Abstract
HIV-1 Nef is an accessory protein necessary for HIV-1 virulence and rapid AIDS development. Nef promotes viral replication and infection by connecting CD4 and several other cell surface receptors to the clathrin adaptor protein AP2, resulting in the internalization and degradation of the receptors interacting with Nef. We investigated how Nef can mediate constitutive receptor endocytosis through the interaction of the dileucine motif in its C-terminal flexible loop (C-loop) with AP2, whereas AP2 binding of the transmembrane receptors usually results in an equilibrated (recycled) endocytosis. Our results indicated that in addition to the dileucine motif, there is a second motif in the Nef C-loop involved in the Nef–AP2 interaction. Nef-mediated CD4 downregulation was impaired when the residue in the hydrophobic region in the Nef C-loop (LL165HPMSLHGM173) was mutated to a basic residue K/R or an acidic residue E/D or to the rigid residue P, or when M168L170, L170H171, or G172M173 was mutated to AA. A pull-down assay indicated that AP2 was not coprecipitated with Nef mutants that did not downregulate CD4. Molecular modeling of the Nef C-terminal flexible loop in complex with AP2 suggests that M168L170 occupies a pocket in the AP2 σ2 subunit. Our data suggest a new model in the Nef–AP2 interaction in which the hydrophobic region in the Nef C-loop with the dileucine (L164L165) motif and M168L170 motif binds to AP2(σ2), while the acidic motif E174 and D175 binds to AP2(α), which explains how Nef through the flexible loop connects CD4 to AP2 for constitutive CD4 downregulation.
Introduction
Nef is a 27- to 35-kDa multifunctional HIV-1 and SIV accessory protein necessary for HIV-1 virulence and rapid AIDS development.1–3 One of the prominent pathological activities of Nef is to promote viral replication and infection by connecting CD4 and several other cell surface receptors to the clathrin adaptor protein complexes (APs), resulting in the internalization and lysosomal degradation of the receptors that interacted with HIV-1 Nef.4–8 The mechanism of Nef-mediated receptor endocytosis is well elucidated in the studies of Nef-mediated CD4 downregulation.1–4 Nef is myristoylated at a Gly residue (G2) in the N-terminus, which mediates the membrane association of Nef.9
In CD4 downregulation, the Nef motif W57L58 binds to the cytoplasmic tail of CD4 whereas the dileucine motif (ENNSL164L165) in the Nef C-terminal flexible loop (C-loop) interacts with the clathrin adaptor protein complex AP2, connecting CD4 to the clathrin-coated vesicles for endocytosis.4,10–18 However, it is unclear why the AP2 interaction of the dileucine motif in the Nef C-terminal flexible loop resulted in the constitutive endocytosis of Nef-connected receptors whereas non-Nef-mediated cell surface receptor endocytosis interacting with AP2 through their own dileucine motif usually resulted in a recycled endocytosis without stimulation.19–22 For example, cell surface CD4 is expressed at a stable level and strong downregulation of CD4 was observed with PMA-induced phosphorylation of the serine residues proximal to the dileucine motif in the CD4 tail.19 Upon receptor engagement downregulation of the TCR/CD3 complex occurs with the phosphorylation of the serine residue upstream of the dileucine motif in CD3γ.20 We investigated whether mechanisms other than the classic dileucine–AP2 interaction are involved in the Nef–AP2 interaction and Nef-mediated CD4 downregulation. By using systematic mutagenesis and molecular modeling we identified a novel motif M168/L170 in the Nef C-terminal flexible loop that binds into a pocket in AP2 (σ2).
Materials and Methods
Plasmids
Plasmids encoding all HIV-1 Nef mutants described in Fig. 1 were constructed by three rounds of contiguous polymerase chain reaction (PCR) mutagenesis using HIV-1 Nef (NA7) as the templates in the first round of PCR. The PCR products were gel purified and used as the templates in the next round of PCR. The final PCR products were subcloned into pEBB, a mammalian cell expression vector containing an actin promoter, between BamHI–NotI sites. GST-Nef plasmids were generated by PCR subcloning of the Nef mutants into a pGEX 4T-2 vector between EcoRI–BamHI sites. The cDNAs encoding the full-length human AP-2 α/σ2 subunits subcloned in the pFastBac-dual vector were kindly provided by Dr. Stuart Kornfeld of Washington University, School of Medicine.23 All mutations generated in this study were confirmed by DNA sequencing.
FIG. 1.
