Pain and disability are the primary symptoms for patients who suffer from osteoarthritis (OA), representing one of the major health burdens in the industrialized world (reviewed in [1]). Current symptom management approaches (NSAIDs, viscosupplementation, opiates, corticosteroids) are largely inadequate, because of their limited efficacy, particularly for severe OA pain, and the plethora of safety issues with prolonged treatment. Ultimately, uncontrolled pain is the primary motive for total joint replacement (TJR) [2] and even after TJR a significant portion of subjects report persistent pain of unknown origin [3]. Effect sizes of NSAIDs, the most commonly used painkillers in OA, are small to moderate and often close to those of placebo [4, 5]. Serious side effects associated with chronic use of NSAIDs have been extensively documented [6]. Recently, blockade of Nerve Growth Factor (NGF) was reported to be strongly analgesic in knee OA [7] but an unexpected side effect was encountered in seemingly accelerated OA, especially in patients who were taking concomitant NSAID therapy [8]. These issues underscore the significant gaps in our current understanding of OA pain: firstly, the molecular pathways that generate and maintain the pain but also the relationship between joint pathology and pain and whether this changes with disease stage, progression or initiating cause (“trigger”).
Currently Used Animal Models For The Study Of OA-Associated Pain
Filling in these considerable shortcomings in our knowledge will require that clinical research in OA patients is complemented by studies in disease-specific animal models of OA. Clinical studies provide important data on association between clinical symptoms (i.e. pain) and particular tissue pathologies, genetic differences (e.g. SNPs), psychosocial determinants etc, and these can be described “risk factors”. Ascribing a causal relationship between a specific molecular, cellular or pathological event and OA pain, requires therapeutic or prophylactic modification of that factor with a measurable change in the onset, severity or progression of the pain. In the absence of such interventions for patients with OA, defining the key changes that cause OA pain needs to be investigated in pre-clinical models where such factors can be prophylactically or therapeutically targeted (e.g. specific genetic mutations).
The number of research papers specifically aiming to evaluate pain and pain mechanisms in animal models of OA is surprisingly small, relative to the extent of the medical problem it represents. A Pubmed search conducted on March 31 2013 using the search terms “osteoarthritis pain” yielded 13,391 results whereas adding the keyword “animal models” revealed just 240 papers, only 113 of which were original reports on pain in OA animal models. On the contrary, a search for “animals models of OA” resulted in 1737 papers (3/25/2013), describing a plethora of models, including spontaneous and induced disease (using at least 20 induction methods) in variably aged male and female animals of some 10 different species (reviewed in [9-11]). The majority of these studies aimed to investigate the pathophysiological mechanisms of OA joint pathology and/or test potential disease-modifying therapies. It remains unclear whether any one of the array of models and or species is superior and more predictive of translation to humans, both with regard to disease mechanisms and therapeutic targets. Nevertheless, our understanding of the cellular and molecular pathways that regulate the initiation and progression of structural joint damage in OA has advanced enormously as a result of findings from animal models.
The number of OA models/induction methods used to study pain, and the animals (species, age, gender) in which they have been examined is much more restricted than for studies of structural pathology [12]. The animal models used to study OA pain and the techniques to assess pain in the papers retrieved from the PubMed search, are listed in Table 1. The opportunities and limitations associated with the most commonly used models are discussed, in addition to well-established and emerging techniques for evaluating pain. We will briefly discuss evidence of neuronal degeneration in pre-clinical models, while specific mechanisms of pain uncovered in animal models are reviewed in detail elsewhere in this special issue [13]. We have focused this discussion largely on studies in small animals (mouse, rat, guinea pig) as these represent the most commonly used species for OA pain investigation, as is becoming the case in all pre-clinical medical research (Understanding animal research http://understandinganimalresearch.org.uk/). There is no evidence to suggest that pain outcomes in small animals better replicate human disease than other species used (e.g. dog, sheep, horse), and these larger animals may provide more anatomically and biomechanically useful models of humans, particularly for evaluation of potential non-pharmacological symptom-modifying OA therapies (e.g. surgery, physical therapy). In dogs and horses in particular, pain and disability associated with OA is a significant clinical problem, and thus findings in these species could have a direct therapeutic and economic veterinary impact in addition to translation to human disease.
Table 1.
- Animal Models of OA and changes in nociception/pain reported
| MODEL | SPECIES | Changes in Nociception /Pain Outcomes reported |
|---|---|---|
| MIA | Rat (knee) | - Mechanical hypersensitivity (progressive) in hind paw [84]; - Weight bearing deficit [84]; - Altered gait [85]; - Diminished hind limb grip force [86]; - Cooling hypersensitivity [19]; - Vocalization in response to knee bend [85]; - Conditioned place preference [87] - Locomotive changes, including rearing (assessed by photocell) [88] - Depressed wheel- running [89]; - Altered sleep patterns [90]; |
| Mouse (knee) | - Diminished locomotion during forced exercising [91]; - Mechanical hypersensitivity [92]; |
|
| Guinea Pig (knee) | - Altered weight-bearing [93]; - Mechanical allodynia [93] |
|
| Surgical models (Instability inducing) | Rat ACLT | - Gait changes [94]; - Weight bearing deficits [95] - Mechanical allodynia [95]; |
| Rat MMT | - Weight bearing asymmetry [96] - Mechanical allodynia [96] |
|
| Mouse DMM | - Mechanical allodynia (von Frey) early on, maintained for 16 weeks; absence of thermal allodynia up to 8 weeks post DMM [52] - Late-onset altered behavior on Laboras platform (reversible with indomethacin) [37, 38] - Late-onset weight bearing deficit [38]; |
|
| Mouse partial medial MNX | - Vocalization upon knee compression [75] - No weight bearing deficit [75] - Secondary mechanical allodynia and hypersensitivity [75] - Cold hypersensitivity [75] |
|
| Rabbit Partial MNX | Changes in weight bearing [97] | |
| Dog ACLT | Altered gait and locomotion [98] | |
| Dog Groove model | Altered gait [99] | |
| Sheep MNX, DMM | Altered gait [100] | |
| Horse osteochondral fragment plus exercise (carpus) | Altered gait; reduced mechanical nociceptive threshold (joint flexion) [101] | |
| Obesity-associated OA | Mouse | Changes in locomotion [57] Mechanical allodynia/hypersensitivity [57] Anxiety-like behaviors [57] |
| Other | Rat Collagenase-induced arthritis | Mechanical and thermal allodynia [51] |
| Mouse Collagenase-induced arthritis | Changes in weight distribution [102] |
References in this table represent a selection from the 113 papers revealed by the Pubmed search in addition to hand-selected papers that were missed in the search or appeared after the search date. Abbreviations used: MIA = mono-iodoacetate; ACLT = anterior cruciate ligament transection; MNX = meniscectomy; MMT = medial meniscal tear; DMM - destabilization of the medial meniscus.
Pain assessment in OA models
Evaluating joint pain in animal models of OA is fraught with many practical complications requiring an observant and patient experimenter. The subjectivity in interpreting some of the pain behavioural responses reflects the need for blinded experiments whenever possible. A number of pain behaviour assessment techniques have recently been borrowed from the pain field at large and applied to OA pain measurement. All of these behaviour measures have their own advantages and limitations. As such, multiple different tests should be carried out in order to provide a global measure of OA pain.
