Abstract
Despite significant economic losses resulting from infection with Anaplasma marginale, a tick-transmitted rickettsial pathogen of cattle, available vaccines provide, at best, only partial protection against clinical disease. The green-fluorescent protein expressing mutant of the A. marginale St. Maries strain is a live, marked vaccine candidate (AmStM-GFP1). To test whether AmStM-GFP is safe and provides clinical protection, a group of calves was vaccinated, and clinical parameters, including percent parasitized erythrocytes (PPE), packed cell volume (PCV) and days required to reach peak bacteremia, were measured following inoculation and following tick challenge with wild type St Maries strain (AmStM). These clinical parameters were compared to those obtained during infection with the A. marginale subsp. centrale vaccine strain (A. centrale) or wild type AmStM. AmStM-GFP resulted in similar clinical parameters to A. centrale, but had a lower maximum PPE, smaller drop in PCV and took longer to reach peak bacteremia than wild type AmStM. AmStM-GFP provided clinical protection, yielding a stable PCV and low bacteremia following challenge, whereas A. centrale only afforded partial clinical protection.
Keywords: bovine, tick-borne disease
1. Introduction
Anaplasma marginale is a tick-transmitted rickettsial pathogen of cattle resulting in decreased production due to weight loss, abortion, lower milk yields and death in up to 36% of clinical cases [1]. Despite far-reaching economic impacts there is no vaccine universally accepted as safe and efficacious. Various vaccine strategies based on the immunogenic outer membrane proteins of A. marginale sensu stricto strains have been examined. Blood-derived whole outer membrane (OM) preparations and cross-linked surface proteins provide the best protection from high level bacteremia and anemia, but may not be practical for large scale production [2-4]. Recombinant proteins, DNA vaccines and killed preparations of A. marginale, including inactivated cell-culture derived organisms, have failed to recapitulate the protection seen with OM based vaccines [5-10]. Vaccine induced protection is complex and requires more than antibodies to immunodominant proteins, as studies have repeatedly demonstrated specific seroconversion in the face of failure of clinical protection [5, 10, 11]. Advantages offered by a live vaccine include a full complement of surface antigens in their native conformations, and presentation of new surface protein variants over time.
The Anaplasma marginale ssp. centrale (A. centrale) strain has been used for over a century as a live vaccine against anaplasmosis, and is now widely utilized in Australia, Israel, South Africa and several South American countries to decrease clinical signs associated with exposure to field strains of A. marginale. Because the A. centrale vaccine is blood-based, it is not approved in the United States or European Union due to the inherent risk of transmission of known or emerging blood-borne pathogens along with the vaccine, as previously demonstrated in a batch of vaccine contaminated with bovine leucosis virus [12]. A. centrale protects vaccinates from severe clinical disease upon challenge with field strains of A. marginale, with animals generally showing mild signs of anaplasmosis post-vaccination and post-challenge [13, 14]. However, variability in clinical manifestations of anaplasmosis upon infection with the vaccine strain and protection from clinical signs upon challenge with field strains is well documented. Studies in Australia, South Africa, Kenya and Argentina generally demonstrated mild clinical signs post-vaccination and protection against severe disease upon challenge with A. marginale, whereas studies in Zimbabwe, Paraguay and Argentina have shown that the same A. centrale strain provides little to no protection [15-19]. Potential explanations for variable efficacy include dissimilar endemic strains by country and variation in the challenge dose among studies.
