Abstract
Sensory axon integrity and regenerative capacity are important considerations in understanding neuropathological conditions characterized by hyper- or insensitivity. However, our knowledge of mechanisms regulating axon outgrowth are limited by an absence of suitable high-throughput assay systems. The 50B11 cell line generated from rat embryonic dorsal root ganglion neurons offers a promising model for screening assays. Prior characterization shows that these cells express cytoskeletal proteins and genes encoding ion channels and neurotrophin receptors in common with sensory nociceptor neurons. In the present study we further characterized 50B11 cells in regard to their phenotypes and responsiveness to neurotrophic and hormonal factors.
50B11 cells express neuronal cytoplasmic proteins including beta-3 tubulin, peripherin (a marker of unmyelinated neurons), and the pan-neuronal ubiquitin hydrolase, PGP9.5. Only PGP9.5 immunoreactivity was uniformly distributed throughout soma and axons, and therefore presents the best means for visualizing the entire axon arbor. All cells co-express both NGF and GDNF receptors and addition of ligands increased neurite length. 50B11 cells also showed immunoreactivity for the estrogen receptor-α and the angiotensin receptor type II, and both 17-β estradiol and angiotensin II increased outgrowth by differentiated cells.
50B11 cells therefore show features reported previously for primary unmyelinated nociceptor neurons, including responsiveness to classical neurotrophins and hormonal modulators. Coupled with their ease of culture and predictable differentiation, 50B11 cells represent a promising cell line on which to base assays that more clearly reveal mechanisms regulating axon outgrowth and integrity.
Keywords: Dorsal root ganglion, Neuron, Axon, Neurotrophic factors, Hormones, Outgrowth, High throughput screening
1. introduction
Peripheral sensory innervation is critical in transducing environmental information necessary for awareness and protection of the organism. Pathological loss of peripheral axons of sensory dorsal root ganglion (DRG) neurons underlies common forms of neuropathy affecting many patients including diabetics and cancer survivors receiving chemotherapy [20, 40]. Conversely, abnormal proliferation of DRG axons occurs in inflammatory pain syndromes [8, 35]. Thus, appropriate structural geometry of peripheral axons appears integral in ensuring optimal sensory function.
Factors regulating DRG target innervation are incompletely understood, in part because we lack robust assay systems. Primary DRG cultures provide the principal means for assessing factors regulating axon outgrowth in vitro. However, these are limited by low throughput, cellular heterogeneity, and tedious preparation protocols. Attempts to simulate sensory outgrowth in vitro have included PC12 cells and neuroblastomas [30, 31], but with limited success. Immortalized cell lines from rat, mouse and human DRGs include the F11 cell line which fused mouse hybridoma with rat embryonic DRG neurons [27, 28], ND lines generated by fusing neonatal mouse DRG with neuroblastoma cells [39] and HD10.6 lines derived by incorporating a tetracycline-inducible v-myc oncogene into human embryonic DRG neurons [32]. While these lines are useful for electrophysiological, cell signaling and biochemical studies [12, 13, 39], none display axonal morphologies similar to primary cultures, thus limiting their use in studying axonogenesis.
Recently, Höke and colleagues created the 50B11 cell line by electroporating E14.5 rat primary DRG neurons to incorporate the SV40 large T-antigen and human telomerase reverse transcriptase. These cells remain largely undifferentiated under standard culture conditions, but in the presence of forskolin assume neuronal properties [9]. They express features in common with small diameter, nociceptor neurons including axonogenesis and gene expression for some neurotrophin receptors and voltage-gated ion channels [9]. Accordingly, 50B11 cells hold promise as a model for studying axon growth. However, DRG axonogenesis involves interactions among trophic and modulatory factors acting on multiple receptors regulating cytoskeletal proteins, and it remains unclear how closely 50B11 cells replicate outgrowth in primary neurons. We show here that differentiated 50B11 cells display some phenotypic properties and responses to growth factors that are highly similar to DRG neurons.
2. Materials and methods
2.1 Cell Culture, differentiation and treatments
50B11 cells, a gift from Dr. Ahmet Hoke, were plated in 6 or 24 well plastic tissue culture plates in Neurobasal medium (Life Technologies, Gibco) supplemented with FBS (Sigma-Aldrich), B27 (Life Technologies), glucose (Fisher) and glutamine (Sigma-Aldrich) [9]. Cells were plated at different densities including low densities optimal for visualizing individual neurite arbors. 24h after plating cells were differentiated by adding forskolin (Sigma-Aldrich, 75 μM) to the medium. Based on observations by Chen et al. [9] and our preliminary studies, neuronal phenotype was most stable between about 20–36h post-forskolin, and treatment protocols were designed to be completed within this time frame.
