Abstract
Background
Endurance athletes have been using altitude training for decades to improve near sea-level performance. The predominant mechanism is thought to be accelerated erythropoiesis increasing haemoglobin mass (Hbmass) resulting in a greater maximal oxygen uptake ( ). Not all studies have shown a proportionate increase in
). Not all studies have shown a proportionate increase in  as a result of increased Hbmass. The aim of this study was to determine the relationship between the two parameters in a large group of endurance athletes after altitude training.
as a result of increased Hbmass. The aim of this study was to determine the relationship between the two parameters in a large group of endurance athletes after altitude training.
Methods
145 elite endurance athletes (94 male and 51 female) who participated in various altitude studies as altitude or control participants were used for the analysis. Participants performed Hbmass and  testing before and after intervention.
testing before and after intervention.
Results
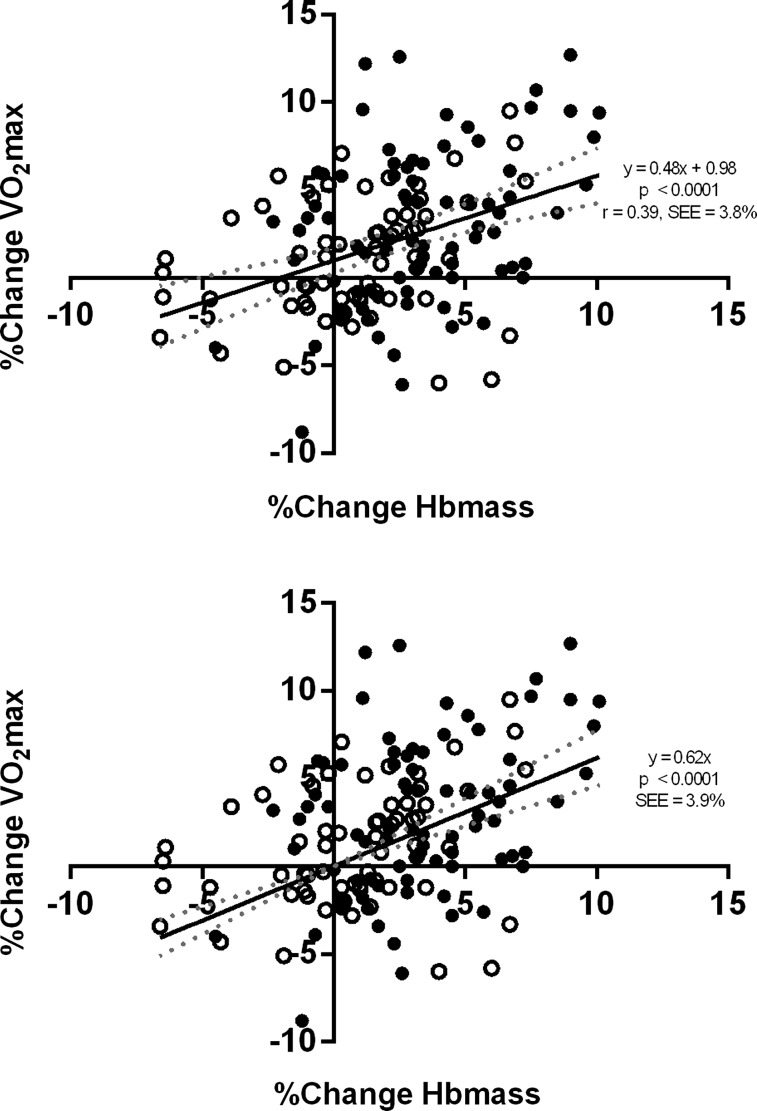
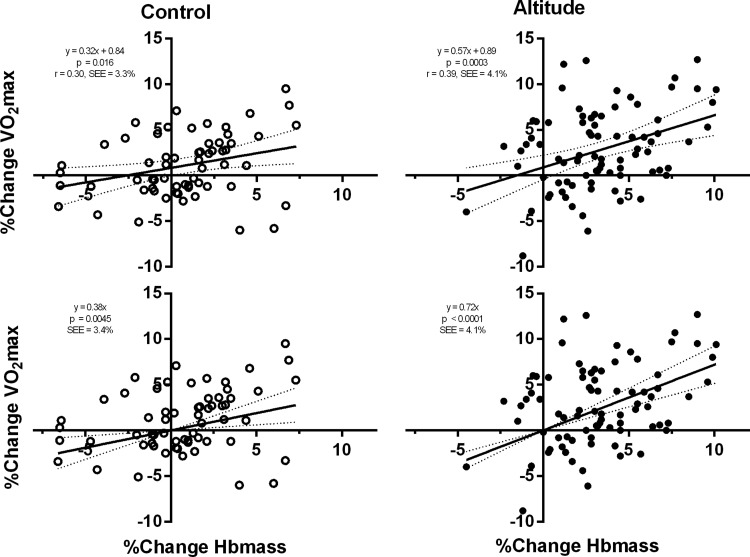
For the pooled data, the correlation between per cent change in Hbmass and per cent change in  was significant (p<0.0001, r2=0.15), with a slope (95% CI) of 0.48 (0.30 to 0.67) intercept free to vary and 0.62 (0.46 to 0.77) when constrained through the origin. When separated, the correlations were significant for the altitude and control groups, with the correlation being stronger for the altitude group (slope of 0.57 to 0.72).
was significant (p<0.0001, r2=0.15), with a slope (95% CI) of 0.48 (0.30 to 0.67) intercept free to vary and 0.62 (0.46 to 0.77) when constrained through the origin. When separated, the correlations were significant for the altitude and control groups, with the correlation being stronger for the altitude group (slope of 0.57 to 0.72).
Conclusions
With high statistical power, we conclude that altitude training of endurance athletes will result in an increase in  of more than half the magnitude of the increase in Hbmass, which supports the use of altitude training by athletes. But race performance is not perfectly related to relative
of more than half the magnitude of the increase in Hbmass, which supports the use of altitude training by athletes. But race performance is not perfectly related to relative  , and other non-haematological factors altered from altitude training, such as running economy and lactate threshold, may also be beneficial to performance.
, and other non-haematological factors altered from altitude training, such as running economy and lactate threshold, may also be beneficial to performance.
