Abstract
Study Design
Descriptive, cross-sectional.
Introduction
Breast cancer (BC) treatments place the nervous system at risk, which may contribute to upper extremity (UE) mechanosensitivity.
Purpose of the Study
To evaluate elbow extension range of motion (EE-ROM) during upper limb neurodynamic testing (ULNT) post-BC treatment.
Methods
ULNT EE-ROM was measured for 145 women post-BC treatment. Women were sub-grouped by presence/absence of pain and lymphedema
Results
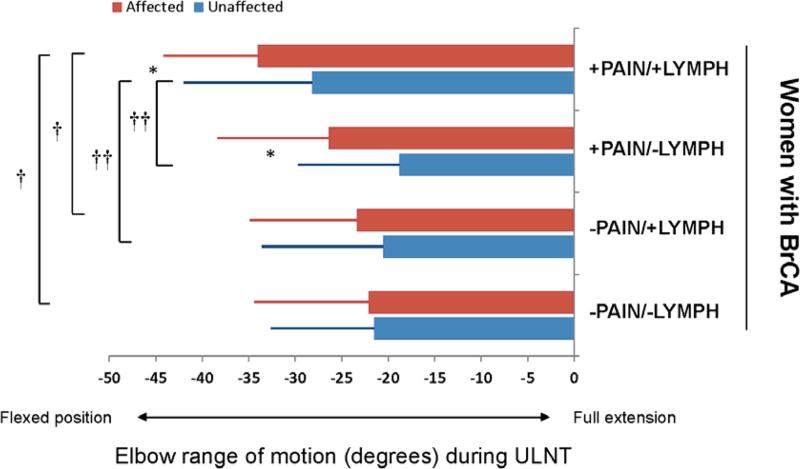
Mean EE-ROM during ULNT1 was −22.3° (SD: 11.9°) on the unaffected limb and −25.99° (SD 13.1°) on the affected limb. The women with pain and lymphedema had the greatest limitation in EE-ROM during ULNT1 testing, particularly of their affected limb (−33.8°, SD 12.9). Symptoms were reported more frequently in the affected chest, shoulder, arm, elbow, and hand. The intensity of symptoms was greater at the affected chest (p=0.046), shoulder (p=0.033) and arm (p=0.039).
Conclusions
Women with lymphedema and pain after BC treatment may present with altered neural mechanosensitivity.
Introduction
Many of the more than 2 million breast cancer survivors in the U.S.,1-3 have upper extremity morbidities associated with their breast cancer treatment, including pain and lymphedema. While breast surgery alone may result in physical impairments, the addition of axillary dissection, radiation, and chemotherapy are associated with increased incidence of morbidity, not only lymphedema, but neuropathy, and reductions in range of motion.4 It is estimated that between 5 and 42% of breast cancer survivors develop lymphedema,5-10 as many as 47% report persistent pain,11 and up to 77% report sensory disturbance in the breast or arm.12 These short and long term consequences have dramatic impact on physical function and quality of life in this population.8,13,14
For example, women who develop breast cancer-related lymphedema experience greater pain and limitation in upper extremity (UE) function, and more restrictions in activity than women without lymphedema.4,13-15 Breast cancer-related lymphedema results from impaired lymph transport due to surgical removal of or radiation-induced damage to axillary lymph nodes and lymphatic channels,16,17 which leads to accumulation of lymph in the UE, chest, or trunk. In addition to pain there are other symptoms associated with lymphedema that are troublesome, including heaviness, ache, or tiredness of the affected limb, jewelry or clothes feeling too tight, swelling in the limb, and difficulty writing.8,18 Complaints of heaviness and ache often associated with lymphedema, and complaints of weakness, sensory disturbance, and pain following breast cancer treatment, may also be associated with injury to peripheral nerves.
Injury to the long thoracic, thoracodorsal, and intercostobrachial nerves has been reported with axillary dissection.19-23 Nerve injury may be a result of positional tractioning, forceful retraction, direct laceration, or contusion of neural tissue during surgery.19 Nerve injury can also be due to entrapment or compression related to post-operative or radiation-induced fibrosis and scarring.19,24 Radiation-induced fibrosis is thought to occur in 3 phases.25 The prefibrotic phase includes marked chronic inflammation, increased vascular permeability, edema formation, and fibroblast proliferation. During the second phase the damaged tissue is composed primarily of activated fibroblasts in a disorganized extracellular matrix with excessive deposition of extracellular matrix proteins and collagen. During the fibroatrophic phase, there is loss of parenchymal cells and retraction of the fibrous tissue which is dense and poorly vascularized.26 Though relatively uncommon, radiation-induced brachial plexus neuropathy in breast cancer survivors has been described.27,28 Damage is thought to be due to direct neuronal damage, microvascular injury and resultant ischemia, or to entrapment or compression from radiation-induced fibrotic changes in surrounding tissues.
Chemotherapy-induced peripheral neuropathy (CIPN) is a common complication of systemic cancer treatments with chemotherapeutic agents.29 A number of factors have been implicated in the pathophysiology of CIPN, including disruption of axoplasmic microtubule-mediated transport, axonal degeneration, and damage to the sensory nerve cell bodies in the dorsal root ganglia.30
Peripheral nerves may become “sensitized” when subjected to trauma and become less tolerant to the physical stresses, such as compression and stretch, imposed upon them during movement. The mechanisms responsible for development of neuropathic pain from cancer treatment (i.e. radiation-induced neuropathy, CIPN, or surgical injury) may also affect the tolerance of the nervous system to movement. For example, taxanes, commonly used in the treatment of breast cancer, are known to lead to impaired axonal transport.31,32 Ellis, et al.33 have demonstrated heightened mechanosensitivity in the sciatic nerve with a rat model of impaired axonal transport. Additionally, peripheral nerves at risk during surgery or radiation may be subjected to higher than normal physical stresses during movement due to compression or restrictions from adhesions and fibrosis.