Cross-protection provided by A. centrale against challenge with field strains of A. marginale is attributed to conserved epitopes [20-22], however there is a much lower degree of conservation between the deduced amino acid sequences of surface proteins of A. centrale and sequenced A. marginale strains than between any two A. marginale strains examined to date. The greater divergence between A. centrale and A. marginale field strains has been demonstrated in molecular studies: a multi-strain sequencing approach to identify conserved vaccine candidates identified 19 expressed genes with >90% identity among 10 U.S. strains of A. marginale. While these sequences all had homologs in A. centrale, they were conserved to a much lesser degree, typically between 60-80% [23, 24]. Additional sequence comparisons revealed more divergence among surface proteins between A. centrale and A. marginale than when comparing just between A. marginale strains: 72.4% versus 95.1% average identity [22]. In contrast, housekeeping proteins had higher identities: 97.3% identity when comparing between A. centrale and A. marginale and 99.7% identity among A. marginale strains [22]. These data suggest that better protection may be afforded by a vaccine strain with greater identity to field strains of A. marginale.
Here we examine whether a transformed and cell culture-derived A. marginale St. Maries strain, more closely related to North American field strains of A. marginale, is an alternative approach for safe and effective vaccination. The green fluorescent protein (GFP)-expressing mutant of A. marginale St. Maries strain (AmStM-GFP) was created by transposon mediated insertion of a 4.5kb construct containing antibiotic resistance genes for selection and Turbo GFP as a marker, and grows more slowly than the parent strain in culture [25, 26]. The stability of the insert has been demonstrated through a complete in vivo transmission cycle [27]. Two advantages of AmStM-GFP as a vaccine compared to A. centrale are its potential to provide better protection due to greater similarity to field strains, and elimination of the risk of delivering emerging pathogens as it is maintained in defined medium in cell culture. In this study we investigate AmStM-GFP as a live, cell culture-based vaccine candidate, and test the hypothesis that infection with AmStM-GFP causes only mild clinical signs and provides clinical protection to vaccinated calves upon challenge with a homologous field strain.
2. Methods
2.1 Cattle inoculation
AmStM-GFP was maintained in ISE6 cells cultured at 34°C as previously described [25, 28, 29]. When passage 27 of AmStM-GFP infected greater than 80% of ISE6 cells in a T75 cell culture flask, as determined by examination of Giemsa-stained cytospin preparations, all cells were re-suspended in 25 ml of media. Three ml aliquots of fresh, intact, unpurified cell culture suspension, each containing 109 organisms, were injected intravenously into the jugular vein of each of five male, age-matched, seronegative Holstein calves: 35277, 35340, 35349, 35352, and 35369.
Unpublished clinical data from animal experiments in which naïve calves were infected with either A. centrale or the St. Maries strain of A. marginale were used in comparisons with AmStM-GFP inoculated calves described above. Five naïve calves were injected with A. centrale-infected stabilate, with inoculums containing 108 organisms (6171, 6175, 6187, and 6188) or 1010 organisms (1302) [30]. A. centrale stabilates were prepared from packed erythrocytes, previously washed 3 times in PBS, resuspended in an equal volume of stabilate buffer (1X PBS and 31.2% DMSO), and then plunged into liquid nitrogen. At the time of intravenous injection, 2mL of stabilate were thawed and mixed with 10mL of Hank’s balanced salt solution. Fourteen naïve calves (951, 956, 988, 995, 1024, 1067 , 1075, 1076, 1200, 1247, 1280, 31794, 31919, 31993) were infected with AmStM by a 7 day tick-transmission using Dermacentor andersoni from the Reynolds Creek stock [31].
All animals were determined to be negative for antibodies to A. marginale by competitive ELISA (VMRD, Pullman, WA) prior to experimental infection [32]. Sera from vaccinated and control animals were tested by cELISA to confirm seroconversion after peak bacteremia.
2.2 AmStM challenge
A naïve calf (36676) was inoculated with AmStM stabilate, and infection was established as evidenced by positive Giemsa-stained blood smears and seroconversion. When animal 36676 was in the persistent phase of infection, approximately 460 ticks were applied for a seven day acquisition feed. Ticks were held at 26°C for seven days to allow for clearance of the blood meal from the mouthparts, then 51 ticks were placed on each of four AmStM-GFP infected calves seven months post-inoculation (35277, 35340, 35349, 35352; AmStM-GFP inoculated calf 35369 was removed from the experiment prior to challenge for unrelated health reasons.) and five additional naïve control calves (35294, 35338, 35356, 35370, 35371) for a seven day transmission feed. Following transmission, cohorts of 10 ticks per calf were confirmed positive for AmStM with levels ranging from 103 to 107 organisms per salivary gland pair.
