Abstract
Performance in athletic activities that include a significant aerobic component at mild or moderate altitudes shows a large individual variation. Physiologically, a large portion of the negative effect of altitude on exercise performance can be traced to limitations of oxygen diffusion, either at the level of the alveoli or the muscle microvasculature. In the lung, the ability to maintain arterial oxyhaemoglobin saturation (SaO2) appears to be a primary factor, ultimately influencing oxygen delivery to the periphery. SaO2 in hypoxia can be defended by increasing ventilatory drive; however, during heavy exercise, many athletes demonstrate limitations to expiratory flow and are unable to increase ventilation in hypoxia. Additionally, increasing ventilatory work in hypoxia may actually be negative for performance, if dyspnoea increases or muscle blood flow is reduced secondary to an increased sympathetic outflow (eg, the muscle metaboreflex response). Taken together, some athletes are clearly more negatively affected during exercise in hypoxia than other athletes. With careful screening, it may be possible to develop a protocol for determining which athletes may be the most negatively affected during competition and/or training at altitude.
Keywords: Altitude, Aerobic Fitness/Vo2 Max, Exercise Physiology, Respiratory
Introduction
It is well established that for an individual athlete training or competing at altitude maximal oxygen uptake (VO2max) will be impaired. It follows that exercise performance in events with a large aerobic component will likewise be impaired at altitude, except for those exercise activities that involve a fast velocity of the body through the reduced density air at altitude (eg, cycling, speed skating—in those events, performance at altitude is often enhanced vs sea level).1 Interestingly, the extent to which performance is impaired at altitude shows a substantial individual variability across the population.2–5 This variation is hardly a new phenomenon, as in the 1970s, Dill and Adams6 noted that highly trained athletes at altitude are paradoxically ‘impaired to an unusual extent’ compared with lesser trained individuals. Since then, physiologists have continued efforts to determine the various factors which predict who may (or may not) be more susceptible to declines in exercise performance at altitude.
During the years, substantial focus has been placed on the role of the lung, ventilation and pulmonary gas exchange limitations on exercise impairment at altitude. Certainly, oxygen delivery to the periphery is dependent on various factors that occur downstream from the lung. However, for this review, we will focus primarily on the role that pulmonary gas exchange and specifically arterial oxyhaemoglobin saturation (SaO2, or SpO2 when measured by oximetry) maintenance plays in predicting the decline in exercise performance at mild, moderate and the lower range of high altitude.
Baseline VO2max and the decline in VO2max at altitude
Across the general population, from sedentary ‘couch potatoes’ to highly trained endurance athletes, a strong relationship exists between VO2max at sea level and the decline in VO2max at altitude (pearson r value range 0.56–0.94).2–4 For example, between groups of trained versus untrained individuals, ΔVO2max between sea level and altitude is as much as 5 mL/kg/min or 3.3% greater in trained individuals at 3500 m.7 The explanation for this phenomenon resides at the level of the lung, as there is also a significant negative correlation between SaO2, measured either in normoxia or hypoxia, and the decline in VO2max.2 4 7–9 Therefore, individuals who are least able to maintain SaO2 likely end up being the ones with the largest drop in VO2max. Certainly at altitude, the decline in the partial pressure of oxygen (PO2) in the inspired air leads to a decline in PO2 down the cascade from the atmosphere, to the alveoli, to the arterial blood and finally into the capillary. As arterial PO2 dips to the shoulder of the oxyhaemoglobin dissociation curve (eg, an arterial PO2 of ∼75 mm Hg), a small decline in PO2 leads to a relatively large decline in SaO2. Why would highly trained endurance athletes experience a larger decline in arterial PO2 and SaO2 during exercise compared with lesser trained individuals?