Seventeen hours after initiating forskolin-induced differentiation, cells were treated with nerve growth factor (NGF, 50ng/ml recombinant mNGF, Peprotech), glial cell line-derived neurotrophic factor (GDNF, 50ng/ml recombinant hGDNF, Peprotech), estrogen (17β-estradiol, 20 nM, Sigma-Aldrich) or angiotensin II (ANGII, 100 nM, Sigma-Aldrich). Cells were maintained for 20h and fixed with 4% paraformaldehyde.
2.2 Immunostaining
Fixed cells were washed and incubated in blocking solution containing 1% BSA (Sigma-Aldrich) and 5% normal donkey serum (Millipore) in phosphate buffered saline (Sigma-Aldrich) containing 0.3% Triton X-100 (Sigma-Aldrich) for 1h at room temperature, and immunostained for PGP9.5 (1:700, rabbit antiserum, Serotec), βIII-tubulin (1:400, mouse antiserum, Millipore), peripherin (1:200, chicken antiserum Millipore), TrkA (1:200, rabbit antisera, Millipore), GFRα1 (1:200, goat antiserum, R&D Systems), GFRα2 (1:200, goat antiserum, R&D Systems), estrogen receptor alpha (ERα) (1:200, rabbit antiserum, Santa Cruz Biotech), and ANGII receptor type 2 (AT2, 1:200, rabbit antiserum, Alamone Labs). Donkey IgG (1:200 to 1:400, Jackson Immunoresearch) tagged with Cy3 or Alexa 488 was directed against host primary antibodies. All antibodies were diluted in PBS containing 0.3% Triton X-100 and 5% normal donkey serum. Antibody specificities were confirmed by preabsorption and omission controls in our lab and others [2, 3, 7, 8, 35].
2.3 Quantitation of neurite outgrowth
For each treatment group in each experiment, 75–100 individual neurons were imaged. Neurons were counted from randomly collected images (about 15–20 per well). Single neurons with minimal or no overlapping of neurite arbors with adjacent cells were analyzed using NIH ImageJ software with the NeuronJ Plugin. Distances from soma perimeter to neurite tips were measured by tracing arbors, and expressed as the summed length of outgrowth and as length of the longest axon. All data are presented as mean +/- the standard error of the mean, andreatment effects were compared by Mann-Whitney rank sum tests.
3. Results
3.1 Characterization of axonal markers in differentiated 50B11 cells
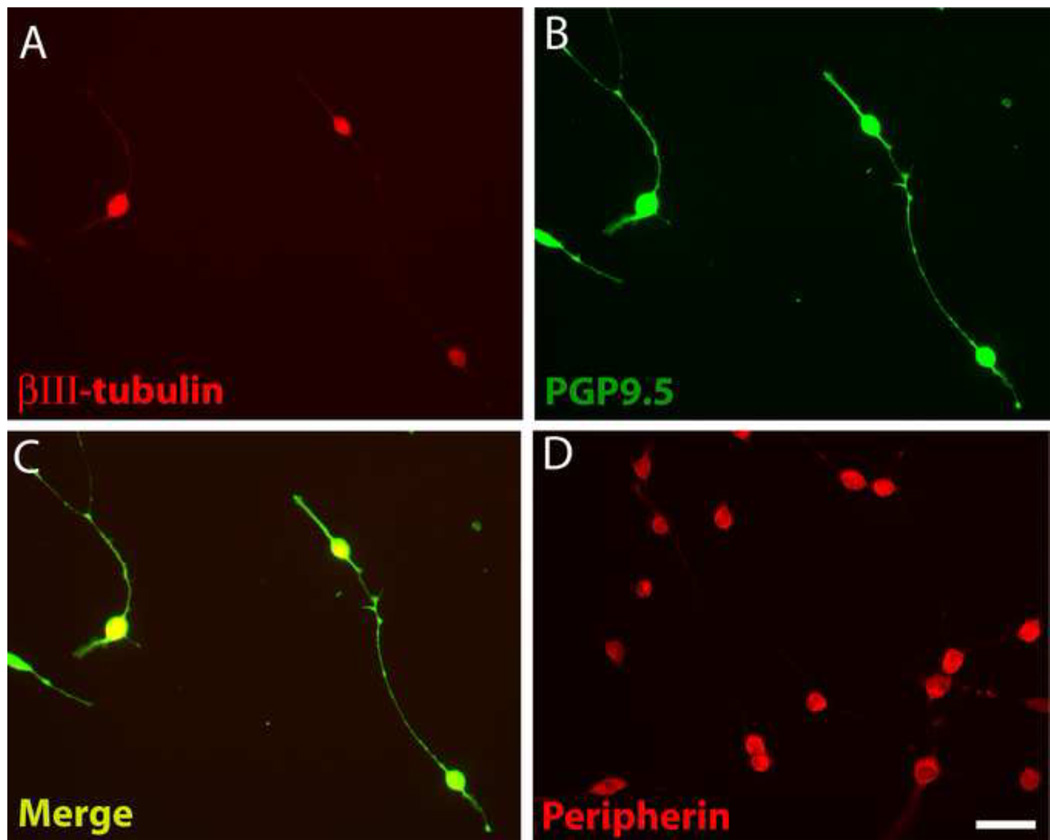
Differentiated 50B11 cells acquire many phenotypic features characteristic of DRG neurons including expression of the cytoskeletal proteins βIII-tubulin and hypophosphorylated neurofilament H [9]. We confirmed that 50B11 cells express βIII-tubulin, which was most intense in the cell body and fainter in the neurites (Fig. 1A). PGP9.5, a ubiquitin hydrolase expressed in all intact axons [22], has been used extensively for staining nerve fibers in vitro and in vivo [38]. PGP9.5-immunoreactivity (ir) was intense within cell bodies but also bright throughout the axons including finer processes (Fig.1B). PGP9.5 showed consistent colocalization with βIII-tubulin within all differentiated cells (Fig. 1C), and lower expression in undifferentiated cells.