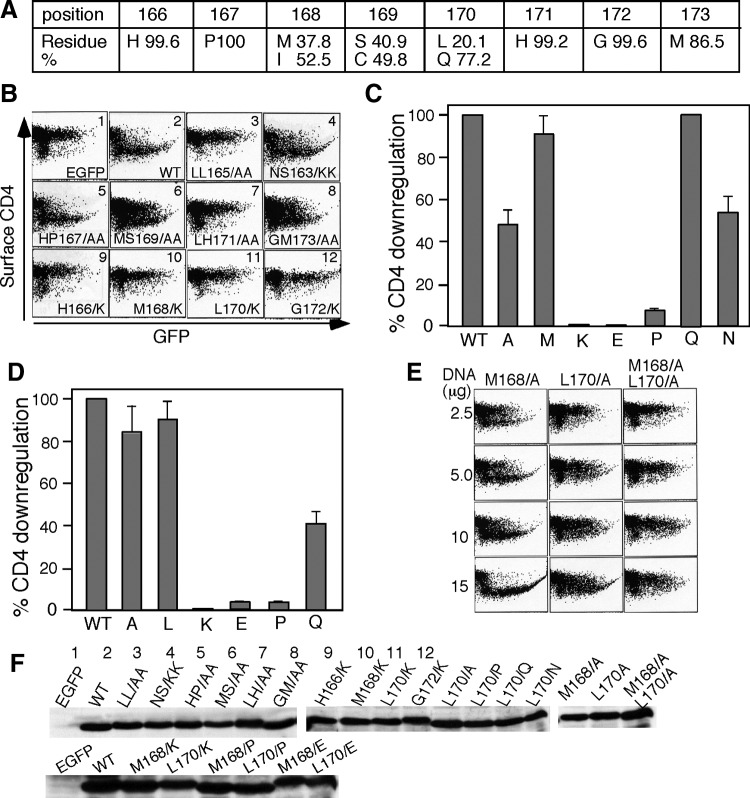
Mutations in the hydrophobic region (H166PMSLHGM173) in HIV-1 Nef C-loop-abrogated Nef-mediated CD4 downregulation. (A) L164L165 downstream sequence H166PMSLHGM173 is generally conserved among 259 Nef sequences. (B) FACS analysis shows CD4 downregulation by the Nef mutants indicated in each panel. Nef plasmids (10 (g) and pEGFP (2 (g) were cotransfected into the CD4 T cells by electroporation. The cells were surface stained with anti-CD4 PE for 2D FACS. (C) Effects of L170 mutations on Nef-mediated CD4 downregulation. L170 was mutated to Ala, Met, Lys, Glu, Pro, Gln, and Asn, respectively. The percentage of CD4 downregulation is calculated as described in Materials and Methods. The results are an average of three independent experiments. (D) Effects of M168 mutations on Nef-mediated CD4 downregulation. M168 was mutated to Ala, Leu, Lys, Glu, Pro, and Gln, respectively. (E) Comparison of the CD4 downregulation by Nef mutant M168/A, L170/A, and M168L170/AA. Nef plasmids of 2.5, 5.0, 10, or 15 (g and pEGFP of 2.5 (g were cotransfected into CD4 cells and analyzed as described in B. (F) Cellular expressions of the Nef mutants indicated at the top of each lane and numbered as shown in B. Whole cell lysates of 106 transfected cells were resolved on SDS–PAGE and western blotted with anti-Nef antibody.
Antibodies
PE-conjugated monoclonal antibody (mAb) of anti-CD4 (Leu3a) was purchased from Becton Dickinson (San Diego, CA). Anti-HIV-1 Nef rabbit serum was obtained from the NIH AIDS Research and Reference Reagent Program. Anti-γ-adaptin (AP1) clone 100/3 and anti-α-adaptin (AP2) clone 100/2 mAb were from Sigma. HRP-conjugated antirabbit IgG and antimouse IgG were from GE Healthcare (UK).
Cell culture, DNA transfection, and FACS analysis of CD4 downregulation
BYCD4 hybridoma cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS). Nef plasmids were transfected into BYCD4 cells by electroporation as previously described.15 Sixteen to 24 h after the transfection, cells were surfaced stained with PE-conjugated anti-CD4 mAbs at 1:100 dilution in phosphate-buffered saline (PBS) for 30 min on ice. The cells were fixed in 2% paraformaldehyde and subjected to FACS analysis on a FACScan (Becton Dickinson). The FACS data are plotted on a log scale. The amount of the CD4 downregulation is the steady-state medium CD4 staining minus the CD4 staining in cells transfected with wt Nef. The percentage (%) of CD4 downregulation is the ratio of CD4 downregulation between wt Nef (100%) and the Nef mutants.