Scientific thinking on the response of SaO2 during exercise in healthy individuals has undergone substantial change over time. The concept that oxygen transport by the pulmonary system was sufficient to maintain SaO2 at or very near to the resting levels during submaximal and maximal exercise was the established belief among early physiologists.10 Later, conflicting data emerged documenting considerable reductions in SaO2 during heavy exercise in select numbers of endurance-trained men,11 12 and subsequent work by Dempsey et al13 established the incidence of exercise-induced arterial hypoxaemia (EIH) in a group of highly trained distance runners. However, the prevalence of EIH is far from a universal phenomenon, and the finding of EIH within an individual athlete may be strongly dependent on the techniques used to determine arterial PO2 and SaO2 (eg, muscle temperature correction, arterial blood gas sampling vs. oximetry measures).14 The mechanism behind arterial oxyhaemoglobin desaturation during exercise seems to be greater pulmonary gas exchange limitations, secondary to some combination of inadequate hyperventilation, arterial-venous shunting, greater ventilation—perfusion (V/Q) mismatch or diffusion limitations.15 16 It is believed that each of these mechanisms plays a role in arterial hypoxaemia, with the specific magnitude of contribution of each mechanism differing both (1) across individuals, and (2) within individuals, dependent on factors such as exercise workload, training status, altitude acclimatisation status, pulmonary mechanics and more. However, on average, ∼20% of the variation in arterial PO2 between individuals during exercise is due to variations in the hyperventilatory response, with the remaining ∼80% of the variance in arterial PO2 roughly divided evenly between V/Q mismatch and diffusion limitations.16 In the case of diffusion limitations within the athletic population, the limitation does not seem to derive from a clinical issue with the alveolar—capillary barrier, but rather from a decreased transit time of the erythrocyte across the pulmonary capillary.17 18 When the endurance athlete trains chronically, this leads to an increase in stroke volume and cardiac output; however, pulmonary capillary blood volume remains unchanged.19 As a result, the erythrocyte must transverse the pulmonary capillary at a faster rate during maximal exercise, the available time for oxygen to diffuse from the alveoli to the erythrocyte is reduced, and in some (but not all) highly trained endurance athletes, this reduced erythrocyte transit time is shorter than the time needed for haemoglobin to become fully saturated with O2.17 Ultimately, independent of the mechanism behind desaturation, endurance-trained athletes with significant pulmonary gas exchange limitations, a given decline in inspired PO2 with altitude results in a greater decline in SaO2, and thus a greater reduction in skeletal muscle oxygen delivery and VO2max compared with lesser trained individuals.2 4 7
Does a threshold altitude exist for aerobic impairment?
An extension of this phenomenon of greater susceptibility for altitude mediated declines in performance within select endurance-trained athletes also applies to the threshold altitude for aerobic impairment. Original thinking on the topic from the 1960s held that there was no significant decline in VO2max up to an altitude of 1524 m.20 In the 1980s, the same research laboratory revised the threshold altitude for aerobic impairment down to 1219 m.21 However, the first study only had a sample size of six participants, and the latter study used recreational runners who were of mostly average fitness. Based on these data, Terrados et al22 theorised that the threshold altitude for aerobic impairment may not be universal for all, and their group ultimately showed that trained athletes demonstrate a reduction in VO2max at a much lower threshold altitude (900 m) than untrained individuals (1200 m). Gore et al9 demonstrated a significant aerobic impairment at an even lower altitude of 580 m, and a regression of data from 11 different studies on endurance-trained athletes show a linear reduction in VO2max with ascent from sea level.23 Our research group took these analyses one step further, by dividing endurance-trained athletes into cohorts of EIH and non-EIH, based on oximetry estimates of SaO2 during maximal exercise at sea level.8 Despite having sea level VO2max values that were not different (EIH=71.1±5.3 vs non-EIH=67.2±7.6 mL/kg/min), the EIH athlete group demonstrated a significant decline in VO2max of 4.2% at a simulated mild altitude of 1000 m, whereas the non-EIH athlete group had no change in VO2max at 1000 m. In total, these data suggest that SaO2 maintenance, and not baseline VO2max levels per se, is a primary limiting factor determining VO2max decline with exposure to acute altitude.
What about exercise performance?
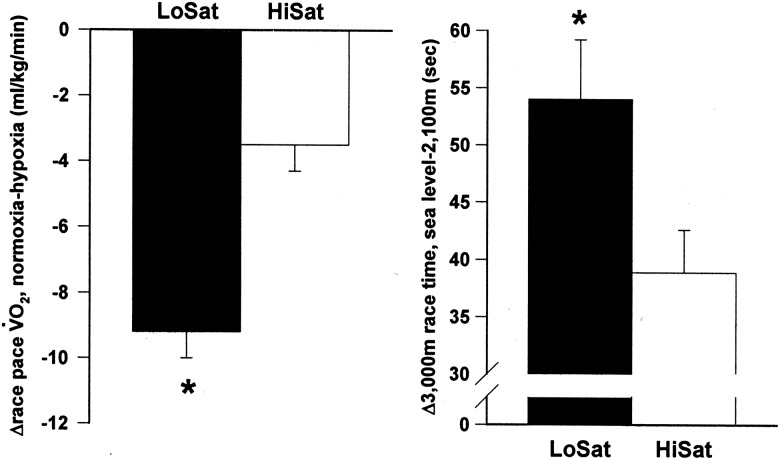
Although understanding the mechanism behind VO2max decline at altitude is important, for the competitive endurance athlete, performance is the primary outcome of interest. Endurance athletes in sports such as distance running, cycling, swimming and cross country skiing do not hold VO2max competitions—they race against each other. It is well established that the ability to consume oxygen at a high rate is strongly linked to endurance exercise performance.24–26 However, does the link from SaO2 maintenance to VO2max maintenance at altitude extend one step further to include maintenance of competitive performance outcomes? In an attempt to understand this relationship better, we examined 26 elite US distance runners (17 M, 9 W), each lifetime sea level residents and all but one ranked among the top 50 elite US distance runners in their primary event in the year of the study.5 SaO2 was estimated using oximetry during a constant speed, simulated race pace exercise bout on a treadmill at sea level, and 3000 m time trials were completed on a standard 400 m track at sea level and at 2100 m, 48 h after arrival to 2500 m. As expected, group 3000 m performance was slower at altitude (Δ3000 m time=48.5±12.7 s). However, when participants were divided into LoSat (<91%) and HiSat (>93%) groups, on the basis of oximetry estimates of arterial SaO2 values during the sea level race pace treadmill bout, the LoSat group demonstrated a significant worsening of performance at 2100 m compared with the HiSat group (figure 1).