Purpose
In light of this shared theoretical etiology and overlapping symptomatic complaints, it is important to recognize the unique symptoms of altered mechanosensitivity in women following breast cancer treatment and whether this presentation is altered in the presence of lymphedema. Our hypotheses are that 1) following breast cancer treatment women will have impaired mechanosensitivity in the affected UE compared to their unaffected UE and 2) this impairment will be even greater in the women with lymphedema and pain. The results of this study will provide valuable information to clinicians who treat women with upper limb impairments following breast cancer treatment. The aims of this study were to 1) evaluate the mechanosensitivity of the UE nervous system in women following breast cancer treatment and 2) to compare mechanosensitivity between subgroups of women after breast cancer treatment (defined by presence or absence of pain and lymphedema).
Methods
Participants
Participants consisted of 145 women over the age of 18 who had completed active breast cancer treatment at least 6 months previously. Women were excluded for bilateral breast cancer, current UE infection, lymphangitis, pre-existing lymphedema, recurrence of breast cancer, or pre-existing neuromuscular or musculoskeletal conditions that would preclude UE testing. Women were recruited through the National Lymphedema Network website, San Francisco Bay area hospitals, San Francisco Bay area breast cancer or lymphedema support groups, and breast cancer conferences. The women were required to have no history of UE trauma, cervical radiculopathy, breast cancer, lymphedema, upper quadrant neurovascular entrapment, or UE peripheral nerve injury. Participants were required to be able to read, speak, and understand English. The University of California, San Francisco (UCSF) Committee on Human Research and the Clinical and Translational Science (CTSI) Clinical Research Center Advisory Committee approved both studies. Written informed consent was obtained prior to testing and the rights of participants were protected.
Procedures
Participants in this cross-sectional study attended a single evaluation session at the UCSF CTSI Clinical Research Center. One investigator (BS) completed all testing.
Subjective measures
Participants completed a 28-item Demographic Profile questionnaire. Information was collected regarding age, income, ethnicity, gender, menopausal status, Karnofsky Performance Status, and co-morbidities. The women completed the Norman Questionnaire, a validated self-report measure used to monitor symptoms of UE lymphedema34 and the Lymphedema and Breast Cancer Questionnaire to collect data regarding sins and symptoms at the time of testing, during the month prior, and during the year prior.35 Pain was evaluated using the Breast Symptoms Questionnaire (BSQ) including information on the occurrence of pain and other symptoms in the breast and UE (swelling, numbness, strange sensations, hardness). Participants rated the intensity of their average and worst pain, in the past week, using a numeric rating scale (NRS) that ranged from 0 (no pain) to 10 (worst imaginable pain). Participants were also asked to rate any symptoms in the UE using the same NRS. The NRS is a valid and reliable measure of pain intensity in adults with cancer.36
Objective measures
A 12 inch goniometer was used to measure shoulder and elbow range of motion (ROM), following standardized procedures reported by Norkin.37 Circumferential measurements were used to objectively document UE limb volume. A flexible tape measure was used for segmental measurement of circumference of each UE beginning at the ulnar styloid, and at 10 centimeter intervals proximal to this point up to a maximum of 40 centimeters. Volume was calculated from the circumference measurements using the following formula for volume of a truncated cone: V = 1/12Π Σ h (C12 + C1C2 + C22), where h is the length of each measured segment and C is the circumference at each end of that segment.38
Neural tolerance to movement was assessed through neurodynamic testing. The upper limb neurodynamic test 1 (ULNT1)39 was utilized in this study as it has the highest reliability compared to other variations.40 The ULNT1 consists of motions known to apply increased strain on the UE neural pathway from the brachial plexus to the distal peripheral nerve branches.41-43 Measurement of the last motion during ULNT1 sequencing, elbow extension, represents a measure of the overall tolerance of the neural tissue to movement when under greater relative loading (elongation). To insure that the limitation of elbow extension during ULNT1 was truly related to neural tissue sensitization, the findings were compared to elbow extension range of motion with the shoulder in 0 degrees of flexion, abduction, and rotation and wrist/hand in 0 degrees of flexion/extension, positions in which the neural tissue is comparatively under less loading (more slack).
Testing was performed with the participant supine, without a pillow under the head or knees, legs uncrossed, arms at side, and head in neutral rotation. The ULNT1 consisted of blocking shoulder girdle elevation with pressure from examiner's hand and forearm. Then, with the participant's forearm in neutral pronation/supination, the shoulder was taken into 90° of abduction with the elbow flexed at 90°. The shoulder was then moved into 70-90° of external rotation, the maximum allowed by the most restricted shoulder. The forearm was then supinated followed by wrist and finger extension and finally elbow extension. Testing was stopped at the elbow ROM at which the shoulder girdle attempted to elevate, resistance to movement prevented further motion, or the participant reported submaximal pain, previously defined as the maximal discomfort the participant was prepared to tolerate.44 Elbow extension ROM was recorded, as was participant's report of symptom quality, location, and intensity.
Statistical analysis
Sample size calculations were performed a priori, using an alpha of 0.05 and power of 0.80. The sample size estimate of 120 was determined prospectively for the parent breast cancer symptom study, based on correlation coefficient of 0.25 for regression analysis.
