Abstract
Background:
Our previous research investigated the ability of [F-18]fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging results to predict outcome in patients with sarcoma. Tumor uptake of FDG before and after neoadjuvant chemotherapy was predictive of patient outcome. With this background, a prospective clinical study was designed to assess whether tumor FDG uptake levels in the middle of neoadjuvant chemotherapy added additional prognostic information to pre-therapy imaging data.
Methods:
Sixty-five patients with either bone or soft-tissue sarcoma were treated with neoadjuvant-based chemotherapy according to the standard clinical practice for each tumor group. All patients had FDG PET studies before therapy, mid-therapy (after two cycles of chemotherapy), and before resection. Tumor FDG uptake (SUVmax, the maximum standardized uptake value) at each imaging time point, tumor type (bone or soft-tissue sarcoma), tumor size, and histopathologic grade were recorded for each patient. The time from the pre-therapy FDG PET study to events of local tumor recurrence, metastasis, or death were extracted from the clinical records for comparison with the imaging data. Univariate and multivariate analyses of the imaging and clinical data were performed.
Results:
Univariate and multivariate data analyses showed that the difference (measured as the percentage reduction) between the pre-therapy and mid-therapy maximum tumor uptake values added prognostic value to patient outcome predictions independently of other patient variables.
Conclusions:
The utility of a tumor pre-therapy FDG PET scan as a biomarker for the outcome of patients with sarcoma was strengthened by a mid-therapy scan to evaluate the interim treatment response.
Level of Evidence:
Prognostic Level I. See Instructions for Authors for a complete description of levels of evidence.
Peer Review
This article was reviewed by the Editor-in-Chief and one Deputy Editor, and it underwent blinded review by two or more outside experts. The Deputy Editor reviewed each revision of the article, and it underwent a final review by the Editor-in-Chief prior to publication. Final corrections and clarifications occurred during one or more exchanges between the author(s) and copyeditors.
[F-18]fluorodeoxyglucose (FDG) positron emission tomography (PET) has made gains in its establishment as a biomarker in cancer imaging1. Useful for staging disease and identifying tumor response to therapy in most common cancers, it has become an established part of cancer management. Sarcomas are one of the less frequently occurring malignancies, and many patients with this disease still have poor outcomes despite current therapy. Assessment of tumor response on the basis of criteria involving changes in tumor size is not particularly helpful in these tumors2. Our previous research investigated the ability of tumor uptake of FDG, measured with PET, to predict patient outcome3. Furthermore, we established that comparison of tumor FDG uptake before and after neoadjuvant therapy was also predictive of patient outcome4-6.
With this background, a prospective clinical study was designed to assess whether tumor FDG uptake in the middle of neoadjuvant chemotherapy was as predictive of patient outcome as the levels prior to therapy and at the end of neoadjuvant therapy. The goal in this study was to establish the usefulness of the mid-therapy scan as a biomarker of tumor response, enabling an oncology patient care team to use this information to guide treatment decisions and also allowing its use in evaluation of new therapies.
Materials and Methods
Patients
Participating patients were seen in the clinics for either bone or soft-tissue sarcoma and were treated with doxorubicin-based neoadjuvant chemotherapy according to the standard clinical practice for each tumor group. Patients were enrolled in the study from 1995 to 2005 and were selected for inclusion on the basis of their willingness to participate. Inclusion criteria were the presence of untreated primary sarcoma, planned chemotherapy and surgical resection, the ability to provide informed consent, and the ability to lie on the imaging table. All patients provided consent prior to participation in the institution-approved protocol. Postoperative radiation and/or chemotherapy were administered according to the standard clinical practice for each tumor group.
Study Imaging Protocol
All patients had PET studies of FDG uptake before therapy, in the middle of therapy (after two cycles of chemotherapy), and before resection. Tumor uptake (SUVmax, the maximum standardized uptake value) at each imaging time point, tumor type (bone or soft-tissue sarcoma), tumor size, and histopathologic grade were recorded for each patient. Clinical data were also recorded for each patient. For this analysis, the time from the pre-therapy FDG PET study to events of local tumor recurrence, metastasis, or death were extracted from the clinical records.