Figure 1.
Differences between LoSat and HiSat groups in (left) the change in race pace VO2 between normoxia and hypoxia (16.3% O2) and (right) the change in 3000 m race time between sea level and an altitude of 2100 m. Values are means±SE. *Significantly different from HiSat, p≤0.05. Reprinted with permission from Wolter Kluwer Health.
These data would suggest that prescreening SaO2 during heavy or maximal exercise may help to predict who may or may not be more negatively affected at altitude than an average response. Certainly, SaO2 will not be an absolute predictor of the magnitude of performance decline at altitude. However, 71% of the time our LoSat and HiSat designations correctly predicted whether an athlete would experience a performance decline at altitude that was more or less than the group mean.5 For the elite athlete, for whom training and competitive efforts must be regulated very tightly, this information may be quite beneficial. Coaches could, in theory, make modifications in training loads while at altitude, or in team sports, make personnel decisions about playing time in competitions at altitude based (in part) on the ability to maintain SaO2 during exercise.
Role of the ventilatory response to exercise
One key question that follows is whether an athlete demonstrates a low SaO2 during exercise, and if SaO2 maintenance is important to altitude performance, are there any treatments or interventions available to mitigate the fall in SaO2? In other words, if an athlete desaturates, is there anything that can be done? Of the factors involved in the development of EIH, perhaps the only one that is under at least some level of voluntary control by the athlete is the ventilatory response to exercise. Athletes with EIH consistently show a reduced ventilatory drive during exercise compared with non-EIH athletes13 27 28 evidenced in our data as a reduced VE/VO2 and end-tidal PO2 and higher end-tidal PCO2 in EIH versus non-EIH athletes.5 8 Athletes demonstrating EIH have also been shown to have lower hypoxic ventilatory responses at rest, compared with non-EIH athletes.27 The fact that differing ventilatory responses to exercise and hypoxia exist across the athletic population is important, as one of the only strategies available for the athlete to defend SaO2 during exercise is to increase ventilation, to maximise alveolar PO2 (PAO2). Our own data have shown that oximetry estimates of SaO2 during exercise in a highly endurance-trained cohort is significantly correlated to VE/VO2 (r=0.61, p<0.01).8 This significant correlation between SpO2 and VE/VO2 has also been shown to exist across trained and untrained individuals exercising submaximally at a range of simulated altitudes between 1000 and 4500 m.28
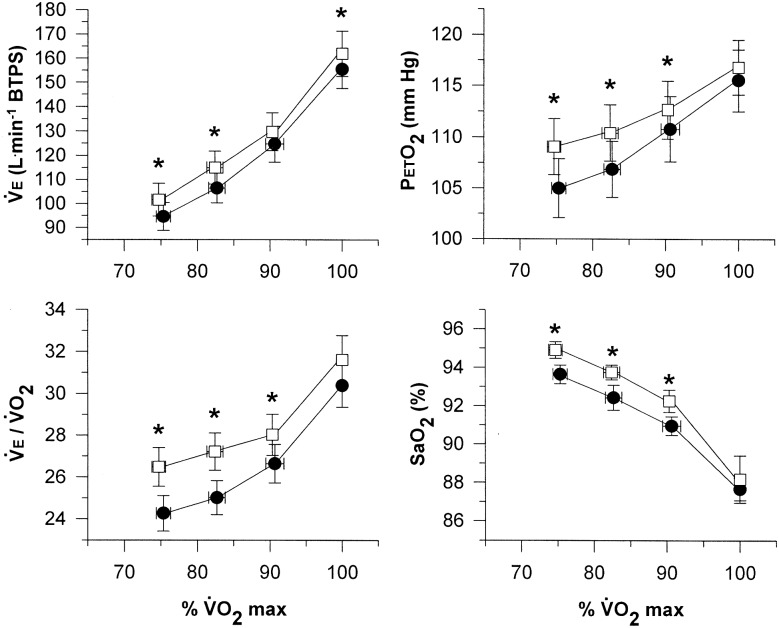
We asked if an inadequate hyperventilatory response contributes in some part to the low SaO2 exhibited by EIH athletes during heavy exercise, could the desaturation be mitigated by stimulating ventilation? PAO2 has been increased in EIH athletes using a mild hyperoxic inspirate, which reduced the hypoxaemia and increased VO2max.29 In theory, a similar response could be achieved by increasing VE. Interestingly, caffeine in moderate doses has a long history as a ventilatory stimulant, showing effects on both central and peripheral chemosensitivity.30 31 In a cohort of eight caffeine naïve athletes with EIH (VO2 max=69.2±4 mL/kg/min; oximetry estimates of SaO2 at VO2max=88.0±1.7%), we found that a moderate dose of caffeine (8 mg/kg body weight, 90 min prior to exercise) significantly