Immunoblotting
Immunoblotting was performed as described previously.18 HIV-1 Nef was blotted with anti-HIV-1 Nef rabbit serum (1:10,000) (NIH). AP1 and AP2 were blotted with anti-AP1 (γ) and anti-AP2 (α) mAb (1: 500). The immunoblotting was done at 4°C overnight followed by HRP-conjugated secondary Abs at room temperature (RT) for 1 h. Films were developed using ECL.
In vitro analysis of Nef–AP2 interaction
The GST-Nef proteins were generated in Escherichia coli BL21 (DE3) cells transformed with the GST-Nef plasmids and isolated by using glutathione-agarose beads (Piece). [35S]AP2 (α) and [35S]AP1 (γ) were in vitro transcribed and translated by using the TNT T7 Quick Coupled Transcription/Translation System (Promega) in the presence of [35S]methionine (PerkinElmer). The AP2 (α/σ2) complex was generated in Sf9 cells (Armyworm) transfected with the bacmids of AP2 (α/σ2) by using the published methods.23 For the in vitro pull-down assay, GST-Nef proteins (∼5 μg) were incubated with the AP2 proteins for 2–4 h at 4°C in 0.5 ml of Triton X-100 buffer [0.5% Triton X-100, 20 mM Tris–HCl (pH 8.0), 150 mM NaCl, 5 mM MgCl2, 2 mM EDTA, and protease inhibitor cocktail from Sigma]. The protein complexes were precipitated with glutathione-agarose beads and washed once with Triton X-100 buffer and three times with PBS. The precipitated proteins were resolved by SDS–PAGE and autoradiography to detect [35S]AP2 (α) and [35S]AP1 (γ) or immunoblotted with anti-AP2 (α) to detect the α chain of the AP2 (α/σ2) complex. It was also blotted with anti-Nef to determine the amounts of GST-Nef proteins in the precipitated complexes.
Results
The hydrophobic region downstream of the dileucine motif (L164L165) is involved in Nef-mediated CD4 downregulation
A survey with the randomly selected 259 naturally occurring Nef sequences in the online HIV sequence database (Los Alamos National Laboratory) indicated that the hydrophobic region downstream of the dileucine motif (LL165HPMSLHGM173) in the Nef C-loop is generally conserved (Fig. 1A). To determine whether this region is involved in Nef-mediated CD4 downregulation, we introduced a series of single or double mutations into the region and analyzed the effects of these mutations on Nef-mediated CD4 downregulation.
Figure 1B shows that CD4 was downregulated by wt Nef (panel 2), which was completely impaired by the dileucine mutation (L164L165/AA) (panel 3) but was not impaired by the double mutations of the dileucine upstream residues N162S163 to AA (N162S163/AA) or to KK (N162S163/KK) (panel 4 and data not shown). However, double mutations of the dileucine downstream residues L170H171 to AA (L170H171/AA) (panel 7) completely impaired Nef-mediated CD4 downregulation and the double mutations of M168S169 and G172M173 to AA impaired Nef-mediated CD4 downregulation by ∼60% and ∼80%, respectively (panels 6 and 8). Nef-mediated CD4 downregulation was also impaired when any single residue in the region was mutated to the positively charged residue K/R or the negatively charged residue E/D (Fig. 1B, panels 9–12, Fig. 1C and D, and data not shown). CD4 downregulation was also impaired when M168, L170, H171, G172, or M173 was mutated to the rigid residue P (Fig. 1C and D and data not shown).
The western blotting analysis indicated that the cellular expressions of the Nef mutants were at similar levels (bottom panels in Fig. 1F). The results indicated that the L164L165 downstream hydrophobic region in the Nef C-loop is involved in Nef-mediated CD4 downregulation.
To analyze more specifically the role of the hydrophobic residues L170 and M168 in Nef-mediated CD4 downregulation, L170 and M168 were mutated to different amino acids. Figure 1C shows that Nef-mediated CD4 downregulation was completely impaired by the mutation of L170/K, L170/E, or L170/P, and was ∼50% impaired with the mutation of L170/A, but was not impaired with the mutation of L170 to the hydrophobic residue M (L170/M), suggesting that L170 is involved in a hydrophobic interaction. However, CD4 downregulation was also not impaired when L170 was mutated to Q or ∼40% impaired when mutated to N despite the fact that both Q and N are not hydrophobic residues. The volume of the side chain of Q (85.6 Å3) is closer to that of L (100.8 Å3) and M (103.9 Å3) than the side chain of N (63.7 Å3) is.24 Therefore, it suggests that in addition to the chemical characteristics of the side chain, a snug steric fit of L170 into the pocket may also play an important role in the interaction, and that mutation to Q results in no effect because Q170 well fills in the binding pocket.