Keywords: Aerobic fitness/Vo2 Max, Altitude, Exercise, Endurance, Elite performance
Introduction
The effects of training at moderate altitude on subsequent performance at altitude and near sea-level became important at the 1968 Mexico City Olympic Games (2300 m), and has subsequently been researched extensively. It is now common practice for elite endurance athletes across a range of sports to use altitude training to improve performance near sea-level. The prevailing paradigm of adaptation to hypoxia is that the lower partial pressure of oxygen associated with moderate altitude induces erythropoietin (EPO) production in the kidneys, which in turn stimulates the production of red blood cells in the bone marrow, facilitating an increase in maximal oxygen uptake ( ) and potentially improving endurance performance.1–3 Since
) and potentially improving endurance performance.1–3 Since  depends on cardiac output and the arteriovenous O2 difference, all factors that influence these physiological capacities may exert limiting effects on endurance capacity.4 The most important factor related to blood supply is the total blood volume, which may limit venous return and thus the stroke volume, as well as haemoglobin mass (Hbmass), which along with the capacity of muscles to extract and use O2 determines the O2-transport capacity and therefore the arteriovenous O2 difference.5
depends on cardiac output and the arteriovenous O2 difference, all factors that influence these physiological capacities may exert limiting effects on endurance capacity.4 The most important factor related to blood supply is the total blood volume, which may limit venous return and thus the stroke volume, as well as haemoglobin mass (Hbmass), which along with the capacity of muscles to extract and use O2 determines the O2-transport capacity and therefore the arteriovenous O2 difference.5
Although some authors have explicitly related the change in near sea-level performance after an altitude training camp (hypoxic intervention) to the change in serum EPO1
2 at altitude, the correlation for the change in  versus the change in red blood cell volume yielded an r2=0.14.1 Therefore, 86% of the variance in
versus the change in red blood cell volume yielded an r2=0.14.1 Therefore, 86% of the variance in  is attributable to factors other than the change in Hbmass. Further, it is important to be aware that
is attributable to factors other than the change in Hbmass. Further, it is important to be aware that  is not the sole determinant of performance.6 Among elite athletes, other factors such as exercise economy and the fractional utilisation of
is not the sole determinant of performance.6 Among elite athletes, other factors such as exercise economy and the fractional utilisation of  are also important determinants of endurance performance.6 In addition to the increase in Hbmass and the subsequent increase in near sea-level
are also important determinants of endurance performance.6 In addition to the increase in Hbmass and the subsequent increase in near sea-level  , altitude training can enhance muscle efficiency, probably at a mitochondrial level, and change the muscle proteins involved in acid–base control; it can also increase the capacity for flux of lactate, bicarbonate and hydrogen ions from muscle to blood, all of which can be associated with better performance in endurance athletes and team sport athletes.7
8 A detailed review of non-haematological adaptations to hypoxia that can improve near sea-level endurance performance has been published recently.8 Given an adequate hypoxic dose (high enough, long enough and for enough hours/day),9 there will be an increase in Hbmass,
, altitude training can enhance muscle efficiency, probably at a mitochondrial level, and change the muscle proteins involved in acid–base control; it can also increase the capacity for flux of lactate, bicarbonate and hydrogen ions from muscle to blood, all of which can be associated with better performance in endurance athletes and team sport athletes.7
8 A detailed review of non-haematological adaptations to hypoxia that can improve near sea-level endurance performance has been published recently.8 Given an adequate hypoxic dose (high enough, long enough and for enough hours/day),9 there will be an increase in Hbmass,  and possibly performance.8 However, there is evidence that although altitude training increases Hbmass, there may also be a parallel reduction in cardiac output, vascular regulation or some other mechanism that can limit the increase in
and possibly performance.8 However, there is evidence that although altitude training increases Hbmass, there may also be a parallel reduction in cardiac output, vascular regulation or some other mechanism that can limit the increase in  .10
11
.10
11
When evaluating the increases in Hbmass and  resulting from altitude training, factors such as age, sex, training status and type of sport all must be considered. Many research studies have also used small sample sizes and a major strength of the current study is pooling data across many research studies to increase the sample size and to encompass a range of measures across athletes varying in the aforementioned characteristics. Recently, there has also been a controversy about whether altitude training, particular live high–train low (LHTL), is effective in improving Hbmass,
resulting from altitude training, factors such as age, sex, training status and type of sport all must be considered. Many research studies have also used small sample sizes and a major strength of the current study is pooling data across many research studies to increase the sample size and to encompass a range of measures across athletes varying in the aforementioned characteristics. Recently, there has also been a controversy about whether altitude training, particular live high–train low (LHTL), is effective in improving Hbmass,  and endurance performance.12 Therefore, the aim of the current study was to investigate the effectiveness of altitude (both live high–train high (LHTH) and LHTL) in increasing Hbmass and
and endurance performance.12 Therefore, the aim of the current study was to investigate the effectiveness of altitude (both live high–train high (LHTH) and LHTL) in increasing Hbmass and  and the strength of the relationship between changes in Hbmass and
and the strength of the relationship between changes in Hbmass and  in a large number of elite endurance athletes from different sports after different forms of altitude training. The substantial number of participants pooled for analysis offers more statistical power than individual smaller studies with modest sample sizes.
in a large number of elite endurance athletes from different sports after different forms of altitude training. The substantial number of participants pooled for analysis offers more statistical power than individual smaller studies with modest sample sizes.
Methods
Subjects
The current study used elite endurance athletes from 10 separate studies spanning a 6-year period across four different sports (cycling, running, triathlon and race walking). The study comprised a total of 145 participants (94 males and 51 females),  67.7±7.2 (71.9±4.6 male and 61.4±5.9 female) mL/min/kg (mean±SD). The participants were at a minimum ‘nationally ranked’ athletes, but most had represented Australia in international competition. All participants were part of various altitude studies that were approved by the Australian Institute of Sport Ethics Committee. A summary of each of the 10 studies is listed in table 1.
67.7±7.2 (71.9±4.6 male and 61.4±5.9 female) mL/min/kg (mean±SD). The participants were at a minimum ‘nationally ranked’ athletes, but most had represented Australia in international competition. All participants were part of various altitude studies that were approved by the Australian Institute of Sport Ethics Committee. A summary of each of the 10 studies is listed in table 1.
Table 1.