Figure 1.
Differentiated 50B11 cells show cytoplasmic proteins that may be useful in analyzing neuronite outgrowth. A. Staining for βIII-tubulin is prominent in the cell body but diminished in distal processes. B. PGP9.5 immunostaining delineates soma and axons in good detail. C. An overlay of A and B shows colocalization of these neuronal markers. D. Peripherin immunohistochemistry shows strong expression in the soma but little in processes. Bar in E=50μm for all panels.
Peripherin is a type III intermediate filament protein that selectively marks unmyelinated axons in vivo and in vitro [15, 16]. Expression in differentiated 50B11 cells was largely restricted to the soma (Fig. 1D) and weak in undifferentiated cells. When stained for PGP9.5, cells were found to typically have 2 or 3 neurites (Fig. 1B) but occasionally extended more as described previously [9].
3.2 Receptor expression in 50B11 cells
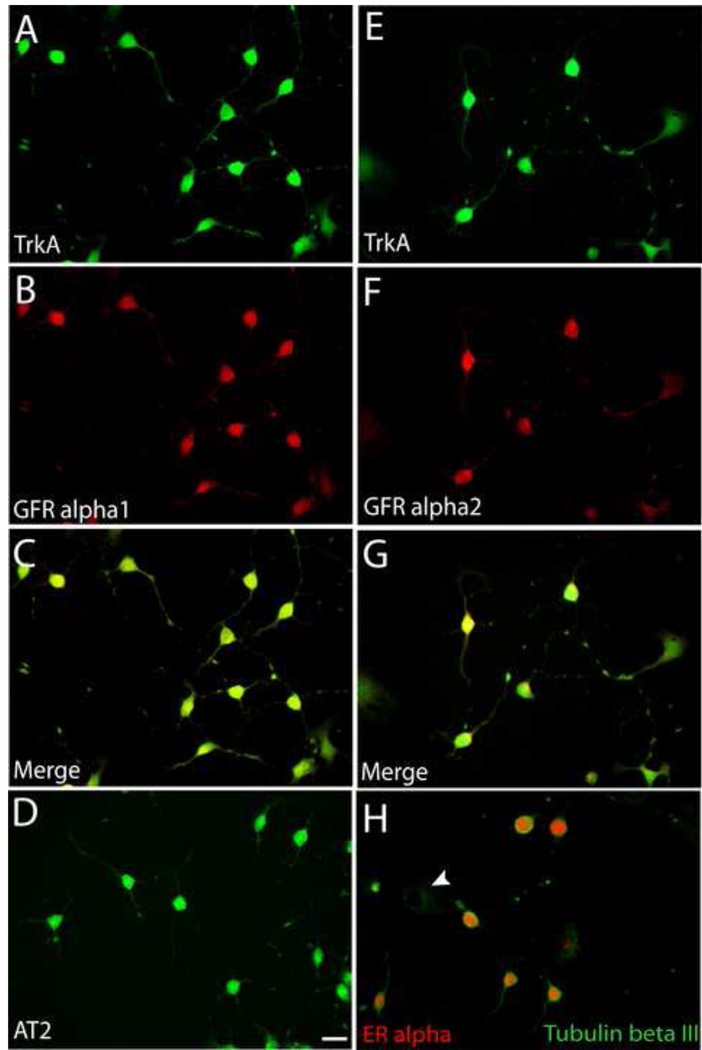
Differentiated 50B11 cell cultures show mRNA for trkA, p75, c-RET, and GFR alpha1 [9]. We performed immunocytochemistry to localize trkA, GFRα1 and GFRα2 protein in these cells. Differentiated 50B11 cells express these receptor subtypes (Fig. 2A, B, E, F). However, co-immunostaining showed that all differentiated cells uniformly express both NGF and GDNF-family receptors (Fig. 2C & G) and cannot be subdivided based on unique receptor expression.
