Abstract
Background
The performance of coital diaries and clinic-based interviews to measure sexual behaviour were compared during a pilot study for a Phase III microbicide trial.
Methods
In Mwanza 59 women were enrolled for 4 weeks and provided with 20 placebo gels. Weekly, women were given coital diaries (CD) to complete daily. At the final clinic visit, women attended a face-to-face interview (Clinic FFI) about their sexual behaviour, and gel use was accounted for (GA). Comparisons were made of CD, Clinic FFI and GA data. In-depth interviews following clinic visits elicited reasons for discrepancies in reports.
Results
Twice as many sex acts during one week were recorded in the CD (median 4) compared with the clinic FFI (median 2). At the clinic FFI more women reported using the gel for each sex act (84% v. 40%; p<0.001) and vaginal washing for each sex act (98% v. 56%; p<0.001) compared with the CD. Over four weeks 16.4% of women recorded sex during menstruation in CDs compared with 1.8% at the clinic visit (p=0.01). The median number of gels used reported in the CDs was the same as the GA (10) with 59% agreement on the number used within +/−2 gels. Reasons for misreporting during clinic FFI were reported to have been poor recall, embarrassment or misunderstanding. Inaccuracies in CDs were attributed to misunderstanding or poor recording.
Conclusions
CDs elicited higher recording of sex acts and lower reporting of gel use than clinic FFIs which has implications for measuring adherence during clinical trials. With clear instructions and support, coital dairies should be considered in future microbicide trial design.
Keywords: Sexual behaviour, coital diaries, clinical trials, microbicides
Background
In clinical trials of HIV prevention technologies there is a need to produce valid and reliable indicators of exposure to risk of HIV transmission among trial participants [1]. Coital diaries are increasingly used by social scientists to collect sexual behaviour data [2]. Some studies suggest that they produce better quality data than retrospective questionnaires as diaries allow data to be collected at short intervals (usually daily) [2, 3]. They have also been found to reduce errors in self-reported sexual behaviour caused by embarrassment and recall bias [4-6]. Their use in clinical trials could therefore be an important way of improving the accuracy of data collected.
The Microbicides Development Programme (MDP) commenced a Phase III clinical trial of a vaginal microbicide gel (PRO 2000/5) in six sites in sub-Saharan Africa in September 2005. Mwanza, Tanzania is one of the sites and recruits participants who work in bars, restaurants, guesthouses, and as food and alcohol vendors, because they have been found to be at higher risk of HIV than the general population [7-9]. As part of a feasibility study, conducted between 2001 and 2004 to explore whether Mwanza was a suitable site for a clinical trial, a coital diary (CD) was developed. A sub-study was conducted to compare these locally developed, project-specific, CDs with face-to-face structured interviews conducted in the respondents home in measuring key sexual behaviours reported by the women [4]. The impact of varying levels of support from researchers on CD data was also evaluated. This study provided evidence of higher validity of data from CDs than from face-to-face interviews. Specifically, sexual behaviours that were socially stigmatised in the Mwanza context (including sex with a casual partner, sex during menstruation) were reported more frequently, and by more women, in the CDs than at face-to-face interviews. Furthermore, CD results were found to be more consistent in the face of differing levels of researcher support.
As collecting sexual behaviour data in a clinic setting is standard for most HIV prevention trials this paper will present findings from a comparison of CD data and sexual behaviour data collected in research clinics in preparation for the MDP301 clinical trial and will discuss the implications of these findings for data collection procedures in such trials.
Methods
Coital diary development
The purpose of the coital diary was to capture key behaviours associated with sex which are important to measure in relation to microbicide use. As well as measuring condom use the diary was also designed to capture vaginal washing and vaginal inserts which may impact on the effectiveness of the microbicide gel. The CD design was developed by a team of social scientists who pre-tested five diary designs, using a mixture of pictures drawn by local artists and written text. These designs were refined following five focus group discussions with women participants, attended by two local artists who adjusted their drawings accordingly. A largely pictorial diary was chosen, given the level of illiteracy among feasibility study participants (20%) and the acceptability and good understanding of this design. The pictures used in the pilot study CD were similar to those presented in Allen et al [4] with extra pictures depicting placebo gel use, discussion of gel use with partner and placebo gel timing (see Figure 1). Each CD booklet consisted of 7 pages, each page representing 1 day and consisting of three sets of the pictures (allowing recording of up to 21 sex acts a week). When a woman had sex she was asked to tick either the vaginal or anal sex picture and to tick (or cross) each behaviour associated with that act. A box to denote ‘no sex’ was also included on each page.