PET Imaging
Standard FDG PET images were made with use of a PET Advance scanner (GE Healthcare, Waukesha, Wisconsin) according to standard clinical procedures and as described previously3. The SUVmax value for the tumor regions of interest in each patient image was determined as described previously3. SUVdiff, the percentage change in tumor SUVmax, was calculated as the difference between the square-root-transformed SUVmax values in the pre-therapy and mid-therapy scans divided by the pre-therapy value.
Statistical Analysis
Patient death was considered the primary end point, with secondary end points involving tumor progression (either local recurrence or metastases). Clinical records were used to determine patient disease status. The event-free survival time was defined as the time (in months) from the pre-therapy FDG PET study to local tumor recurrence, metastasis, or death. Patients who were alive and without evidence of disease at the last clinic visit were considered disease-free survivors in the analysis. Patients with metastases at the time of study entry were excluded from the analysis. Initial analysis of tumor SUV and size data showed a skewed distribution; a square-root transformation was applied to those variables to address this issue. Continuous variables were standardized. The reported hazard ratios correspond to a one-standard-deviation change in the covariate. A p value of 0.05 was considered significant.
The question of interest is whether the maximum uptake of FDG by tumors at mid-therapy provides additional prognostic information beyond that provided by the tumor pre-therapy SUV data and other prognostic variables currently used in standard clinical practice3. SUVdiff, the percentage change in tumor SUVmax, was calculated as the difference between the SUVmax values in the pre-chemotherapy and mid-chemotherapy scans divided by the pre-chemotherapy value. (As previously noted, SUVmax values were square-root-transformed.) The SUVmax and SUVdiff data from the imaging time points and other prognostic variables were analyzed with use of a Cox regression model to understand the prognostic capability of this additional imaging information7. Patient survival, progression-free survival (free of distant and local progression), and local-progression-free survival were studied with use of univariate and multivariate Cox regression models. A set of eight prognostic variables were considered in the analysis; in addition to tumor pre-therapy SUVmax and SUVdiff, these were tumor histopathologic grade, sex, age, tumor size, tumor site, and sarcoma type.
The analyses considered all possible multivariate Cox regression models that contained at least one of the eight prognostic variables (a total of 255 models). A leave-out-one cross-validation procedure was used to assess which of these models provided the most reliable predictive relationship with each type of patient outcome (patient survival, progression-free survival, and local-progression-free survival). Cox model fits were evaluated on the basis of the likelihood information measure (−2 × log-likelihood) that has been established as appropriate for Cox regression analysis7,8. In addition, models were scored with use of the Akaike Information Criterion measure used with the Cox model, and the Hartell concordance statistic is also reported9. Note that model-fit statistics (likelihood or concordance) for the data subset used for validation are expected to be less favorable than those obtained with the full data set. The discrepancy between the two diminishes as the reliability of the model improves and as the sample size increases. Detailed results regarding the model selection are given in the Appendix.
We focused on the model selected by the cross-validation process for the patient survival analysis. The variables selected for this model were then used to define corresponding multivariate Cox models for progression-free survival and local-progression-free survival. We compared the cross-validation error in each of the latter two models with the error in the optimal cross-validated model for the same end point. The results for our final set of models are reported in detail with use of the standard methodology for Cox regression8. All continuous variables were scaled so that the hazard ratios presented indicate the hazard associated with a one-standard-deviation increase in the comparison covariate. Progression-free survival and local-progression-free survival were assessed with the same model that was chosen for patient survival; this model was then augmented by each of the remaining covariates and assessed for a further significant increase in prognostic utility. Survival curves are shown to illustrate the differences in predicted risk for death or progression associated with high and low pre-therapy SUVmax values and SUVdiff values in each of the models8,9.
Source of Funding
The patient FDG PET scanning, the corresponding author (J.F.E.), and the statistician coauthors (J.O’S., F.O’S.) were supported by National Institutes of Health (NIH) grant R01 CA65537.
Results
Seventy-nine patient imaging studies were available for this analysis. One patient was removed from the analysis because tumor SUVmax data were missing, and thirteen patients were removed because they had metastases at the time of study entry, resulting in a final sample size of sixty-five patients (Table I). Two of the patients who were included in the analysis did not have accurate tumor size information available, and tumor size was estimated from the scans. Figs. 1-A and 1-B show an example of tumor response on PET imaging. The median duration of patient follow-up was 3.4 years (range, 0.34 to 8.14 years). Disease progression occurred in thirty-one of these patients, there were twenty-five deaths, and twenty patients had local disease recurrence.