Figure 2.
Expression of growth factor receptors by 50B11 cells. Cells were differentiated for 20h and stained for receptor proteins. A. All cells showing neuron-like morphologies showed immunostaining for the NGF receptor, trkA. B. Cells also showed strong immunoreactivity for the GDNF receptor, GFRα1. C. A merged image shows that all differentiated neuron-like cells express both trkA and GFR alpha1. D. Differentiated cells show immunoreactivity for the ANGII receptor, AT2. E. TrkA staining of differentiated cells. F. Immunostaining of the same field as E shows GFRα2. G. Merged image of E and F shows colocalization of trkA and GFRα2. H. Differentiated tubulin βIII positive cells display predominantly nuclear immunoreactivity for estrogen receptor α whereas undifferentiated cells display little or no ERα. Bar in D =50μm for all panels.
Hormones also affect axon outgrowth in primary DRG neuron cultures. ANGII for example promotes DRG axon growth via AT2 receptor signaling [7]. Estrogen also induces axonogenesis via ERα by increasing AT2 signaling [7]. 24h after forskolin-induced differentiation, 50B11 cells showed AT2-ir throughout the soma extending into neurites (Fig. 2D); undifferentiated cells weakly expressed AT2. Differentiated 50B11 cells also showed prominent nuclear ERα-ir, which was low or absent in undifferentiated cells (Fig. 2H).