increased submaximal and maximal exercise ventilation compared with placebo.32 VE/VO2, end-tidal PO2 and oximetry estimates of SaO2 all significantly increased during submaximal exercise after caffeine; however, none of these variables were different between treatments during maximal exercise and VO2max was not different between caffeine and placebo (figure 2). The data suggest that the pharmacological effect of caffeine on exercise ventilation and arterial saturation maintenance are workload dependent. Although existing evidence suggests a decrease in the gain of the ventilatory response to various stimuli with increasing workload (eg, inspired CO2, increased dead space, hypoxia inspirate33 34), we believe that the approaching (or frank achievement) of mechanical expiratory flow limitation may strongly affect the ventilatory response to exercise and potentially, the downstream effects on arterial oxygenation.35 36
Figure 2.
Minute ventilation, ventilatory equivalent for O2, end-tidal partial pressure of oxygen, and arterial oxyhaemoglobin saturation during graded exercise with placebo (filled circles) and caffeine (8 mg/kg body weight, open squares). Values are means±SE. *Significantly different from placebo at the same percentage of maximal oxygen uptake, p<0.05. Reprinted with permission from Wolters Kluwer Health.
Mechanical limitations to expiratory flow
Untrained individuals are typically able to increase both VE and VE/VO2 during maximal exercise in hypoxia compared with normoxia.2 4 7 28 However, the ventilatory requirement of highly trained endurance athletes during heavy exercise is substantially greater than the ventilation produced by untrained individuals.34 37 As a result, the highly trained endurance athlete may have little reserve to increase ventilation during hypoxic exercise at heavy or maximal workloads. In fact, many athletes reach some degree of mechanical limitation to expiratory flow during heavy exercise.34 38 Specifically, the effort-independent portion of the maximal flow-volume relationship during expiration is met by some portion of the tidal breath. In this case, athletes who demonstrate significant expiratory flow limitation may be at a disadvantage at altitude compared with non-flow limited athletes, as they will lack the mechanical reserve to increase ventilation to defend PAO2 and SaO2.
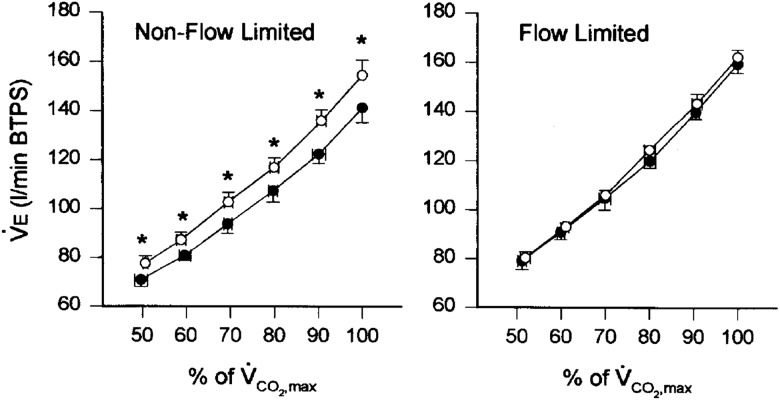
To test the role of expiratory flow limitation on the ventilatory and gas exchange responses to hypoxia, we examined a cohort of highly trained endurance athletes who performed maximal exercise in normoxia and mild hypoxia.39 In participants who were non-flow limited, meaning none of their tidal flow volume loop during maximal exercise encroached on the maximal flow volume envelope, VE at VO2max was significantly increased by 9.8% in mild hypoxia versus sea level (figure 3). However, in participants who were grouped as flow limited, with a mean 56±11% (range 43–70%) of their tidal flow volume loop meeting or exceeding the maximal flow volume boundary, VE at VO2max was not significantly different between normoxia (159.5±9.4 L/min) and mild hypoxia (162.3±6.0 L/min). Even when the flow limited athletes were challenged with the double stimuli of both caffeine and a hypoxic inspirate, they still did not increase VE during maximal exercise over values obtained with ingestion of a placebo and a normoxic inspirate. Thus, the ability to increase exercise ventilation in hypoxia to attempt to defend SaO2 is strongly influenced by the mechanical ventilatory reserve available.