Calf 1302 was challenged intravenously with 109 AmStM blood stabilate-derived organisms thirteen months following inoculation with A. centrale.
2.3 Animal monitoring
Following inoculation and challenge, blood samples were collected throughout the period of detectable bacteremia. Blood samples were analyzed by microscopic examination of Giemsa-stained blood smears to determine the level of bacteremia expressed as percent parasitized erythrocytes (PPE) and capillary tube centrifugation to determine packed cell volume (PCV) as a measure of anemia.
Animal experiments were approved by the Institutional Animal Care and Use Committee at Washington State University, USA, in accordance with institutional guidelines based on the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
2.4 Quantitative PCR
To determine the dose of AmStM-GFP in the inoculum and infection levels in the ticks used for transmission, genomic DNA was extracted from cell culture suspension and tick salivary glands post-transmission using the Puregene DNA Purification cell kit (Qiagen). Quantitative real time PCR of msp5, a conserved single-copy gene, was performed with SybrGreen (Invitrogen), and used to determine the number of organisms in each sample. For quantitative amplification, forward 5’-ATA CCT GCC TTT CCC ATT GAT GAG GTA CAT-3’ and reverse 5’-AGG CGA AGA AGC AGA CAT AAA GAG CGT-3’ primers were used. Standard curves were constructed by amplification of a serial dilution of msp5 cloned into PCR4-Topo (Invitrogen), and amplified simultaneously with genomic DNA samples. Amplification consisted of an initial 2 min at 50°C and 10 min at 95°C, followed by 45 cycles of melting at 95°C for 15 s, and annealing and extension at 60°C for 1 min, and a final extension at 72°C for 7 min.
2.5 Southern analysis
To detect the presence of AmStM-GFP in vaccinated animals during persistent infection (7 months post inoculation) when the organism is undetectable in blood smears, nested PCR followed by Southern blot targeting gfp was performed. Reaction A of the nested PCR was performed with the following primers: GFP F (ATG GAG ATC GAG TGC CGC A) and GFP R (CGG TGT TGC TGT GAT CCT CCT). GFP F2 (ATG ACC AAC AAG ATG AAG AGC ACC A) and GFP R2 (CCG TCC TCG TAC TTC TCG) were used for reaction B of nested PCR and for production of the digoxigenin-labeled probe using the PCR DIG probe synthesis kit (Roche). Amplification for reaction A consisted of thirty five cycles of denaturing at 95°C for 15 s, annealing at 60°C for 15 s and extension at 72°C for 30 s, followed by a 7 min extension at 72°C and holding at 4°C. Reaction B differed with a lower annealing temperature of 58.5°C and reduced extension time of 15 s. The Southern blot was performed according to the DIG application manual (Roche).
2.6 Statistics
For days to peak and minimum PCV, mean differences were compared between groups using ANOVA for overall differences between the groups and pairwise comparisons controlling for multiple comparisons using Tukey’s test. Because maximum PPE was not normally distributed, median values were compared using the Kruskal-Wallis procedure controlling for multiple comparisons using the Bonferroni-Dunn procedure in WinPepi software [33].