Figure 1D shows the effects of M168 mutations on Nef-mediated CD4 downregulation. Similar to L170 mutations, CD4 downregulation was completely impaired by the mutation of M168/K, M168/E, or M168/P and was not impaired by the mutation of M168/L, suggesting that M168 is also involved in the hydrophobic interaction. Different from mutant L170/Q, CD4 downregulation was ∼50% impaired by the mutation of M168/Q. We compared the CD4 downregulation in cells transfected with different doses of Nef plasmids of L170/A, M168/A, or L170M168/AA. Figure 1D shows that Nef-mediated CD4 downregulation was Nef-dose dependent. CD4 was only partially downregulated with the transfection of 2.5 μg, 5 μg, or 10 μg plasmids of M168/A but was completely downregulated with the transfection of 15 μg of Nef M168/A. With the 10 and 15 μg plasmid transfections, ∼50 and ∼70% of CD4 were downregulated by Nef mutant L170/A. In contrast, CD4 was not downregulated when transfected with 15 μg of M168L170/AA or even 30 μg of M168L170/AA (not shown). The systematic mutational analysis suggested that both the hydrophobic character and steric characteristics of the side chains in the L164L165 downstream region may contribute to the Nef C-loop binding to AP2.
Abrogation of CD4 downregulation by mutations in the hydrophobic region is due to impairment of the Nef–AP2 interaction
It is well documented that Nef-mediated CD4 downregulation is AP2 dependent.4,10–18 To confirm that the above mutations that impaired the CD4 downregulation abrogate the Nef–AP2 interaction, we analyzed the Nef–AP2 interaction by an in vitro pull-down assay (see Materials and Methods). The E. coli-generated fusion proteins of GST-Nef (wt), GST-Nef (L164L165/AA), and GST-Nef (M168S169/KK) were incubated with the [35S]AP2 (α) subunit or [35S]AP1 (γ) subunit and the protein complexes were precipitated with glutathione agarose beads. Figure 2A shows that ∼10-fold more of the [35S]AP2 (α) protein was pulled down by the GST-Nef (wt) than by the GST-Nef (M168S169/KK) protein. In contrast, almost equal amounts of [35S]AP1 (γ) proteins were coprecipitated with the GST-Nef (wt) and the GST-Nef (M168S169/KK). The results indicated that the M168S169/KK mutation impaired the Nef–AP2 interaction but not the Nef–AP1 interaction. The Nef–AP1 interaction is known not to be required in Nef-mediated CD4 downregulation.4,11
FIG. 2.
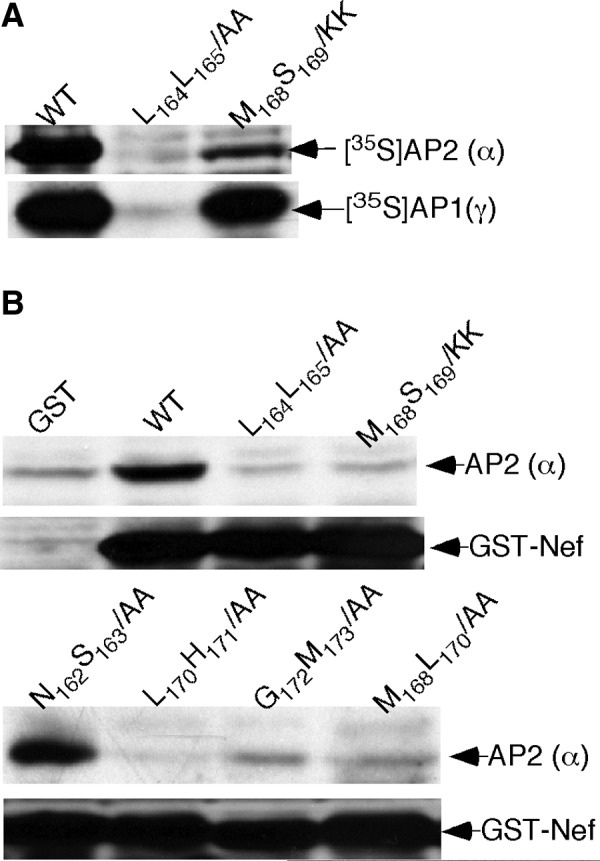
In vitro pull-down assay of Nef–AP2 interaction. (A) Coprecipitation of the [35S]AP2 (and the [35S]AP1 (subunit with GST-Nef proteins. [35S]AP2 (and [35S]AP1 (were generated by in vitro transcription and translation. The proteins were incubated with the E. coli-generated proteins of GST-Nef (wt) or GST-Nef (M168S169/KK). The protein complexes were precipitated with glutathione-agarose beads, resolved on SDS–PAGE, and autoradiographed. (B) Coprecipitation of the AP2 α/σ2 dimer with GST-Nef proteins. The AP2 α/σ2 dimer was generated in sf9 cells. The dimer was incubated with equal amounts of the proteins of GST, GST-Nef (wt), GST-Nef (L164L165/AA), GST-Nef (M168S169/KK), GST-Nef (N162S163/AA), GST-Nef (L170H171/AA), GST-Nef (G170M173/AA), and GST-Nef (M168L170/AA). The protein complexes pulled down with glutathione beads were immunoblotted with anti-AP2 (α) and by anti-Nef (HIV), respectively.