TABLE I.
Patient Characteristics
| Characteristic | No. of Patients |
| Age at diagnosis | |
| Pediatric, 10-20 yr | 22 |
| Adult, 21-66 yr | 43 |
| Tumor site | |
| Upper extremity | 8 |
| Lower extremity | 36 |
| Pelvis | 14 |
| Trunk | 7 |
| Tumor diagnosis* | |
| Ewing sarcoma | 10 |
| Osteosarcoma | 15 |
| Fibrosarcoma | 1 |
| Leiomyosarcoma | 7 |
| Liposarcoma | 6 |
| MPNST | 5 |
| Sarcoma NOS | 13 |
| Synovial sarcoma | 8 |
MPNST = malignant peripheral nerve sheath tumor; NOS = not otherwise specified.
FDG PET images showing an example of the treatment response in a patient with a large Ewing tumor in the left pelvis. FDG-avid lung metastases are also present (arrow).
Fig. 1-A.
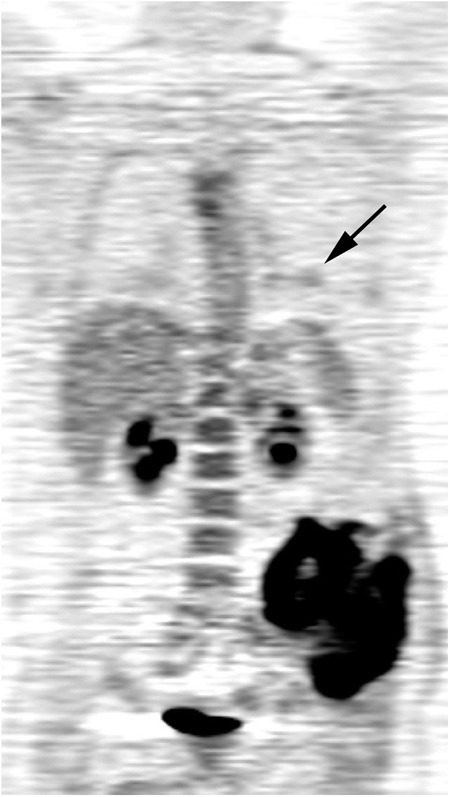
FDG SUVmax = 11.5 before treatment.
Fig. 1-B.
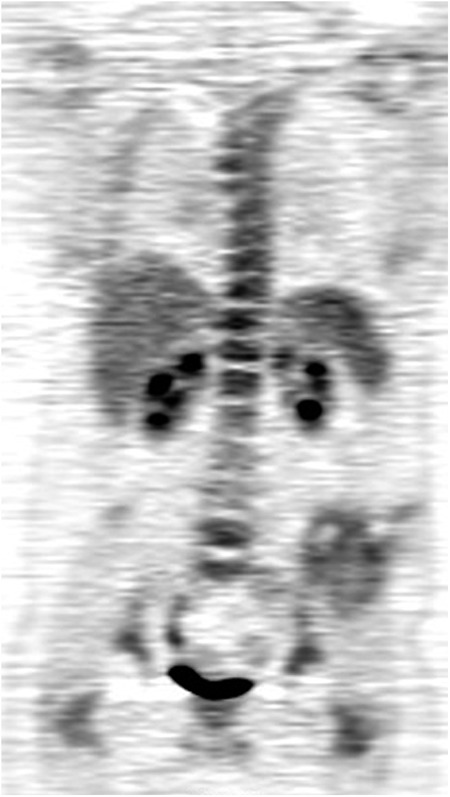
Tumor uptake of FDG after treatment is much lower; SUVmax = 4.4.
In the univariate analyses, tumor size, SUVdiff, tumor site, and tumor type showed significant associations with patient survival (Table II). However, when all covariates were included in the multivariate model, none was significantly associated with patient survival. This was due to high collinearity, which is a common issue in multivariate modeling. However, the model selected by cross-validation revealed that, when the effects of multicollinearity and over-fitting from the five least effective prognostic variables were removed, the associations of pre-therapy SUVmax, SUVdiff, and tumor site with patient survival were significant (p = 0.04, p = 0.01, and p = 0.04, respectively). Tumor type and size, although excellent prognostic variables when used singly, did not add information to the multivariate model, implying overlap between these variables and the variables already present in the model (Table III). Although pre-therapy SUVmax was not a key prognostic factor in the univariate analysis, it became important when included along with SUVdiff and site in the multivariate analysis.
