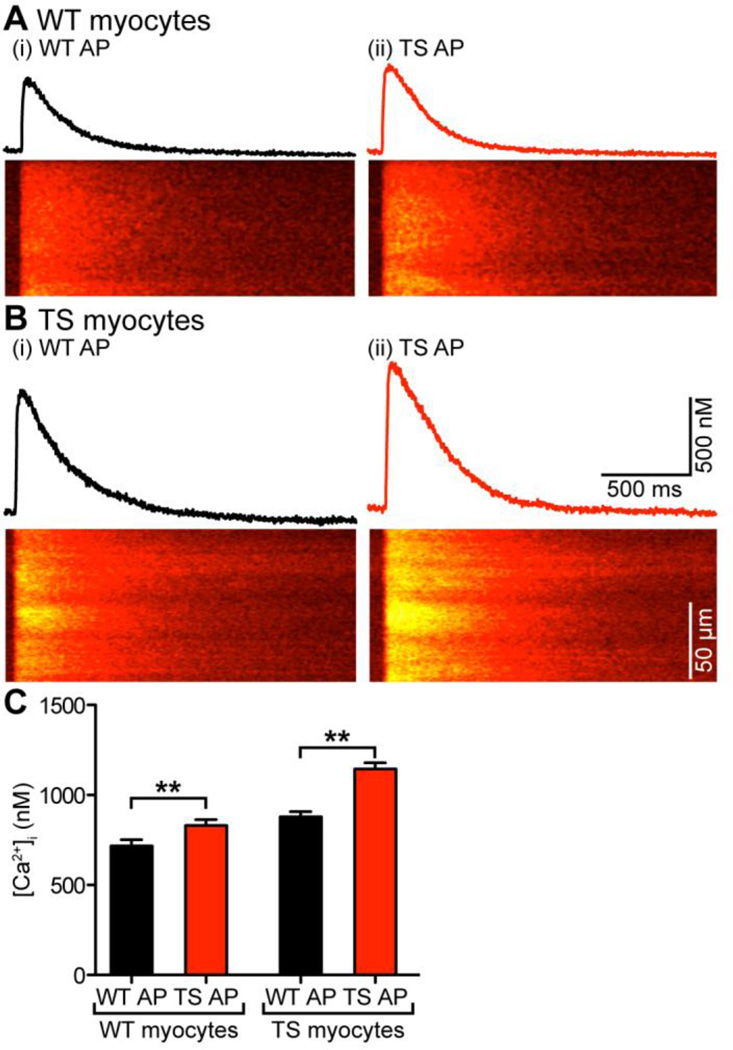
Figure 7A–B shows a set of confocal line-scan images and [Ca2+]i transients from representative WT and TS cells depolarized with WT and TS APs. As expected, WT cells stimulated with a WT AP had smaller [Ca2+]i transients (717 ± 35 nM, n = 13) compared to TS cells stimulated with a TS AP (1144 ± 35 nM, n = 13; P < 0.01). In addition, we found that WT cells stimulated with TS APs had larger [Ca2+]i transients than when stimulated with WT APs, and TS cells stimulated with WT APs had smaller [Ca2+]i transients than when stimulated with TS APs. Interestingly, however, the amplitudes of these transients were not recapitulations of WT or TS cells. Rather, the [Ca2+]i transient of WT cells depolarized with a WT AP (717 ± 35 nM, n = 13) was smaller in amplitude compared to the [Ca2+]i transients of TS cells depolarized with a WT AP (878 ± 29 nM, n = 11; P < 0.05). Similarly, the [Ca2+]i transient of TS cells depolarized with a TS AP (1144 ± 35 nM, n = 13) was larger in amplitude compared to the [Ca2+]i transients of WT cells depolarized with a TS AP (831 ± 33 nM, n = 13; P < 0.05) (Figure 7C). These data indicate that differences in AP waveform contribute to, but do not sufficiently account for the differences in [Ca2+]i transients observed between WT and TS cells.
Figure 7. Differences in AP waveform contributes to differences in [Ca2+]i between WT and TS myocytes.
A–B, [Ca2+]i transients and confocal line-scan images from a representative WT (A) and TS myocyte (B) stimulated with the WT AP (i) or TS AP (ii). C, bar plot of the mean ± S.E.M. of the amplitude of the [Ca2+]i transient in WT and TS myocytes stimulated with the WT or TS APs.