3. Results and Discussion
To determine safety of AmStM-GFP as a vaccine, clinical parameters were compared between animals inoculated with AmStM-GFP versus AmStM or A. centrale. When comparing animals inoculated with cultured AmStM-GFP with those inoculated with non-cultured AmStM, all measured clinical parameters were significantly different. Infection with AmStM-GFP resulted in a lower peak PPE, a smaller drop in PCV, and took longer to reach peak PPE as compared to AmStM (Fig. 1A and 1C). The bacteremia levels measured as PPE were higher in AmStM infected animals than in AmStM-GFP infected animals with non-overlapping ranges of 3.1 to 14.8% and 0.52 to 1.7%, respectively (p < 0.01) (Fig. 2A). Anemia as measured by PCV was less severe in AmStM-GFP inoculated animals than in AmStM inoculated animals (p < 0.01) (Fig. 2B). The time required to reach peak infection was longer for AmStM-GFP than AmStM with non-overlapping ranges of 41-50 days and 22-37 days, respectively (p < 0.01) (Fig. 2C). Our data is consistent with previously reported data that AmStM-GFP grows more slowly than the parent strain in culture, and takes longer to reach peak bacteremia in the bovine host [25, 27]. At no point in the 10 month experiment did any animals experience a reversion to virulence of AmStM-GFP.
Figure 1.
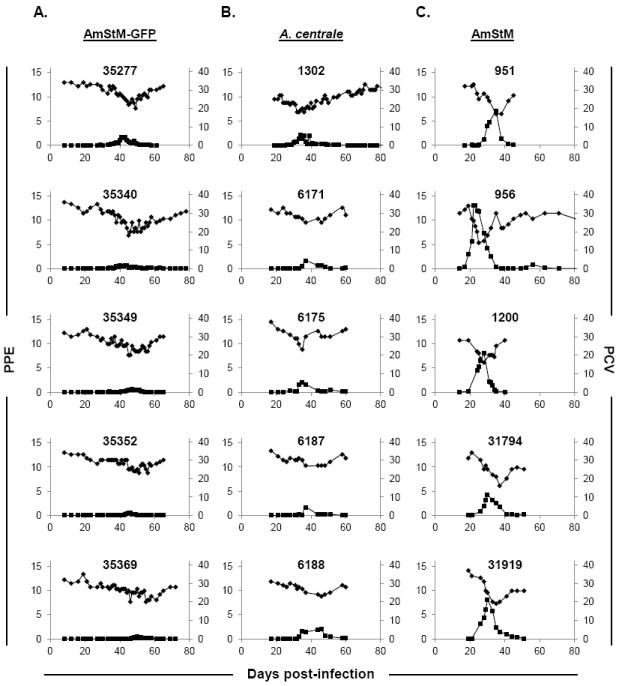
PPEs and PCVs of AmStM-GFP needle-inoculated (A) (n=5), A. centrale needle-inoculated (B) (n=5), and AmStM tick transmitted calves (C) (n=14; 5 represented) during acute anaplasmosis. Animal identification numbers are indicated on each panel. Bacteremia (left axis, ■) is reported as percent parasitized erythrocytes as determined by microscopic evaluation of Giemsa-stained blood smears. PCV (right axis, ◆) was used to evaluate anemia during infection. The x-axis indicates days post-infection where day 0 is the day of needle inoculation (A, B) or the day of tick application (C). Axes values have been standardized to allow comparison between graphs.
Figure 2.