TABLE II.
Univariate Analysis for Patient Survival
| Variable | Hazard Ratio* | 95% Confidence Interval | P Value |
| Pre-therapy SUVmax | 1.25 | 0.87 to 1.79 | 0.23 |
| SUVdiff | 0.62 | 0.4 to 0.97 | 0.03 |
| Tumor size | 1.75 | 1.1 to 2.8 | 0.02 |
| Age | 1.6 | 0.9 to 2.84 | 0.11 |
| Sex | 1.11 | 0.5 to 2.45 | 0.8 |
| Tumor type (bone vs. soft-tissue sarcoma) | 0.32 | 0.12 to 0.86 | 0.02 |
| Tumor grade | 1.01 | 0.46 to 2.23 | 0.98 |
| Tumor site (truncal vs. extremity) | 2.63 | 1.18 to 5.83 | 0.02 |
For a one-standard-deviation change in the covariate.
TABLE III.
Multivariate Analysis for Patient Survival
| Variable | Hazard Ratio* | 95% Confidence Interval | P Value |
| Pre-therapy SUVmax | 1.48 | 1.018 to 2.147 | 0.04 |
| SUVdiff | 0.54 | 0.328 to 0.872 | 0.01 |
| Tumor site (truncal vs. extremity) | 2.37 | 1.059 to 5.292 | 0.04 |
For a one-standard-deviation change in the covariate.
The univariate analysis of progression-free survival (Table IV) revealed a pattern similar to that for patient survival. SUVdiff and tumor size, type, and site were significantly associated with disease progression. When the multivariate analysis model chosen for patient survival was applied to progression-free survival, pre-therapy SUVmax was not a significant predictor, but it was retained as it is integral to the question of interest. The remaining covariates were then added individually and assessed for further prognostic potential. In the multivariate analysis, SUVdiff and tumor site had significant associations with outcome (Table V). No other covariates were found to add any significant prognostic advantage to the original model, although sarcoma type showed a borderline association (p = 0.06).
TABLE IV.
Univariate Analysis for Progression-Free Survival
| Variable | Hazard Ratio* | 95% Confidence Interval | P Value |
| Pre-therapy SUVmax | 1.09 | 0.76 to 1.55 | 0.65 |
| SUVdiff | 0.61 | 0.41 to 0.91 | 0.01 |
| Tumor size | 1.63 | 1.07 to 2.5 | 0.02 |
| Age | 1.42 | 0.86 to 2.33 | 0.17 |
| Sex | 1.41 | 0.68 to 2.9 | 0.36 |
| Tumor type (bone vs. soft-tissue sarcoma) | 0.27 | 0.11 to 0.65 | 0.004 |
| Tumor grade | 0.78 | 0.39 to 1.59 | 0.5 |
| Tumor site (truncal vs. extremity) | 3.08 | 1.51 to 6.29 | 0.002 |
For a one-standard-deviation change in the covariate.
TABLE V.
Multivariate Analysis for Progression-Free Survival
| Variable | Hazard Ratio* | 95% Confidence Interval | P Value |
| Pre-therapy SUVmax | 1.35 | 0.925 to 1.962 | 0.12 |
| SUVdiff | 0.53 | 0.333 to 0.835 | 0.006 |
| Tumor site (truncal vs. extremity) | 2.93 | 1.428 to 6.003 | 0.003 |
For a one-standard-deviation change in the covariate.
In the univariate analysis of local-progression-free survival, pre-therapy tumor SUVmax, size, and site had significant associations with disease progression (Table VI). The associations for SUVdiff and tumor type were of borderline significance. Applying the multivariate model with pre-therapy SUVmax, SUVdiff, and site, all variables had significant associations with local-progression-free survival (Table VII). No additional variables contributed significantly to the model.
TABLE VI.