Individual study summary
| Year | Sport | Study N (M/F) | Altitude type (N) | Level (m) | Duration (d) | Time at altitude |
 (mL/min/kg) (mL/min/kg) |
Test mode (Ergometer) |
|---|---|---|---|---|---|---|---|---|
| 2006 (13) | Cycling | 11 (11/0 ) | LHTL (11) | 3000 | 21 | 14 h/day | 65.32±5.29 | Cycle (lode bike) |
| 2007 (14) | Cycling | 9 (9/0) | LHTH (5) CON (4) |
2700 | 21 | 24 h/day | 72.16±4.66 | Cycle (lode bike) |
| 2007 (17) | Running | 32 (21/11) | LHTL (18) CON (14) |
3000 | 21 | 14 h/day | 71.60±6.05 | Run (treadmill) |
| 2008 (18) | Running | 17 (13/4) | LHTL+TH (8) IHT (9) |
3000+2200 2200 |
21+9 9 |
14 h/day+60 min 60 min |
67.91±7.47 | Run (treadmill) |
| 2008 (19) | Walking | 16 (8/8) | LHTL (6) CON (10) |
3000 | 21 | 14 h/d | 62.17±7.44 | Race walk (treadmill) |
| 2009 (16) | Triathlon | 18 (14/4) | LHTL (5) IHE (8) CON (5) |
3000 3500–6000 |
17 17 |
14 h/day 60 min |
72.49±4.74 | Run (treadmill) |
| 2010 (15) | Cycling | 9 (0/9) | LHTL (5) CON ( 4) |
3000 | 26 | 14 h/day | 62.20±4.68 | Cycle (tode bike) |
| 2011 | Walking | 10 (4/6) | LHTH (5) CON (5) |
1850 | 28 | 64.98±6.59 | Race walk (treadmill) | |
| 2011 | Walking | 6 (2/4) | LHTL (3) CON (3) |
3000 | 28 | 14 h/day | 60.73±6.60 | Race walk (treadmill) |
| 2012 | Walking | 17 (12/5) | LHTH (7) LHTH (10) |
3000/1350 1350 |
21 21 |
9 h/day/15 h/day 24 h/day |
66.50±6.17 | Race walk (treadmill) |
There were a total of 83 altitude athletes and 62 control athletes.
 values means±SD.
values means±SD.
CON, control; F, female; IHE, intermittent hypoxic exposure; IHT, intermittent hypoxic training; LHTL, live high–train low; LHTH, live high–train high; M, male.
Experimental overview
The current study investigated the relationship between changes in Hbmass and  after various forms of altitude training, with the minimum requirement that both Hbmass and
after various forms of altitude training, with the minimum requirement that both Hbmass and  were measured pre-altitude and post-altitude. The altitude exposures included (1) classical LHTH at various locations (1350–2700 m), (2) simulated LHTL (3000 m, 14 h/day) in the altitude house at the Australian Institute of Sport (AIS, Canberra, Australian Capital Territory, Australia) or using altitude tents, (3) intermittent hypoxic training (IHT) in the altitude house at the AIS and (4) intermittent hypoxic exposure (IHE) using hypoxic breathing devices. A total of n=83 athletes completed altitude training interventions (1 and 2) and were treated as the Altitude group. The data from control participants and from altitude protocols that would not normally be expected to induce an increase in Hbmass (IHE and IHT alone) were (n=62) all treated as control data. Details of participants, type of altitude, dose of altitude, exposure duration and average
were measured pre-altitude and post-altitude. The altitude exposures included (1) classical LHTH at various locations (1350–2700 m), (2) simulated LHTL (3000 m, 14 h/day) in the altitude house at the Australian Institute of Sport (AIS, Canberra, Australian Capital Territory, Australia) or using altitude tents, (3) intermittent hypoxic training (IHT) in the altitude house at the AIS and (4) intermittent hypoxic exposure (IHE) using hypoxic breathing devices. A total of n=83 athletes completed altitude training interventions (1 and 2) and were treated as the Altitude group. The data from control participants and from altitude protocols that would not normally be expected to induce an increase in Hbmass (IHE and IHT alone) were (n=62) all treated as control data. Details of participants, type of altitude, dose of altitude, exposure duration and average  are presented in table 1. The studies included were a combination of seven published13–19 and three previously unpublished works.
are presented in table 1. The studies included were a combination of seven published13–19 and three previously unpublished works.
Maximal oxygen uptake
An incremental protocol to volitional exhaustion was used to determine  . Protocols were specific to each sport and have been described in full previously.13–19 Expired ventilation samples were collected using a custom-built open-circuit indirect calorimetry system with associated in-house software for determination of oxygen uptake.20 The same open-circuit indirect calorimetry system was used in all studies. Additionally, the typical error (TE, SD of the difference scores divided by √2) for
. Protocols were specific to each sport and have been described in full previously.13–19 Expired ventilation samples were collected using a custom-built open-circuit indirect calorimetry system with associated in-house software for determination of oxygen uptake.20 The same open-circuit indirect calorimetry system was used in all studies. Additionally, the typical error (TE, SD of the difference scores divided by √2) for  established in our laboratory for this system was 2.1–2.4%,19
21 which includes the combination of biological and analytical error.
established in our laboratory for this system was 2.1–2.4%,19
21 which includes the combination of biological and analytical error.
Haemoglobin mass
Total Hbmass was measured with the optimised 2 min carbon monoxide (CO) rebreathing test adapted from Schmidt and Prommer22 for all the studies included in the analysis. Briefly, a CO dose of ∼1.2 mL/kg body weight was administered and rebreathed for 2 min. Capillary fingertip blood samples were taken before the start of the test and at ∼7 min post administration of the CO dose. Blood samples were measured a minimum of five times for determination of %HbCO using an OSM three hemoximeter (Radiometer, Copenhagen). Hbmass was calculated from the mean change in HbCO before and after rebreathing CO. This parameter was measured prior to the intervention period and within 1 week after the completion of the intervention period. The TE of Hbmass was 1.8%,18 1.9%,13 2.0%,14 15 17 2.2%19 and 2.4%.16
Statistical analysis
A two-tailed Pearson correlation was used to compare the correlation between percentage changes in Hbmass and  with statistical significance set at p<0.05. Linear regression analyses were also performed on the percentage changes in Hbmass and
with statistical significance set at p<0.05. Linear regression analyses were also performed on the percentage changes in Hbmass and  , and provided a regression equation, goodness of fit (r2) and significance of the slope (p value). Analyses were conducted both with the intercept allowed to vary freely and forced through the origin. Linear regression was also conducted for a cross-sectional comparison on the cumulative raw data (pre and post) of Hbmass (g/kg) and
, and provided a regression equation, goodness of fit (r2) and significance of the slope (p value). Analyses were conducted both with the intercept allowed to vary freely and forced through the origin. Linear regression was also conducted for a cross-sectional comparison on the cumulative raw data (pre and post) of Hbmass (g/kg) and  (mL/min/kg). All analyses were performed using Prism software (2007) V.5.01 (GraphPad Software Inc., San Diego, California, USA).