Statistical analyses were performed using IBM SPSS Statistics, version 20 (IBM Corporation, Somer, NY). For participant characteristics, means and standard deviations for interval data were obtained and analysis of variance was used to test for significance of differences for normally distributed data. Chi square analysis was used to assess significance of differences in proportions for nominal and categorical variables. Paired t-tests were performed for within group comparisons. Within groups effect sizes for differences between limbs were calculated as the difference in means/standard deviation of the difference.
Analysis of variance (ANOVA) was performed to evaluate within and between groups differences, followed by post hoc analysis including Bonferroni correction. The participants were subdivided into those with the following complications: 1) pain in affected limb with presence of lymphedema (+Pain/+LE), 2) pain in the affected limb without lymphedema (+Pain/–LE), 3) no pain in the affected limb with presence of lymphedema (–Pain/+LE), and 4) no pain in the affected limb without lymphedema (–Pain/–LE). Non-parametric statistics (related samples Wilcoxon Signed Rank test) were used to compare symptom intensity during ULNT between limbs within the women with BC group due to non-normal distribution of responses. The frequencies of symptom report in the following upper limb regions are reported; chest, shoulder, arm, elbow, forearm, wrist, hand, thumb, 2nd digit, 3rd digit, 4th digit and 5th digit.
Results
Participant characteristics
Of the 145 participants, 74 reported unilateral UE breast cancer-related lymphedema, previously diagnosed by their health care provider. This was used to categorize the participants into the lymphedema or non-lymphedema groups. Participant characteristics, including limb volume calculated from circumference, are presented in Table 1. 90.3% of the women were right handed, 7.6% were left handed, and 2.1% described themselves as ambidextrous. The breast cancer was on the side of the dominant limb in 74 women (49%).
Table 1.
Participant Characteristics: reported as Mean (SD) or Percentage (number of women)
| + Pain + Lymphedema n = 32 | + Pain − Lymphedema n = 23 | − Pain + Lymphedema n = 42 | − Pain − Lymphedema n = 48 | Overall significance of differences among groups (p) | |
|---|---|---|---|---|---|
| Age (years) | 56.31 (10.68) | 54.91 (9.91) | 58.12 (9.46) | 55.29 (8.26) | 0.460† |
| Body mass index | 29.35 (7.36) | 25.59 (5.59) | 25.77 (4.61) | 25.38 (4.16) | 0.007† |
| Exercises regularly | 81% (26) | 91% (21) | 83% (35) | 88% (42) | 0.730‡ |
| Currently working | 56% (18) | 52% (12) | 62% (26) | 60% (29) | 0.742‡ |
| Hand dominance R/L/Both | 29/2/1 | 19/4/0 | 38/4/0 | 45/1/2 | 0.248‡ |
| Affected breast R/L | 18/14 | 7/16 | 20/22 | 22/46 | 0.303‡ |
| Type of breast cancer surgery | BCS: 78% (25) Mast: 22% (7) |
BCS: 52% (12) Mast: 48% (11) |
BCS: 50% (21) Mast: 50% (21) |
BCS: 54% (26) Mast: 46% (22) |
0.090‡ |
| Sentinel node biopsy | 69% (22) | 78% (18) | 43% (18) | 75% (36) | 0.004 ‡ |
| Axillary node dissection | 78% (25) | 78% (18) | 93% (38) | 58% (28) | 0.004 ‡ |
| No. of nodes removed | 12.62 (8.69) | 10.48 (6.37) | 14.81 (8.99) | 7.66 (6.32) | < 0.001† |
| Chemotherapy | 72% (23) | 70% (16) | 73% (30) | 67% (32) | 0.918‡ |
| Radiation therapy | 81% (26) | 70% (16) | 79% (33) | 69% (33) | 0.520‡ |
| Radiation to axilla | 28% (9) | 22% (5) | 33% (14) | 17% (8) | 0.302‡ |
| Inter-limb volume difference (ml) | Affected – unaffected 204.05 (253.82) |
Affected –
unaffected −9.01 (85.03) |
Affected – unaffected 252.32 (326.33) |
Affected –
unaffected −7.31 (66.84) |
< 0.001† |
One way ANOVA
Chi square
Women were grouped by presence or absence of UE pain and lymphedema. Significant differences were found among the groups for BMI (p = 0.007). Post hoc analysis for BMI revealed significant differences between the +Pain/+LE group and both the –Pain/–LE group (p = 0.009) and the –Pain/+LE group (p = 0.030).
Significant differences were found for numbers of women who had sentinel node biopsy (p = 0.004), numbers of women who had axillary node dissection (p = 0.004), and for the number of nodes removed (p < 0.001). Women in both lymphedema groups had a statistically significantly more lymph nodes removed than the women without lymphedema. (p = < 0.001 and 0.044). Statistically significant differences were also found for interlimb volume differences in both lymphedema groups (p = < 0.001 and 0.003).
Range of motion
Upper extremity ROM findings are presented in Table 2. Analysis of variance revealed statistically significant differences among groups for shoulder abduction on the affected side (p = 0.083), elbow extension on the unaffected side (p = 0.021), and ULNT1 elbow extension on both sides (p = 0.013 unaffected side, and p = 0.01 affected side). The statistically significant post-hoc multiple between group comparisons are presented in Table 3.
Table 2.