Univariate Analysis for Local-Progression-Free Survival
| Variable | Hazard Ratio* | 95% Confidence Interval | P Value |
| Pre-therapy SUVmax | 1.49 | 1.01 to 2.2 | 0.04 |
| SUVdiff | 0.64 | 0.39 to 1.06 | 0.08 |
| Tumor size | 2.19 | 1.3 to 3.66 | 0.003 |
| Age | 1.41 | 0.77 to 2.61 | 0.26 |
| Sex | 1.05 | 0.43 to 2.53 | 0.92 |
| Tumor type (bone vs. soft-tissue sarcoma) | 0.40 | 0.14 to 1.09 | 0.07 |
| Tumor grade | 0.96 | 0.4 to 2.33 | 0.94 |
| Tumor site (truncal vs. extremity) | 3.51 | 1.45 to 8.49 | 0.005 |
For a one-standard-deviation change in the covariate.
TABLE VII.
Multivariate Analysis for Local-Progression-Free Survival
| Variable | Hazard Ratio* | 95% Confidence Interval | P Value |
| Pre-therapy SUVmax | 1.82 | 1.186 to 2.786 | 0.006 |
| SUVdiff | 0.46 | 0.254 to 0.849 | 0.013 |
| Tumor site (truncal vs. extremity) | 3.24 | 1.33 to 7.886 | 0.01 |
For a one-standard-deviation change in the covariate.
Risks for decreased patient, progression-free, and local-progression-free survival in the multivariate Cox regression analyses derived from the data are shown in Tables III, V, and VII. For all end points, the risk assessment was significantly improved (p = 0.01) by including SUVdiff in a multivariate model with pre-therapy SUVmax and tumor site compared with a model with SUVmax and tumor site alone. The results of the analyses point to improvement in the assessment of prognosis provided by the SUVdiff variable. On average, for every 18% (one standard deviation) increase in the SUVdiff variable, there was an associated halving of the risk of death (i.e., the hazard ratio was approximately 0.5). This hazard ratio was quite stable across the patient survival, progression-free survival, and local-progression-free survival end points (0.56, 0.53, and 0.46, respectively). The fit statistics for the optimal models selected by the cross-validation had full-data set data likelihood values of 178.17, 212.27, and 141.29 for the same three end points, respectively; the corresponding cross-validated values were on the order of 1% to 5% higher (180.19, 221.92, and 143.47). Sample concordance statistics for the risk factors in these three Cox models were 0.69, 0.74, and 0.71, and these values decreased to 0.67, 0.71, and 0.70 in the cross-validation analysis, showing the relatively small deviation between the training-sample and cross-validation performance. This is a reflection of the model stability. The optimal cross-validated model for patient survival included three variables: pre-therapy SUVmax, SUVdiff, and tumor site. Although this was not the optimal model for progression-free survival or local-progression-free survival, its cross-validation error was close to that of the optimal model in both cases (see Appendix). For all three outcomes, the variables selected for the optimal cross-validation model include SUVdiff. This highlights the prognostic importance of mid-therapy FDG PET imaging information relative to any other available prognostic variable. Although the analysis used transformed data, the model was tested and yielded similar results for the raw (untransformed) data, indicating robustness of the model and suggesting simple application to the clinical setting. One standard deviation in the percentage reduction from before therapy to mid-therapy in the raw data was approximately 27%, and the median percentage reduction in the raw data was approximately 36%.
The Cox model analysis for patient survival revealed that the total risk for a patient outcome was a weighted sum of the risks estimated on the basis of the PET imaging time points plus an adjustment depending on whether the site of the tumor is extremity or truncal. The coefficients for the model are the natural logs of the hazard ratios; thus, from Table III, the PET-predicted risk for decreased patient survival was quantified as 0.39 × (pre-therapy SUVmax) − 0.62 × SUVdiff; the site contribution to the overall risk was 0.86 if the tumor was truncal. Within our data, the PET-predicted risk values had a distribution with a mean of 0.56 and a standard deviation of 0.60. This distribution (including the locations of risk values one standard deviation above and below the median) is illustrated in a histogram of PET-predicted risk values in the Appendix.