(mL/min/kg). All analyses were performed using Prism software (2007) V.5.01 (GraphPad Software Inc., San Diego, California, USA).
Results
Changes in Hbmass and 
The correlation between percentage changes in Hbmass and  was significant for the altitude and control groups combined, with a slope (95% CI) of 0.48 (0.30 to 0.67) when the intercept was free to vary, and a slope of 0.62 (0.46 to 0.77) when the intercept was constrained through the origin (figure 1). The correlation was also significant for the altitude and control groups, separately (figure 2). For the altitude group, the slope was 0.57 (0.27 to 0.87) when free to vary and when constrained through the origin, the slope was 0.72 (0.51 to 0.92). The corresponding values for the Control group were 0.32 (0.06 to 0.58) and 0.38 (0.12 to 0.64). The Altitude group increased Hbmass by 3.3±3.0% (mean±SD) and
was significant for the altitude and control groups combined, with a slope (95% CI) of 0.48 (0.30 to 0.67) when the intercept was free to vary, and a slope of 0.62 (0.46 to 0.77) when the intercept was constrained through the origin (figure 1). The correlation was also significant for the altitude and control groups, separately (figure 2). For the altitude group, the slope was 0.57 (0.27 to 0.87) when free to vary and when constrained through the origin, the slope was 0.72 (0.51 to 0.92). The corresponding values for the Control group were 0.32 (0.06 to 0.58) and 0.38 (0.12 to 0.64). The Altitude group increased Hbmass by 3.3±3.0% (mean±SD) and  by 2.7±4.4%; the corresponding changes in the Control group were 0.8±3.0% and 1.1±3.4%.
by 2.7±4.4%; the corresponding changes in the Control group were 0.8±3.0% and 1.1±3.4%.
Figure 1 .
Linear regression (and 95% CI) for changes between preintervention and postintervention for Hbmass and  , n=145. Regression slope (solid line) and 95% CIs (dashed line) are shown. Top panel is for intercept free to vary, and bottom panel intercept constrained through the origin. Open circles are control participants and filled circles are altitude participants.
, n=145. Regression slope (solid line) and 95% CIs (dashed line) are shown. Top panel is for intercept free to vary, and bottom panel intercept constrained through the origin. Open circles are control participants and filled circles are altitude participants.
Figure 2 .
Linear regression (and 95% CI) for changes between preintervention and postintervention for Hbmass and  , control (n=62), altitude (n=83). Regression slope (solid line) and 95% CIs (dashed line) are shown. Top panels are for intercept free to vary, and bottom panels for intercept constrained through the origin. Left-side graphs are control groups (including IHE and IHT alone) and right-side graphs are altitude groups.
, control (n=62), altitude (n=83). Regression slope (solid line) and 95% CIs (dashed line) are shown. Top panels are for intercept free to vary, and bottom panels for intercept constrained through the origin. Left-side graphs are control groups (including IHE and IHT alone) and right-side graphs are altitude groups.
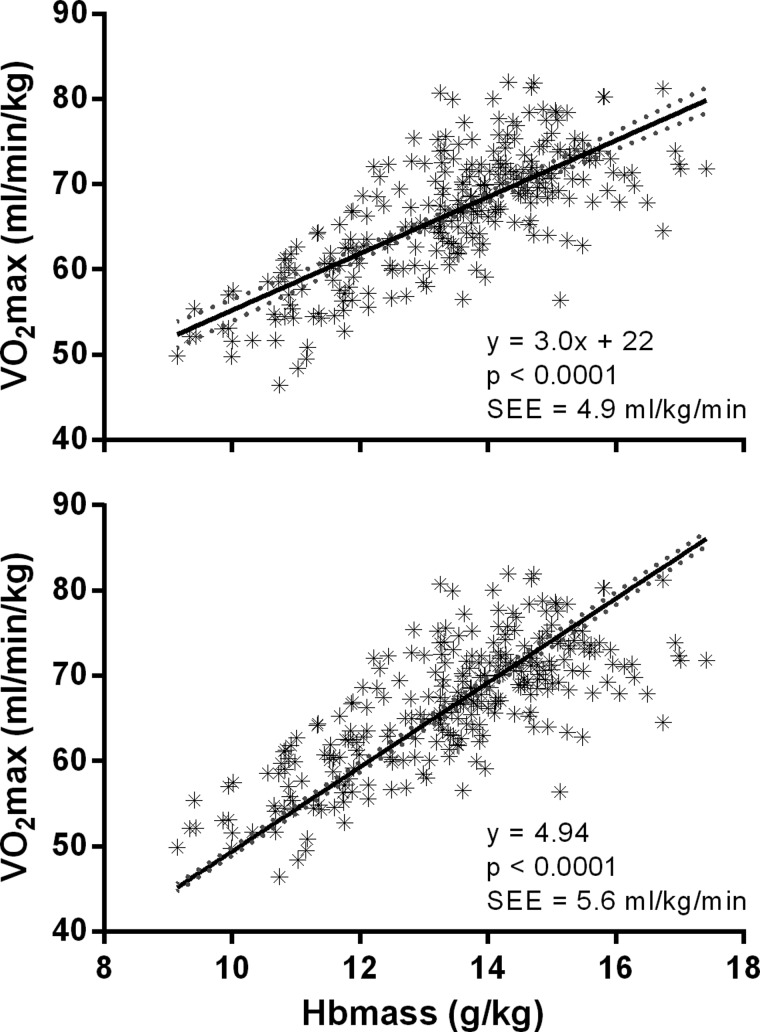
Cross-sectional relationship between Hbmass and 
When the intercept was able to vary, the linear regression of cumulative raw data (control and altitude, pre and post) of Hbmass and  was as follows
was as follows
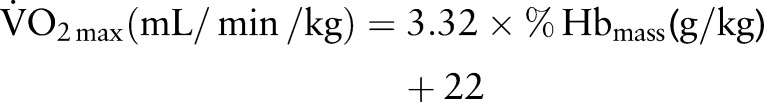 |
The slope was significant (p<0.0001, r=0.75) with 95% CI of 2.98 to 3.66. When constrained through the origin, the corresponding slope was 4.94 (4.89 to 4.99; figure 3).