Figure 3.
Minute ventilation in normoxia (filled circles) and hypoxia (open circles) at different levels of exercise in non-flow limited (left panel) and flow limited (right panel) groups. Values are means±SE. *Significantly different from normoxia at the same percentage of maximal oxygen uptake, p≤ 0.05. Reprinted with permission from Elsevier.
It should be noted that in the endurance athlete population, our data consistently show that expiratory flow limitation during heavy exercise is not usually caused by a constrained maximal flow volume envelope. Rather, flow limited athletes typically have a stronger ventilatory response to exercise than non-flow limited athletes. 34 For example, our data show that during maximal exercise VE/VO2 and end tidal PO2 are consistently higher and end tidal PCO2 lower in flow limited versus non-flow limited athletes,39 40 which is counter to what is typically seen in individuals who are flow limited due to disease or ageing.36 Owing to the strong ventilatory response to exercise, we often refer to athletes with expiratory flow limitation as ‘flow maximizers’ rather than flow limited. By comparison, non-flow limited athletes often have substantial ventilatory reserve during heavy exercise in hypoxia, but still do not increase VE further to defend the fall in SaO2.39 40 Why this is the case is not clear, but it may have to do with the metabolic cost associated with ventilation and potential negative factors associated with high amounts of respiratory muscle work.41
Metabolic cost of ventilation and the metaboreflex response
At any exercise workload, even if an athlete is flow limited, there are options available to increase ventilation. The athlete could: (1) increase expiratory pressure at the beginning of expiration to take advantage of the higher flows available at higher lung volumes or (2) increase end expiratory lung volume, shifting the tidal volume closer to total lung capacity, where higher expiratory flows can be achieved.34 While these two ventilatory strategies are available to the athlete, many ‘choose’ not to do either, as both options involve increased work by the respiratory muscles, an associated increase in the metabolic cost of breathing and increased dyspnoea.34 41 Additionally, increasing respiratory muscle work at high ventilations has been associated with a sympathetic metaboreflex response, where vasoconstriction causes blood flow to the locomotor muscles to decrease, likely in an effort to prioritise blood flow to the respiratory musculature.42–44 Therefore, while increasing ventilation during heavy exercise in hypoxia may be seen as a mechanism to defend PAO2 and SaO2, locomotor muscle oxygen delivery may be compromised, however this has not been rigorously tested. Similarly, although non-flow limited athletes have mechanical room to increase exercise ventilation to defend PO2 and SaO2 in hypoxia, they may modulate work output (eg, in a self-paced performance trial) to avoid higher levels of ventilatory work and dyspnoea. This was the case in unpublished data from our laboratory showing that non-flow limited athletes demonstrate a greater performance decline in 17% O2 during a 5km all-out cycle performance trial, compared with a flow limited group. We believe that the flow limited athletes may simply be accustomed to higher levels of ventilatory work which maximises their available mechanical room for ventilation, and thus were not as affected by a hypoxic stimulus (from a ventilatory standpoint) during a maximal effort performance trial.
Screening of the individual response to exercise at altitude
For the athlete and coach preparing for either training or competition at altitude in a sport with an aerobic component, are there steps that can be taken at sea level, prior to departure, to determine the individual response to acute altitude exposure? For select sporting activities, time trials or other direct performance measures could be completed using a normoxic and a hypoxic inspirate. It is to be remembered that most hypoxic delivery models at sea level utilise normobaric hypoxia, which may produce differing physiological responses than the hypobaric hypoxia that the athletes will experience at terrestrial altitude.45 However, for team-based sport athletes (the focus of these conference proceedings), logistically there are perhaps few options for a direct method for pretesting actual performance decline at altitude. However, based on the data presented in this review, it can be emphasized that there are measures that could be completed at sea level, which combined would perhaps provide an indication to the degree which the athlete tolerates exercise at altitude.
Measures of arterial SaO2 during exercise
With the strongest correlating factor to VO2max declines at altitude being the degree of pulmonary gas exchange limitations, measuring SpO2 non-invasively during exercise appears to be a useful tool. A progressive exercise test, ideally using the same mode of exercise (running, cycling, cross country skiing, etc) that the athlete makes use of in competition, as well as a range of efforts that includes the expected workloads of training and competition, should be utilised. Although there is not a hard desaturation threshold established, athletes who desaturate more than others will likely be more negatively affected at altitude. Keeping in mind the limitations associated with pulse oximetry (eg, motion artifact),46 those demonstrating an SpO2 less than ∼92% during sea level exercise, being already on the shoulder of the oxyhaemoglobin dissociation curve, will likely experience larger declines in VO2max at altitude compared with athletes who maintain SpO2 at higher levels (for an example of typical EIH and non-EIH athlete responses, see table 1). If available, measures of PAO2 during exercise can add additional insight, as athletes with low PAO2 measures during heavy or maximal exercise are often (but not always) the ones who show the most hypoxaemia.8 13 27 28 Should it be possible to complete a second test using a hypoxic inspirate equivalent to the altitude of training/competition, the decline in VO2max and SaO2, as well as the increase in VE and heart rate would give an even more refined data set on which to predict performance decline at altitude.