Both [35S]AP2 (α) and [35S]AP1 (γ) were not pulled down by the Nef dileucine mutant (L164L165/AA), indicating that the dileucine motif is involved in both AP1 and AP2 interactions.11,13 Recent studies indicated that the Nef–AP2 interaction resided on the AP2 α–σ2 dimer23,25,26 (Fig. 3). Therefore, we performed more pull down assays by using the AP2 α–σ2 dimer and various GST-Nef mutants. Figure 2B shows that the AP2 α–σ2 dimer was coprecipitated with GST-Nef (wt) and GST-Nef (N162S163/KK) but was essentially not coprecipitated with GST-Nef (L164L165/AA), GST-Nef (M168S169/KK), GST-Nef (L170H171/AA), GST-Nef (M168,L170/A,A), and GST-Nef (L170M173/A,A). The GST-Nef mutants that did not pull down the AP2 dimer were also impaired in CD4 downregulation as shown by Fig. 1. The anti-Nef immunoblotting indicated that comparable amounts of GST-Nef proteins were precipitated with glutathione beads in these pull-down assays (data not shown). The results confirmed that lacking the Nef–AP2 interaction caused the impairment of Nef-mediated CD4 downregulation due to mutations in the L164L165 downstream hydrophobic region.
FIG. 3.
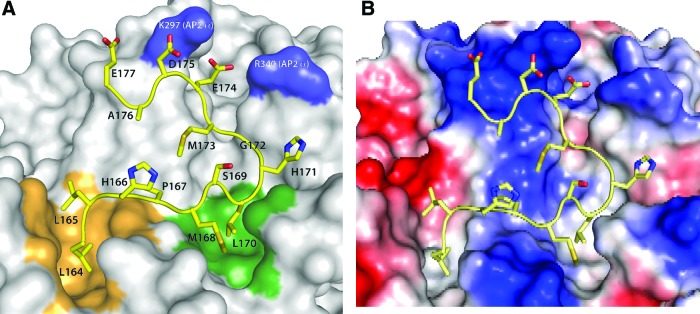
Structural model of the Nef C-terminal loop interaction with the AP2 complex. (A) The Nef loop is shown in yellow and the AP2 surface in gray. The dileucine motif pocket is colored orange, the new L170 binding pocket is shown in green, and the basic patch involved in Nef binding to AP2 is shown in blue. (B) The electrostatics properties of the same region are shown for AP2. The surface of AP2 is colored based on the electrostatics potential computed from the protein structure. Blue indicates regions of positive electrostatic potential and red regions indicate regions of negative electrostatic potential. White indicates regions that are relatively neutral. Color images available online at www.liebertpub.com/aid
Molecular modeling identifies novel binding between the M168/L170 motif in Nef and a specific pocket (L170 pocket) in AP2 (σ2)
To address whether a specific interaction between the hydrophobic region of the Nef C-loop and AP2 exists, we performed molecular modeling by docking the C-loop on AP2 complexes. It was carried out in three steps, each modeling one fragment of the loop between S163 and E177. First, the S163LLHPM168 segment of the Nef loop (including the L164L165 dileucine motif) was docked into the crystal structure of AP225 using the AutoDock program.27 The top scoring docking solutions confirmed that the Nef dileucine motif binds into the previously identified dileucine motif binding pocket in AP225 (Fig. 3, orange patch). This model of the S163LLHPM168 Nef segment interaction was extended by docking the P167MSLHGM173 segment into the AP2 structure. Solutions were selected among the highest scoring poses based on their overlap with the S163LLHPM168 solution over the P167M168 pair. The two docking solutions were joined using program MODELLER.28 Subsequently the joined S163–M168 segment model was extended to include the E174DAE177 segment, using the loop modeling protocol in MODELLER.29 The top scoring solution selected with the DOPE score30 of MODELLER was chosen as the final model for the Nef C-terminal loop S163– E177 segment.