Figure 1.
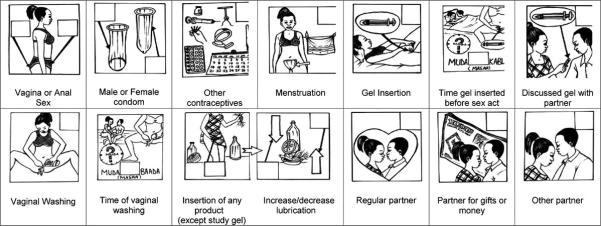
Pilot study design
Fifty-nine women were enrolled in the 4-week pilot study between September and November 2004 and provided with 20 placebo gels and a supply of condoms. They were advised to insert one gel within 1 hour before sex, and to use a condom throughout each sex act. The study design is summarised in Table 1. Each woman was provided with a CD on a weekly basis, either at her home or place of work, by a fieldworker and advised to complete one page every day. The fieldworker taught the woman how to use the diary on the first visit and provided support at each subsequent weekly visit to complete the diary correctly. At the end of 4 weeks the women attended a community-based clinic for review and also participated in a face-to-face interview (FFI) with a counsellor about their sexual behaviour over the past week, or four weeks if they had not reported any sex in the previous week. For the clinic visit, the participants were asked to return both used (empty) and unused (unopened) gel applicators for “gel accountability” (GA). Following the clinic visit, the fieldworker recorded all sexual behaviour reported in the CDs and clinic FFI on a comparison sheet. An in-depth interview (IDI) was conducted within 5 days of the clinic visit in order to explore women’s experiences of the placebo gel, the study procedures, and to explore any discrepancies in their reports of sexual behaviour in the CD, clinic FFI and during the ID according to the comparison sheet.
Table 1. Sexual behaviour data collection.
| Day | Methods | Purpose | Data collected |
|---|---|---|---|
|
| |||
| Day 0 | Visit by researcher | Enrolment to pilot study, informed consent, explain and give first week’s CD | None |
|
| |||
| Day 1-7 | Daily completion of pictorial coital diary (CD) | Measure sexual behaviour |
|
|
| |||
| Day 7 | Visit by researcher | Collect first week’s CD, give support or advice about the CD, and give woman the CD for Week 2. | |
|
| |||
| Day 8-14 | Daily completion of CD | Measure sexual behaviour | |
|
| |||
| Day 14 | Visit by researcher | Collect second week’s CD, give support or advice about the CD, and give woman the CD for Week 3. | |
|
| |||
| Day 15-21 | Daily completion of CD | Measure sexual behaviour | |
|
| |||
| Day 21 | Visit by researcher | Collect third week’s CD, give support or advice about the CD, and give woman the CD for Week 4. | |
|
| |||
| Day 22-28 | Daily completion of CD | Measure sexual behaviour | |
|
| |||
| Day 28 | Visit by researcher | Collect fourth week’s CD | |
|
| |||
| Day 28 | Face-to-face interview at clinic visit Gel accountability | Measure sexual behaviour and number of placebo gel applicators used | |
|
| |||
| Day 29-34 | In-depth interview | Comparison of methods and acceptability of study procedures | |
Ethics clearance for this research was obtained from the National Medical Research Coordinating Committee in Tanzania and the Ethics Committee of the London School of Hygiene and Tropical Medicine in UK. A trained nurse interviewer provided detailed information about the study to women at their first clinic visit. Informed consent was obtained from all participants prior to enrolment. All IDIs were conducted in Swahili, and voice-recorded with the participant’s permission.
Analysis
Of the 59 women recruited to the pilot study, 57 attended the final clinic visit. CD data were missing for one of these women, so the findings are presented for 56 women (95%). All behaviours (sex acts, placebo gel use, condom use, vaginal washing, vaginal insertion of non-gel product, and menstruation) recorded in the CD for the fourth week were compared with equivalent data from the clinic FFI for the past week. Sex with a regular partner was recorded at both the clinic FFI and in the CDs, but sex with ‘partners for gifts or money’ and ‘other partners’ were not recorded in a similar way in the clinic FFI and therefore not comparable. Timings for insertion of placebo gel and vaginal washing were poorly recorded by the participants in the CDs; therefore these data are not presented. The median numbers of sex acts reported on the two instruments were compared using a non-parametric sign test. Differences in the proportions of women always (100%) or not always (<100%) reporting placebo gel use, condom use, vaginal washing, sex with a regular partner and sex during menstruation were compared using McNemars paired chi-squared test. Comparison of reported placebo gel use data at the clinic FFI and placebo gel accountability data (GA) was not possible because of the different time periods (7 days versus 4 weeks). Therefore only agreement between placebo gel use data from the 4 weeks of coital diaries and the equivalent period from the placebo gel accountability data was analysed using a weighted Kappa test with weights defined as 1 for +/− 2 places from the diagonal (agreement) and 0 elsewhere.