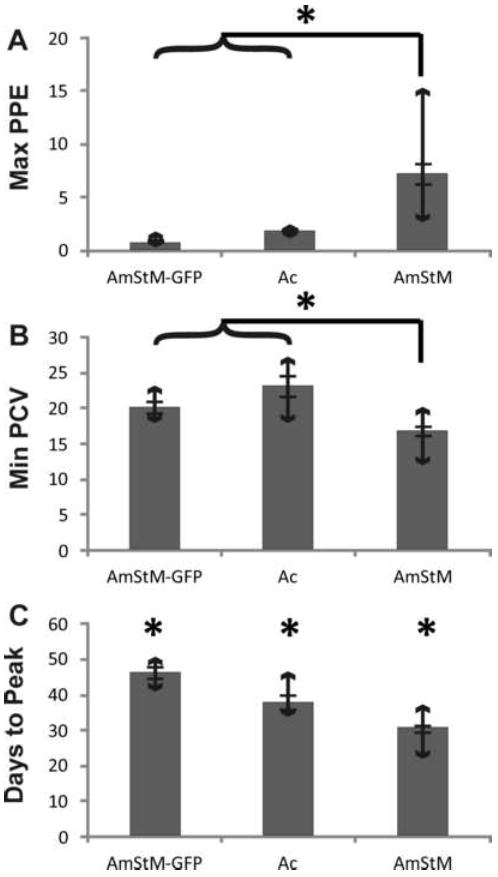
Clinical parameters of groups of naïve calves needle inoculated with AmStM-GFP (n=5), A. centrale (Ac) (n=5) or infected by tick transmission of AmStM (n=14). The group mean is represented by a gray bar, with error bars depicting the standard error. Bars with arrows indicate the range of values for each group. Statistical significance is indicated by an asterisk (*).
When comparing between naïve animals needle inoculated with AmStM-GFP or A.centrale stabilate, only one of three clinical parameters was significantly different. AmStM-GFP infected cattle took longer to achieve peak bacteremia than those infected with A. centrale (41-50 days vs. 34-46 days) (p < 0.01) (Fig. 2C). The maximum PPEs and minimum PCVs between groups were not significantly different (Fig. 2A and 2B), and the infection profiles were similar (Fig. 1A and 1B). The post-vaccination infection profiles indicate that AmStM-GFP results in similar levels of bacteremia and anemia as the A. centrale vaccine strain.
Following tick challenge of four AmStM-GFP inoculated animals with wild type AmStM, PCV and PPE were monitored for evidence of transmission to assess protection. PCV fluctuated within the normal range from 26 to 36 throughout 90 days of monitoring (Fig. 3A). Six individual blood smears, representing all four challenged calves at various time points from day 22 to day 52 post-challenge, were positive with calculated PPEs ranging from 0.006% to 0.03%. These positive blood smears were preceded and followed by negative smears on adjacent days and did not correspond to a decrease in PCV. These results are contrasted with those obtained after vaccination with A. centrale both in this study (Fig. 3C) and in previously published studies [13, 30], where a characteristic peak of bacteremia associated with a drop in PCV is seen after challenge with A. marginale. The A. centrale vaccinated animal had measurable bacteremia and associated mild anemia following challenge with AmStM, in contrast to the near absence of microscopically detectable bacteremia observed in the AmStM-GFP vaccinated group (Fig. 3C). That A. centrale vaccinated animals become infected with the challenge strain, but are protected from high infection levels and severe anemia has been shown repeatedly [13, 18]. The five control calves were successfully infected by tick challenge as evidenced by seroconversion and development of the expected infection profiles (Fig. 3B). The presence of the vaccine strain was confirmed by molecular methods in all four calves seven months post-vaccination, despite the absence of detectable parasitemia by Giemsa-stained blood smears (Fig. 4).
Figure 3.
Clinical parameters following challenge with AmStM. (A) Calves inoculated with AmStM-GFP (n=4), (B) naïve calves (n=5) and (C) A. centrale inoculated calf (n=1). Animal identification numbers are indicated on each panel. The x-axis indicates days post-challenge where day 0 is the day of tick application (A, B) or needle challenge (C). Bacteremia (left axis, ■) is reported as percent parasitized erythrocytes as determined by microscopic evaluation of Giemsa-stained blood smears. PCV (right axis, ◆) was used to evaluate anemia during infection. Axes values have been standardized to allow comparison between graphs, with the exception of the left Y-axis for 35338, 35356 and 35371 in B, which were altered to accommodate higher PPE values.
Figure 4.
Southern analysis of gfp positive animals during persistent infection. Southern blot following nested PCR targeting gfp in genomic DNA extracted from calf blood seven months post-vaccination: (+) positive plasmid control, (-) negative water control, (M) DNA Molecular Weight Marker VIII, DIG-labeled (Roche), (1) calf #35277, (2) calf #35340, (3) calf #35349, (4) calf #35352.