The resulting model is compatible with the mutational data for the Nef sequence presented here and with the identification of the basic patch formed by K297 and R340 in AP2 (Fig. 3, blue patch), which is required for Nef binding.26 The model identifies a new binding pocket in the σ2 subunit of AP2 occupied by L170 and partially by M168 (Fig. 3A, green patch). Figure 3B illustrates the electrostatic properties of this region showing that the two motifs L164L165 and M168L170 bind to regions that are electrostatically more neutral. The L170 pocket is predicted to contribute to the binding of the hydrophobic region in the Nef C-loop based on the deleterious effects of mutations in L170 and M168L170 on CD4 downregulation shown in Fig. 1C and D. The L170 pocket in AP2 (σ2) is critical for Nef binding likely because it complements the hydrophobic and steric characteristics of the side chains in the M168L170 motif (Fig. 1C and D), which makes it a good target for the development of small-molecule inhibitors that block the Nef–AP2 interaction.
Discussion
To understand how Nef, through its interaction with the AP2 complex, mediates constitutive CD4 downregulation in the absence of phosphorylation signals, we investigated whether a region other than the dileucine motif in the Nef C-terminal loop is also involved in the AP2 interaction. The Ala screening (single amino acid mutated to Ala) in the C-loop suggested that the conserved hydrophobic region downstream of the dileucine motif from H166 to M173 may be involved (data not shown). We then made the double mutations to AA or the single mutation to the charged residue K/R or D/E. The results indicated that these mutations in the Nef dileucine downstream region impaired Nef-mediated CD4 downregulation whereas similar mutations upstream of the dileucine motif, such as N162S163 to KK, did not have an effect (Fig. 1). The pull-down assays confirmed that the mutations that impaired Nef-mediated CD4 downregulation abrogated the Nef–AP2 interaction (Fig. 2). The results thus indicated that the LL downstream region played an essential role in the Nef–AP2 interaction required for Nef-mediated CD4 downregulation.
Two characteristics of the LL downstream region seem to be important for its interaction with AP2—the hydrophobic and/or steric complementarities of the side chains and the local conformation of the C-loop. The region from L164 to M173 is the only hydrophobic region in the Nef C-loop. L170, M168, and M173 are very hydrophobic while the other residues H, S, and G are at an intermediate level on the hydrophobicity scale.31 Introduction of the charged residues is expected to greatly affect the overall hydrophobicity of this region, thus disrupting the interaction with AP2. The introduction of proline mutations in the area from M168 to M173, which likely results in the alteration of the local conformation, also impaired CD4 downregulation (Fig. 1). The naturally occurring Nef residue170 is ∼20% L and 80% Q. Mutation of Nef (NA7) L170 to Q did not impair Nef-mediated CD4 downregulation (Fig. 1C). Q is not hydrophobic but is similar to L in shape and volume, suggesting steric complementarity also plays a role in the interaction. Consistent with this proposal, the molecular modeling suggests that both L and Q are well adapted to the L170 pocket in AP2 (σ2) (Fig. 3 and data not shown). The natural occurring Nef sequences from M168 to L170 did not appear to be fully conserved (Fig. 1A). But these alterations are either conserved in the hydrophobicity (M168/I) or conserved in the steric structure (S169/C. L170/Q). This explains why the region is not seen as a fully conserved motif but still serves as an essential binding site between Nef and AP2.
The molecular modeling of the Nef C-terminal flexible loop complex with AP2 indicated that the residues M168 and L170 bind into a pocket (L170 pocket) in AP2 (σ2) (Fig. 3). The studies thus identified a second binding site between the Nef C-loop and AP2 (σ2). In agreement with the previous report,26 the modeling also suggested that the acidic residues E174 and D175 interact with a basic patch in AP2 (α) (Fig. 3). We propose that Nef interacts with AP2 through a region in the C-terminal flexible loop with the double binding of the dileucine motif L164L165 and hydrophobic motif M168L170 in two pockets of AP2 (σ2) while the acidic motif E174D175 binds to the basic patch in AP2 (α). The new model may better explain how the flexible Nef C-loop stably connects CD4 to AP2 for the constitutive CD4 downregulation.