Findings
Coital diaries compared to face-to-face interviews
Of the 56 women, six reported having no sex in the week previous to the clinic visit on both data collection methods. Ten women reported sex acts in either the CD or the clinic FFI, but not on both. Of these 5 women reported at least one act in the CD but none at clinic FFI and 5 women reported at least one act at clinic FFI but none in the CD. For the 40 women who reported at least one sex act by both methods, only 6 (15%) reported exactly the same number of sex acts, placebo gel use and condom use in both the CD and at the clinic FFI.
Overall, the 56 women recorded nearly twice as many sex acts during the past week in the coital diary compared with those reported for the same recall period at the clinic FFI, with a median of 4 per woman recorded in the CD compared with 2 per woman reported at the clinic FFI (p=0.002; Table 2). Table 2 shows that compared to data recorded in CDs, a significantly higher proportion of women reported always using the gel and always carrying out vaginal washing at the clinic FFI. The findings also revealed that at the clinic women reported significantly higher proportions of sex acts with gel use or followed by vaginal washing than they recorded in the CDs. There was no significant difference between condom use reported at the clinics and in the CDs.
Table 2. Comparison of sexual behaviour reported in the CD and clinic-based interview during the 7 days before clinical review.
| Sexual Behaviour during 7 days before clinical review | Coital Diary | Clinic questionnaire | ||
|---|---|---|---|---|
| Number of women | 56 | 56 | ||
|
| ||||
| Number of women with no sex acts | 11 | 11 | ||
|
| ||||
| Number of sex acts reported | 223 | 114 | ||
|
| ||||
| Median number of sex acts reported per woman | 4 | 2 | p=0.002 | |
|
| ||||
| % of women reporting placebo gel use * | always | 40.0% | 84.4% | p<0.001** |
| sometimes | 44.4% | 11.1% | ||
| never | 15.6% | 4.4% | ||
|
| ||||
| Number of sex acts with reported gel use | 134 (60.1%) | 103 (90.4%) | p<0.001*** | |
|
| ||||
| % of women reporting condom use * | always | 35.6% | 44.4% | p=0.29** |
| Sometimes | 26.7% | 11.1% | ||
| Never | 37.8% | 44.4% | ||
|
| ||||
| Number of sex acts with reported condom use | 118 (52.9%) | 60 (52.6%) | p=0.99*** | |
|
| ||||
| % of women reporting vaginal washing * | always | 55.6% | 97.8% | p<0.001** |
| sometimes | 37.8% | 2.2% | ||
| never | 6.7% | 0.0% | ||
|
| ||||
| Number of sex acts with reported vaginal washing | 165 (74.0%) | 113 (99.1%) | p<0.001*** | |
|
| ||||
| % of women reporting sex with regular partner* | always | 73.3% | 88.9 % | p=0.11** |
| sometimes | 17.8% | 8.9% | ||
| never | 8.9% | 2.2% | ||
|
| ||||
| Number of sex acts reported with regular partner | 191 (85.7%) | 104 (91.2%) | p=0.15*** | |
|
| ||||
| % of women reporting vaginal insertion (non-gel)* | never | 100% | 100% | |
excludes women who did not report any sex acts on specified data collection method
paired comparison of always v. not always in 40 women who reported at least 1 sex act on both data collection methods
comparison of proportion of acts with specified feature (e.g. gel use) reported per-woman using sign test
For the total four weeks, ever having sex during menstruation was recorded by only 1.8% of women at the clinic FFI compared to 16.4% in the CDs (p=0.01).
Gel use reported in the coital diary compared with gel accountability
Of the 56 women who attended the clinic visit, data on placebo gel use were available for all 4 weeks of CDs for 53 women. The median number of gel applicators reported to have been used over 4 weeks in the CDs and the median number of used applicators returned at the clinic visit were the same (10) with 59% agreement on the number of gels used within +/−2 placebo gels (Kappa 0.47 (p<0.001)). The 41% disagreement was accounted for by 28% of women reporting more gels used in the CD and 13% reporting less gels used in the CD. Eight (8) women reported using more than 20 placebo gel applicators in the coital diary despite only being provided with 20 applicators.