Range of motion (Means and 95 CI; and effect sizes for within groups differences)
| Range of motion (In degrees) | + Pain + Lymphedema n = 32 | + Pain − Lymphedema n = 23 | − Pain + Lymphedema n = 42 | − Pain − Lymphedema n = 48 | Significance (ANOVA) p |
|---|---|---|---|---|---|
| Shoulder abduction | U: 156.3 (149.2, 163.5) | U: 160.5 (151.6, 170.0) | U: 159.2 (153.0, 165.4) | U: 163.4 (158.3, 168.4) | 0.446 |
| A: 136.3 (126.8, 145.6) | A: 143.5 (132.7, 154.2) | A: 141.6 (131.7, 151.6) | A: 157.8 (150.7, 165,0) | 0.003 | |
| Mean Difference | 20.1 (11.6, 28.6)* | 17.1 (8.6, 25.6)* | 17.6 (8.6, 25.6)* | 5.5 (0.5, 10.6)* | 0.020 |
| Effect size | 0.86 | 0.87 | 0.60 | 0.32 | |
| Shoulder ER | U: 87.8 (83.9, 91.7) | U: 93.4 (90.0, 96.8) | U: 90.1 (87.2, 93.1) | U: 92.7 (90.0, 95.4) | 0.083 |
| A: 85.2 (81.0, 89.3) | A: 88.0 (82.3, 93.7) | A: 86.0 (81.1, 91.0) | A: 89.9 (87.0, 92.6) | 0.360 | |
| Difference | 2.7 (−0.9, 6.2) | 5.4 (0.8, 9.9)* | 4.1 (−0.2, 84) | 2.9 (0.2, 5.6)* | 0.780 |
| Effect size | 0.27 | 0.51 | 0.30 | 0.32 | |
| Elbow extension | U: −0.8 (−3,0, 1.4) | U: 2.2 (0.5, 3.8) | U: 1.3 (0.2,2.2) | U: 2.0 (1.0, 3.0) | 0.021 |
| A: 0.6 (−1.0, 2.2) | A: 2.0 (0.5, 3.5) | A: 1.2 (0, 2.4) | A: 1.9 (0.8, 3.0) | 0.451 | |
| Mean Difference | −1.4 (−2.8, 0.02) | 0.2 (−0.2, 0.6) | 0.1 (−0.6, 0.8) | 0.2 (−0.6, 0.9) | 0.043 |
| Effect size | −0.37 | 0.20 | 0.03 | 0.06 | |
| Wrist extension | U: 71.3 (69.2 73.3) | U: 72.6 (68.8, 76.4) | U: 73.4 (71.1, 75.8) | U: 74.0 (72.1, 75.9) | 0.374 |
| A: 70.0 (66.5, 73.4) | A: 72.0 (68.0, 76.1) | A: 71.8 (69.5, 74.1) | A: 73.9 (71.2, 76.0) | 0.233 | |
| Mean Difference | 1.3 (−1.8, 4.5) | 0.5 (−1.2, 2.2) | 1.6 (−0.04, 3.3) | 0.1 (−1.2, 1.5) | 0.641 |
| Effect size | 0.15 | 0.14 | 0.32 | 0.03 | |
| ULNT elbow extension | U: −28.2 (−31.9, −24.5) | U: −18.8 (−24.1, −13.5) | U: −20.5 (−24.2, −16.8) | U: −21.5 (−25.2, −17.9) | 0.013 |
| A: −33.8 (−38.6, −29.1) | A: −26.4 (−32.3, −20.5) | A: −23.7 (−27.9, −19.5) | A: −22.5 (−25.8, −19.2) | 0.001 | |
| Mean Difference | −5.6 (−10.4, −0.8)* | −7.6 (−11.0, −4.2)* | −3.2 (−5.5, 0.08) | −0.93 (−3.9, 2.0) | 0.065 |
| Effect size | −0.43 | −1.00 | −0.31 | −0.10 |
CI: Confidence Interval
p < 0.05
Table 3.
Upper extremity range of motion multiple comparisons
| Movement | Side | Post hoc comparison | Difference between groups Degrees ROM Mean/SE | 95% Confidence Interval | Significance (p) (ANOVA) |
|---|---|---|---|---|---|
| Shoulder abduction | Affected | Overall: 0.003 | |||
| + pain / + lymphedema vs − pain / − lymphedema | 21.59 (6.22) | 38.23, 4.95 | 0.004 | ||
| − pain / − lymphedema vs − pain / + lymphedema | 16.18 (5.76) | 31.58, 0.77 | 0.034 | ||
| Difference between sides | Overall: 0.020 | ||||
| + pain / + lymphedema vs − pain / − lymphedema | 14.58 (5.25) | 0.53, 28.63 | 0.037 | ||
| Elbow extension | Unaffected | Overall: 0.021 | |||
| + pain / + lymphedema vs − pain / − lymphedema | 2.83 (0.97) | 5.43, 0.22 | 0.026 | ||
| Difference between sides | Overall: 0.043 | ||||
| None | NA | ||||
| ULNT elbow extension | Unaffected | Overall: 0.013 | |||
| + pain / + lymphedema vs + pain / − lymphedema | 9.38 (3.23) | 18.02, 0.83 | 0.026 | ||
| + pain / + lymphedema vs − pain / + lymphedema | 7.67 (2.77) | 15.09, 0.225 | 0.039 | ||
| Affected | Overall: 0.001 | ||||
| + pain / + lymphedema vs − pain / − lymphedema | 11.38 (2.90) | 3.61, 19.15 | 0.001 | ||
| + pain / + lymphedema vs − pain / + lymphedema | 10.11 (2.99) | 18.12, 2.11 | 0.006 | ||
For the entire group (n = 145), elbow extension during ULNT1 was −22.3° (SD: 11.9°) on the unaffected limb and −25.99° (SD 13.1°) on the affected limb. Sub-group analysis revealed statistically significant differences in ULNT1 elbow extension among groups for both the unaffected and affected limbs (Tables 2 and 3; Figure 1). The women with pain and lymphedema had the greatest limitation in elbow extension during ULNT1 testing, compared to the other groups, and the women with no pain or lymphedema had the least restriction. Within group differences between the unaffected and affected limbs were statistically significant in the pain groups, both with and without lymphedema. However, among groups interlimb differences did not reach statistical significance. (Table 2 and Figure 1)
Figure 1. Range of motion during ULNT.
