Significance
Experimental animals are usually raised in small, so-called standard cages, depriving them of numerous natural stimuli. We show that raising mice in an enriched environment, allowing enhanced physical, social, and cognitive stimulation, preserved a juvenile brain into adulthood. Enrichment also rejuvenated the visual cortex after extended periods of standard cage rearing and protected adult mice from stroke-induced impairments of cortical plasticity. Because the local inhibitory tone in the visual cortex of adult enriched mice was not only significantly reduced compared with nonenriched animals but at juvenile levels, the plasticity-promoting effect of enrichment is most likely mediated by preserving low juvenile levels of inhibition into adulthood and thereby, extending sensitive phases of enhanced neuronal plasticity into an older age.
Abstract
Ocular dominance (OD) plasticity in mouse primary visual cortex (V1) declines during postnatal development and is absent beyond postnatal day 110 if mice are raised in standard cages (SCs). An enriched environment (EE) promotes OD plasticity in adult rats. Here, we explored cellular mechanisms of EE in mouse V1 and the therapeutic potential of EE to prevent impairments of plasticity after a cortical stroke. Using in vivo optical imaging, we observed that monocular deprivation in adult EE mice (i) caused a very strong OD plasticity previously only observed in 4-wk-old animals, (ii) restored already lost OD plasticity in adult SC-raised mice, and (iii) preserved OD plasticity after a stroke in the primary somatosensory cortex. Using patch-clamp electrophysiology in vitro, we also show that (iv) local inhibition was significantly reduced in V1 slices of adult EE mice and (v) the GABA/AMPA ratio was like that in 4-wk-old SC-raised animals. These observations were corroborated by in vivo analyses showing that diazepam treatment significantly reduced the OD shift of EE mice after monocular deprivation. Taken together, EE extended the sensitive phase for OD plasticity into late adulthood, rejuvenated V1 after 4 mo of SC-rearing, and protected adult mice from stroke-induced impairments of cortical plasticity. The EE effect was mediated most likely by preserving low juvenile levels of inhibition into adulthood, which potentially promoted adaptive changes in cortical circuits.
Ocular dominance (OD) plasticity induced by monocular deprivation (MD) is one of the best studied models of experience-dependent plasticity in the mammalian cortex (1, 2). OD plasticity in primary visual cortex (V1) of C57BL/6J mice is maximal at 4 wk of age, declines after 2–3 mo, and is absent beyond postnatal day 110 (PD110) if animals are raised in standard cages (SCs) (3–6). In 4-wk-old mice, 4 d of MD are sufficient to induce an OD shift to the open eye; therefore, neurons in the binocular V1, which are usually dominated by the contralateral eye in rodents (3, 7), become activated more equally by both eyes (5, 8). This juvenile OD shift is predominantly mediated by a decrease in the visual cortical responses to the deprived eye (1, 9–11), whereas significant OD shifts in older animals up to PD110 need 7 d of MD and are mediated primarily by increased open-eye responses in V1. Raising animals in an enriched environment (EE) gives them the opportunity of enhanced physical, social, and cognitive stimulation and influences brain physiology and behavior in many ways (12, 13). It has been shown previously that EE enhances visual system development in rats (14) and mice (15–17), increases levels of the brain-derived neurotrophic factor and serotonin (18), reduces both extracellular GABA levels (18, 19) and the density of ECM perineuronal nets (PNNs) (19), and promotes OD plasticity in adult and aging rats (18–21). Here, we explored cellular mechanisms of EE in V1 of mice and the therapeutic potential of EE to prevent impairments of plasticity after a cortical stroke. Furthermore, we studied whether EE would prolong the sensitive phase for OD plasticity into adulthood and also restore this form of plasticity in mice that were raised in SC until PD110 (i.e., in animals that were already beyond their sensitive phase for OD plasticity). Despite pharmacological detection of in vivo GABA levels, suggesting that EE reduces intracortical inhibition, direct electrophysiological evidence is still missing. We therefore recorded GABA, AMPA, and NMDA currents in slices from EE- and SC-raised mice and also tested the efficacy of diazepam injections to abolish OD plasticity of EE mice in vivo. Finally, we studied whether raising mice in EE would protect them from lesion-induced impairments of OD plasticity. Our results show that raising mice in EE preserved OD plasticity into late adulthood rejuvenated the brain after 3 mo of SC-rearing, and protected adult mice from stroke-induced impairments of cortical plasticity. Our electrophysiological measurements and diazepam treatment indicate that the plasticity-promoting effect of EE was primarily mediated by reduced intracortical inhibition compared with SC-raised mice. These results suggest EE as a preventive intervention to enhance and preserve plasticity in adulthood and after a cortical lesion.