3.3 NGF and GDNF promote neurite outgrowth in 50B11 cells
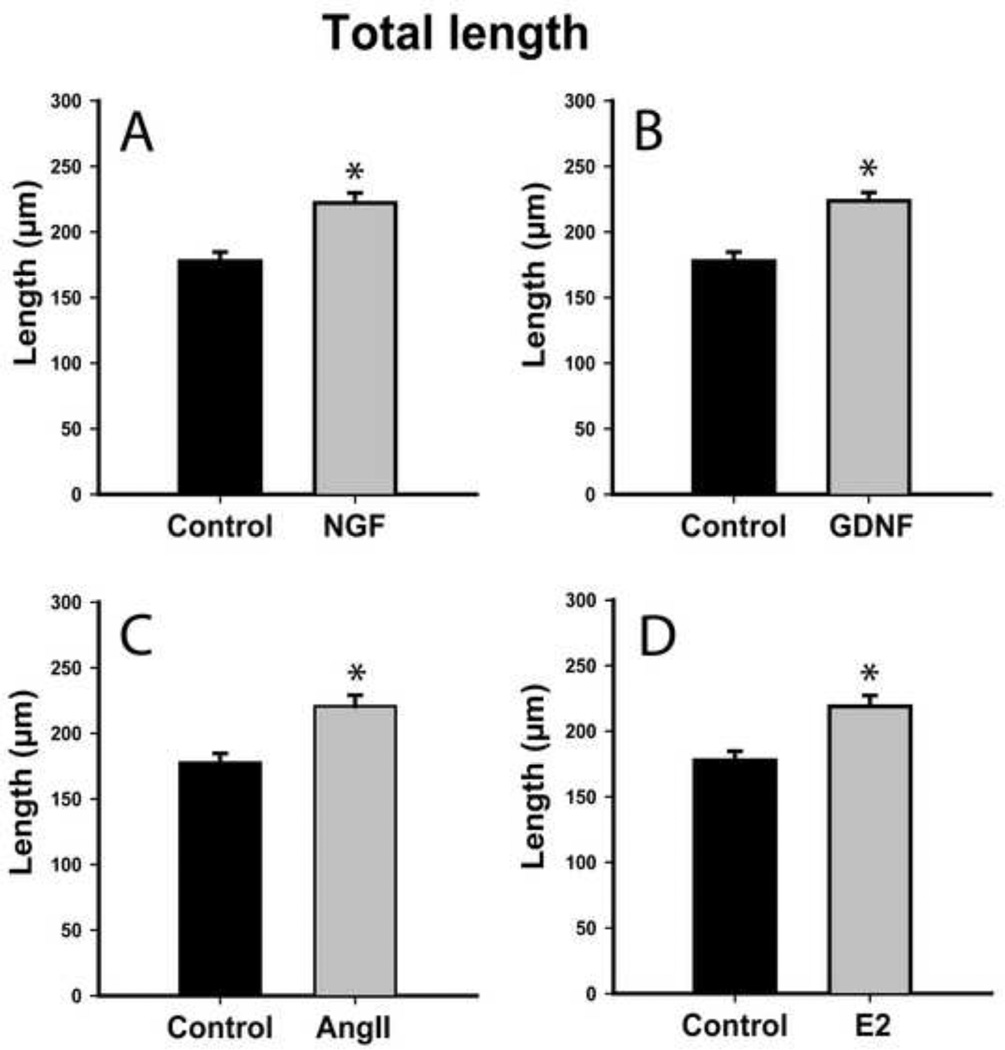
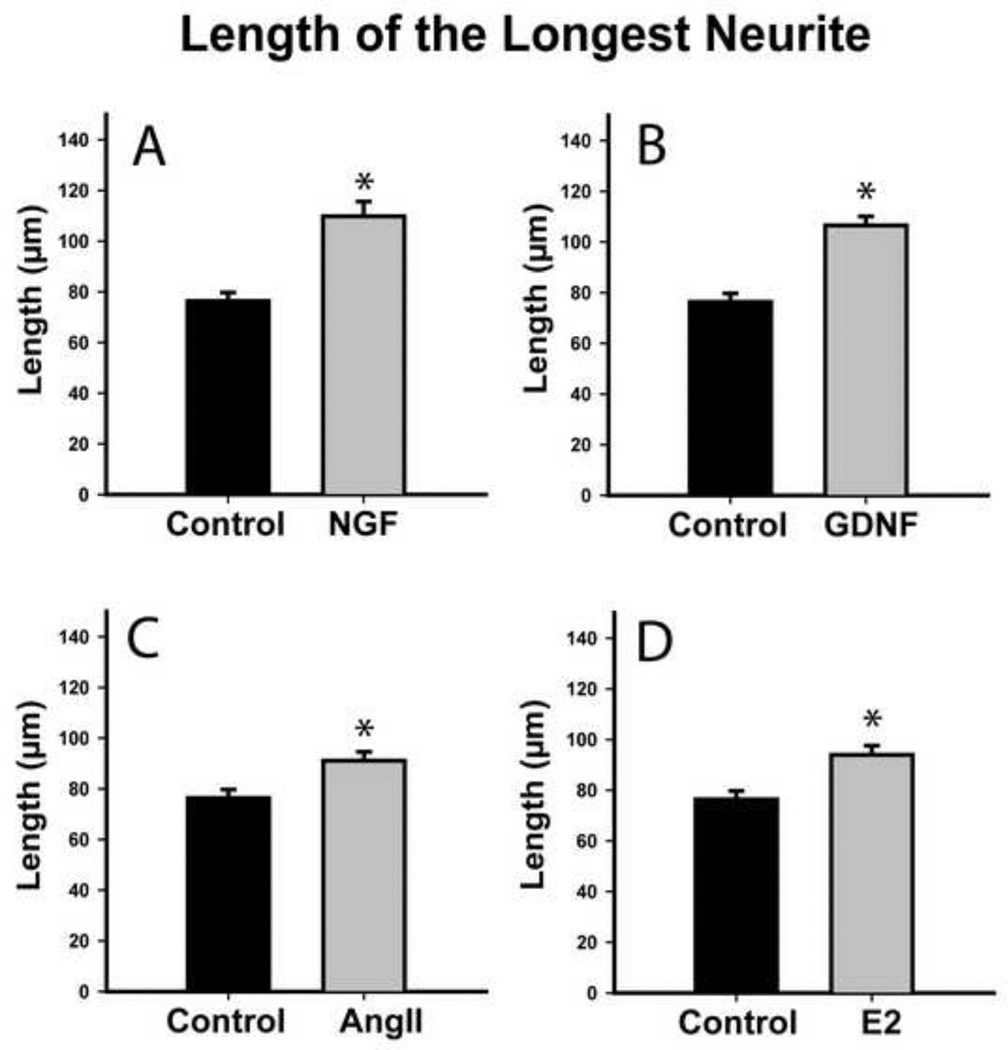
NGF and GDNF regulate neurotrophin receptor gene expression in 50B11 cells [9]. We assessed whether they also induce neurite outgrowth. Total neurite outgrowth at 20h increased from 178±7 μm in controls to 222±8 μm after NGF treatment (Fig. 3A, p≤0.001), while longest neurite length increased from 76±3 μm controls to 110±6 μm after NGF (Fig. 4A, p≤0.001). GDNF also increased total outgrowth to 224±7 μm and longest neurite length to 107±4 μm (Figs. 3B, 4B, p≤0.001), which was statistically comparable to NGF.
Figure 3.
Both neurotrophic and hormonal factors increase total neurite length in differentiated 50B11 cells. Total length of all processes emerging from each differentiated neuron 20h after treatment were summed and compared to untreated controls. A. Treatment with 50 ng/ml Nerve Growth Factor (NGF) increased axon length. B. Treatment with 50 ng/ml Glial cell line Derived Neurotrophic Factor (GDNF) also increased total outgrowth from 50B11 cells to an extent similar to NGF. C. Angiotensin II (AngII, 100nM) increased total neurite length. D. Estrogen (E2, 20nM) also increased neurite length. Data are presented as mean +/−s.e.m. *p≤0.001 vs. Control.
Figure 4.