Elbow extension range of motion in degrees during upper limb neurodynamic testing (ULNT). Full elbow extension to neutral is defined as 0°. Negative numbers represent a limitation in attaining full extension (flexed position). The affected limb and unaffected limb are presented for women with breast cancer (BC) and were subdivided into those with the following complications: 1) pain in affected limb with presence of lymphedema (+Pain/+LE), 2) pain in the affected limb without lymphedema (+Pain/−LE), 3) no pain in the affected limb with presence of edema (−Pain/+LE), and 4) no pain in the affected limb without lymphedema (−Pain/−LE). Group mean values are presented with standard deviation error bars. The following identify statistical differences; asterisks (*) indicate significant within group differences between limbs, cross (†) indicates significant between group differences for the affected limb, double cross (††) indicates significant between group differences for the unaffected limb.
Symptomatic response
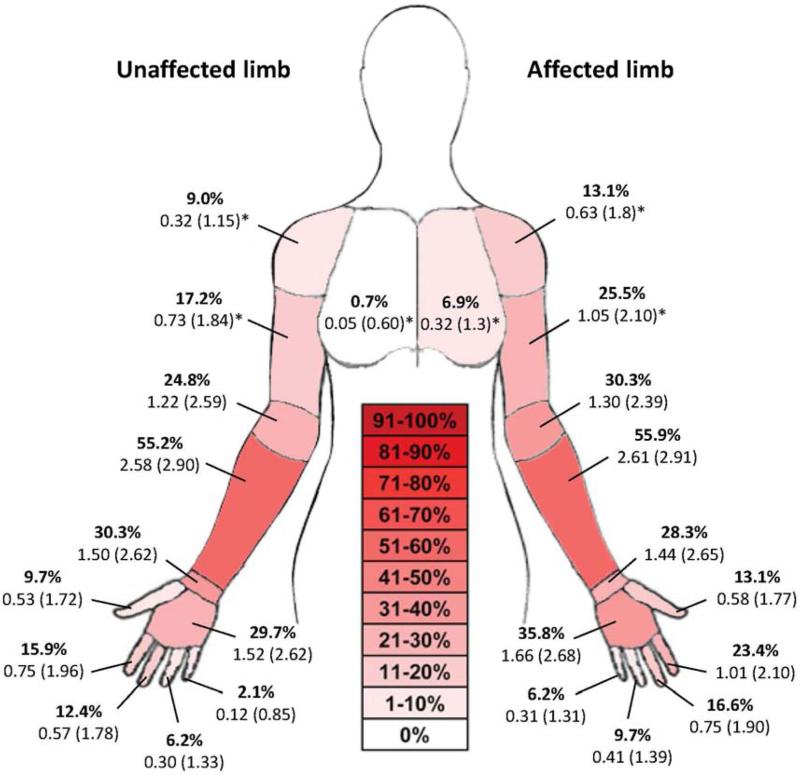
Symptoms were reported more frequently in the affected limb compared to the unaffected limb in women with BC for the chest, shoulder, arm, elbow, hand, thumb and 2nd digit (Figure 2). The forearm was the most frequent region for reports of symptoms on both the affected and unaffected sides. The intensity of symptoms was greater on the affected limb compared to the unaffected limb for the chest (p=0.046), shoulder (p=0.033) and arm (p=0.039). Figure 2 presents frequency and intensity of symptoms in the breast cancer group (n = 145).
Figure 2. Symptomatic response in women with breast cancer during ULNT1.
The percent of women reporting symptoms in the final test position for the ULNT1 are presented (bolded) for each of the following regions: chest, shoulder, arm, elbow, forearm, wrist, hand, and each of the digits. The intensity of symptoms reported on a 0 to 10 point numeric rating scale are reported in mean (SD). Significant between limb differences are notated with an asterisk (*) based upon the related samples Wilcoxon Signed Rank test.
Discussion
The primary aim of this analysis was to evaluate upper limb mechanosensitivity based on elbow extension ROM during ULNT1 in women after breast cancer treatment. The findings of this preliminary study provide support for including assessment of mechanosensitivity to the evaluation of physical impairments following breast cancer treatment, particularly for those women with pain and lymphedema. Meaningful differences were found for ULNT1 on the affected side between women with pain and lymphedema and the women without pain (with or without lymphedema). Interestingly, reductions in elbow extension during ULNT1 were also found on the unaffected side in women with pain and lymphedema.