Our analysis can be used to evaluate survival patterns for different scenarios. Figure 2 shows the estimated patterns (for each end point) for patients with PET-predicted risks above and below the median. The survival curves in the remaining figures in the Appendix were constructed by using a derived estimate for the reference survival curve in the Cox model8; they are not standard Kaplan-Meier curves for subgroups of patients. In contrast, the Kaplan-Meier curves for two or four subgroups within a cohort of only sixty-five patients would exhibit much more variability (because those curves do not take into account the Cox model). The curves in the Appendix illustrate the usefulness of the model in understanding projected patient survival. They compare survival for patients for whom the quantified PET-predicted risk might be considered high in value and for those with low risk, demonstrating remarkable differences between the predicted survival experiences of these patients.
Fig. 2.
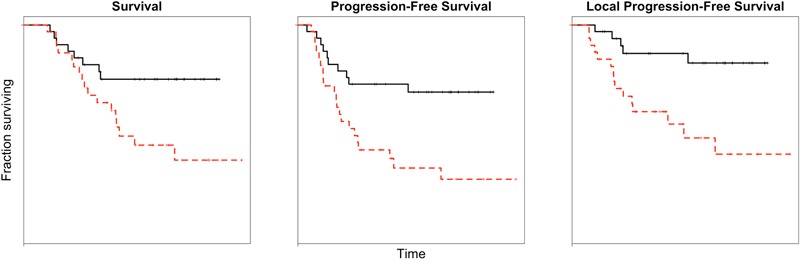
Kaplan-Meier curves of the patient group for three survival end points. The black (solid) lines represent patients with lower risk (below the median) as defined by the survival models. The red (dashed) lines represent patients with higher risk (above the median).
Discussion
Tumor pre-therapy FDG SUVmax and SUVdiff combined as strong predictors of patient outcome and can be considered in further analyses. The results for the reduced model, in which factors with little influence on the survival estimates were removed from the analysis, showed the effects of SUVdiff to be highly significant across all outcome types. Pre-therapy SUVmax has a significant effect on both patient survival and local-progression-free survival, with a trend toward an effect on progression-free survival. It is encouraging to have such strong results in a relatively small data set, in which nearly one-half of patient outcomes were censored. Tumor type and site were also associated with a risk for poor outcome, consistent with previous reports and clinical experience10. Patients with bone sarcoma generally have a better outcome than patients with high-grade soft-tissue sarcoma, and extremity tumors pose less risk for a poor outcome compared with those in truncal sites. Another variable that was significantly associated with survival in the univariate models for all outcomes was tumor size. Tumor size is recognized as a significant prognostic factor in the planning of treatment for soft-tissue sarcoma tumors10. Consequently, histologically intermediate-grade soft-tissue sarcomas that are >5 cm in diameter are considered in the same high-risk category for reduced survival as histologically high-grade tumors.
A unique feature of the present study is the addition of a subanalysis of local-progression-free survival. The ability to predict local tumor progression can likely be used clinically to plan whether or not neoadjuvant treatment should include local radiation in addition to combination chemotherapy. Surgical resection options, including limb salvage, can also possibly be considered with more precision on the basis of these results. The high rate of local recurrence in the patient group reflects the distribution of tumor types and sites. The majority of the patients were adults with high-risk soft-tissue sarcomas located in the extremities. The data suggest that patients treated with limb salvage resection following chemotherapy may need additional treatment such as radiation therapy for optimal long-term local control of the tumor bed.
Previously published reports on the ability of FDG PET to quantify therapy response have made important contributions to the use of this imaging modality in the care of patients with sarcoma. These reports have largely, and appropriately, focused on validation of the imaging results through assessment of the association between tumor FDG uptake and the presence or absence of tumor necrosis. The presence of a high level of tumor necrosis in sarcomas is thought to be a strong predictor of long-term treatment response11-14. In one study, tumor FDG SUV changes contributed additional information to assessment of the treatment response with magnetic resonance imaging (MRI)15. In another study, the SUVdiff value was found to be more accurate for assessing the response than either the presence of substantial tumor necrosis or the RECIST (Response Evaluation Criteria in Solid Tumors) when these were applied to the same group of soft-tissue sarcomas16. Benz et al. reported that a reduction in tumor FDG uptake of >35% from the pre-therapy value was predictive of histologically assessed treatment response. These results established specific criteria for post-therapy treatment response assessment in clinical practice17. In bone sarcomas, Cheon et al. found that the MR-based volume change, used in combination with pre-therapy and post-therapy tumor FDG SUV data, was associated with the histologic response of the tumor18. Cheon et al. demonstrated the contribution that complementary imaging modalities can make to these challenging tumor response assessments. Dimitrakopoulou-Strauss et al. used multiparameter FDG kinetic analysis to demonstrate that the tumor FDG metabolic rate was associated with the histologic response19.