Table 1.
Response of a representative EIH and non-EIH participant to maximal exercise in normoxia and mild hypoxia (0.187 O2)
| VO2max (mL/kg/min) | SaO2 (%) | VE (L/min) | PETO2 (mm Hg) | PETCO2 (mm Hg) | |
|---|---|---|---|---|---|
| EIH Normoxia |
76.2 | 88.6 | 129.1 | 112.1 | 43.0 |
| EIH Mild hypoxia |
71.9 | 83.0 | 140.4 | 101.8 | 36.4 |
| Non-EIH Normoxia |
79.8 | 95.1 | 147.4 | 125.9 | 31.4 |
| Non-EIH Mild hypoxia |
80.3 | 92.8 | 150.2 | 109.0 | 31.4 |
Data from reference8.
PETCO2, end-tidal CO2 partial pressure; PETO2, end-tidal O2 partial pressure; SaO2, oximetry estimate of arterial oxyhaemoglobin saturation; VE, minute ventilation; VO2max, maximal oxygen uptake.
Hypoxic ventilatory response
While it has been shown that individuals with a high hypoxic ventilatory response have better performances and less acute mountain sickness symptoms at extreme altitudes,46 we have not found a significant relationship between the isocapnic HVR at rest and the decline in VO2max or performance with acute altitude exposure.5 8 32 However, a lack of a correlation between HVR and ΔVO2max or Δperformance from sea level to moderate altitude in our studies may simply be the result of a small distribution of HVR values. Most highly trained athletes (including a large portion of the cohort of athletes studied in our laboratory) display substantially blunted peripheral chemoresponsiveness with HVR values that are quite homogeneous.47 For example, of 53 endurance athletes studied in our laboratory, 89% displayed HVR values between 0.03 and 0.45 L/min/%SaO2, a narrow range of values which makes a correlational analysis between HVR and performance decline at altitude less meaningful. Anecdotally, in contrast to the data from mountaineers at high altitudes,46 our group has noted that the few athletes who demonstrate relatively strong hypoxic ventilatory responses (ie, greater than ∼0.5 L/min/%SaO2) typically demonstrate the most dyspnoea and perceived exertion during training on arrival and throughout training at moderate altitude. As a result, these athletes tend to struggle more with workouts and may train at lower workloads in an effort to avoid large amounts of ventilatory work. In this case, it may very well be that athletes with high HVRs are better off staying at sea level to train, with a better chronic sea level training response outweighing any positive effects of altitude acclimatisation; however, this would need to be confirmed with direct examination.
Expiratory flow limitation
A measure of expiratory flow limitation may be useful in characterising how an athlete may respond to exercise and training in hypoxia. Owing to many, if not most flow limited athletes within the athletic population reach or approach flow limited status due to a vigorous ventilatory response to exercise which they are accustomed to even at sea level, added sensations of dyspnoea during hypoxic exercise may be less than in non-flow limited athletes. At least with acute exposure to altitude, non-flow limited athletes may reduce work output during training, in an effort to keep ventilation and dyspnoea at manageable levels.
What are the new findings?
Arterial oxyhaemoglobin saturation (SaO2) is strongly linked to the ability to maintain maximal oxygen uptake at altitude.
By extension, the ability to defend SaO2 is also strongly linked to the ability to maintain performance in aerobic activities at altitude.
Pharmacologically stimulating ventilation increases end-tidal PO2 and SaO2 during submaximal exercise, but not during maximal exercise where many athletes reach a mechanical limit for expiratory flow.
How might it impact on clinical practice in the near future?
Screening responses such as SaO2 or SpO2 during exercise, expiratory flow limitation and hypoxic ventilatory response at rest may provide coaches and athletes valuable information about how they might individually respond to training or competition at altitude.
This information could allow team physicians to make recommendations on how to modify intensity or duration of practice bouts at altitude, as well as playing time during altitude-based competitions.
Acknowledgments
The author expresses gratitude to colleagues Benjamin Levine, Jim Stray-Gundersen and Joel Stager for their partnership in the original studies referenced as part of this review.