4. Discussion
We performed a detailed biophysical analysis of the functional consequences of CaV1.2-TS channel expression on EC coupling in adult ventricular myocytes. On the basis of these data, we propose a mechanistic model for how CaV1.2-TS channels alter [Ca2+]i and EC coupling in ventricular myocytes. Our data indicate that expression of CaV1.2-TS increases resting and AP-evoked Ca2+ influx into ventricular myocytes. This is associated with an increase in diastolic [Ca2+]i and SR Ca2+ load that likely augments the frequency and amplitude of spontaneous Ca2+ sparks in TS cells. Accordingly, AP-evoked [Ca2+]i transients were larger in TS than in WT myocytes. Furthermore, we found that there is a non-linear relationship between the expression of CaV1.2-TS channel and Ca2+ wave frequency, suggesting that relatively low levels of CaV1.2-TS expression induces a disproportionally large increase in the probability of arrhythmogenic SR Ca2+ release events in ventricular myocytes.
The mouse model of TS we generated expresses WT CaV1.2 channels and mutant CaV1.2 channels with the glycine at position 406 substituted by an arginine, which promotes mode 2 gating of CaV1.2-TS channels [9]. Recent studies suggest two potential mechanisms by which the G406R substitution alters CaV1.2-TS gating. Erxleben et al. [9] suggested that this G406R mutation creates a new phosphorylation site for the CaMKII. In their experiments in HEK293 cells, CaMKII was critical for mode 2 gating by CaV1.2-TS channels. Thiel et al. [14] reached a similar conclusion using cultured ventricular myocytes. Others, however, suggest that phosphorylation by CaMKII may not be necessary for CaV1.2-TS channels to have a slower rate of inactivation than WT channels [13, 22].
Cheng et al. [13] proposed an alternative model for CaV1.2-TS channel dysfunction during Timothy syndrome. In this model, the anchoring protein AKAP150 and CaV1.2-TS form a complex that is necessary for aberrant CaV1.2-TS channel gating and arrhythmias. CaV1.2-TS channels likely interact with AKAP150 via leucine zipper motifs in the C-terminals of these proteins [23]. AKAP150 functions like an allosteric modulator of CaV1.2-TS channels, increasing CaV1.2-TS currents by stabilizing the open conformation (i.e., mode 2 gating) and increasing the probability of coupled gating between CaV1.2-TS channels [13, 16, 24]. The longer openings of CaV1.2-TS channels are due, at least in part, to decreased voltage-dependent inactivation of these channels [11]. This leads to increased Ca2+ influx, AP prolongation, cardiac hypertrophy, and arrhythmias. Coupled gating of CaV1.2-TS channels presumably occurs because AKAP150 promotes physical interactions of adjacent channels via their C-tails [23–25]. Thus, a combination of frequent, longer, and coupled openings of CaV1.2-TS channels increases Ca2+ influx even with shorter APs. Furthermore, although increasing K+ channel conductance is likely to decrease Ca2+ influx, it may not be sufficient to eliminate the pathology.
Our data suggest that the impact of CaV1.2-TS channels on myocyte [Ca2+]i extends beyond the AP. Indeed, we found that resting and diastolic [Ca2+]i was higher in TS than in WT myocytes. In quiescent myocytes, [Ca2+]i is largely determined by the balance between Ca2+ extrusion and influx through the sarcolemma. The two primary pathways of Ca2+ influx are CaV1.2 channels and the Na+/Ca2+ exchanger (NCX) in its “reverse mode” of operation. Ca2+ is extruded by the NCX in its forward mode and, to a much lower extent, by the sarcolemmal Ca2+ pump [26, 27]. Because at the diastolic potential of ventricular myocytes (−80 mV) the open probability of WT CaV1.2 channels is very low, Ca2+ influx through these channels is also minimal. Consistent with this, we found that application of nifedipine does not alter resting [Ca2+]i in WT ventricular myocytes. However, CaV1.2-TS channels have a higher level of activity than WT channels even at diastolic membrane potentials [13, 24]. Accordingly, nifedipine-sensitive Ca2+ influx — presumably via CaV1.2-TS channels — contributes to higher resting [Ca2+]i in TS than in WT myocytes. Thus, while in WT myocytes resting [Ca2+]i is largely determined by the NCX and to a lesser extent the sarcolemmal Ca2+ pump, in TS cells CaV1.2-TS channels represent a new Ca2+ “leak” pathway at rest. Yet, our analysis indicate that this Ca2+ leak is likely due to a relatively low number of persistently open CaV1.2-TS channels open at rest, as the rate of Ca2+ influx at these potentials is orders of magnitude lower than during the AP.