4. Conclusions
This study describes a live, culture-based vaccine for anaplasmosis using a marked strain of A. marginale. Several features of this potential vaccine are noteworthy: 1) Cell culture-based vaccines eliminate the risk of pathogen transmission; 2) Transformation of AmStM has produced a stable, marked, slower growing strain that can be distinguished from field strains (AmStM-GFP was detectable in all four vaccinated calves with negative blood smears seven months post-infection) [25, 27]; 3) Because of the persistent nature of A. marginale infection, only a single dose of live AmStM-GFP is required for protection [27]; 4) The findings of this study indicate that AmStM-GFP provides protection against disease following homologous challenge. Further trials are warranted to determine if protection is extended to heterologous challenge. A drawback of this potential vaccine is that it carries an antibiotic resistance marker, but this could be replaced by an inconsequential marker in future trials. These initial studies are a proof of concept for the basis of future development for this type of vaccine.
Highlights.
AmStM-GFP is a marked, culture derived, live vaccine candidate for anaplasmosis.
AmStM-GFP and A. centrale result in similar levels of bacteremia and anemia.
AmStM-GFP provides protection against disease following homologous challenge.
Acknowledgments
The authors wish to thank Dr. Ulrike G. Munderloh for ISE6 cells and AmStM-GFP, and Ralph Horn and James Allison for technical assistance. This work was supported by Wellcome Trust grant GR075800M, NIH R01 AI44005, and BARD 4187-09C. GKH was supported by a NIH Postdoctoral Fellowship (T32AI007025).
Footnotes
Abbreviations: AmStM: Anaplasma marginale St. Maries strain; AmStM-GFP: GFP-transformed AmStM ; OM: Outer membrane
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer GH. Anaplasma vaccines. In: Wright, editor. Veterinary protozoan and hemoparasite vaccines. Boca Raton, FL: CRC Press; 1989. pp. 2–29. [Google Scholar]
- 2.Tebele N, McGuire TC, Palmer GH. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect Immun. 1991 Sep;59(9):3199–204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown WC, Shkap V, Zhu D, McGuire TC, Tuo W, McElwain TF, et al. CD4(+) T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect Immun. 1998 Nov;66(11):5406–13. doi: 10.1128/iai.66.11.5406-5413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noh SM, Brayton KA, Brown WC, Norimine J, Munske GR, Davitt CM, et al. Composition of the surface proteome of Anaplasma marginale and its role in protective immunity induced by outer membrane immunization. Infect Immun. 2008 May;76(5):2219–26. doi: 10.1128/IAI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott JR, Palmer GH, Kegerreis KA, Hetrick PF, Howard CJ, Hope JC, et al. Rapid and long-term disappearance of CD4+ T lymphocyte responses specific for Anaplasma marginale major surface protein-2 (MSP2) in MSP2 vaccinates following challenge with live A. marginale. J Immunol. 2005 Jun 1;174(11):6702–15. doi: 10.4049/jimmunol.174.11.6702. [DOI] [PubMed] [Google Scholar]
- 6.Lasmar PV, Carvalho AU, Facury Filho EJ, Bastos CV, Ribeiro MF. Evaluating the effectiveness of an inactivated vaccine from Anaplasma marginale derived from tick cell culture. Rev Bras Parasitol Vet. 2012 Jun;21(2):112–7. doi: 10.1590/s1984-29612012000200008. [DOI] [PubMed] [Google Scholar]