Figure 3.
Linear regression between Hbmass and  in raw units. Regression slope (solid line) and 95% CIs (dashed line) are shown. Data are cumulative for pre-exposure and postexposure to altitude, n = 290. Top panel is for intercept free to vary, and bottom panel intercept constrained through the origin.
in raw units. Regression slope (solid line) and 95% CIs (dashed line) are shown. Data are cumulative for pre-exposure and postexposure to altitude, n = 290. Top panel is for intercept free to vary, and bottom panel intercept constrained through the origin.
Discussion
The main findings of the current study were (1) a significant, very large cross-sectional relationship between Hbmass and  and (2) a significant, moderate correlation between changes in Hbmass and
and (2) a significant, moderate correlation between changes in Hbmass and  in a large group of elite endurance athletes undertaking altitude training. Athletes who were part of LHTL and LHTH altitude interventions increased Hbmass and
in a large group of elite endurance athletes undertaking altitude training. Athletes who were part of LHTL and LHTH altitude interventions increased Hbmass and  by ∼3% such that each 1% change in Hbmass will result in a 0.6–0.7% change in
by ∼3% such that each 1% change in Hbmass will result in a 0.6–0.7% change in  . Even though significant, the correlation was weak and explained only 15% of the variation, indicating that other factors are still important in increasing
. Even though significant, the correlation was weak and explained only 15% of the variation, indicating that other factors are still important in increasing  , apart from an increased Hbmass.15
, apart from an increased Hbmass.15
Cross-sectional relationship between Hbmass and 
The current study yielded a strong relationship between Hbmass and  (r=0.75), with a slope of ∼4.9 when constrained through the origin; this implies that every additional gram of Hb will increase
(r=0.75), with a slope of ∼4.9 when constrained through the origin; this implies that every additional gram of Hb will increase  by ∼4.9 mL/min. Other cross-sectional studies indicate a similarly strong relationship between Hbmass and
by ∼4.9 mL/min. Other cross-sectional studies indicate a similarly strong relationship between Hbmass and  , which is independent of sex or age.23 A close association between absolute Hbmass and
, which is independent of sex or age.23 A close association between absolute Hbmass and  (r=0.72) was reported in 131 males of varying training status.24 Likewise, strong correlations between Hbmass and
(r=0.72) was reported in 131 males of varying training status.24 Likewise, strong correlations between Hbmass and  have been reported across a range of sports and degrees of training status, with the slope of the regression line ∼4 in each instance, although in the present study the slope is closer to 5 when the regression is forced through the origin. In practical terms, this relationship translates to a change in
have been reported across a range of sports and degrees of training status, with the slope of the regression line ∼4 in each instance, although in the present study the slope is closer to 5 when the regression is forced through the origin. In practical terms, this relationship translates to a change in  of ∼4–5 mL/min for every 1 g change in Hbmass.23
25
26 Furthermore, the relationship is consistent with the theoretical calculation of O2 transport during maximal aerobic exercise. The strong relationship between Hbmass and
of ∼4–5 mL/min for every 1 g change in Hbmass.23
25
26 Furthermore, the relationship is consistent with the theoretical calculation of O2 transport during maximal aerobic exercise. The strong relationship between Hbmass and  has two important implications; first, that a high Hbmass is an important prerequisite for a high
has two important implications; first, that a high Hbmass is an important prerequisite for a high  ,23
27 and second, that alterations to Hbmass have the potential to alter
,23
27 and second, that alterations to Hbmass have the potential to alter  and possibly performance.
and possibly performance.
Successful endurance performance is highly correlated with an athlete's  for the event duration (fractional utilisation or lactate threshold)33
34 and exercising with relatively low energy expenditure (good economy).30
33
35 These factors in isolation are not necessarily better predictors of performance than
for the event duration (fractional utilisation or lactate threshold)33
34 and exercising with relatively low energy expenditure (good economy).30
33
35 These factors in isolation are not necessarily better predictors of performance than  , although in elite athletes where an already high
, although in elite athletes where an already high  is present, these factors may become more important. A three-factor model (
is present, these factors may become more important. A three-factor model ( , running economy, lactate threshold) was reported to highly predict the within-subject changes in performance (measured by peak running speed) during a 17-week training period in well-trained distance runners.36 When holding each variable constant, to ascertain the effect of a predictor variable independently of all the other predictor variables,
, running economy, lactate threshold) was reported to highly predict the within-subject changes in performance (measured by peak running speed) during a 17-week training period in well-trained distance runners.36 When holding each variable constant, to ascertain the effect of a predictor variable independently of all the other predictor variables,  was the best predictor of performance with a 1% improvement in
was the best predictor of performance with a 1% improvement in  resulting in a 0.5% improvement in peak running speed, but the running economy was also a moderate predictor of performance.36
resulting in a 0.5% improvement in peak running speed, but the running economy was also a moderate predictor of performance.36
Changes in Hbmass and 
Interestingly, the Hbmass– relationship appears to uncouple somewhat following altitude training, with disproportionate changes being reported in Hbmass and
relationship appears to uncouple somewhat following altitude training, with disproportionate changes being reported in Hbmass and  . A weak association (r=0.32) between changes in Hbmass and
. A weak association (r=0.32) between changes in Hbmass and  was reported following 3 weeks of LHTL simulated altitude training combined with hypoxic training.18 In fact, when the individual data are examined, some athletes displayed substantial increases in Hbmass (>5%) with no change in
was reported following 3 weeks of LHTL simulated altitude training combined with hypoxic training.18 In fact, when the individual data are examined, some athletes displayed substantial increases in Hbmass (>5%) with no change in  , whereas others who experienced minor reductions in Hbmass increased
, whereas others who experienced minor reductions in Hbmass increased  by ∼5%.18 Similarly, despite a ∼4% increase in Hbmass observed in elite runners following ∼400 h of simulated LHTL (∼2900 m), only a trivial change in
by ∼5%.18 Similarly, despite a ∼4% increase in Hbmass observed in elite runners following ∼400 h of simulated LHTL (∼2900 m), only a trivial change in  was observed and, not surprisingly, the relationship between changes in Hbmass and
was observed and, not surprisingly, the relationship between changes in Hbmass and  was also trivial (r=0.04).37 It has been reported that after 24 days of LHTL, the Hbmass increased by 5.3% (∼44 g) in a group of orienteers, accompanied by a 4.1% increase in