We found that docking of the published Nef loop structure model32 into AP2 results in significant steric clashes (data not shown) and the resulting complex is not fully compatible with our mutational data. Because of its high flexibility, the C-loop is likely to adopt different conformations in different complexes. In the resolved structure of the Nef C-loop it interacts with another copy of Nef not with AP2.32 Hence, our model describes how the Nef C-loop interacts with AP2 and it is validated by its compatibility with the mutational data. The loop region from L164 to M173 is the only hydrophobic region in the ∼33 amino acids of the Nef C-loop. The rest of Nef loop is highly charged and predominantly acidic containing 10 acidic residues within two charged patches E149PEKVEE and E174DAEKE at the converging ends opposing each other.32 These electrostatic characteristics of the Nef C-loop and the complementary electrostatic characteristics of AP2 are likely to play an important role in the Nef–AP2 interaction.
Our model (Fig. 3) suggests that the E174DAEKE acidic region may play a role (probably together with the E149PEKVEE region not covered by the model) in the Nef–AP2 interaction by guiding Nef motifs L164L165 and M168L170 into the pockets that are electrostatically more neutral, while the flanking charged regions interact with complementary charged regions in AP2 (Fig. 3B). The Nef–AP2 interaction is, therefore, stabilized by the shape complementarity of the L164L165 and M168L170 motifs in their respective pockets and by the overall electrostatic complementarity of the Nef C-loop and the corresponding binding region in AP2. These characteristics also explain the specificity of the Nef–AP2 interaction. The important role of electrostatics is highlighted by the fact that a single substitution with a charged residue or double substitution with AA in the region disrupted the Nef–AP2 interaction (Figs. 1 and 2). Likely, in addition to the L170M168 motif, the other residues, particularly H171, G172, and M173, in the region may also contribute to the Nef–AP2 interaction through hydrophobic interactions with AP2.
The Nef-specific L170 pocket in AP2 (σ2) may be a good target for the development of Nef inhibitors. So far screening of small-molecule compounds targeting Nef for AIDS treatment has not been successful.32–36 Nef, lacking an active catalytic center or a substrate-binding pocket such as those found in proteases and kinases, is not a good target for small molecules to bind and interfere with function. On the contrary, a noncharged site, such as the L170 pocket, is likely to be more drugable and amenable to structure-based small-molecule screening. A small polar/apolar compound containing a polar group at one side and a hydrophobic group at the other side that binds in the L170 pocket might effectively block the Nef–AP2 interaction. Since the L170 pocket is Nef specific unlike the classic dileucine motif, blocking the L170 pocket may result in fewer side effects.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arora VK. Fredericksen BL. Garcia JV. Nef: Agent of cell subversion. Microbes Infect. 2002;4(2):189–199. doi: 10.1016/s1286-4579(01)01527-1. [DOI] [PubMed] [Google Scholar]
- 2.Geyer M. Fackler OT. Peterlin BM. Structure–function relationships in HIV-1 Nef. EMBO Rep. 2001;2(7):580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roeth JF. Collins KL. Human immunodeficiency virus type 1 Nef: Adapting to intracellular trafficking pathways. Microbiol Mol Biol Rev. 2006;70(2):548–563. doi: 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia JV. Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350(6318):508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz O. Marechal V. Le Gall S. Lemonnier F. Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2(3):338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 6.Collins KL. Chen BK. Kalams SA. Walker BD. Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391(6665):397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 7.Bell I. Ashman C. Maughan J. Hooker E. Cook F. Reinhart TA. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J Gen Virol. 1998;79(Pt 11):2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 8.Swigut T. Shohdy N. Skowronski J. Mechanism for down-regulation of CD28 by Nef. EMBO J. 2001;20(7):1593–1604. doi: 10.1093/emboj/20.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geyer M. Munte CE. Schorr J. Kellner R. Kalbitzer HR. Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein. J Mol Biol. 1999;289(1):123–138. doi: 10.1006/jmbi.1999.2740. [DOI] [PubMed] [Google Scholar]