Footnotes
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chapman RF, Levine BD. The effects of hypo- and hyperbaria on performance. In: Garrett WE, Kirkendall JT. eds Exercise and sport science. Philadelphia: Lippincott Williams & Wilkins, 2000:447–58 [Google Scholar]
- 2.Gavin TP, Derchak PA, Stager JM. Ventilation's role in the decline in VO2max and SaO2 in acute hypoxic exercise. Med Sci Sports Exerc 1998;30:195–9 [DOI] [PubMed] [Google Scholar]
- 3.Young AJ, Cymerman A, Burse RL. The influence of cardiorespiratory fitness on the decrement in maximal aerobic power at high altitude. Eur J Appl Physiol 1985;54:12–15 [DOI] [PubMed] [Google Scholar]
- 4.Lawler J, Powers SK, Thompson D. Linear relationship between VO2max and VO2max decrement during exposure to acute hypoxia. J Appl Physiol 1988;64:1486–92 [DOI] [PubMed] [Google Scholar]
- 5.Chapman RF, Stager JM, Tanner DA, et al. Impairment of 3000-m run time at altitude is influenced by arterial oxyhemoglobin saturation. Med Sci Sports Exerc 2011;43:1649–56 [DOI] [PubMed] [Google Scholar]
- 6.Dill DB, Adams WC. Maximal oxygen uptake at sea level and at 3,090-m altitude in high school champion runners. J Appl Physiol 1971;30:854–9 [DOI] [PubMed] [Google Scholar]
- 7.Mollard P, Woorons X, Letournel M, et al. Role of maximal heart rate and arterial O2 saturation on the decrement of VO2max in moderate acute hypoxia in trained and untrained men. Int J Sports Med 2007;28:186–92 [DOI] [PubMed] [Google Scholar]
- 8.Chapman RF, Emery M, Stager JM. Degree of arterial desaturation in normoxia influences VO2max decline in mild hypoxia. Med Sci Sports Exerc 1999;31:658–63 [DOI] [PubMed] [Google Scholar]
- 9.Gore CJ, Hahn AG, Scroop GC, et al. Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol 1996;80:2204–10 [DOI] [PubMed] [Google Scholar]
- 10.Shepard RH. Effect of pulmonary diffusing capacity on exercise tolerance. J Appl Physiol 1958;12:487–8 [DOI] [PubMed] [Google Scholar]
- 11.Holmgren A, Linderholm H. Oxygen and carbon dioxide tensions of arterial blood during heavy and exhaustive exercise. Acta Physiol Scand 1958;44:203–15 [DOI] [PubMed] [Google Scholar]
- 12.Rowell LB, Taylor HL, Wang Y, et al. Saturation of arterial blood with oxygen during maximal exercise. J Appl Physiol 1964;19:284–6 [DOI] [PubMed] [Google Scholar]
- 13.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 1984;355:161–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scroop GC, Shipp NJ. Exercise-induced hypoxemia: fact or fallacy? Med Sci Sports Exerc 2010;42:120–6 [DOI] [PubMed] [Google Scholar]
- 15.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol 1999;87:1997–2006 [DOI] [PubMed] [Google Scholar]
- 16.Hopkins SR. Exercise induced arterial hypoxemia: the role of ventilation-perfusion inequality and pulmonary diffusion limitation. Adv Exp Med Biol 2006;588:17–30 [DOI] [PubMed] [Google Scholar]
- 17.Hopkins SR, Belzberg AS, Wiggs BR, et al. Pulmonary transit time and diffusion limitation during heavy exercise in athletes. Respir Physiol 1996;103:67–73 [DOI] [PubMed] [Google Scholar]
- 18.Warren GL, Cureton KJ, Middendorf WF, et al. Red blood cell pulmonary capillary transit time during exercise in athletes. Med Sci Sports Exerc 1991;23:1353–61 [PubMed] [Google Scholar]
- 19.Dempsey JA, Gledhill N, Reddan WG, et al. Pulmonary adaptation to exercise: effects of exercise type and duration, chronic hypoxia and physical training. Ann N Y Acad Sci 1977;301:243–61 [DOI] [PubMed] [Google Scholar]
- 20.Buskirk ER, Kollias J, Akers RF, et al. Maximal performance at altitude and on return from altitude in conditioned runners. J Appl Physiol 1967;23:259–66 [DOI] [PubMed] [Google Scholar]
- 21.Squires RW, Buskirk ER. Aerobic capacity during acute exposure to simulated altitude, 914 to 2286 meters. Med Sci Sports Exerc 1982;14:36–40 [DOI] [PubMed] [Google Scholar]
- 22.Terrados N, Mizuno M, Andersen H. Reduction in maximal oxygen uptake at low altitudes; role of training status and lung function. Clin Physiol 1985;5(Suppl 3):75–9 [DOI] [PubMed] [Google Scholar]