- 7.de la Fuente J, Kocan KM, Garcia-Garcia JC, Blouin EF, Claypool PL, Saliki JT. Vaccination of cattle with Anaplasma marginale derived from tick cell culture and bovine erythrocytes followed by challenge-exposure with infected ticks. Vet Microbiol. 2002 Oct 22;89(2-3):239–51. doi: 10.1016/s0378-1135(02)00206-7. [DOI] [PubMed] [Google Scholar]
- 8.Kocan KM, Halbur T, Blouin EF, Onet V, de la Fuente J, Garcia-Garcia JC, et al. Immunization of cattle with Anaplasma marginale derived from tick cell culture. Vet Parasitol. 2001 Dec 3;102(1-2):151–61. doi: 10.1016/s0304-4017(01)00519-2. [DOI] [PubMed] [Google Scholar]
- 9.Albarrak SM, Brown WC, Noh SM, Reif KE, Scoles GA, Turse JE, et al. Subdominant antigens in bacterial vaccines: AM779 is subdominant in the Anaplasma marginale outer membrane vaccine but does not associate with protective immunity. PLoS One. 2012;7(9):e46372. doi: 10.1371/journal.pone.0046372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Andrade GM, Machado RZ, Vidotto MC, Vidotto O. Immunization of bovines using a DNA vaccine (pcDNA3.1/MSP1b) prepared from the Jaboticabal strain of Anaplasma marginale. Ann N Y Acad Sci. 2004 Oct;1026:257–66. doi: 10.1196/annals.1307.040. [DOI] [PubMed] [Google Scholar]
- 11.Noh SM, Zhuang Y, Futse JE, Brown WC, Brayton KA, Palmer GH. The immunization-induced antibody response to the Anaplasma marginale major surface protein 2 and its association with protective immunity. Vaccine. 2010 May 7;28(21):3741–7. doi: 10.1016/j.vaccine.2010.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers RJ, Dimmock CK, de Vos AJ, Rodwell BJ. Bovine leucosis virus contamination of a vaccine produced in vivo against bovine babesiosis and anaplasmosis. Aust Vet J. 1988 Sep;65(9):285–7. doi: 10.1111/j.1751-0813.1988.tb16144.x. [DOI] [PubMed] [Google Scholar]
- 13.Shkap V, Leibovitz B, Krigel Y, Molad T, Fish L, Mazuz M, et al. Concomitant infection of cattle with the vaccine strain Anaplasma marginale ss centrale and field strains of A. marginale. Vet Microbiol. 2008 Aug 25;130(3-4):277–84. doi: 10.1016/j.vetmic.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Palmer GH, Rurangirwa FR, Kocan KM, Brown WC. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999 Jul;15(7):281–6. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 15.Abdala AA, Pipano E, Aguirre DH, Gaido AB, Zurbriggen MA, Mangold AJ, et al. Frozen and fresh Anaplasma centrale vaccines in the protection of cattle against Anaplasma marginale infection. Rev Elev Med Vet Pays Trop. 1990;43(2):155–8. [PubMed] [Google Scholar]
- 16.Turton JA, Katsande TC, Matingo MB, Jorgensen WK, Ushewokunze-Obatolu U, Dalgliesh RJ. Observations on the use of Anaplasma centrale for immunization of cattle against anaplasmosis in Zimbabwe. Onderstepoort J Vet Res. 1998 Jun;65(2):81–6. [PubMed] [Google Scholar]
- 17.Brizuela CM, Ortellado CA, Sanabria E, Torres O, Ortigosa D. The safety and efficacy of Australian tick-borne disease vaccine strains in cattle in Paraguay. Vet Parasitol. 1998 Mar 31;76(1-2):27–41. doi: 10.1016/s0304-4017(97)00047-2. [DOI] [PubMed] [Google Scholar]
- 18.Bock RE, de Vos AJ. Immunity following use of Australian tick fever vaccine: a review of the evidence. Aust Vet J. 2001 Dec;79(12):832–9. doi: 10.1111/j.1751-0813.2001.tb10931.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuttler KL. Comparative response to premunization using attenuated Anaplasma marginale virulent A. marginale and A. centrale in different age groups. Trop Anim Health Prod. 1972;4(4):197–203. doi: 10.1007/BF02360111. [DOI] [PubMed] [Google Scholar]