I. Electrophysiology
A powerful but technically demanding method of quantifying joint nociception involves recording from neurones in the pain pathway. When peripheral nerves become sensitized through local release of algogenic agents in OA joints, the frequency of firing of these nociceptors is dramatically increased. This in turn causes plasticity changes in second order neurones in the dorsal horn of the spinal cord leading to central sensitization. By recording from these pain-transmitting neurones it is possible to build an elegant picture of the changing neurophysiological properties of the nervous system during OA.
Early experiments in which single unit recordings were made from joint primary afferent neurones showed that C and Aδ fibres possess mechanogated ion channels [14]. That is to say, these sensory nerves express ion channels that only open in response to mechanical movement of the joint leading to the generation of neural impulses and the production of mechanosensation. The first recordings from OA joint mechanoceptors were carried out in the rat mono-iodoacetate (MIA) model, in which it was found that joint mechanosensory nerves become sensitized in response to joint degeneration [15]. While MIA itself does not sensitize peripheral nerve endings, the resultant degeneration and concomitant production of chemical mediators activate joint nociceptors in a concentration-dependent manner [16]. Other studies have investigated nociceptor activity in the Dunkin-Hartley guinea pig model of spontaneous OA in which the sensory nerves are hyperactive, even at rest [17]. Interestingly, it was discovered that the severity of joint destruction in this model did not correlate with nociceptor firing rate, highlighting a disconnect between disease and symptom. Part of the reason for this uncoupling could be due to the decline in the number of thinly myelinated neurones in OA joints [17] or an alteration in the sensitivity of OA joint nociceptors to algogenic mediators [18].
In addition to OA promoting peripheral sensitization, it has recently been shown that second-order neurones in the dorsal horn of the spinal cord also become hypersensitive following joint destruction. Electrophysiological recording of neurones located in laminae V-VI in the dorsal horn of MIA-injected rats revealed enhanced responsiveness to mechanical stimulation of their peripheral receptive fields [19]. It should be noted, however, that although the knee joint was rendered osteoarthritic, the test mechanical stimuli were applied to the hindpaw and not the joint. Therefore, a direct link between joint afferent activity and central sensitization has yet to be confirmed. Nevertheless, central sensitization would amplify nociceptive signals arising from the periphery leading to enhanced pain perception in diseased joints. Furthermore, these plasticity changes in the spinal cord could mean that joint pain may still be experienced in the absence of any peripheral input. Other studies have shown that microglia contribute to central sensitization in OA joints and that inhibition of these satellite cells could be a useful means of managing pain in diseased joints [20].
II. Evoked Pain Behaviour
The majority of pain behaviour tests used in the laboratory employ some sort of evoked response to an external environmental stimulus. These stimuli can be mechanical, thermal or chemical; however, only mechanical stimulation bears any real relevance to arthritic pain in a clinical sense. Evoked pain behaviour experiments are typically carried out on rodents as these animals are easy to handle and readily respond to sensory testing. Rats are the species of choice as they are easier to handle and are less susceptible to stress-induced analgesia compared to mice [21]. Mice are significantly more active than rats and are therefore more problematic when it comes to these types of pain tests, which typically require the animal to be in a state of rest. Animal restraint is counterproductive here as the test subject will exhibit stress-induced analgesia. The exploratory behaviour of rodents can be tempered somewhat by habituating the animal to the test apparatus over hours or days prior to measurement. Mice require more habituation than rats and investigator patience is certainly key here. Habituation to the test environment will also minimize the incidence of startle responses in which the animal simply reacts to a novel stimulus rather than a true pain behaviour. Repeat animal handling is beneficial in rats as this makes the animals more relaxed and amenable to sensory testing. Excessive handling of mice, however, is not advised as these animals become increasingly stressed with persistent human interaction. Finally, the test laboratory should be quiet, warm and free of perfumes as any sort of extraneous stimulus can cause stress or promote startle responses. As mentioned previously, mechanically-evoked responses are most germane for arthritis pain testing and shall be the main focus here.
von Frey hair algesiometry
Mechanical allodynia is commonly measured by application of von Frey hairs to the dorsal, glabrous surface of the hindpaw and determining a threshold for mechanosensitivity. Since arthritis is usually induced in the knee and the von Frey hairs are applied to the paw, this technique is really measuring referred pain or secondary allodynia in these models. The original hairs used by Maximillian von Frey in 1896 were taken from various animals (e.g. squirrel, badger and swine), but nowadays calibrated nylon filaments are used. These monofilaments are of various thicknesses and consequently bend with a discrete force when pressed against the skin. During the training period, animals are habituated to a Perspex container with a wire mesh floor and the von Frey hair is repeatedly applied to the paw. This approach reduces the likelihood of false positive responses that could be attributed to startle effects. Three different approaches to determining mechanosensitivity have been developed. The first involves choosing a mid-range von Frey hair and determining whether it produces a true withdrawal response. If positive then a thinner filament is chosen and again applied to the hindpaw. If the animal does not respond to the filament, a thicker von Frey hair is chosen instead. In this manner the mechanical threshold is ascertained. The second method uses an up-down approach as originally described by Dixon then subsequently refined by Chaplan and colleagues [22]. In this regression analysis approach the mechanical threshold is inferred from response versus non-response observations. The final method uses 3 filaments with either low, medium or high bending forces and the number of positive responses to 10 applications of each von Frey hair is recorded.
More recently, an automated von Frey hair algesiometer has been developed which uses a motorized controller to apply a single filament with increasing force. A mirror is used to align the filament so that it will push against the metatarsal region of the hindpaw. The animal should be stationary and not exploring prior to filament application. A positive reaction to the mechanical stimulus involves a rapid withdrawal possibly followed by licking of the paw.
Vocalization
Many mammals communicate their mood, condition and identity by vocalizing. Each vocalization has a distinct signature based on acoustic frequency and duration, which encodes the physiological and psychological well-being of the animal. While audible squeaks in response to a noxious stimulus indicate a nociceptive response, rodents can also emit ultrasonic chirps which underlie a more affective component of pain [23, 24]. These ultrasonic calls have a frequency between 18-32kHz, are between 300-4000ms in duration and 65-85dB in sound pressure [25, 26]. Monitoring vocalization as a means of interpreting rodent pain is complicated by the fact that ultrasonic chirps are also emitted following copulation, submission, “tickling”, and the presence of a predator. Thus, ultrasonic vocalizations are context-specific and are open to a degree of subjective interpretation. Nevertheless, ultrasonic vocalization has been used as an affective pain readout and can be ameliorated by opioid treatment [23]. Animal models of arthritis have been found to emit audible calls as well as ultrasonic chirps in response to noxious stimulation of the affected joint [27, 28]. As for other types of evoked pain behaviour, animals must be habituated to the test environment so as to avoid any startle effects or stress-induced vocalization.