- 23.Wehrlin JP, Hallen J. Linear decrease in VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol 2006;96:404–12 [DOI] [PubMed] [Google Scholar]
- 24.Robinson S, Edwards HT, Dill DB. New records in human power. Science 1937;85:409–10 [DOI] [PubMed] [Google Scholar]
- 25.Bassett DR, Howley ET. Limiting factors for maximal oxygen uptake and determinants of exercise performance. Med Sci Sports Exerc 2000;32:70–84 [DOI] [PubMed] [Google Scholar]
- 26.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol 2008;586:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harms CA, Stager JM. Low chemoresponsiveness and inadequate hyperventilation contribute to exercise-induced hypoxemia. J Appl Physiol 1995;79:575–80 [DOI] [PubMed] [Google Scholar]
- 28.Woorons X, Mollard P, Pichon A, et al. Moderate exercise in hypoxia induces a greater arterial desaturation in trained than untrained men. Scan J Med Sci Sports 2007;17:431–6 [DOI] [PubMed] [Google Scholar]
- 29.Powers SK, Lawler J, Dempsey JA, et al. Effects of incomplete pulmonary gas exchange on VO2max. J Appl Physiol 1989;66:2491–5 [DOI] [PubMed] [Google Scholar]
- 30.D'Urzo AD, Jhirad R, Jenne H, et al. Effect of caffeine on ventilatory responses to hypercapnia, hypoxia, and exercise in humans. J Appl Physiol 1990;68:322–8 [DOI] [PubMed] [Google Scholar]
- 31.Rall TW. Central nervous system stimulants: the methylxanthines. In: Gilman AG, Goodman LD, After Rall TW, Murad FA, eds Pharmacological basis of therapeutics. 7th edn New York: MacMillian, 1985:589–603 [Google Scholar]
- 32.Chapman RF, Stager JM. Caffeine stimulates ventilation in athletes with exercise-induced hypoxemia. Med Sci Sports Exerc 2008;40:1080–6 [DOI] [PubMed] [Google Scholar]
- 33.McParland C, Mink J, Gallagher CG. Respiratory adaptations to dead space loading during maximal incremental exercise. J Appl Physiol 1991;70:55–62 [DOI] [PubMed] [Google Scholar]
- 34.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 1992;73:874–86 [DOI] [PubMed] [Google Scholar]
- 35.McClaran SR, Wetter TJ, Pegelow DF, et al. Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol 1999;86:1357–66 [DOI] [PubMed] [Google Scholar]
- 36.Babb TG. Exercise ventilatory limitation: the role of expiratory flow limitation. Exer Sport Sci Rev 2013;41:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward SA. Ventilatory control in humans: constraints and limitations. Exp Physiol 2007;92:357–66 [DOI] [PubMed] [Google Scholar]
- 38.Grimby G, Saltin B, Wilhelmsen L. Pulmonary flow-volume and pressure-volume relationship during submaximal and maximal exercise in young well-trained men. Bull Physio-pathol Resp 1971;7:157–72 [PubMed] [Google Scholar]
- 39.Chapman RF, Emery M, Stager JM. Extent of expiratory flow limitation influences the increase in maximal exercise ventilation in hypoxia. Respir Physiol 1998;113:65–74 [DOI] [PubMed] [Google Scholar]
- 40.Derchak PA, Stager JM, Tanner DA, et al. Expiratory flow limitation confounds ventilatory response during exercise in athletes. Med Sci Sports Exerc 2000;32:1873–9 [DOI] [PubMed] [Google Scholar]
- 41.Aaron EA, Seow KC, Johnson BD, et al. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol 1992;72:1818–25 [DOI] [PubMed] [Google Scholar]
- 42.Dempsey JA, Harms CA, Ainsworth DM. Respiratory muscle perfusion and energetics during exercise. Med Sci Sports Exerc 1996;28:1123–8 [DOI] [PubMed] [Google Scholar]
- 43.Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 1997;82:1573–83 [DOI] [PubMed] [Google Scholar]
- 44.Harms CA, Wetter TJ, St Croix CM, et al. Effects of respiratory muscle work on exercise performance. J Appl Physiol 2000;89:131–8 [DOI] [PubMed] [Google Scholar]
- 45.Millet GP, Faiss R, Pialoux V. Point: hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J Appl Physiol 2012;112: 1783–4 [DOI] [PubMed] [Google Scholar]
- 46.Schoene RB, Lahiri S, Hackett PH, et al. Relationship of hypoxic ventilatory response to exercise performance on Mount Everest. J Appl Physiol 1984;56:1478–83 [DOI] [PubMed] [Google Scholar]
- 47.Byrne-Quinn E, Weil JV, Sodal IE, et al. Ventilatory control in the athlete. J Appl Physiol 1971;30:91–8 [DOI] [PubMed] [Google Scholar]