was also trivial (r=0.04).37 It has been reported that after 24 days of LHTL, the Hbmass increased by 5.3% (∼44 g) in a group of orienteers, accompanied by a 4.1% increase in  (∼145 mL/min), which in terms of the mean data appears in line with the expected increase in
(∼145 mL/min), which in terms of the mean data appears in line with the expected increase in  /g of Hbmass.38 Indeed, the relationship between the change scores was ∼0.7 when the group was divided into men (r=0.75) and women (r=0.68); however, when the group data are combined, the relationship becomes much weaker (r=0.35, p=0.29). Clark et al13 report a trivial correlation between changes in Hbmass and
/g of Hbmass.38 Indeed, the relationship between the change scores was ∼0.7 when the group was divided into men (r=0.75) and women (r=0.68); however, when the group data are combined, the relationship becomes much weaker (r=0.35, p=0.29). Clark et al13 report a trivial correlation between changes in Hbmass and  in well-trained cyclists following 21 days of simulated LHTL (r=0.09, p=0.32); however, the slope of the regression line appears to indicate that a 1% increase in Hbmass is associated with a 0.8% increase in
in well-trained cyclists following 21 days of simulated LHTL (r=0.09, p=0.32); however, the slope of the regression line appears to indicate that a 1% increase in Hbmass is associated with a 0.8% increase in  . The only study to report a significant (albeit weak) correlation (r=0.4, p=0.02) between changes in red cell volume (measured using Evan's Blue) and
. The only study to report a significant (albeit weak) correlation (r=0.4, p=0.02) between changes in red cell volume (measured using Evan's Blue) and  was in a group of collegiate runners following 4 weeks of LHTL, where 5% and 9% increases in red cell volume and
was in a group of collegiate runners following 4 weeks of LHTL, where 5% and 9% increases in red cell volume and  were reported.1
were reported.1
The current study suggests that the relationship between increases in Hbmass and  is slightly stronger in athletes undertaking altitude training compared to control athletes, although the variance explained was ∼15%, which is similar to that reported previously (r2=0.14)1 for a 9% increase in red cell volume associated with a 5% increase in
is slightly stronger in athletes undertaking altitude training compared to control athletes, although the variance explained was ∼15%, which is similar to that reported previously (r2=0.14)1 for a 9% increase in red cell volume associated with a 5% increase in  . When participants (n=18) received recombinant EPO injections (50 IU/kg 3×/week) for a period of 25 d, they increased
. When participants (n=18) received recombinant EPO injections (50 IU/kg 3×/week) for a period of 25 d, they increased  more proportionately with the increase in Hbmass (r2=0.28) when compared with the current data set,39 adding support to this apparent uncoupling of the Hbmass–
more proportionately with the increase in Hbmass (r2=0.28) when compared with the current data set,39 adding support to this apparent uncoupling of the Hbmass– relationship with altitude training. On the other hand, when non-athletic, altitude adapted participants return to near sea-level, they show a
relationship with altitude training. On the other hand, when non-athletic, altitude adapted participants return to near sea-level, they show a  similar to lowlanders despite a 13% increase in Hbmass.40 The lack of adaptation in
similar to lowlanders despite a 13% increase in Hbmass.40 The lack of adaptation in  during training at hypoxia may be related to impairment in vascular regulation and reduced cardiac output after altitude training.10
11 Interestingly, 23 consecutive nights of LHTL simulated altitude exposure (3000 m) depressed
during training at hypoxia may be related to impairment in vascular regulation and reduced cardiac output after altitude training.10
11 Interestingly, 23 consecutive nights of LHTL simulated altitude exposure (3000 m) depressed  by 7% with only a trivial increase in Hbmass.41 This, along with other non-haematological adaptations that occur with training at altitude, may explain why
by 7% with only a trivial increase in Hbmass.41 This, along with other non-haematological adaptations that occur with training at altitude, may explain why  does not increase more proportionately with the increase in Hbmass. Finally, it should be considered that even with a small TE of measurement for
does not increase more proportionately with the increase in Hbmass. Finally, it should be considered that even with a small TE of measurement for  and Hbmass, it is quite likely that in some of the studies with smaller samples, as well as in the current study, these errors contribute to an obfuscation of the relationship between the two. However, the results of the current study are quite likely to be more robust, given the relatively large sample size and hence greater statistical power, as well as using only one method for measuring Hbmass.
and Hbmass, it is quite likely that in some of the studies with smaller samples, as well as in the current study, these errors contribute to an obfuscation of the relationship between the two. However, the results of the current study are quite likely to be more robust, given the relatively large sample size and hence greater statistical power, as well as using only one method for measuring Hbmass.
The differences in the changes in the Hbmass– relationship between the altitude and control athletes (figure 2) are most likely a result of individual variation.17 However, using the component estimates of TE, the combined error of measurement for changes between successive measures of Hbmass and
relationship between the altitude and control athletes (figure 2) are most likely a result of individual variation.17 However, using the component estimates of TE, the combined error of measurement for changes between successive measures of Hbmass and  at the 95% level could be as large as ∼±8% (1.96×√2×(√(22+2.22)), which is a consequence of analytical and biological variation in both tests. Factors such as illness, training (or detraining), fatigue and iron stores may all potentially affect changes in Hbmass and
at the 95% level could be as large as ∼±8% (1.96×√2×(√(22+2.22)), which is a consequence of analytical and biological variation in both tests. Factors such as illness, training (or detraining), fatigue and iron stores may all potentially affect changes in Hbmass and  .42
43 Interestingly, for the control group, there was a scatter of data (for changes in Hbmass and
.42
43 Interestingly, for the control group, there was a scatter of data (for changes in Hbmass and  ) about the origin in positive and negative directions suggestive of measurement error, whereas the Altitude group showed a preponderance of points in the positive/positive quadrant for both measures, inferring that altitude is an effective intervention to increase Hbmass and hence
) about the origin in positive and negative directions suggestive of measurement error, whereas the Altitude group showed a preponderance of points in the positive/positive quadrant for both measures, inferring that altitude is an effective intervention to increase Hbmass and hence  . In addition, the significant relationship for changes in Hbmass and
. In addition, the significant relationship for changes in Hbmass and  found for the Control group most likely reflects not just measurement error but also real changes in these markers secondary to factors such as training/detraining.
found for the Control group most likely reflects not just measurement error but also real changes in these markers secondary to factors such as training/detraining.