Pressure application measurement (PAM) device
Evoked pain responses typically involve applying the noxious stimulus to the hindpaw and since the majority of arthritis models centre around the knee, these experiments are measuring secondary allodynia. To circumvent this limitation of evoked pain techniques, a pressure application measurement (PAM) device can be used to apply the stimulus directly to the knee. The instrument consists of a calibrated force sensor which is worn on the thumb of the experimenter. With the animal gently restrained, the PAM device can be pressed against the joint of interest and the peak force required to elicit a withdrawal response is indicative of mechanosensitivity. Experiments using rodent models of joint inflammation have found that the PAM device gives a robust measure of knee joint pain with high inter-experimenter agreement. So far, the PAM device has not been tested on rodent models of OA.
III. Gait analysis
Patients living with OA often exhibit abnormal movement patterns primarily due to altered joint kinematics. While some of these gait changes in OA are due to deterioration in joint congruency, compensatory movement to minimize joint loading and pain is also likely to play a part. While monitoring animal movement following arthritis induction could reveal some interesting insights into pain perception, a couple of caveats need to be considered. Firstly, it is difficult to interpret whether any observable gait changes are due to OA pain or a consequence of altered joint biomechanics. Secondly, since rodents are prey animals they tend to disguise any gait deficiencies because in the wild they would be a prime target for predation. Arthritis-induced gait changes in higher order mammals (e.g. dogs and cats) is more pronounced than in rodents making these animals better suited for kinematic studies.
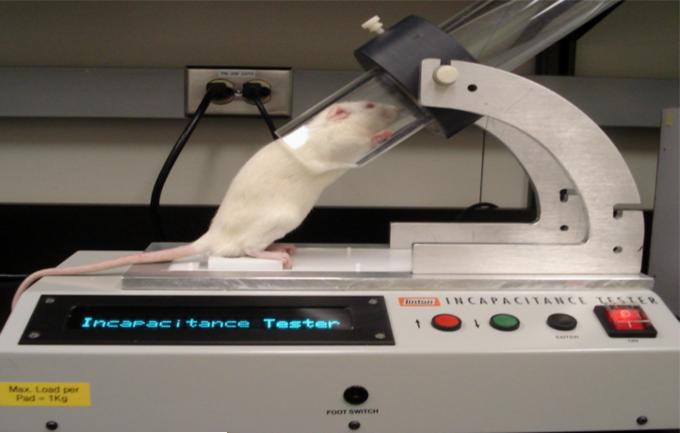
Two types of joint function have been used to assess pain in OA animals viz. static weight bearing and dynamic gait analysis. In the former approach, hindlimb weight bearing between an arthritic and non-arthritic contralateral hindlimb are measured by an incapacitance meter. This technique involves training an animal to stand with each hindlimb resting on individual force plates. The amount of body weight distributed between the two hindlimbs is averaged over a 3-5 second period. Weight bearing deficits have been observed in various models of OA including the MIA model [29-31], and following joint instability [32]. The standard receptacle used to restrain rodents for incapacitance measurement has the disadvantage that animals tend to lean on the sides of the box thereby dissipating some of their body weight away from the force plates. We and others have modified the system to take advantage of the fact that rodents like to hide in drainpipes. The modified apparatus uses a tube into which the rat will readily stand so that total body weight is now directed towards the force plates (Fig. 1). While the incapacitance tester has been found to produce consistent and reproducible measurements in rats, its use in mice is less robust due to the hyperactive and overly exploratory nature of this species.
Fig. 1.
Modification of the rodent incapacitance meter showing a rat in the correct resting position. The advantage of the tube is that it encourages the animal to stand with its weight directed onto the force plates.
Several approaches are available to assess gait in a freely moving animal. The first simply involves dipping the paws of the animal in India ink and then allowing it to run in a straight line across paper. Rudimentary gait parameters such as stride length and paw area can be easily quantified using this method. These principles have been automated in the Catwalk apparatus wherein a high speed digital camera records the movement of a rodent as it traverses a glass platform. Ferland et al. recently reported that the swing time of MIA treated and unstable joints were significantly greater than control animals and this effect could be reversed acutely with a cyclooxygenase-2 inhibitor [33]. The Catwalk technique has been modified to replace the glass platform with a transparent Perspex treadmill belt. The use of a treadmill standardizes the speed at which the animal walks and this forced movement approach has been validated in rodent models of arthritis [34, 35].
IV. Spontaneous pain behaviours
A tremendous amount of pain behaviour information can be gleaned from simply observing OA animals over a period of time. Animals in chronic pain tend to be withdrawn, hypo-locomotive, exhibit shallow breathing and become hypotensive. These spontaneous pain behaviours are thought to be more clinically relevant than evoked pain responses; however, obtaining scientifically robust measures of spontaneous pain is laborious and open to subjective interpretation.
Activity-based assessment
In an attempt to standardize spontaneous pain data capture, a number of automated protocols have recently been developed. The LABORAS system, for example, analyses distinct vibration signatures generated by freely moving rodents to create a record of behaviours, which includes grooming, feeding, climbing and rearing. The system consists of a rodent cage of specific dimensions with a food hopper and water bottle at distinct locations in the cage. The animal cage sits on a triangular platform with highly sensitive force transducers at the apices of the platform. Using sophisticated software, it is possible to create a detailed time-course of animal behaviours which can be later analysed offline. Readings are best performed overnight as rodents are nocturnal and are therefore more active during this time. The advantage of the LABORAS system is that there is very little direct intervention by the animal tester, no need for extensive habituation to any apparatus and the animal behaviours are spontaneous. The technique has been successfully used in rodent models of arthritis which generally showed reduced locomotion, rearing and climbing behaviour [36-38]. Whether these changes in behaviour are due to pain per se or a consequence of fatigue or general ill-health is open to interpretation.
Facial expressions
One of the challenging aspects in the clinical assessment of pain is how to determine pain levels in non-communicative patients (e.g. babies, dementia patients). In this realm, facial expressions have been used as a means of interpreting pain severity by analysing facial features such as cheek raise or eye tightening. These principles were successfully applied to animal models of pain including inflammatory joint pain [39]. Using automated frame grabbing software, still images were captured from videos of rat faces and scored for pain features including orbital tightening, nose/cheek flattening, ear angulation, and whisker positioning. This rat grimace scale was found to be highly accurate, reliable and reproducible between blinded scorers. The main limitation of the technique is that it is most effective for measuring acute pain responses and therefore may not be suitable for OA pain assessment.
Involvement of Neural Degeneration in OA
In the treatment of OA pain, it has been known for a long time that some patients are unresponsive to classical analgesics such as NSAIDs and opioids. Since these drugs are primarily used to treat inflammatory pain it only recently dawned on us that these OA patients could possibly be experiencing neuropathic pain. Evidence from our own laboratory showed that the peripheral nervous system is responsible for some of the vascular disturbances associated with degenerative joint disease [40] and these vasomotor changes were due to heightened release of neurogenic mediators such as calcitonin gene-related peptide. Interestingly, these neuropeptides can also sensitize joint afferents and cause pain [30] [41] [42]. Further investigation revealed that the sensory nerves innervating injured joints were punctate, contorted and full of pain producing neuropeptides [43] [44]. This pattern of innervation is consistent with a peripheral neuropathy providing some of the first evidence that the pain found in degenerating joints could have a neuropathic component. This was later corroborated by the observation that gabapentin, which is used to treat neuropathic pain, can reduce afferent hypersensitivity in arthritic joints [45]. Neuronal tracer studies have also shown that OA joint afferents undergo a progressive degeneration as evidenced by heightened expression of the nerve injury marker activating transcription factor-3 [46]. These findings highlight that OA pain is a lot more complicated than originally thought. Mixed inflammatory and neuropathic components to the disease mean that targeted therapies are likely to be ineffective in alleviating OA pain. A treatment strategy which tackles both aspects of the disease depending on different disease states may have a better chance of managing the debilitating symptoms of OA.