- 10.Aiken C. Konner J. Landau NR. Lenburg ME. Trono D. Nef induces CD4 endocytosis: Requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76(5):853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg M. DeTulleo L. Rapoport I. Skowronski J. Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8(22):1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- 12.Craig HM. Pandori MW. Guatelli JC. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95(19):11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg ME. Bronson S. Lock M. Neumann M. Pavlakis GN. Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16(23):6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresnahan PA. Yonemoto W. Ferrell S. Williams-Herman D. Geleziunas R. Greene WC. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8(22):1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 15.Jin YJ. Cai CY. Zhang X. Zhang HT. Hirst JA. Burakoff SJ. HIV Nef-mediated CD4 down-regulation is adaptor protein complex 2 dependent. J Immunol. 2005;175(5):3157–3164. doi: 10.4049/jimmunol.175.5.3157. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri R. Lindwasser OW. Smith WJ. Hurley JH. Bonifacino JS. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol. 2007;81(8):3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin YJ. Zhang X. Cai CY. Burakoff SJ. Alkylating HIV-1 Nef —a potential way of HIV intervention. AIDS Res Ther. 2010;7:26. doi: 10.1186/1742-6405-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai CY. Zhang X. Sinko PJ. Burakoff SJ. Jin YJ. Two sorting motifs, a ubiquitination motif and a tyrosine motif, are involved in HIV-1 and simian immunodeficiency virus Nef-mediated receptor endocytosis. J Immunol. 2011;186(10):5807–5814. doi: 10.4049/jimmunol.1003506. [DOI] [PubMed] [Google Scholar]
- 19.Shin J. Dunbrack RL., Jr Lee S. Strominger JL. Phosphorylation-dependent down-modulation of CD4 requires a specific structure within the cytoplasmic domain of CD4. J Biol Chem. 1991;266(16):10658–10665. [PubMed] [Google Scholar]
- 20.Dietrich J. Hou X. Wegener AM. Geisler C. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 1994;13(9):2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittrich E. Haft CR. Muys L. Heinrich PC. Graeve L. A di-leucine motif and an upstream serine in the interleukin-6 (IL-6) signal transducer gp130 mediate ligand-induced endocytosis and down-regulation of the IL-6 receptor. J Biol Chem. 1996;271(10):5487–5494. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich J. Hou X. Wegener AM. Pedersen LO. Odum N. Geisler C. Molecular characterization of the di-leucine-based internalization motif of the T cell receptor. J Biol Chem. 1996;271(19):11441–11448. doi: 10.1074/jbc.271.19.11441. [DOI] [PubMed] [Google Scholar]
- 23.Doray B. Lee I. Knisely J. Bu G. Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18(5):1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harpaz Y. Gerstein M. Chothia C. Volume changes on protein folding. Structure. 1994;2(7):641–649. doi: 10.1016/s0969-2126(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 25.Kelly BT. McCoy AJ. Spate K, et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456(7224):976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhuri R. Mattera R. Lindwasser OW. Robinson MS. Bonifacino JS. A basic patch on alpha-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex. J Virol. 2009;83(6):2518–2530. doi: 10.1128/JVI.02227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodsell DS. Morris GM. Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J Mol Recognit. 1996;9(1):1–5. doi: 10.1002/(sici)1099-1352(199601)9:1<1::aid-jmr241>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Eswar N. John B. Mirkovic N, et al. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 2003;31(13):3375–3380. doi: 10.1093/nar/gkg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiser A. Do RK. Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9(9):1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen MY. Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15(11):2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose GD. Wolfenden R. Hydrogen bonding, hydrophobicity, packing, and protein folding. Annu Rev Biophys Biomol Struct. 1993;22:381–415. doi: 10.1146/annurev.bb.22.060193.002121. [DOI] [PubMed] [Google Scholar]
- 32.Horenkamp FA. Breuer S. Schulte A, et al. Conformation of the dileucine-based sorting motif in HIV-1 Nef revealed by intermolecular domain assembly. Traffic. 2011;12(7):867–877. doi: 10.1111/j.1600-0854.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 33.Betzi S. Restouin A. Opi S, et al. Protein-protein interaction inhibition (2P2I) combining high throughput and virtual screening: Application to the HIV-1 Nef protein. Proc Natl Acad Sci USA. 2007;104(49):19256–19261. doi: 10.1073/pnas.0707130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olszewski A. Sato K. Aron ZD, et al. Guanidine alkaloid analogs as inhibitors of HIV-1 Nef interactions with p53, actin, and p56lck. Proc Natl Acad Sci USA. 2004;101(39):14079–14084. doi: 10.1073/pnas.0406040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emert-Sedlak L. Kodama T. Lerner EC, et al. Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds. ACS Chem Biol. 2009;4(11):939–947. doi: 10.1021/cb900195c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dikeakos JD. Atkins KM. Thomas L, et al. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol Biol Cell. 2011;21(19):3279–3292. doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]