In-depth Interviews
During IDIs, some women also reported different sexual behaviour during the past week than in the CD reports and clinic FFI reports. However, on probing, women said that difficulties in recall at both the in-depth interview and the clinic FFI were the reason for this. When probed about inconsistencies between the CD reports and clinic FFI, although they did not directly refer to the reasons for each-and-every inconsistency, women acknowledged that they found it difficult to report accurately in either, or both, of the data collection methods, although the majority attributed the mistakes to the clinic interview. Women suggested that the inaccuracies during the clinic FFI were due to their poor understanding of the questions; poor recall of each-and-every sexual act, condom or placebo gel use; embarrassment about the questions being asked; or that the counsellor did not understand or record their responses correctly. Where women acknowledged that they made mistakes in their own recordings in the CDs, they attributed this to poor understanding of the CD during the first week; not completing the CD on a daily basis as instructed; or because they used one placebo gel applicator for two sex acts but recorded two placebo gels used.
Discussion
This study found higher reports of sex acts recorded in the coital diary which suggests that women may be embarrassed to record high sexual activity in face-to-face interviews. Moreover, the stigmatised act of having sex whilst menstruating was rarely reported at the clinics but more frequently in the CDs. Other studies have found higher reporting of sex acts or socially stigmatised acts in diaries[4, 10, 11]. This suggests that diaries can be a useful tool to identify and measure behaviours which are rarely reported (e.g. anal sex and sex during menstruation) but which have important implications for the development of effective and acceptable microbicides.
This study also found higher reporting of gel use and vaginal washing at the clinic, which suggests socially desirability bias in face-to-face clinic interviews. As the gels were provided by the clinic staff with instructions to use them with every sex act, it is possible that the women wanted to report that they used the gels to ‘please’ the staff. The reason for higher reports at the clinic of washing before or after sex is more complex. This has been found to be a widely-accepted behaviour amongst women in Mwanza, most of whom consider it to be a good thing to do, so higher reporting at the clinic suggests that women expect that vaginal washing is socially sanctioned by health workers. Condom use is a more difficult issue. Allen et al. [4] found significantly higher reports of condom use in the CDs than face-to-face interviews, whilst this study found little difference between coital diary reports and clinic interview reports. However, Allen et al. [4] found only 38% of women ever reporting condom use in CDs compared with 62% of women in this study, where women were counselled to use condoms with the gel. Ramjee et al [12] suggest that high reports of condom use in diaries could reflect over- reporting when condom counselling is provided as part of the research clinic procedures. However, this effect may also apply to condom reporting at clinic interviews.
The implication of these findings for microbicide clinical trials is that data that are routinely collected at clinic visits to measure adherence, such as number of sex acts, gel and condom use may be subject to important inaccuracies. As well as issues of stigma and social desirability, this study has revealed that data collected at clinic interviews were reported by the participants to have been associated with greater problems of recall due to being asked to remember sexual behaviour in the last week or 4 weeks, misunderstandings by the participants about the information they were being asked for or embarrassment of the participants related to the questions asked.
This study also confirmed that coital diaries do not provide a gold standard for measuring sexual behaviour data. The study found that CDs may be inaccurate due to misunderstandings of the diary and errors in recording by the participants. For example, comparison of reports of placebo gel use in the CDs with placebo gel accountability showed evidence of both under and over reporting. To improve accuracy of data, the coital diary should be easy to complete, not time consuming, participants should be provided with clear instructions and intensive follow up support, and missing data should be minimised by checking diaries with participants [4, 10-13].
In order to improve accuracy of sexual behaviour data collected during clinical trials, two approaches can be taken. Where funding allows, all women should be provided with well-designed CDs to be completed daily, with weekly support by the research team. Ideally CDs should be completed over the course of the trial to gain a more accurate measure of condom use and gel adherence. However, this can be costly in terms of resources such as staff and transport to find participants. Where funding is insufficient to permit this, a simple aide memoire could be developed, which could be completed by the women daily, between clinic visits, and used to aid recall at the face-to-face interview. This could be a simplified version of a coital diary, set on one sheet of paper or printed on their gel box, with columns for sex, condom and gel use and rows where women can tick every time they have sex and use a condom or gel. Moreover, in-depth interviews with a sample of participants are important in examining the validity of quantitative data.