Considerations when studying pain-related behaviors and pathways in animal models of OA
1) Are pain-related behaviours and associated pathways dependent on the model used to induce arthritis?
A recent review [12] discussed the heterogeneity of OA models with respect to changes in different joint tissues, such as degree of inflammation, joint instability, progression and extent of cartilage damage, and osteophytosis - thus highlighting how different approaches to induce OA-like changes in small animals may be used to model different aspects of the heterogeneous human syndrome we call “osteoarthritis” [47]. When using animal models to elicit joint pain and explore its cellular and molecular mechanisms, it is still unclear whether there are differences between different models, and what ultimately the implications may be for translation to human disease. There is emerging evidence that different methods of inducing arthritis may be associated with distinct pain behaviours. Few papers report side-by-side comparison of different models within the same lab – but when this is done, pain behaviours show different patterns. For instance, a paper comparing pain behaviours following intra-articular mono-iodoacetate (MIA) injection versus partial meniscectomy (MNX) in the rat [32] revealed that MIA rats displayed persistent robust secondary mechanical allodynia and hyperalgesia, whereas partial MNX was associated with milder and slower-onset allodynia, without hyperalgesia. In addition, MIA rats had more marked reduction in weight bearing on the ipsilateral limb throughout the 4-week study. The overall severity of joint damage was similar in both models, and thus the authors concluded that “the type of joint damage rather than the absolute extent is important in generating a behavioural pain response” [32]. Using different models that differentially display specific aspects of structural joint pathology should enable this hypothesis to be tested. For instance, collagenase-induced OA is associated with synovial inflammation [48] more so than the DMM (destabilization of the medial meniscus) model [49] and as such there are differential disease-modifying effects when these two models are compared in genetically-modified mice [50]. Interestingly, mechanical allodynia is a feature of both models, whereas thermal hypersensitivity (to heat) in the hind paw can be detected in collagenase-induced arthritis [51] but not in the DMM model (when mechanical and thermal hypersensitivity were assessed side by side in the same laboratory [52]). In general, models of inflammatory arthritis are strongly associated with thermal hyperalgesia, e.g. carrageenan-induced arthritis [53] and collagen-induced arthritis [54]. As different molecular and neuronal pathways are engaged in mediating these types of hypersensitivity, this clearly offers an opportunity for comparative analysis of pathways of pain generation associated with different aspects of OA. At this time, there is no information on whether spinal and supraspinal pathways of pain processing differ between different OA models. These results from different models point to the need for careful and precise interpretation of data from pre-clinical studies and its translation to different subtypes or stages of human OA.
Very few reports in the literature assess pain in models other than MIA and instability-provoked models. Obesity is a major risk factor for development and progression of knee OA [55] as well as for knee pain [56] – yet, obesity is seldom utilized as a model for OA induction. One report investigated the effects of dietary obesity in a one-year study in C57Bl/6 mice [57]. High-fat diet led to symptomatic features of OA, including hyperalgesia and anxiety-like behaviors, in association with OA-like changes in the knee and impaired musculoskeletal force generation and motor function compared with controls. Age is the other major risk factor for OA, yet most studies in small animals are performed in younger mice. In the DMM model, surgery on 12 month-old mice results in markedly more severe OA than in 12-week old mice [58], but comparative data on pain behaviors are not available. One study examined the relationship between age, joint nociception, and joint pathology in naturally occurring OA in Dunkin Hartley guinea pigs [17]. The level of joint pathology correlated well with increasing age, whereas joint nociception, assessed by electrophysiological recording from knee joint afferents, was not correlated with OA severity.
Finally, almost all reported studies on OA pain have studied animal models using the knee joint (Table 1) - gait abnormality/lameness associated with induced carpal OA in the horse is the most common exception. Consensus recommendations for pharmacological therapy of knee and hip OA (rather than physical or topical treatments which may be impacted by divergent anatomy) did not define or identify any treatments that were effective in one joint but not the other [5]. Comparisons of the efficacy of some treatments for symptomatic OA relief do suggest there may be joint specific differences e.g. intra-articular corticosteroids have a longer lasting effect in hip OA [59] compared with knee OA [60]. There are however very few direct comparative studies in people where the effects of a given therapy in different joints are reported – in an evaluation of the efficacy of rofecoxib no significant difference associated with joint location (hip versus knee) was found [61]. Whether there are differences in the pain pathways in different joints has not been directly evaluated in animal studies. It is noteworthy that in Collagen VI null mice, hips show increased age-associated OA (cartilage erosion) compared with wild type mice [62] while knees show decreased OA (cartilage erosion and subchondral bone thickening) [63]. This may suggest there are differences in the underlying molecular mechanisms of OA between joints, and suggest analogous joint-specific pain mechanisms could also be identified.
2) Are pain-behaviours correlated with structural changes?
The discordance between radiographic severity and pain in patients has been well documented, particularly for knee OA [64]. The availability of MRI to study specific features of OA changes has identified pathological changes, including bone marrow lesions (BML) and synovitis, that show much stronger correlation with the severity of existing pain as well as incident pain [65], providing new insights into the origins of joint pain. Studies in animal models are clearly lagging behind in this field, although the ability to assess specific histological changes in animals should in theory offer a tremendous opportunity. Animal models have been used to test disease-modifying efficacy of compounds that target proteins implicated in OA pathogenesis. An increasing number of these studies also address concomitant analgesic effects, as listed in Table 2.
Table 2.