Signs of increased neural mechanosensitivity during upper limb neurodynamic tests include subjective sensory responses, resistance to movement, and limitations or asymmetries in range of motion.45 In this study, within group (interlimb) ULNT1 elbow extension differences were minimal for the entire sample (n=145). While the interlimb differences were small, they were larger and statistically significant in both groups of women with pain, such that women with pain and lymphedema had ~6° interlimb difference and women with pain but no lymphedema had ~8° interlimb difference in elbow extension during ULNT1, compared to a 1 to 3° difference in the no-pain groups. In addition, there were more frequent reports of symptoms provoked in multiple areas on the affected limb during ULNT1 with higher intensity levels in the chest, shoulder and arm. When elbow extension was tested with increased preloading of the nervous system (during ULNT1), elbow extension ROM was clearly reduced. In comparison, none of the subgroups had a significant interlimb difference in isolated elbow extension range of motion tested prior to ULNT1 assessment. This reduction in elbow extension during ULNT1 may, in part, be related to an increase in local muscle activation as a protective muscle guarding response to the loading of the nervous system during ULNT1 as has been demonstrated in previous studies. 46,47
Reductions were also seen for isolated shoulder abduction range of motion, with the largest reductions again in the women with both pain and lymphedema. It is important to note that isolated upper extremity range of motion was assessed prior to ULNT1 procedures and did not provoke subjective sensory responses in the upper extremity. Since shoulder abduction is also a component of the ULNT1 procedures, this could have influenced the outcome. However, even when tested in positions of less shoulder abduction, a position that would reduce the stress on the nervous system, the women with pain still had the most restricted ULNT1 elbow extension. These findings lend support to the conclusion that the limitations in elbow extension during ULNT1 may, at least in part, be due to increased mechanosensitivity while the restricted isolated shoulder abduction may be due to local tissue changes but not exclusively neural involvement.
Of equal importance, our study highlights that both upper extremities show reductions in isolated upper extremity range of motion and ULNT1 elbow extension for the women with pain and lymphedema, compared to the other groups. This suggests that interlimb differences in this population may not be a reliable comparison to identify the magnitude of increased mechanosensitivity. If comparison to an individual's pre-cancer treatment ROM is not possible, then comparisons to bilateral UE normative data may provide a more useful comparison, in addition to symptomatic responses during ULNT1. A previous study involving a group of asymptomatic individuals found 11.7° (SD 13.0°) restrictions of elbow extension during ULNT1 when tested to submaximal pain as was done in the present study.48 In comparison, the women with pain and lymphedema in the present study have on average almost three times the restriction on their affected limb and over twice the restriction on their unaffected side.
The magnitude of bilateral reductions in range of motion was an unexpected finding and warrants further consideration as to the mechanism behind such impairments. Our findings are consistent with the findings of Kelley and Jull (1998),49 who evaluated mechanosensitivity of UE neural tissue in 20 women with unilateral BC, before and 6 weeks after BC surgery. Shoulder abduction ROM during the Upper Limb Tension Test 2 was significantly reduced on the surgical side. As in our study, bilateral reductions were observed which the authors suggested might be related to additional central mechanisms.
Lymphedema and neuropathic pain are common sequelae of breast cancer treatment. They may share etiology and may occur concomitantly. Dominick et al.50 found a strong association between higher body mass index (BMI), removal of 11 or more nodes, and surgery plus radiation, and the development of lymphedema. Our findings support this previous literature. However, while more women in the lymphedema subgroups received radiation therapy, the difference did not reach statistical significance. Pain following breast cancer treatment may be a consequence of surgery, radiation, or chemotherapy. More invasive surgery and subsequent radiation therapy were found to be significantly related to the development of post-mastectomy pain.51,52 Although the surgical trauma was unilateral in the present study, the presence of pain in the affected limb seemed to be the biggest factor associated with bilateral range of motion reductions both in isolated joints and during ULNT1. Axillary lymph node dissection with chemotherapy and radiation therapy is associated with significant increased prevalence of chronic pain.53 The role of chemotherapy in the development of CIPN is well established. There were no between-groups differences in the numbers of women who received chemotherapy in this study. The average time since initial cancer diagnosis and treatment was over 4 years thus CIPN symptoms may have resolved in many of the women by the time of testing. While the present study was cross sectional and causation cannot be inferred, it is possible that the presence of pain induced a centrally mediated response to restrict motion bilaterally throughout the upper extremities as a protective mechanism. This is purely speculation at this time and further investigation is necessary to evaluate the mechanisms responsible for the documented findings.
The ULNT1 is thought to load the peripheral nerves in the limb as well as other non-neural structures, such as vasculature, fascia, muscles, and joints. The addition of movement of a distant joint (e.g. adding movement of the wrist or neck at the end of the ULNT1 test) could have assisted in identifying if the symptomatic response documented was from a neurological origin, termed structural differentiation.54 The primary aim of the study was not to classify participants as positive or negative on the ULNT1, but to describe differences in ROM and symptomatic responses with the basic test maneuver. The addition of structural differentiation would have strengthened the current study and should be considered for future research. Additional differentiation could be achieved with the use of ultrasound or magnetic resonance imaging and these objective imaging techniques should be considered for future research.
We provide support for our hypothesis that breast cancer treatment and the presence of treatment-related pain and lymphedema may be associated with reductions in elbow extension during ULNT1 testing. However, due to this study's cross sectional design we cannot infer causation. An additional limitation is that past diagnosis of lymphedema by the participant's health care provider was used to determine categories of lymphedema. The reliability and accuracy of those measurement methods and diagnostic criteria used for this previous diagnosis were unknown and were likely variable. Similarly, we were not able to control for variability in post-operative treatment of upper limb impairments among participants. Despite these limitations, the results of the present study demonstrate the need to consider testing ULNT1 in this population, particularly in terms of guiding therapeutic intervention.
To our knowledge, this is only one of two studies that have evaluated neural mechanosensitivity in women following breast cancer treatment, and the only study comparing subgroups of women with and without pain and lymphedema. Results of this study serve as a foundation for future research on neural mechanosensitivity in this population of women with breast cancer. Future studies should include a closely age-matched control group as previous work has identified an impact of age on sensory responses during ULNT155 and structural differentiation at the end of the ULNT1. Additionally, it would be important to know if the neural sensitivity were secondary to disruptions in axonal transport related to the chemotherapy, increased extra-neural pressure from the lymphedema, myofascial compression related to fibrosis, or trauma to the brachial plexus related to lymph node dissection. It would also be important to determine if early and ongoing neural gliding exercises as part of the rehabilitation program could decrease the neural mechanosensitivity.