Our results show that the TS phenotype not only increases cytosolic [Ca2+] directly via increased flux through TS channels, it also increases the SR load and causes the SR to be more leaky. As noted above, CaMKII signaling has been implicated in SR leak, has been found to be activated in TS [14, 28, 29]. Bradshaw et al. [30] found that CaMKII activation is relatively insensitive to resting [Ca2+]. Indeed, they found that a [Ca2+]i ≈ 3.2 µM is needed to activate 50% CaMKII in vitro. Consistent with this, the Bers group [31, 32] found that the majority of CaMKII activation in ventricular myocytes was found in areas of local high [Ca2+]i, such as the dyadic cleft. Thus, we speculate that these high Ca2+ areas would be both more abundant and richer in Ca2+ in TS due to increased Ca2+ sparklets [13, 24], Ca2+ spark activity, higher Ca2+ transients, and Ca2+ wave activity. CaMKII activation in these areas could further augment SR leak and increase mode 2 gating of TS and WT channels in TS cells, creating a feed-forward mechanism that could increase the probability of arrhythmogenic changes in Ca2+ signaling. Furthermore, it is intriguing to speculate that the seemingly contradictory reports [13, 22] on the role of CaMKII on CaV1.2-TS channel function may reflect differences in local Ca2+ signaling and thus the activity of this kinase. Future experiments should examine this issue in detail.
Our data suggest that SR Ca2+ release had a profound impact on the kinetics of ICa in TS cells, greatly accelerating the rate of inactivation of this current. This is important because SR Ca2+ release increased CDI, limiting Ca2+ influx into TS myocytes. Because CDI is unaffected in TS channels [11], the faster rate of inactivation is likely due to the faster CDI of WT and TS CaV1.2 channels in TS cells. Note, however, that while ICa inactivates at a much lower rate in TS than in WT in the absence of EC coupling, with SR Ca2+ release enabled, TS ICa inactivates faster than WT cells, albeit to a higher sustained level. We propose that SR Ca2+ release forms part of a negative feedback mechanism that decreases Ca2+ influx in TS cells by increasing CDI of WT and TS CaV1.2 channels.
An important observation in this study is that the relationship between the level of expression of CaV1.2-TS channels and the probability of Ca2+ wave occurrence was non-linear. This suggests that even low levels of these channels are sufficient to induce maximal changes in [Ca2+]i. A potential mechanism by which a relatively small number of CaV1.2 channels could have a disproportionally large effect on [Ca2+]i was proposed by Dixon et al. [33], who found that CaV1.2-TS channels can physically interact with WT CaV1.2 channels. When they do, WT channel activity increases to a level similar to that of TS channels. In this context, even a small level of expression of functional TS channels could have a disproportionally large effect on Ca2+ influx by making adjoined WT channels function like TS channels. Fusion of channels increases ICa by increasing the open probability of adjoining channels. It is intriguing to speculate that in heterozygous humans expressing G406R CaV1.2-TS channels, where these channels account for only ~12% of CaV1.2 channels, coupling of WT and TS channels amplifies Ca2+ influx and thus increases the probability of SR Ca2+ overload and arrhythmias [12].
Furthermore, the relationship between [Ca2+]i and Ca2+ overload has been well characterized and shown to be highly nonlinear. A two-fold increase in diastolic [Ca2+]i could lead to up to an 84-fold increase in pathological Ca2+ activity such as Ca2+ waves [34]. This non-linear relationship between CaV1.2-TS expression and Ca2+ waves arises from an increase in basal [Ca2+]i, SR Ca2+ content, and Ca2+ spark properties such as frequency and amplitude as well as the non-linear combinatorics of these properties’ influence (e.g., RyR Ca2+ sensitivity) on Ca2+ wave development. In line with these results, we have found that even low levels of TS expression lead to a ≈1.4-fold increase in diastolic [Ca2+]i, a ≈3-fold increase in Ca2+ spark frequency, and a ≈10-fold increase in Ca2+ wave frequency. Furthermore, it is important to note that potential changes in the electrical properties of the TS cells (e.g., decreased inward rectifying K+ currents) could conspire with higher [Ca2+]i to increase the probability of arrhythmogenic voltage fluctuations in these cells.