- Effect of putative DMOADs on joint structure and pain assessments
| Target/Compound | Model | Effect on Joint Pathology | Changes in Nociception/Pain Outcomes reported | Ref. | Comments |
|---|---|---|---|---|---|
| MMP-13 (selective inhibitor) | Rat MIA 2-week follow up | Cartilage protection | Prevention of changes in weight-bearing | [103] | In the same paper, the same compound had chondroprotective effects in the rat MMT model, but effects on associated pain were not analyzed. |
| ADAMTS-4/ADAMTS-5 selective inhibitor | Rat MMT 13-week follow-up | Cartilage protection | Prevention of changes in weight-bearing | [104] | |
| ADAMTS-5 KO mice | Mouse DMM 8-week follow-up | No OA-like pathology | No development of secondary mechanical allodynia (unlike wild type mice, which develop progressive allodynia) | [52] | |
| Cathepsin K (selective inhibitor) | Guinea Pig spontaneous 1-month follow-up | Decreased urinary CTXII (marker of type II collagen degradation) | Reduced mechanosensitivity (elctrophysiologically determined) in response to noxious and non-noxious joint movement | [105] | Cathepsin K inhibition has recently gathered attention as a promising target for structure modification in OA, and selective inhibitors have proven efficacious in canine, mouse, and rabbit models of OA [106, 107]. |
| Biphosphonates: | |||||
| zoledronate | Rat MIA | Protective effect against all MIA-induced joint changes | Ameliorated changes in weight-bearing | [108] | The effect of prophylactic and therapeutic zoledronate at different time point post MIA were compared |
| tiludronate | Dog ACLT, 8-week follow-up | Some beneficial effects on joint changes (including subchondral bone and synovitis) | Positive effect on gait changes and joint symptoms (a composite numerical rating scale (NRS), visual analog scale, and electrodermal activity) | [98] | Treatment commenced at the time of surgery |
| Angiogenesis blocker, PPI-2458 | Rat MMT 5-week follow-up | Reduced joint damage and synovitis | Reduced changes in weight-bearing | [70] | This compound is a fumagillin analog that triggers growth arrest of endothelial cells in the G1 phase. |
| Intra-articular recombinant human lubricin with a truncated mucin-like domain (LUB-1) | Rat MMT 5-week follow-up | Reduced cartilage degradation (no changes in subchondral bone) | Ameliorated reduced weight-bearing on operated limb | [109, 110] | IA administration of LUB-1 started one week after surgery |
| GM-CSF blocking antibody | Mouse CoiA 6-week follow-up | Reduced cartilage damage Reduced synovitis | Ameliorated changes in weight-bearing | [102] | Antibodies were efficacious in a therapeutic and in a prophylactic protocol |
Abbreviations: MIA - monoiodoacetate-induced arthritis, MMT - medial meniscal transection, DMM = destabilization of the medial meniscus, ACLT - anterior (or cranial in dog) cruciate ligament transection, MNX = meniscectomy, CoiA = collagenase-induced arthritis.
Subchondral bone has received much attention as a putative target for modulating OA-associated pain. A recent randomized placebo-controlled clinical trial of intravenous zoledronic acid demonstrated significant bone marrow lesion size reduction as well as pain reduction at 6 months [66]. One particular histopathological feature that has been documented in several animal models as well as the human OA joint is increased angiogenesis in the synovium, the menisci, and at the osteochondral junction. The latter is manifested as channels that extend from the subchondral bone into non-calcified articular cartilage (reviewed in [67, 68]. Blood vessel growth may contribute to inflammation and, because of its role in endochondral ossification, to structural disease progression – but neovascularisation may also be linked to pain, because it is accompanied by growth of sensory nerves that penetrate noncalcified articular cartilage, osteophytes and the inner regions of menisci [69]. Thus, angiogenesis may provide a target for OA pain [70]. A broad-spectrum MMP-inhibitor also reduced joint damage, osteochondral angiogenesis and pain behaviors [71].
Most studies exploring structure-modifying effects of potential disease modifying osteoarthritic drugs (DMOADs) and concomitant analgesic effects start the treatment protocol at the onset of the model – thus they are essentially prophylactic studies, and therefore do not provide us with real insight into the relationship between progression of OA and pain mechanisms. To address the issue of whether disease-modification or halting progression of existing OA will also modulate pain, more studies are needed where treatment is started in a therapeutic protocol.
3) Do pain-behaviours and associated pathways change over time in animal models of OA?
Clinical research assessing how pain in OA changes over time as the disease progresses has been limited, but it is an increasingly important focus in the field [72]. Neuropathic elements are recognized in patients with advanced disease, such as a burning sensation, “pins and needles,” and sensory deficits [73]. Thus, it is likely that neuronal and molecular pathways involved in OA pain and its perception, evolve over time. The use of slowly progressive models of OA will enable longitudinal study of pain behaviors and associated pathways. Despite OA being a chronic progressive disease, very few studies assess pain behaviours in animal models at different time points. There is a temporal pattern not only in the severity of structural damage in OA joints, but also the pathophysiological pathways that are active at different times that may impact the efficacy of disease modifying drugs depending on when they are administered. This was recently observed where zoledronic acid inhibited cartilage degradation in the rat MNX model when administered in the early bone-resorptive phase but not later in established disease [74]. To date, few similar temporal studies of pain severity and molecular regulation associated with changing structural pathology in the joint have been conducted.
In one study, female C57BL/6 mice developed pain hypersensitivity following partial medial meniscectomy in two phases [75]. An early phase, 1-2 weeks after surgery, appeared to be associated with postoperative inflammation and was responsive to diclofenac. In a later phase, approximately 7 weeks after surgery, hypersensitivity, including vocalization in response to knee pressure, was no longer responsive to diclofenac, but responded to morphine. Pain levels during the later phase fluctuated and could be unmasked by the non-selective opioid receptor antagonist, naloxone, indicating that reduced pain was due to endogenous opioids. Induction of the endogenous opioid system was also reported to delay the onset of pain behaviors following DMM surgery [38]. In this model, the temporal onset of pain behaviors appeared to correlate with concomitant changes in the innervating DRG [37]. After DMM surgery, mice developed early-onset secondary mechanical allodynia that was maintained for 16 weeks. Eight weeks post-surgery, MCP-1 and CCR2 mRNA, protein, and signaling activity were temporarily up-regulated in the innervating DRG and this correlated with the presentation of movement-provoked pain behaviors, which were maintained up to 16 weeks. From 8 weeks onward, macrophages infiltrated the DRG.
These reports suggest that long-term models can be used to study early and late phases of OA development and associated pain and will enable identification of targets that are optimized for these different phases. This was demonstrated in the mouse DMM model where NGF was induced in the joints during both post-operative (day 3) and advanced OA (16weeks) pain, but not in the non-painful stage of disease (8 weeks post-surgery) [76]. The soluble NGF receptor, TrkAd5, was highly effective at suppressing pain in both phases. Induction of NGF in the post-operative phase of pain was TNF-dependent as anti-TNF reduced NGF expression in the joint and abrogated pain. However, TNF was not regulated in the late OA joints where pain was not affected by anti-TNF therapy. Fucoidan, by suppressing cellular infiltration into the joint, was able to suppress post-operative, but not late OA pain.
4) Are findings in animal models translatable to human OA?
All the issues described above affect the potential translation of findings from pre-clinical studies to clinical trials and ultimately patient therapy. A recent study highlighted the problems with “translatability” of analgesic effects in OA. A randomised, placebo-controlled clinical trial with an irreversible fatty acid amide hydrolase-1 (FAAH) inhibitor modulated endocannabinoids but failed to achieve effective analgesia in patients with pain due to OA of the knee [77]. In contrast, the same highly selective inhibitor had shown efficacy in an inflammatory arthritis model and ameliorated mechanical hyperalgesia of the knee 14 days after MIA injection (significantly increased joint compression thresholds) [78]. In addition, the FAAH inhibitor URB597 has been shown to reduce afferent nerve hypersensitivity in the Dunkin-Hartley guinea pig model of naturally-occcurring OA indicating that endocannabinoids are effective at reducing joint nociception [79]. These contrasting findings in humans and animal models prompted the authors to conclude that “the disconnect between species needs further study”. However, the disconnect between outcomes in the pre-clinical versus clinical trials, may also be because the disease models used do not reflect well the cellular or molecular mechanisms responsible for the symptoms in human OA. It is difficult to evaluate translatability of experimental drugs for OA pain, due to the scarcity of published data.