Conclusion
Mechanosensitivity of the upper extremity peripheral nerves should be assessed in women following treatment for breast cancer. This assessment of mechanosensitivity should be bilateral and correlated clinically to symptom presentation. Women with lymphedema and pain may be more likely to present with altered neural mechanosensitivity and associated reductions in upper extremity range of motion. Therapists need to integrate findings of neural mechanosensitivity into the rehabilitation program for patients who present with pain and lymphedema following treatment for breast cancer.
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NIH 1R21 NR0101282U) and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131, and by a University of California Graduate Student Research Award. Dr. Smoot is partially supported by the BIRCWH K12 (K12HD052163 NICHD/NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA: a cancer journal for clinicians. 2006 Mar-Apr;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA: a cancer journal for clinicians. 2006 May-Jun;56(3):168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 3.Bani HA, Fasching PA, Lux MM, et al. Lymphedema in breast cancer survivors: assessment and information provision in a specialized breast unit. Patient Educ Couns. 2007 Jun;66(3):311–318. doi: 10.1016/j.pec.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002 Oct 15;20(20):4242–4248. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Deo SV, Ray S, Rath GK, et al. Prevalence and risk factors for development of lymphedema following breast cancer treatment. Indian journal of cancer. 2004 Jan-Mar;41(1):8–12. [PubMed] [Google Scholar]
- 6.Nardone L, Palazzoni G, D'Angelo E, et al. Impact of dose and volume on lymphedema. Rays. 2005 Apr-Jun;30(2):149–155. [PubMed] [Google Scholar]
- 7.Johansson K, Holmstrom H, Nilsson I, Ingvar C, Albertsson M, Ekdahl C. Breast cancer patients’ experiences of lymphoedema. Scandinavian journal of caring sciences. 2003 Mar;17(1):35–42. doi: 10.1046/j.1471-6712.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 8.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009 Jan 20;27(3):390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008 Nov 10;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008 Jul 20;26(21):3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 11.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA : the journal of the American Medical Association. 2009 Nov 11;302(18):1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 12.Andersen KG, Jensen MB, Kehlet H, Gartner R, Eckhoff L, Kroman N. Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer: Cyclophosphamide, epirubicin and fluorouracil compared with docetaxel + epirubicin and cyclophosphamide. Acta Oncol. 2012 Nov;51(8):1036–1044. doi: 10.3109/0284186X.2012.692884. [DOI] [PubMed] [Google Scholar]
- 13.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005 Nov;13(11):904–911. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 14.Smoot B, Wong J, Cooper B, et al. Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 2010 Jun;4(2):167–178. doi: 10.1007/s11764-010-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawes DJ, Meterissian S, Goldberg M, Mayo NE. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med. 2008 Aug;40(8):651–658. doi: 10.2340/16501977-0232. [DOI] [PubMed] [Google Scholar]
- 16.Mortimer PS. The pathophysiology of lymphedema. Cancer. 1998 Dec 15;83(12 Suppl American):2798–2802. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003 Jan;44(1):43–57. [PubMed] [Google Scholar]
- 18.Cidon EU, Perea C, Lopez-Lara F. Life after breast cancer: dealing with lymphoedema. Clinical Medicine Insights. Oncology. 2011;5:9–14. doi: 10.4137/CMO.S6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducic I, Seiboth LA, Iorio ML. Chronic postoperative breast pain: danger zones for nerve injuries. Plast Reconstr Surg. 2011 Jan;127(1):41–46. doi: 10.1097/PRS.0b013e3181f9587f. [DOI] [PubMed] [Google Scholar]
- 20.Torresan RZ, Cabello C, Conde DM, Brenelli HB. Impact of the preservation of the intercostobrachial nerve in axillary lymphadenectomy due to breast cancer. The breast journal. 2003 Sep-Oct;9(5):389–392. doi: 10.1046/j.1524-4741.2003.09505.x. [DOI] [PubMed] [Google Scholar]
- 21.Zin T, Maw M, Oo S, Pai D, Paijan R, Kyi M. How I do it: Simple and effortless approach to identify thoracodorsal nerve on axillary clearance procedure. Ecancermedicalscience. 2012;6:255. doi: 10.3332/ecancer.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agee N, Bouton ME, Vanderhoof JW. Successful repair of the long thoracic nerve after complete transection during axillary lymph node dissection. The American surgeon. 2009 Mar;75(3):266–268. [PubMed] [Google Scholar]
- 23.Vecht CJ, Van de Brand HJ, Wajer OJ. Post-axillary dissection pain in breast cancer due to a lesion of the intercostobrachial nerve. Pain. 1989 Aug;38(2):171–176. doi: 10.1016/0304-3959(89)90235-2. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. British journal of cancer. 2005 Jan 31;92(2):225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Seminars in radiation oncology. 2007 Apr;17(2):99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell R, Cotran R. Tissue Repair: Cell Regeneration and Fibrosis. In: Kumar S, Cotran R, Robbins S, editors. Robbins Basic Pathology. 7th ed. Saunders; Philadelphia: 2006. pp. 61–78. [Google Scholar]