The present study was designed to examine the ability of mid-therapy PET measurement of FDG uptake by tumors to predict the risk for a poor outcome on the basis of statistical analyses validated by the actual patient outcomes in the study group. This is distinctly different from the goals of the studies cited above, which involved the association of tumor FDG uptake with tumor histologic response as identified in the resected specimen. The significant hazard ratios for the pre-therapy SUVmax value and SUVdiff value obtained in the present study confirmed that these measures have a dose effect on the clinical outcome. The results demonstrated that clinical FDG PET scans made mid-therapy can be used to further stratify patients with sarcoma according to the risk for a poor outcome, with greater certainty compared with pre-therapy observations of tumor metabolic activity alone.
The results remained relevant for the raw (untransformed) data, and the procedure that was used to define the types of clinical outcome in this study can easily be instituted in a clinical setting. The median SUVdiff value for the raw data was 35.7%, and a difference that is smaller implies a higher risk for a poor outcome. The median pre-therapy SUVmax value for the raw data was 7.3. A decision scheme for treatment could be constructed from the data in this study, using the median as a cutoff point between “high” and “low” risk. For example, a patient with a low pre-therapy tumor SUVmax is at low risk for a poor outcome (Fig. 2). A patient who has a high pre-therapy SUVmax and little change in tumor uptake with therapy may consider discontinuing that line of treatment, as the models would predict a high risk for a poor outcome. Conversely, a patient with a high pre-therapy SUVmax but a high SUVdiff could be encouraged to continue treatment, since better survival would be predicted according to the presented models. We suggest using the SUV data as indicators of risk, rather than as cutoff points for decision-making, since the tumor pre-therapy SUVmax values and SUVdiff values are part of a continuum in the biological behavior of the tumor and its response to treatment.
Use of FDG PET data for sarcoma assessment in clinical applications presumes consistent implementation of standard PET procedures. Such consistent implementation has been described previously for the use of FDG PET as a biomarker for treatment response assessment20. It also points the way to use of FDG PET as a marker for early response assessment in new combination therapies or new therapy trials in which changes in tumor uptake predict the clinical outcome. The patient outcomes in the present study validate these results.
Sarcomas are often very large, with heterogeneous metabolism within an individual tumor. As a group, they are histologically and clinically diverse. We hypothesize that responses to neoadjuvant treatment may also vary according to tumor type and size, with some tumors requiring more therapy to achieve a response that is indicative of efficacy and predictive of prolonged survival, and this would be a subject for future studies.
Appendix
Figures showing a histogram of risk values in the patient group and predicted survival curves according to SUV values, a table showing the optimal model and chosen model for each survival outcome, and an appendix outlining the derivation of those models are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Figures showing a histogram of risk values in the patient group and predicted survival curves according to SUV values, a table showing the optimal model and chosen model for each survival outcome, and an appendix outlining the derivation of those models
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Podoloff DA, Advani RH, Allred C, Benson AB, 3rd, Brown E, Burstein HJ, Carlson RW, Coleman RE, Czuczman MS, Delbeke D, Edge SB, Ettinger DS, Grannis FW, Jr, Hillner BE, Hoffman JM, Kiel K, Komaki R, Larson SM, Mankoff DA, Rosenzweig KE, Skibber JM, Yahalom J, Yu JM, Zelenetz AD. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. 2007 May;5(Suppl 1):S1-22; quiz S23-2 [PubMed] [Google Scholar]