5) Standardisation of pain assessment techniques and OA models is needed
The ethical and scientific need to standardize the conduct and reporting of research using animals both in general and in OA in particular, has previously been discussed with regard to the introduction and use of the ARRIVE guidelines [12, 80]. It is worth re-emphasizing, however, that in order to facilitate advances in our knowledge through direct comparison of outcomes between different research groups and ultimately meta-analyses, consistent conduct and reporting is mandatory. The ARRIVE and other guidelines are an excellent tool to facilitate consistency in experimental design and reporting of studies using animals. Guidelines for histopathology scoring (see collected articles in Osteoarthritis Cartilage. 2010 Oct;18 Suppl 3) may improve comparative evaluation of structural OA pathology. Standardization of imaging and laboratory (e.g. serum biomarkers) methodology has become routine in human OA research and clinical trials, and some similar normalization guidelines for use in animal studies are available (e.g. for μCT [81]). To date there has been little attempt to compare and standardize OA models, or the pain outcome measures and methodology between different research groups or laboratories. As noted above, a number of the pain outcome measures particularly in small animals are somewhat qualitative and may therefore be operator-dependent. Greater investments in standardization of pain outcome measures would not only reduce intra-experimental variance limiting the number of experimental animals required and the need to replicate, thus fulfilling the central “3-Rs” tenant of animal ethics, but would also facilitate inter-laboratory comparisons and the potential to conduct systematic reviews and further our understanding and potential treatment of OA pain.
Intrinsic differences in such things as specific pathogen-free status of an animal house, cage type and size, available or required anaesthetics and analgesics may all affect the OA structural disease severity, progression and pain. Marked differences can be observed in structural disease severity in a single species with different knee joint instability surgeries [82, 83], suggesting that comparison of pain outcomes even between surgical knee instability models may be problematic. Even comparing supposedly a single model between laboratories may not be appropriate when for example what is defined and referenced as surgical destabilization of the medial meniscus (DMM) in mice as described by Glasson et al. [83] in some papers also includes a hemi-meniscectomy and/or a medial collateral ligament transection. How such subtle differences may affect OA pathophysiology, progression and pain is presently unknown. This same caution is necessary even when interpreting the effect of sham surgery, where for example some researchers may only incise the skin while others do a comparable arthrotomy and surgical manipulation of the joint just not including ligament transection.
Even for the model most widely used to study pain, MIA, there can be marked differences due to methodology. There is a wide range of doses in use for the MIA model, from 1 to 4.8 mg MIA, with dose-dependent effects on joint damage, pain behaviors, and nociceptor sensitization reported [16, 29, 31]. A recent study comparing the pathophysiological sequellae of 1 and 2 mg MIA [19] reported that the higher dose was associated with greater hindpaw mechanical hypersensitivity than the lower dose, in the presence of the same degree of cartilage proteoglycan loss. Two mg, but not 1 mg, MIA produced an increase in the expression of the injury marker ATF-3 in DRG cells, a reduction in intra-epidermal nerve fiber density in plantar hindpaw skin, and ipsilateral spinal cord microgliosis, all markers of neural injury. This demonstrates that intra-articular 2 mg MIA inflicts significant axonal injury to DRG cells, including those that innervate tissues outside of the knee joint. The authors concluded that this neuropathic component, if it can be attributed to neurotoxicity, would call into question the utility of MIA at doses greater than 1 mg for the translational study of OA pain [19].
Conclusions
Animal models offer great potential to unravel the complex pathophysiology of OA pain, its molecular and temporal regulation, and a critical pathway for developing and testing symptom-modifying therapeutic interventions. However, a number of issues remain to be resolved to optimize pre-clinical OA-pain research and to optimize the outcome translation of clinical trials and patient therapies. In particular, the research in this field should strive for better standardization of OA animal models and pain outcome measures, to study the longitudinal pattern and temporal changes in pain/pain mechanisms, and to better understand how pain outcomes in our models relate to specific molecular biochemical and structural changes in the joint. Retrospective testing of any clinically beneficial symptom-modifying therapies in multiple animal models and using multiple pain assessment modalities will help to validate translational strategies into subsets of OA patients.
Acknowledgements
AM. Malfait acknowledges funding from the National Institutes of Health - NIAMS (RO1AR060364 and R01AR064251) and from the Arthritis Foundation. J. McDougall is funded by the Canadian Institutes of Health Research, and the Nova Scotia Health Research Fund. C. Little acknowledges funding from the following sources for research on the pathogenesis of osteoarthritis: National Health and Medical Research Council of Australia (grants APP384414 and APP1045890), Arthritis Australia and The Hillcrest Foundation through Perpetual Philanthropies. The funding sources had no role in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors were involved in collecting data, reviewing the literature and drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Conflict of Interest
Dr. Little provides consulting services on preclinical animal models of OA to both Universities and pharmaceutical companies. Dr. McDougall has acted as a consultant for several pharmaceutical companies on the neurophysiology of joint pain. AM Malfait is an Associate Editor for Osteoarthritis and Cartilage and C. Little is on the Editorial Board.