- 20.Molad T, Brayton KA, Palmer GH, Michaeli S, Shkap V. Molecular conservation of MSP4 and MSP5 in Anaplasma marginale and A. centrale vaccine strain. Vet Microbiol. 2004 May 20;100(1-2):55–64. doi: 10.1016/j.vetmic.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Shkap V, Molad T, Brayton KA, Brown WC, Palmer GH. Expression of major surface protein 2 variants with conserved T-cell epitopes in Anaplasma centrale vaccinates. Infect Immun. 2002 Feb;70(2):642–8. doi: 10.1128/IAI.70.2.642-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnes JT, Brayton KA, LaFollett M, Norimine J, Brown WC, Palmer GH. Identification of Anaplasma marginale outer membrane protein antigens conserved between A. marginale sensu stricto strains and the live A. marginale subsp. centrale vaccine. Infect Immun. 2011 Mar;79(3):1311–8. doi: 10.1128/IAI.01174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herndon DR, Palmer GH, Shkap V, Knowles DP, Jr, Brayton KA. Complete genome sequence of Anaplasma marginale subsp. centrale. J Bacteriol. 2010 Jan;192(1):379–80. doi: 10.1128/JB.01330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dark MJ, Al-Khedery B, Barbet AF. Multistrain genome analysis identifies candidate vaccine antigens of Anaplasma marginale. Vaccine. 2011 Jul 12;29(31):4923–32. doi: 10.1016/j.vaccine.2011.04.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felsheim RF, Chavez AS, Palmer GH, Crosby L, Barbet AF, Kurtti TJ, et al. Transformation of Anaplasma marginale. Vet Parasitol. 2010 Feb 10;167(2-4):167–74. doi: 10.1016/j.vetpar.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierlé S, Hammac GK, Palmer GH, Brayton KA. Transcriptional pathways associated with the slow growth phenotype of transformed Anaplasma marginale. BMC Genomics. 2013 doi: 10.1186/1471-2164-14-272. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noh SM, Ueti MW, Palmer GH, Munderloh UG, Felsheim RF, Brayton KA. Stability and tick transmission phenotype of gfp-transformed Anaplasma marginale through a complete in vivo infection cycle. Appl Environ Microbiol. 2011 Jan;77(1):330–4. doi: 10.1128/AEM.02096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munderloh UG, Blouin EF, Kocan KM, Ge NL, Edwards WL, Kurtti TJ. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J Med Entomol. 1996 Jul;33(4):656–64. doi: 10.1093/jmedent/33.4.656. [DOI] [PubMed] [Google Scholar]
- 29.Munderloh UG, Liu Y, Wang M, Chen C, Kurtti TJ. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994 Aug;80(4):533–43. [PubMed] [Google Scholar]
- 30.Galletti MF, Ueti MW, Knowles DP, Jr, Brayton KA, Palmer GH. Independence of Anaplasma marginale strains with high and low transmission efficiencies in the tick vector following simultaneous acquisition by feeding on a superinfected mammalian reservoir host. Infect Immun. 2009 Apr;77(4):1459–64. doi: 10.1128/IAI.01518-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scoles GA, Broce AB, Lysyk TJ, Palmer GH. Relative efficiency of biological transmission of Anaplasma marginale (Rickettsiales: Anaplasmataceae) by Dermacentor andersoni (Acari: Ixodidae) compared with mechanical transmission by Stomoxys calcitrans (Diptera: Muscidae) J Med Entomol. 2005 Jul;42(4):668–75. doi: 10.1603/0022-2585(2005)042[0668:REOBTO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Knowles D, Torioni de Echaide S, Palmer G, McGuire T, Stiller D, McElwain T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J Clin Microbiol. 1996 Sep;34(9):2225–30. doi: 10.1128/jcm.34.9.2225-2230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