Neurotrophins and hormones increase length of the longest neurite in differentiated 50B11 cells. The length of the longest neurite from each cell was measured 20h after treatment. A. NGF (50 ng/ml) increased maximum neurite length. B. GDNF (50ng/ml) also increased axonal length. C. Angiotensin II (AngII, 100nM) increased maximum neurite length. Estrogen (E2, 20nM) induced significantly longer axons in treated neurons as compared to controls. Data are presented as mean +/−s.e.m. *p≤0.001 vs. Control.
3.4 Angiotensin II and estrogen increase neurite growth in 50B11 cells
At concentrations optimal for increasing outgrowth in primary DRG cultures [7], ANGII increased total arbor length from 178±7 μm to 221±8 μm (Fig. 3C, p≤0.001) and longest neurite length from 76±3 μm to 91±4 μm (Fig. 4C, p≤0.001). 50B11 cells also responded to optimal E2 concentration [7] by increasing total arbor length to 219±8 μm (Fig. 3D, p≤0.001) and longest neurite length to 94±4 μm (Fig. 4D, p≤0.001). E2 and ANGII effects were comparable. Similarly, increases in overall outgrowth were comparable to those induced by NGF and GDNF. Neurotrophins were more effective than ANGII in increasing maximum axon length (NGF vs ANGII, p=0.03; GDNF vs ANGII, p=0.002); while NGF was comparable to E2 in increasing maximum axon length, GDNF was more effective than E2 (p=0.004).
4. Discussion
The utility of the 50B11 cells in studying neurodegeneration is recognized [9, 26] and this study illustrates their potential for studying axon outgrowth. Axon growth and maintenance are dependent upon specific cytoskeletal proteins, many of which can be used as selective markers. βIII tubulin is a neuron-specific marker used extensively for quantifying axons both in vitro and in vivo [14, 18, 19]. However, in differentiated 50B11 cells βIII-tubulin-ir was intense within cell bodies and proximal neurites but less so in distal neurites; this may represent intrinsic differences between 50B11 cells and DRG neurons, or may be due to the relatively short culture period such that βIII-tubulin may not have reached optimal levels for axon visualization.
Peripherin-ir was also abundant in the cell body, providing further evidence that the 50B11 cells display a phenotype consistent with sensory nociceptors. However, fluorescence intensity was insufficient to fully elucidate axons. This may again reflect culture duration, as peripherin arrives relatively late to distal regenerating axons [17].
In contrast, differentiated 50B11 cells showed high levels of PGP9.5 throughout soma and neurites. PGP 9.5, a neuron-specific ubiquitin hydrolase, is believed to play a role in cleaving ubiquitin-protein bonds, which is an important function within growing neurite tips where protein turnover via the ubiquitin-proteasome pathway is high [36]. Indeed, PGP9.5 comprises 5–10% of cytoplasmic proteins in primary neurons [10], and high levels are apparently retained in 50B11 cells including neurites. Accordingly, the high PGP9.5-ir in 50B11 cells make it useful for visualizing neurite extensions and most optimal for morphological analysis.
In postnatal DRG, distinct populations of small-diameter neurons are defined by their neurotrophin dependencies; some require NGF for survival while others require members of the GDNF family of ligands [29]. Chen et al. demonstrated mRNA encoding NGF and GDNF receptors in differentiated 50B11 cell cultures [9]. Our immunofluorescence studies demonstrate NGF and GDNF receptor protein synthesis. However, both receptors co-localized uniformly in individual 50B11 cells. This may not be altogether surprising as 50B11 cells are derived from d14.5 embryonic DRG neurons. At this stage, DRG neural crest cells have undergone preliminary differentiation [24], and all small neurons are trkA positive. Under the subsequent influence of Runx1, some cells lose trkA and acquire Ret expression, with these 2 populations becoming ‘peptidergic’ (i.e., calcitonin gene-related peptide-ir) and ‘non-peptidergic’ (isolectin IB4-positive) populations, respectively [24] (although mature rat DRG neurons continue to coexpress receptor RNAs [21]). It seems likely that the normal developmental program is altered during immortalization, resulting in cells expressing both receptor types. These findings suggest that differentiated 50B11 cells can potentially respond to both families of neurotrophic proteins.
Our studies show that, in differentiated 50B11 cells, both trkA and GFRα pathways do induce axon outgrowth. Both NGF and GDNF comparably increased total arbor length and the length of the longest axon. Hence, unlike mature subpopulations of DRG neurons, 50B11 cells exhibit an ability to respond to both classes of neurotrophins.