References
- 1.Neogi T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthritis Cartilage. 2013 doi: 10.1016/j.joca.2013.03.018. (current issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, Badley EM. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med. 2000;342:1016–22. doi: 10.1056/NEJM200004063421405. [DOI] [PubMed] [Google Scholar]
- 3.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152:566–72. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M, Dieppe P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:1255–62. doi: 10.1016/j.joca.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:476–99. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 7.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13:790–98. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Little CB, Smith MM. Animal Models of Osteoarthritis. Current Rheumaotlogy Reports. 2008;4:175–82. [Google Scholar]
- 10.Smith M, Little C. Experimental Models of Osteoarthritis. In: Moskowitz R, Altman R, Hochberg M, Buckwalter J, Goldberg V, editors. Osteoarthitis: Diagnosis and Medical/Surgical Management. Lipincott Williams & Wilkins; Philadelphia: 2007. pp. 107–25. [Google Scholar]
- 11.Little CB. Experimental models of osteoarthritis. OARSI online primer. http://primeroarsiorg/
- 12.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis”--the need for consensus? Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:261–67. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R-X, Ren K, Dubner R. Osteoarthritis pain mechanisms: Basic studies in animal models. Osteoarthritis Cartilage. 2013 doi: 10.1016/j.joca.2013.06.013. (current issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heppelmann B, McDougall JJ. Inhibitory effect of amiloride and gadolinium on fine afferent nerves in the rat knee: evidence of mechanogated ion channels in joints. Exp Brain Res. 2005;167:114–18. doi: 10.1007/s00221-005-0040-z. [DOI] [PubMed] [Google Scholar]
- 15.Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP6-28 reduces nociception in an animal model of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:1155–62. doi: 10.1016/j.joca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009;465:184–88. doi: 10.1016/j.neulet.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 17.McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain. 2009;141:222–32. doi: 10.1016/j.pain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 18.McDougall JJ, Schuelert N. Age alters the ability of substance P to sensitize joint nociceptors in guinea pigs. J Mol Neurosci. 2007;31:289–96. doi: 10.1385/jmn:31:03:289. [DOI] [PubMed] [Google Scholar]
- 19.Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DL. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One. 2012;7:e33730. doi: 10.1371/journal.pone.0033730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagar DR, Burston JJ, Hathway GJ, Woodhams SG, Pearson RG, Bennett AJ, Kendall DA, Scammell BE, Chapman V. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Molecular pain. 2011;7:88. doi: 10.1186/1744-8069-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–22. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- 22.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 23.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 24.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 25.van der Poel AM, Noach EJ, Miczek KA. Temporal patterning of ultrasonic distress calls in the adult rat: effects of morphine and benzodiazepines. Psychopharmacology (Berl) 1989;97:147–48. doi: 10.1007/BF00442236. [DOI] [PubMed] [Google Scholar]
- 26.Wohr M, Borta A, Schwarting RK. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: a dose-response study in the rat. Neurobiol Learn Mem. 2005;84:228–40. doi: 10.1016/j.nlm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Calvino B, Besson JM, Boehrer A, Depaulis A. Ultrasonic vocalization (22-28 kHz) in a model of chronic pain, the arthritic rat: effects of analgesic drugs. Neuroreport. 1996;7:581–84. doi: 10.1097/00001756-199601310-00049. [DOI] [PubMed] [Google Scholar]
- 28.Han JS, Bird GC, Li W, Jones J, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. Journal of neuroscience methods. 2005;141:261–69. doi: 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11:821–30. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 30.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123:98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Pomonis JD, Boulet JM, Gottshall SL, Phillips S, Sellers R, Bunton T, Walker K. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114:339–46. doi: 10.1016/j.pain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97:603–10. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Berryman ER, Harris RL, Moalli M, Bagi CM. Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact. 2009;9:89–98. [PubMed] [Google Scholar]
- 35.Plaas A, Li J, Riesco J, Das R, Sandy JD, Harrison A. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis research & therapy. 2011;13:R46. doi: 10.1186/ar3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graepel R, Leung G, Wang A, Villemaire M, Jirik FR, Sharkey KA, et al. Murine autoimmune arthritis is exaggerated by infection with the rat tapeworm, Hymenolepis diminuta. Int J Parasitol. 2013 doi: 10.1016/j.ijpara.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20602–7. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inglis JJ, McNamee KE, Chia SL, Essex D, Feldmann M, Williams RO, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008;58:3110–9. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 39.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Molecular pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDougall JJ, Ferrell WR, Bray RC. Neurogenic origin of articular hyperemia in early degenerative joint disease. Am J Physiol. 1999;276:R745–52. doi: 10.1152/ajpregu.1999.276.3.R745. [DOI] [PubMed] [Google Scholar]
- 41.McDougall JJ, Hanesch U, Pawlak M, Schmidt RF. Participation of NK1 receptors in nociceptin-induced modulation of rat knee joint mechanosensitivity. Exp Brain Res. 2001;137:249–53. doi: 10.1007/s002210000650. [DOI] [PubMed] [Google Scholar]
- 42.Pawlak M, Schmidt R, Hanesch U. Sensitization of articular afferents to mechanical stimuli by SP is mediated by NK1 receptors. 9th World Congress on Pain Vienna, Austria. 1999;518 1999. [Google Scholar]
- 43.McDougall JJ, Bray RC, Sharkey KA. Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints. Anat Rec. 1997;248:29–39. doi: 10.1002/(SICI)1097-0185(199705)248:1<29::AID-AR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 44.McDougall JJ, Yeung G, Leonard CA, Bray RC. A role for calcitonin gene- related peptide in rabbit knee joint ligament healing. Can J Physiol Pharmacol. 2000;78:535–40. [PubMed] [Google Scholar]
- 45.Hanesch U, McDougall J, Pawlak M. Inhibitory effects of gabapentin on rat articular afferent mechanosensitivity. Regulatory Peptides. 2000;89:63. [Google Scholar]
- 46.Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driban JB, Sitler MR, Barbe MF, Balasubramanian E. Is osteoarthritis a heterogeneous disease that can be stratified into subsets? Clin Rheumatol. 2010;29:123–131. doi: 10.1007/s10067-009-1301-1. [DOI] [PubMed] [Google Scholar]
- 48.van der Kraan PM, Vitters EL, van Beuningen HM, van de Putte LB, van den Berg WB. Degenerative knee joint lesions in mice after a single intra-articular collagenase injection. A new model of osteoarthritis. J Exp Pathol (Oxford) 1990;71:19–31. [PMC free article] [PubMed] [Google Scholar]
- 49.Blom A, van Lent P, van den Bosch M, Cats H, van den Hoogen F, van der Kraan P, van den Berg W. Transcriptomics on synovial specimen of early human (CHECK) and experimental OA to identify pathways and processes associated with cartilage damage. Osteoarthritis Cartilage. 2013;21:S42. [Google Scholar]
- 50.van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis and rheumatism. 2012;64:1466–76. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
- 51.Lee CH, Wen ZH, Chang YC, Huang SY, Tang CC, Chen WF, et al. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: association with attenuation of N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:1485–93. doi: 10.1016/j.joca.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:572–80. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–73. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 54.Inglis JJ, Notley CA, Essex D, Wilson AW, Feldmann M, Anand P, Williams R. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis and rheumatism. 2007;56:4015–23. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- 55.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Annals of the rheumatic diseases. 1994;53:565–8. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jinks C, Jordan K, Croft P. Disabling knee pain--another consequence of obesity: results from a prospective cohort study. BMC Public Health. 2006;6:258. doi: 10.1186/1471-2458-6-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis research & therapy. 2010;12:R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis and rheumatism. 2012;64:705–717. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atchia I, Kane D, Reed MR, Isaacs JD, Birrell F. Efficacy of a single ultrasound- guided injection for the treatment of hip osteoarthritis. Annals of the rheumatic diseases. 2011;70:110–6. doi: 10.1136/ard.2009.127183. [DOI] [PubMed] [Google Scholar]
- 60.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006:CD005328. doi: 10.1002/14651858.CD005328.pub2. [DOI] [PubMed] [Google Scholar]