In conclusion, given the importance of collecting valid sexual behaviour data in microbicide trials to better understand gel efficacy, coital dairies represent an innovative, locally-appropriate, user-directed method for collecting this information, and should be considered as a research tool in microbicide trial design.
Acknowledgements
Our special thanks go to the women of Mwanza who participated in the pilot study and in particular to members of the MDP Mwanza team and the Community Advisory Committee. We thank our colleagues at the National Institute for Medical Research, Mwanza, Tanzania and the African Medical and Research Foundation, Mwanza, Tanzania for their support and assistance in carrying out this research.
Source of support: This research forms part of the Microbicides Development Programme, funded by the UK Department of International Development and the UK Medical Research Council
References
- 1.Wolff B. Draft protocol for monitoring risk behaviour for HIV transmission during Phase III vaccine trials. 2000 Report to UNAIDS.
- 2.Coxon AP. Parallel accounts? Discrepancies between self-report (diary) and recall (questionnaire) measures of the same sexual behaviour. AIDS Care. 1999;11(2):221–234. doi: 10.1080/09540129948108. [DOI] [PubMed] [Google Scholar]
- 3.Leigh BC, Gillmore MR, Morrison DM. Comparison of diary and retrospective measures for recording alcohol consumption and sexual activity. J Clin Epidemiol. 1998;51(2):119–27. doi: 10.1016/s0895-4356(97)00262-x. [DOI] [PubMed] [Google Scholar]
- 4.Allen CA, Lees SS, Desmond N, et al. Validity of coital diaries in the Microbicides Development Programme feasibility study among women at high risk of HIV in Mwanza, Tanzania. Sexually Transmitted Infections. 2007;83:490–497. doi: 10.1136/sti.2007.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catania J, Gibson D, Chitwood D, et al. Methodological problems in AIDS behavioral research, influences on measurement error and participation bias in studies of sexual behaviour. Psychological Bulletin. 1990;108:339–362. doi: 10.1037/0033-2909.108.3.339. [DOI] [PubMed] [Google Scholar]
- 6.Graham CA, Catania JA, Brand A, et al. Recalling sexual behavior: a methodological analysis of memory recall bias via interview using the diary as the gold standard. J Sex Res. 2003;40(4):325–32. doi: 10.1080/00224490209552198. [DOI] [PubMed] [Google Scholar]
- 7.Vallely A, Kasindi S, Hambleton I, et al. Tanzania-Baseline Characteristics of an Occupational Cohort and Reattendance at 3 Months. Sexually Transmitted Diseases. 2007;34(9):638–643. doi: 10.1097/OLQ.0b013e3180325120. [DOI] [PubMed] [Google Scholar]
- 8.Kapiga S, Sam N, Shao J, et al. HIV-1 epidemic among female bar and hotel workers in Northern Tanzania: Risk factors and opportunities for prevention. JAIDS. 2002;29(4):409–417. doi: 10.1097/00126334-200204010-00013. [DOI] [PubMed] [Google Scholar]
- 9.Watson-Jones D, Weiss H, Rusizoka M, et al. Risk factors for herpes simplex virus type 2 and HIV among women at high risk in northwestern Tanzania: preparing for an HSV-2 intervention trial. JAIDS. 2007;46(5):631–642. doi: 10.1097/QAI.0b013e31815b2d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson A, Morris C, Kariuki C. Using diaries to measure parameters of transactional sex: an example from the Trans-Africa highway in Kenya. Cult Health Sex. 2006;8(2):175–185. doi: 10.1080/13691050600665006. [DOI] [PubMed] [Google Scholar]
- 11.McAuliffe TL, DiFranceisco W, Reed BR. Effects of question format and collection mode on the accuracy of retrospective surveys of health risk behavior: a comparison with daily sexual activity diaries. Health Psychology. 2007;26(1):60–7. doi: 10.1037/0278-6133.26.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Ramjee G, Weber AE, Morar MS. Recording sexual behavior: comparison of recall questionnaires with a coital diary. Sexually Transmitted Diseases. 1999;26(7):374–80. doi: 10.1097/00007435-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hays MA, Irsula B, McMullen SL, et al. A comparison of three daily coital diary designs and a phone-in regimen. Contraception. 2001;63(3):159–66. doi: 10.1016/s0010-7824(01)00183-4. [DOI] [PubMed] [Google Scholar]