When acutely exposed to hypoxia, it is not just  that is affected. All the functional systems of the body are affected, including the central nervous system, respiratory system, cardiovascular system and muscles, a process that is mediated at the tissue level through rapid oxygen sensing.44 The transcription factor hypoxia-inducible factor-1 (HIF-1), present in every tissue of the body, is the global regulator of oxygen homeostasis and plays a critical role in the acute cardiovascular and respiratory responses to hypoxia.45 Improvement in many of these responses may be a factor in their variable response to increasing
that is affected. All the functional systems of the body are affected, including the central nervous system, respiratory system, cardiovascular system and muscles, a process that is mediated at the tissue level through rapid oxygen sensing.44 The transcription factor hypoxia-inducible factor-1 (HIF-1), present in every tissue of the body, is the global regulator of oxygen homeostasis and plays a critical role in the acute cardiovascular and respiratory responses to hypoxia.45 Improvement in many of these responses may be a factor in their variable response to increasing  after exposure to altitude.
after exposure to altitude.
In summary, the current data indicate that increases in Hbmass were significantly, albeit weakly, correlated with increases in  in elite endurance athletes after altitude training interventions with an approximate 1% increase in Hbmass resulting in a 0.6–0.7% increase in
in elite endurance athletes after altitude training interventions with an approximate 1% increase in Hbmass resulting in a 0.6–0.7% increase in  . However, since the relationship is not perfect, other factors must be considered, which affect changes in
. However, since the relationship is not perfect, other factors must be considered, which affect changes in  , in addition to other responses from altitude that may impact on performance.
, in addition to other responses from altitude that may impact on performance.
What are the new findings?
This study confirms the strong cross-sectional relationship between haemoglobin mass (Hbmass) and maximal oxygen uptake (
 ) in a sample of 145 elite athletes.
) in a sample of 145 elite athletes.Adequate moderate altitude exposure is effective for increasing Hbmass and
 in elite endurance athletes by ∼3%. But the correlation between these changes is weak and explains less than one-sixth of the variation, indicating that other factors are still important in increasing
in elite endurance athletes by ∼3%. But the correlation between these changes is weak and explains less than one-sixth of the variation, indicating that other factors are still important in increasing  after altitude training, apart from an increased Hbmass.
after altitude training, apart from an increased Hbmass.
How might it impact on clinical practice in the near future?
Altitude training can be undertaken as a helpful method to improve endurance performance in already highly trained athletes, but it should be incorporated as part of an annual plan.
Race performance may improve post-altitude as a result of increased haemoglobin mass and maximal oxygen uptake, but other non-haematological factors are also quite likely important. Therefore, altitude training studies should not be limited to measuring these two variables alone.
Acknowledgments
The authors would like to acknowledge Dr Sally Clark, Dr Clare Humberstone-Gough, Dr Eileen Robertson, Dr David Martin and Dr David Pyne for their contribution to various studies used for the current manuscript.
Footnotes
Contributors: PUS was involved in the conception and design, acquisition of data, analysis, interpretation of data, drafting of the article and approved the final version. LAG-L was involved in the acquisition of data, analysis and interpretation of data, and critically revised the article and approved the final version. WFS was involved in the conception and design, and critically revised the article and approved the final version. CJG was involved in the conception and design, analysis and interpretation of data, and critically revised the article and approved the final version.
Competing interests: None.
Ethics approval: Australian Institute of Sport Ethics Committee (for all 10 studies).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Levine BD, Stray-Gundersen J. ‘Living high-training low’: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 1997;83:102–12 [DOI] [PubMed] [Google Scholar]
- 2.Levine BD, Stray-Gundersen J, Gore CJ, et al. Point: counterpoint: positive effects of intermittent hypoxia (live high:train low) on exercise are/are not mediated primarily by augmented red cell volume. J Appl Physiol 2005;99:2053–5; discussion 2055–2058 [DOI] [PubMed] [Google Scholar]
- 3.Stray-Gundersen J, Chapman RF, Levine BD. ‘Living high-training low’ altitude training improves sea level performance in male and female elite runners. J Appl Physiol 2001;91:1113–20 [DOI] [PubMed] [Google Scholar]
- 4.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev 2000;28:10–14 [PubMed] [Google Scholar]
- 5.Schmidt W, Heinicke K, Rojas J, et al. Blood volume and hemoglobin mass in endurance athletes from moderate altitude. Med Sci Sports Exerc 2002;34:1934–40 [DOI] [PubMed] [Google Scholar]
- 6.di Prampero PE. The energy cost of human locomotion on land and in water. Int J Sports Med 1986;7:55–72 [DOI] [PubMed] [Google Scholar]
- 7.Billaut F, Gore CJ, Aughey RJ. Enhancing team-sport athlete performance: is altitude training relevant? Sports Med 2012;42:751–67 [DOI] [PubMed] [Google Scholar]
- 8.Gore CJ, Clark SA, Saunders PU. Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc 2007;39:1600–9 [DOI] [PubMed] [Google Scholar]
- 9.Wilber RL. Current trends in altitude training. Sports Med 2001;31:249–65 [DOI] [PubMed] [Google Scholar]
- 10.Favret F, Richalet JP, Henderson KK, et al. Myocardial adrenergic and cholinergic receptor function in hypoxia: correlation with O2 transport in exercise. Am J Physiol Regul Integr Comp Physiol 2001;280:R730–8 [DOI] [PubMed] [Google Scholar]
- 11.Reboul C, Tanguy S, Dauzat M, et al. Altitude negates the benefits of aerobic training on the vascular adaptations in rats. Med Sci Sports Exerc 2005; 37:979–85 [PubMed] [Google Scholar]