To conclude, CaV1.2-TS channels exhibit greater Ca2+ flux, which increases [Ca2+] in both the cytosol and the SR. This Ca2+-overloaded state leads to increases in Ca2+ spark frequency and amplitude, AP-evoked [Ca2+]i transients, and probability of Ca2+ waves. The increase in CaV1.2-TS Ca2+ flux also leads to increased SR Ca2+ load, thus increasing EC coupling gain. The non-linear relationship between CaV1.2-TS expression and Ca2+ waves shows that even low levels of CaV1.2-TS can induce dramatic effects on cell Ca2+ levels. These conditions combine to create a cell environment prone to arrhythmogenic spontaneous SR Ca2+ release.
Supplementary Material
Highlights.
Sarcolemmal Ca2+ “leak” and diastolic [Ca2+]i are higher in TS than in WT cells.
Sarcoplasmic reticulum Ca2+ load is higher in TS than in WT myocytes.
Ca2+ release increases the rate of inactivation of CaV1.2 currents in TS myocytes.
Few CaV1.2---TS are sufficient to induce maximal change in [Ca2+]i.
Ca2+---overload in TS creates increased arrhythmogenic events.
Acknowledgements
We thank Ms. Jennifer Cabarrus for technical assistance, and Drs. Jose Mercado and Claudia Moreno for editing the manuscript. This study was supported by NIH grant HL085686 and the ARCS Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none declared
References
- 1.López-López JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- 2.Niggli E, Lederer WJ. Voltage-independent calcium release in heart muscle. Science. 1990;250:565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- 3.Santana LF, Cheng H, Gomez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- 4.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 5.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 7.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 9.Erxleben C, Liao Y, Gentile S, Chin D, Gomez-Alegria C, Mori Y, et al. Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices. Proc Natl Acad Sci U S A. 2006;103:3932–3927. doi: 10.1073/pnas.0511322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 11.Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc Natl Acad Sci U S A. 2008;105:2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintron M, Scott JD, et al. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ Res. 2011;109:255–261. doi: 10.1161/CIRCRESAHA.111.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiel WH, Chen B, Hund TJ, Koval OM, Purohit A, Song LS, et al. Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation. 2008;118:2225–2234. doi: 10.1161/CIRCULATIONAHA.108.788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shioya T. A simple technique for isolating healthy heart cells from mouse models. J Physiol Sci. 2007;57:327–335. doi: 10.2170/physiolsci.RP010107. [DOI] [PubMed] [Google Scholar]
- 17.Rossow CF, Dilly KW, Santana LF. Differential Calcineurin/NFATc3 Activity Contributes to the Ito Transmural Gradient in the Mouse Heart. Circ Res. 2006;98:1306–1313. doi: 10.1161/01.RES.0000222028.92993.10. [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 19.Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annual review of cell and developmental biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 21.Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarotskyy V, Gao G, Peterson BZ, Elmslie KS. The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation. J Physiol. 2009;587:551–565. doi: 10.1113/jphysiol.2008.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, et al. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, Robinson CV, et al. Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc Natl Acad Sci U S A. 2011;108:6426–6431. doi: 10.1073/pnas.1014400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers DM, Bassani JW, Bassani RA. Competition and redistribution among calcium transport systems in rabbit cardiac myocytes. Cardiovascular research. 1993;27:1772–1777. doi: 10.1093/cvr/27.10.1772. [DOI] [PubMed] [Google Scholar]
- 27.Balke CW, Egan TM, Wier WG. Processes that remove calcium from the cytoplasm during excitation- contraction coupling in intact rat heart cells. J Physiol. 1994;474:447–462. doi: 10.1113/jphysiol.1994.sp020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 29.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 30.Bradshaw JM, Kubota Y, Meyer T, Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc Natl Acad Sci U S A. 2003;100:10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95:4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Q, Saucerman JJ, Bossuyt J, Bers DM. Differential integration of Ca2+-calmodulin signal in intact ventricular myocytes at low and high affinity Ca2+-calmodulin targets. J Biol Chem. 2008;283:31531–31540. doi: 10.1074/jbc.M804902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon RE, Yuan C, Cheng EP, Navedo MF, Santana LF. Ca2+ signaling amplification by oligomerization of L-type Cav1.2 channels. Proc Natl Acad Sci U S A. 2012;109:1749–1754. doi: 10.1073/pnas.1116731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.