- 61.Detora LM, Krupa D, Bolognese J, Sperling RS, Ehrich EW. Rofecoxib shows consistent efficacy in osteoarthritis clinical trials, regardless of specific patient demographic and disease factors. The Journal of rheumatology. 2001;28:2494–2503. [PubMed] [Google Scholar]
- 62.Alexopoulos LG, Youn I, Bonaldo P, Guilak F. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis and rheumatism. 2009;60:771–9. doi: 10.1002/art.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christensen SE, Coles JM, Zelenski NA, Furman BD, Leddy HA, Zauscher S, et al. Altered trabecular bone structure and delayed cartilage degeneration in the knees of collagen VI null mice. PLoS One. 2012;7:e33397. doi: 10.1371/journal.pone.0033397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunter DJ, Guermazi A, Roemer F, Neogi T. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage. 2013 doi: 10.1016/j.joca.2013.05.017. (current issue) [DOI] [PubMed] [Google Scholar]
- 66.Laslett LL, Dore DA, Quinn SJ, Boon P, Ryan E, Winzenberg TM, Jones G. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Annals of the rheumatic diseases. 2012;71:1322–8. doi: 10.1136/annrheumdis-2011-200970. [DOI] [PubMed] [Google Scholar]
- 67.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–8. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 68.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51:204–11. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Annals of the rheumatic diseases. 2007;66:1423–8. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis and rheumatism. 2011;63:2700–10. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- 71.Mapp PI, Walsh DA, Bowyer J, Maciewicz RA. Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:593–600. doi: 10.1016/j.joca.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis-an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:415–22. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 73.Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–54. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Yu DG, Yu B, Mao YQ, Zhao X, Wang XQ, Ding HF, et al. Efficacy of zoledronic acid in treatment of teoarthritis is dependent on the disease progression stage in rat medial meniscal tear model. Acta Pharmacol Sin. 2012;33:924–34. doi: 10.1038/aps.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knights CB, Gentry C, Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain. 2012;153:281–92. doi: 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 76.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–92. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–46. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, et al. Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther. 2011;338:114–24. doi: 10.1124/jpet.111.180257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuelert N, Johnson MP, Oskins JL, Jassal K, Chambers MG, McDougall JJ. Local application of the endocannabinoid hydrolysis inhibitor URB597 reduces nociception in spontaneous and chemically induced models of osteoarthritis. Pain. 2011;152:975–81. doi: 10.1016/j.pain.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 80.Percie du Sert N. Maximising the output of osteoarthritis research: the ARRIVE guidelines. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:253–5. doi: 10.1016/j.joca.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 82.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005;13:632–41. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370:236–40. doi: 10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 85.Ferreira-Gomes J, Adaes S, Mendonca M, Castro-Lopes JM. Analgesic effects of lidocaine, morphine and diclofenac on movement-induced nociception, as assessed by the Knee-Bend and CatWalk tests in a rat model of osteoarthritis. Pharmacol Biochem Behav. 2012;101:617–24. doi: 10.1016/j.pbb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Marker CL, Pomonis JD. The monosodium iodoacetate model of osteoarthritis pain in the rat. Methods Mol Biol. 2012;851:239–48. doi: 10.1007/978-1-61779-561-9_18. [DOI] [PubMed] [Google Scholar]
- 87.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, et al. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011;493:72–5. doi: 10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagase H, Kumakura S, Shimada K. Establishment of a novel objective and quantitative method to assess pain-related behavior in monosodium iodoacetate-induced osteoarthritis in rat knee. J Pharmacol Toxicol Methods. 2012;65:29–36. doi: 10.1016/j.vascn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, et al. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98:35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silva A, Araujo P, Zager A, Tufik S, Andersen ML. Sex differences in sleep pattern of rats in an experimental model of osteoarthritis. European journal of pain. 2011;15:545–53. doi: 10.1016/j.ejpain.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Whitehead RA, Puil E, Ries CR, Schwarz SK, Wall RA, Cooke JE, et al. GABA(B) receptor-mediated selective peripheral analgesia by the non-proteinogenic amino acid, isovaline. Neuroscience. 2012;213:154–60. doi: 10.1016/j.neuroscience.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 92.Ogbonna AC, Clark AK, Gentry C, Hobbs C, Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. European journal of pain. 2013;17:514–26. doi: 10.1002/j.1532-2149.2012.00223.x. [DOI] [PubMed] [Google Scholar]
- 93.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007;87:349–59. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 94.Fu SC, Cheuk YC, Hung LK, Chan KM. Limb Idleness Index (LII): a novel measurement of pain in a rat model of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:1409–16. doi: 10.1016/j.joca.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 95.Wen ZH, Tang CC, Chang YC, Huang SY, Hsieh SP, Lee CH, et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1192–1202. doi: 10.1016/j.joca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 96.Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:1041–8. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Hashizume M, Koike N, Yoshida H, Suzuki M, Mihara M. High molecular weight hyaluronic acid relieved joint pain and prevented the progression of cartilage degeneration in a rabbit osteoarthritis model after onset of arthritis. Mod Rheumatol. 2010;20:432–8. doi: 10.1007/s10165-010-0299-1. [DOI] [PubMed] [Google Scholar]
- 98.Moreau M, Rialland P, Pelletier JP, Martel-Pelletier J, Lajeunesse D, Boileau C, et al. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis research & therapy. 2011;13:R98. doi: 10.1186/ar3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frost-Christensen LN, Mastbergen SC, Vianen ME, Hartog A, DeGroot J, Voorhout G, et al. Degeneration, inflammation, regeneration, and pain/disability in dogs following destabilization or articular cartilage grooving of the stifle joint. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:1327–35. doi: 10.1016/j.joca.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 100.Cake MA, Read RA, Corfield G, Daniel A, Burkhardt D, Smith MM, Little CB. Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:226–36. doi: 10.1016/j.joca.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Haussler KK, Hill AE, Frisbie DD, McIlwraith CW. Determination and use of mechanical nociceptive thresholds of the thoracic limb to assess pain associated with induced osteoarthritis of the middle carpal joint in horses. Am J Vet Res. 2007;68:1167–76. doi: 10.2460/ajvr.68.11.1167. [DOI] [PubMed] [Google Scholar]
- 102.Cook AD, Pobjoy J, Steidl S, Durr M, Braine EL, Turner AL, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis research & therapy. 2012;14:R199. doi: 10.1186/ar4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, et al. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis and rheumatism. 2009;60:2008–18. doi: 10.1002/art.24629. [DOI] [PubMed] [Google Scholar]
- 104.Glasson SS, Bendele AM, Sum PE, Tam S, Tejada J, Rivera-Bermudez M, et al. Selective aggrecanase inhibition is disease modifying and pain alleviating in a rat meniscal tear model of osteoarthritis. Osteoarthritis Cartilage. 2009;17:S56. [Google Scholar]
- 105.McDougall JJ, Schuelert N, Bowyer J. Cathepsin K inhibition reduces CTXII levels and joint pain in the guinea pig model of spontaneous osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1355–7. doi: 10.1016/j.joca.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 106.Connor JR, LePage C, Swift BA, Yamashita D, Bendele AM, Maul D, Kumar S. Protective effects of a cathepsin K inhibitor, SB-553484, in the canine partial medial meniscectomy model of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:1236–43. doi: 10.1016/j.joca.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 107.Hayami T, Zhuo Y, Wesolowski GA, Pickarski M, Duong le T. Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012;50:1250–9. doi: 10.1016/j.bone.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 108.Strassle BW, Mark L, Leventhal L, Piesla MJ, Jian Li X, Kennedy JD, et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1319–28. doi: 10.1016/j.joca.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Glasson S, Rivera-Bermudez M, Tejada J, Mark L, Strassle B, Whiteside G, et al. Intra-articular Lubricin Supplementation Modifies Disease Progression and Ameliorates Pain in a Rat Model of Osteoarthritis. Trans Orthop Res Soc. 2009;34:1116. [Google Scholar]
- 110.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis and rheumatism. 2009;60:840–7. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]