- 27.Bajrovic A, Rades D, Fehlauer F, et al. Is there a life-long risk of brachial plexopathy after radiotherapy of supraclavicular lymph nodes in breast cancer patients? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2004 Jun;71(3):297–301. doi: 10.1016/j.radonc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Johansson S, Svensson H, Larsson LG, Denekamp J. Brachial plexopathy after postoperative radiotherapy of breast cancer patients--a long-term follow-up. Acta Oncol. 2000;39(3):373–382. doi: 10.1080/028418600750013140. [DOI] [PubMed] [Google Scholar]
- 29.Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp K. The Modifed Total Neuropathy Score: A Clinically Feasible and Valid Measure of Taxane-Induced Peripheral Neuropathy in Women With Breast Cancer. The Journal of Supportive Oncology. 2006;4(8):9–16. [Google Scholar]
- 30.Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Current medicinal chemistry. 2008;15(29):3081–3094. doi: 10.2174/092986708786848569. [DOI] [PubMed] [Google Scholar]
- 31.Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Critical reviews in oncology/hematology. 2008 Jun;66(3):218–228. doi: 10.1016/j.critrevonc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004 Sep;12(9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 33.Ellis R, Hing W, Dilley A, McNair P. Reliability of measuring sciatic and tibial nerve movement with diagnostic ultrasound during a neural mobilisation technique. Ultrasound in medicine & biology. 2008 Aug;34(8):1209–1216. doi: 10.1016/j.ultrasmedbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Physical therapy. 2001 Jun;81(6):1192–1205. [PubMed] [Google Scholar]
- 35.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003 Nov-Dec;52(6):370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003 Feb;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 37.Norkin CC, White DJ. Measurement of Joint Motion, A Guide to Goniometry. 3rd ed. FA Davis; Philadelphia: 2003. [Google Scholar]
- 38.Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Physical therapy. 2002 Dec;82(12):1201–1212. [PubMed] [Google Scholar]
- 39.Butler DS. The sensitive nervous system. Noigroup Publications; Adelaide, Australia: 2000. [Google Scholar]
- 40.Schmid AB, Brunner F, Luomajoki H, et al. Reliability of clinical tests to evaluate nerve function and mechanosensitivity of the upper limb peripheral nervous system. BMC musculoskeletal disorders. 2009;10:11. doi: 10.1186/1471-2474-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nee RJ, Yang CH, Liang CC, Tseng GF, Coppieters MW. Impact of order of movement on nerve strain and longitudinal excursion: a biomechanical study with implications for neurodynamic test sequencing. Manual therapy. 2010 Aug;15(4):376–381. doi: 10.1016/j.math.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Byl C, Puttlitz C, Byl N, Lotz J, Topp K. Strain in the median and ulnar nerves during upper-extremity positioning. J Hand Surg Am. 2002;27A(6):1032–1040. doi: 10.1053/jhsu.2002.35886. [DOI] [PubMed] [Google Scholar]
- 43.Kleinrensink G, Stoeckart R, Vleeming A, Snijders C, Mulder P. Mechanical tension in the median nerve. The effects of joint position. Clin Biomech. 1995;10(5):240–244. doi: 10.1016/0268-0033(95)99801-8. [DOI] [PubMed] [Google Scholar]
- 44.Coppieters M, Stappaerts K, Janssens K, Jull G. Reliability of detecting ‘onset of pain’ and ‘submaximal pain’ during neural provocation testing of the upper quadrant. Physiother Res Int. 2002;7(3):146–156. doi: 10.1002/pri.251. [DOI] [PubMed] [Google Scholar]
- 45.Butler D. The Sensitive Nervous System. Noigroup Publications; Adelaide: 2000. [Google Scholar]
- 46.Legakis A, Boyd BS. The influence of scapular depression on upper limb neurodynamic test responses. The Journal of manual & manipulative therapy. 2012 May;20(2):75–82. doi: 10.1179/2042618611Y.0000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Heide B, Allison GT, Zusman M. Pain and muscular responses to a neural tissue provocation test in the upper limb. Manual therapy. 2001 Aug;6(3):154–162. doi: 10.1054/math.2001.0406. [DOI] [PubMed] [Google Scholar]
- 48.Coppieters MW, Van de Velde M, Stappaerts KH. Positioning in anesthesiology: toward a better understanding of stretch-induced perioperative neuropathies. Anesthesiology. 2002 Jul;97(1):75–81. doi: 10.1097/00000542-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Kelley S, Jull G. Breast surgery and neural tissue mechanosensitivity. The Australian journal of physiotherapy. 1998;44(1):31–37. doi: 10.1016/s0004-9514(14)60361-5. [DOI] [PubMed] [Google Scholar]
- 50.Dominick SA, Madlensky L, Natarajan L, Pierce JP. Risk factors associated with breast cancer-related lymphedema in the WHEL Study. J Cancer Surviv. 2012 Dec 5; doi: 10.1007/s11764-012-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. International journal of fertility and women's medicine. 2006 Mar-Apr;51(2):75–82. [PubMed] [Google Scholar]
- 52.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006 Sep;7(9):626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain. 2008 Sep;9(9):813–822. doi: 10.1016/j.jpain.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Nee RJ, Jull GA, Vicenzino B, Coppieters MW. The validity of upper-limb neurodynamic tests for detecting peripheral neuropathic pain. The Journal of orthopaedic and sports physical therapy. 2012 May;42(5):413–424. doi: 10.2519/jospt.2012.3988. [DOI] [PubMed] [Google Scholar]
- 55.Costantini M, Tunks K, Wyatt C, Zettel H, MacDermid JC. Age and upper limb tension testing affects current perception thresholds. J Hand Ther. 2006 Jul-Sep;19(3):307–316. doi: 10.1197/j.jht.2006.04.015. quiz 317. [DOI] [PubMed] [Google Scholar]