- 2.Evilevitch V, Weber WA, Tap WD, Allen-Auerbach M, Chow K, Nelson SD, Eilber FR, Eckardt JJ, Elashoff RM, Phelps ME, Czernin J, Eilber FC. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008 Feb 1;14(3):715-20 [DOI] [PubMed] [Google Scholar]
- 3.Eary JF, O’Sullivan F, Powitan Y, Chandhury KR, Vernon C, Bruckner JD, Conrad EU. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002 Sep;29(9):1149-54 Epub 2002 Jun 19 [DOI] [PubMed] [Google Scholar]
- 4.Hawkins DS, Rajendran JG, Conrad EU, 3rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002 Jun 15;94(12):3277-84 [DOI] [PubMed] [Google Scholar]
- 5.Hawkins DS, Conrad EU, 3rd, Butrynski JE, Schuetze SM, Eary JF. [F-18]-fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer. 2009 Aug 1;115(15):3519-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuetze SM, Rubin BP, Vernon C, Hawkins DS, Bruckner JD, Conrad EU, 3rd, Eary JF. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005 Jan 15;103(2):339-48 [DOI] [PubMed] [Google Scholar]
- 7.Andersen PK, Borgan Ø, Gill RD, Keiding N. Statistical models based on counting processes. New York: Springer; 1993 [Google Scholar]
- 8.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. John Wiley & Sons; 2002 [Google Scholar]
- 9.Collett D. Modelling survival data in medical research. 2nd ed. Boca Raton: Chapman & Hall/CRC; 2003 [Google Scholar]
- 10.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006 Oct;130(10):1448-53 [DOI] [PubMed] [Google Scholar]
- 11.Jones DN, McCowage GB, Sostman HD, Brizel DM, Layfield L, Charles HC, Dewhirst MW, Prescott DM, Friedman HS, Harrelson JM, Scully SP, Coleman RE. Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med. 1996 Sep;37(9):1438-44 [PubMed] [Google Scholar]
- 12.Iagaru A, Masamed R, Chawla SP, Menendez LR, Fedenko A, Conti PS. F-18 FDG PET and PET/CT evaluation of response to chemotherapy in bone and soft tissue sarcomas. Clin Nucl Med. 2008 Jan;33(1):8-13 [DOI] [PubMed] [Google Scholar]
- 13.Ye Z, Zhu J, Tian M, Zhang H, Zhan H, Zhao C, Yang D, Li W, Lin N. Response of osteogenic sarcoma to neoadjuvant therapy: evaluated by 18F-FDG-PET. Ann Nucl Med. 2008 Jul;22(6):475-80 Epub 2008 Aug 01 [DOI] [PubMed] [Google Scholar]
- 14.Hamada K, Tomita Y, Inoue A, Fujimoto T, Hashimoto N, Myoui A, Yoshikawa H, Hatazawa J. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Ann Nucl Med. 2009 Jan;23(1):89-95 Epub 2009 Feb 11 [DOI] [PubMed] [Google Scholar]
- 15.Bredella MA, Caputo GR, Steinbach LS. Value of FDG positron emission tomography in conjunction with MR imaging for evaluating therapy response in patients with musculoskeletal sarcomas. AJR Am J Roentgenol. 2002 Nov;179(5):1145-50 [DOI] [PubMed] [Google Scholar]
- 16.Evilevitch V, Weber WA, Tap WD, Allen-Auerbach M, Chow K, Nelson SD, Eilber FR, Eckardt JJ, Elashoff RM, Phelps ME, Czernin J, Eilber FC. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008 Feb 1;14(3):715-20 [DOI] [PubMed] [Google Scholar]
- 17.Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Elashoff D, Chow K, Evilevitch V, Eckardt JJ, Phelps ME, Weber WA, Eilber FC. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009 Apr 15;15(8):2856-63 Epub 2009 Apr 07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, Koh JS, Yoo JY, Oh DH, Shin DS, Jeon DG. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009 Sep;50(9):1435-40 Epub 2009 Aug 18 [DOI] [PubMed] [Google Scholar]
- 19.Dimitrakopoulou-Strauss A, Strauss LG, Egerer G, Vasamiliette J, Mechtersheimer G, Schmitt T, Lehner B, Haberkorn U, Stroebel P, Kasper B. Impact of dynamic 18F-FDG PET on the early prediction of therapy outcome in patients with high-risk soft-tissue sarcomas after neoadjuvant chemotherapy: a feasibility study. J Nucl Med. 2010 Apr;51(4):551-8 [DOI] [PubMed] [Google Scholar]
- 20.Shankar LK, Hoffman JM, Bacharach S, Grahm MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute trials. J Nucl Med. 2006 Jun;47(6):1059-66 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
Figures showing a histogram of risk values in the patient group and predicted survival curves according to SUV values, a table showing the optimal model and chosen model for each survival outcome, and an appendix outlining the derivation of those models