Results
EE Extended OD Plasticity into Adulthood.
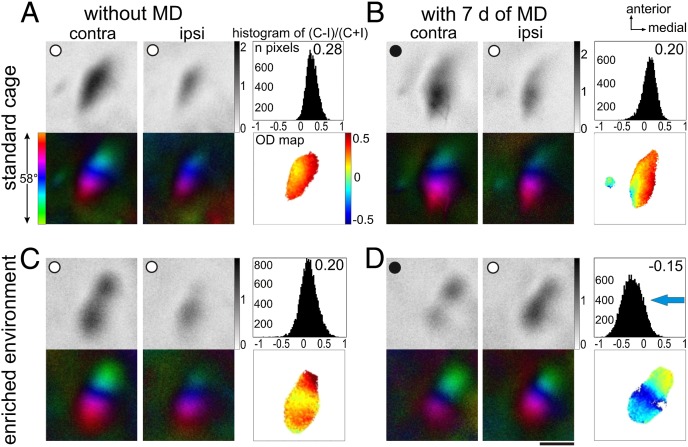
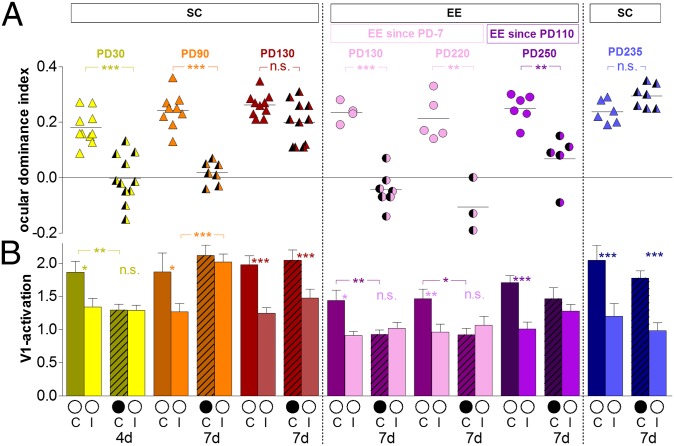
In mice raised in EE from 7 d before birth (PD−7) to PD130, 7-d MD induced a strong and highly significant OD shift to the open eye, which was as strong as usually is only seen in 4-wk-old animals (9, 10). Visually driven activity in V1 could even become dominated by input from the formerly weaker ipsilateral eye (Fig. 1D). In these cases, the activity patch induced by stimulation of the ipsilateral eye was darker than the patch of the contralateral eye, the OD index (ODI) became negative, the ODI histogram was shifted left, and cold colors prevailed in the 2D OD map, indicating ipsilateral eye dominance (Fig. 1D). Quantitative analyses of V1 activation showed that the average ODI decreased significantly from 0.23 ± 0.02 (n = 4) without MD to −0.04 ± 0.02 (n = 7) with MD [age range = PD119–PD129; P < 0.001, Bonferroni-adjusted (B) t test] (Fig. 2A). This OD shift was mediated by a significant decrease of deprived eye responses in V1 (1.44 ± 0.16, n = 4; with MD: 0.92 ± 0.07, n = 7; P < 0.01, t test) (Fig. 2B), whereas open-eye responses remained unchanged (0.91 ± 0.06, n = 4; with MD: 1.02 ± 0.09, n = 7; P > 0.05, t test). In contrast, the OD shift of adult SC-reared mice below PD110 (ODI 0.24 ± 0.02, n = 9; with MD: 0.02 ± 0.02, n = 7; P < 0.01, t test) (Fig. 2A) was mediated by an increase of open-eye responses in V1 (ipsilateral, no MD: 1.27 ± 0.12, n = 9; with MD: 2.02 ± 0.12, n = 7; P < 0.01; contralateral, no MD: 1.87 ± 0.28, n = 9; with MD: 2.11 ± 0.15, n = 7; P > 0.05, t test) (Fig. 2B). Decreases of deprived eye responses in V1 are typically only observed in juvenile mice (PD30) and after 4 d of MD (1.86 ± 0.16, n = 10; with MD: 1.29 ± 0.09, n = 11; P < 0.01, t test), whereas open-eye responses remained unchanged (1.34 ± 0.13, n = 10; with MD: 1.29 ± 0.08, n = 11; P > 0.05, t test) (Fig. 2B). As previously described by Lehmann and Löwel (6) (Fig. 1 A and B and Fig. 2A), OD plasticity was absent in SC-reared mice older than PD110: V1 responses were not significantly different without and with MD (contralateral, no MD: 1.98 ± 0.13, n = 10; with MD: 2.04 ± 0.16, n = 11; ipsilateral, no MD: 1.25 ± 0.08, n = 10; with MD: 1.47 ± 0.13, n = 11).