Factors in addition to classical neurotrophins influence axonal growth. There is accumulating evidence that DRG nociceptor neurons are strongly affected by local or systemic hormonal factors. For example, sustained plasma estrogen elevation increases sensory nociceptor innervation of skin, mesentery, and mammary gland in rats [4, 6]. Estrogen acts via ERα to increase nociceptor levels of AT2 mRNA and protein. AT2 mediates the neuritogenic effects of the hormone ANGII, which is derived from autocrine and paracrine sources [7]. Our findings show that differentiated 50B11 cells, like DRG nociceptors, express both AT2 and ERα receptors, consistent with potential hormonal modulation.
The AT2 receptor and ERα appear to function comparably in both 50B11 cells and DRG neurons. Hence, peripherin-positive DRG neurons show enhanced neurite outgrowth when cultures are treated for 48–72h with either estrogen or ANGII, and the present studies show that both agents are equally effective in inducing 50B11 cell neurite outgrowth. Neither ANGII nor estrogen promoted 50B11 cell outgrowth to the extent obtained with either NGF or GDNF, although estrogen is more effective than NGF in eliciting outgrowth in primary DRG cultures [5]. Nonetheless, 50B11 cells appear to respond to hormones and neurotrophic factors in a manner that is qualitatively similar to that of native DRG nociceptors.
5. Conclusion
Alterations in peripheral sensory innervation are strongly associated with pathological conditions such as peripheral neuropathies where sensory axons are lost [23, 25, 33] and chronic inflammatory pain syndromes characterized by nociceptor axon proliferation [1, 11, 34, 37]. Therefore, signaling pathways responsible for regulating nociceptor axonal architecture are attractive targets for therapeutic intervention to either increase or decrease outgrowth as appropriate. To date, intracellular signaling proteins and their interactions remain incompletely understood, and we lack effective tools to modify outgrowth, in part because of the difficulty in conducting high-throughput screening on primary neuronal cultures. We show here that 50B11 cells constitute a robust assay system for assessing neurite outgrowth in vitro. Moreover, these cells respond to trophic factors and hormones in a manner that is largely similar to DRG neurons. These findings provide validation for the idea that differentiated 50B11 cells provide a useful tool for investigating mechanisms regulating nociceptor axon outgrowth.
Highlights.
50B11 cells share features with nociceptor neurons including neurite formation.
We show that PGP9.5 (but not other markers) accurately defines their axon arbors.
All differentiated cells co-express receptors for both NGF and GDNF.
NGF and GDNF increase neurite outgrowth, as do angiotensin II and estrogen.
50B11 cells provide a potential tool for high throughput analysis of axonogenesis.
ACKNOWLEDGEMENTS
We thank Dr. Ahmet Höke of Johns Hopkins University for his generous gift of the 50B11 cells, Drs. Anuradha Chakrabarty and Dora Krizsan-Agbas for technical guidance, and the staff of the Kansas Intellectual and Developmental Disabilities Research. Funding was provided by NIH NICHD RO1HD049615 with core support from NICHD P30HD002528.
LIST OF ABBREVIATIONS
- ANGII
angiotensin II
- AT2
angiotensin II receptor type 2
- DRG
dorsal root ganglion
- E2
estradiol or estrogen
- ER
estrogen receptor
- GDNF
glial cell line derived neurotrophic factor
- NGF
nerve growth factor
- PGP9.5
protein gene product 9.5
- -ir
immunoreactivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alfredson H. Chronic midportion Achilles tendinopathy: an update on research and treatment. Clin Sports Med. 2003;22:727–741. doi: 10.1016/s0278-5919(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 2.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 3.Barker-Gibb AL, Dougherty KD, Einheber S, Drake CT, Milner TA. Hippocampal tyrosine kinase A receptors are restricted primarily to presynaptic vesicle clusters. J Comp Neurol. 2001;430:182–199. doi: 10.1002/1096-9861(20010205)430:2<182::aid-cne1024>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Blacklock AD, Cauveren JA, Smith PG. Estrogen selectively increases sensory nociceptor innervation of arterioles in the female rat. Brain Res. 2004;1018:55–65. doi: 10.1016/j.brainres.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 5.Blacklock AD, Johnson MS, Krizsan-Agbas D, Smith PG. Estrogen increases sensory nociceptor neuritogenesis in vitro by a direct, nerve growth factor-independent mechanism. Eur J Neurosci. 2005;21:2320–2328. doi: 10.1111/j.1460-9568.2005.04075.x. [DOI] [PubMed] [Google Scholar]