- 12.Siebenmann C, Robach P, Jacobs RA, et al. ‘Live high-train low’ using normobaric hypoxia: a double-blinded, placebo-controlled study. J Appl Physiol 2012;112:106–17 [DOI] [PubMed] [Google Scholar]
- 13.Clark SA, Quod MJ, Clark MA, et al. Time course of haemoglobin mass during 21 days live high:train low simulated altitude. Eur J Appl Physiol 2009;106:399–406 [DOI] [PubMed] [Google Scholar]
- 14.Garvican L, Martin D, Quod M, et al. Time course of the hemoglobin mass response to natural altitude training in elite endurance cyclists. Scand J Med Sci Sports 2012;22:95–103 [DOI] [PubMed] [Google Scholar]
- 15.Garvican LA, Pottgiesser T, Martin DT, et al. The contribution of haemoglobin mass to increases in cycling performance induced by simulated LHTL. Eur J Appl Physiol 2011;111:1089–101 [DOI] [PubMed] [Google Scholar]
- 16.Humberstone-Gough CE, Saunders PU, Bonetti DL, et al. Comparison of live high: train low altitude and intermittent hypoxic exposure. J Sports Sci Med 2013;112:3275–85 [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson EY, Saunders PU, Pyne DB, et al. Reproducibility of performance changes to simulated live high/train low altitude. Med Sci Sports Exerc 2010;42:394–401 [DOI] [PubMed] [Google Scholar]
- 18.Robertson EY, Saunders PU, Pyne DB, et al. Effectiveness of intermittent training in hypoxia combined with live high/train low. Eur J Appl Physiol 2010;110:379–87 [DOI] [PubMed] [Google Scholar]
- 19.Saunders PU, Ahlgrim C, Vallance B, et al. An attempt to quantify the placebo effect from a three-week simulated altitude training camp in elite race walkers. Int J Sports Physiol Perform 2010;5:521–34 [DOI] [PubMed] [Google Scholar]
- 20.Saunders PU, Telford RD, Pyne DB, et al. Improved running economy in elite runners after 20days of simulated moderate-altitude exposure. J Appl Physiol 2004;96:931–7 [DOI] [PubMed] [Google Scholar]
- 21.Saunders PU, Pyne DB, Telford RD, et al. Reliability and variability of running economy in elite distance runners. Med Sci Sports Exerc 2004;36:1972–6 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt W, Prommer N. The optimized CO-rebreathing method—a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 2005;95:486–95 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt W, Prommer N. Impact of alterations in total hemoglobin mass on VO2max. Exerc Sport Sci Rev 2010;38:68–75 [DOI] [PubMed] [Google Scholar]
- 24.Heinicke K, Wolfarth B, Winchenbach P, et al. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med 2001;22:504–12 [DOI] [PubMed] [Google Scholar]
- 25.Gore CJ, Hahn AG, Burge CM, et al. VO2max and haemoglobin mass of trained athletes during high intensity training. Int J Sports Med 1997;18:477–82 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt W, Prommer N. Effects of various training modalities on blood volume. Scand J Med Sci Sports 2008;18(Suppl 1):57–69 [DOI] [PubMed] [Google Scholar]
- 27.Martino M, Gledhill N, Jamnik V. High VO2max with no history of training is primarily due to high blood volume. Med Sci Sports Exerc 2002;34:966–71 [DOI] [PubMed] [Google Scholar]
- 28.Costill DL. The relationship between selected physiological variables and distance running performance. J Sports Med Phys Fitness 1967;7:61–6 [PubMed] [Google Scholar]
- 29.Costill DL, Thomason H, Roberts E. Fractional utilization of the aerobic capacity during distance running. Med Sci Sports 1973;5:248–52 [PubMed] [Google Scholar]
- 30.Conley DL, Krahenbuhl GS. Running economy and distance running performance of highly trained athletes. Med Sci Sports Exerc 1980;12:357–60 [PubMed] [Google Scholar]
- 31.Saltin B, Astrand PO. Maximal oxygen uptake in athletes. J Appl Physiol 1967;23:353–8 [DOI] [PubMed] [Google Scholar]
- 32.Schabort EJ, Killian SC, St Clair Gibson A, et al. Prediction of triathlon race time from laboratory testing in national triathletes. Med Sci Sports Exerc 2000; 32:844–9 [DOI] [PubMed] [Google Scholar]
- 33.di Prampero PE, Capelli C, Pagliaro P, et al. Energetics of best performances in middle-distance running. J Appl Physiol 1993;74:2318–24 [DOI] [PubMed] [Google Scholar]
- 34.Jones AM. The physiology of the world record holder for the women's marathon. Int J Sports Sci Coach 2006;1:101–16 [Google Scholar]
- 35.Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part I: cardiorespiratory aspects. Ann N Y Acad Sci 1977;301:310–22 [DOI] [PubMed] [Google Scholar]
- 36.Saunders PU, Cox AJ, Hopkins WG, et al. Physiological measures tracking seasonal changes in peak running speed. Int J Sports Physiol Perform 2010;5:230–8 [DOI] [PubMed] [Google Scholar]
- 37.Saunders PU, Telford RD, Pyne DB, et al. Improved running economy and increased hemoglobin mass in elite runners after extended moderate altitude exposure. J Sci Med Sport 2009;12:67–72 [DOI] [PubMed] [Google Scholar]
- 38.Wehrlin JP, Zuest P, Hallen J, et al. Live high-train low for 24days increases hemoglobin mass and red cell volume in elite endurance athletes. J Appl Physiol 2006;100:1938–45 [DOI] [PubMed] [Google Scholar]
- 39.Parisotto R, Wu M, Ashenden MJ, et al. Detection of recombinant human erythropoietin abuse in athletes utilizing markers of altered erythropoiesis. Haematologica 2001;86:128–37 [PubMed] [Google Scholar]
- 40.Prommer N, Heinicke K, Viola T, et al. Long-term intermittent hypoxia increases O2-transport capacity but not VO2max. High Alt Med Biol 2007;8:225–35 [DOI] [PubMed] [Google Scholar]
- 41.Gore CJ, Hahn AG, Aughey RJ, et al. Live high:train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiol Scand 2001; 173:275–86 [DOI] [PubMed] [Google Scholar]
- 42.Garvican LA, Martin DT, McDonald W, et al. Seasonal variation of haemoglobin mass in internationally competitive female road cyclists. Eur J Appl Physiol 2010;109:221–31 [DOI] [PubMed] [Google Scholar]
- 43.Wachsmuth NB, Volzke C, Prommer N, et al. The effects of classic altitude training on hemoglobin mass in swimmers. Eur J Appl Physiol 2013;113:1199–211 [DOI] [PubMed] [Google Scholar]
- 44.Rusko HK, Tikkanen HO, Peltonen JE. Altitude and endurance training. J Sports Sci 2004;22:928–44; discussion 945 [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol 2004;96:1173–7; discussion 1170–1172 [DOI] [PubMed] [Google Scholar]