- 6.Blacklock AD, Smith PG. Estrogen increases calcitonin gene-related peptideimmunoreactive sensory innervation of rat mammary gland. J Neurobiol. 2004;59:192–204. doi: 10.1002/neu.10310. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarty A, Blacklock A, Svojanovsky S, Smith PG. Estrogen elicits dorsal root ganglion axon sprouting via a renin-angiotensin system. Endocrinology. 2008;149:3452–3460. doi: 10.1210/en.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarty A, McCarson KE, Smith PG. Hypersensitivity and hyperinnervation of the rat hind paw following carrageenan-induced inflammation. Neurosci Lett. 2011;495:67–71. doi: 10.1016/j.neulet.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Mi R, Haughey N, Oz M, Hoke A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J Peripher Nerv Syst. 2007;12:121–130. doi: 10.1111/j.1529-8027.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day IN, Thompson RJ. UCHL1 (PGP 9.5): neuronal biomarker and ubiquitin system protein. Prog Neurobiol. 2010;90:327–362. doi: 10.1016/j.pneurobio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Di Sebastiano P, Fink T, Weihe E, Friess H, Beger HG, Buchler M. Changes of protein gene product 9.5 (PGP 9.5) immunoreactive nerves in inflamed appendix. Dig Dis Sci. 1995;40:366–372. doi: 10.1007/BF02065423. [DOI] [PubMed] [Google Scholar]
- 12.Fan SF, Shen KF, Scheideler MA, Crain SM. F11 neuroblastoma x DRG neuron hybrid cells express inhibitory mu- and delta-opioid receptors which increase voltagedependent K+ currents upon activation. Brain Res. 1992;590:329–333. doi: 10.1016/0006-8993(92)91116-v. [DOI] [PubMed] [Google Scholar]
- 13.Faravelli L, Arcangeli A, Olivotto M, Wanke E. A HERG-like K+ channel in rat F-11 DRG cell line: pharmacological identification and biophysical characterization. J Physiol. 1996;496(Pt 1):13–23. doi: 10.1113/jphysiol.1996.sp021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feit H, Dutton GR, Barondes SH, Shelanski ML. Microtubule protein. Identification in and transport to nerve endings. J Cell Biol. 1971;51:138–147. doi: 10.1083/jcb.51.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornaro M, Lee JM, Raimondo S, Nicolino S, Geuna S, Giacobini-Robecchi M. Neuronal intermediate filament expression in rat dorsal root ganglia sensory neurons: an in vivo and in vitro study. Neuroscience. 2008;153:1153–1163. doi: 10.1016/j.neuroscience.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein ME, House SB, Gainer H. NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J Neurosci Res. 1991;30:92–104. doi: 10.1002/jnr.490300111. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Qiang M, Luduena RF. The distribution of beta-tubulin isotypes in cultured neurons from embryonic, newborn, and adult mouse brains. Brain Res. 2011;1420:8–18. doi: 10.1016/j.brainres.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Walss-Bass C, Luduena RF. The beta isotypes of tubulin in neuronal differentiation. Cytoskeleton (Hoboken) 2010;67:431–441. doi: 10.1002/cm.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain research. Molecular brain research. 2003;110:52–62. doi: 10.1016/s0169-328x(02)00584-3. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg LM, Alm P, Wharton J, Polak JM. Protein gene product 9.5 (PGP 9.5). A new neuronal marker visualizing the whole uterine innervation and pregnancy-induced and developmental changes in the guinea pig. Histochemistry. 1988;90:9–17. doi: 10.1007/BF00495700. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 24.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 25.McLeod JG. Investigation of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1995;58:274–283. doi: 10.1136/jnnp.58.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melli G, Hoke A. Dorsal Root Ganglia Sensory Neuronal Cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin Drug Discov. 2009;4:1035–1045. doi: 10.1517/17460440903266829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platika D, Baizer L, Fishman MC. Sensory neurons "immortalized" by fusion with neuroblastoma cells. Trans Assoc Am Physicians. 1985;98:301–304. [PubMed] [Google Scholar]
- 28.Platika D, Boulos MH, Baizer L, Fishman MC. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci U S A. 1985;82:3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- 30.Radio NM, Breier JM, Shafer TJ, Mundy WR. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicol Sci. 2008;105:106–118. doi: 10.1093/toxsci/kfn114. [DOI] [PubMed] [Google Scholar]
- 31.Radio NM, Mundy WR. Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008;29:361–376. doi: 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Raymon HK, Thode S, Zhou J, Friedman GC, Pardinas JR, Barrere C, Johnson RM, Sah DW. Immortalized human dorsal root ganglion cells differentiate into neurons with nociceptive properties. J Neurosci. 1999;19:5420–5428. doi: 10.1523/JNEUROSCI.19-13-05420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tague SE, Clarke GL, Winter MK, McCarson KE, Wright DE, Smith PG. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J Neurosci. 2011;31:13728–13738. doi: 10.1523/JNEUROSCI.3637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- 37.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148:1021–1027. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 39.Wood JN, Bevan SJ, Coote PR, Dunn PM, Harmar A, Hogan P, Latchman DS, Morrison C, Rougon G, Theveniau M, et al. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990;241:187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- 40.Younger DS. Peripheral nerve disorders. Prim Care. 2004;31:67–83. doi: 10.1016/S0095-4543(03)00116-7. [DOI] [PubMed] [Google Scholar]