Fig. 1.
EE from birth preserved a strong OD plasticity into adulthood. Optically recorded activity maps after visual stimulation of the contralateral (contra) and ipsilateral (ipsi) eye in the binocular region of mouse V1 in both (A and B) standard and (C and D) enriched cage at PD130. Maps of mice without MD are shown in A and C, and maps of mice after MD (7 d) are shown in B and D. (Upper) Grayscale-coded response magnitude maps and their quantification and (Lower) color-coded polar maps of retinotopy are illustrated. (A and C) In both SC and EE mice without MD, activity patches evoked by stimulation of the contralateral eye were darker than activity patches after ipsilateral eye stimulation, the average ODI was positive, and warm colors prevailed in 2D OD maps, indicating contralateral dominance. (B) Seven days of MD did not induce a significant OD shift in adult SC mice, whereas (D) it induced a strong OD shift to the open eye in adult EE mice; V1 activity even became dominated by input from the ipsilateral eye. SC maps were modified from ref. 6. (Scale bar: 1 mm.)
Fig. 2.
EE extended the sensitive phase for OD plasticity into adulthood and restored plasticity in adult mice raised in SCs. (A) Optically imaged OD indices in control animals and after MD (4 d MD in PD30 mice, 7 d MD in all other groups) of the contralateral eye in SC and EE mice of various groups. Symbols represent ODI values of individuals; means are marked by horizontal lines. (B) V1 activation elicited by stimulation of the contralateral (C) or ipsilateral (I) eye in control animals and after MD (black circle indicates MD eye). In SC mice, OD plasticity after MD was maximal at PD30 and absent beyond PD110 (ODI values of PD30 and PD130 mice were from ref. 6). In contrast, EE raising not only increased OD shifts but created adult animals in which OD shifts were mediated primarily by a reduction of deprived-eye responses in V1. *P < 0.05; **P < 0.01; ***P < 0.001.
To test whether EE expanded the sensitive phase for OD plasticity into older ages, we additionally imaged visual cortical responses in 6- to 9-mo-old EE mice. Again, 7 d of MD induced a very strong OD plasticity: The ODI decreased significantly from 0.21 ± 0.04 (n = 5, PD204–PD261) to −0.11 ± 0.05 after MD (n = 3, PD177–PD196; P < 0.01, t test) (Fig. 2A), and the OD shift was again mediated by a significant decrease of deprived eye responses in V1 (contralateral/ipsilateral 1.47 ± 0.14/0.96 ± 0.12, n = 5; with MD: contralateral/ipsilateral 0.92 ± 0.10/1.06 ± 0.14, n = 3; P < 0.05, t test) (Fig. 2B). Because ODIs of the two EE mice groups (PD130/PD220) were not significantly different (P > 0.05, t test), we pooled values for additional analyses to the EE PD130 + P220 group (no MD: 0.22 ± 0.02, n = 9, PD130–PD261; with MD: −0.06 ± 0.02, n = 10, PD119–PD196). Surprisingly, the average OD shift of the pooled EE mice (PD130 + PD220) was again as strong as in PD30 SC mice (Fig. 2A). Actually, 8 of 10 EE mice showed even an ipsilateral dominance after MD (including the oldest mouse in this group with PD196); in one mouse, V1 was equally dominated by ipsilateral and contralateral eye responses, and in the remaining mouse, V1 was still slightly dominated by the contralateral eye.
EE Restored OD Plasticity in Old Mice.
Because OD plasticity is absent in SC-raised mice beyond PD110, we next tested whether EE would restore OD plasticity in these animals. To this end, we transferred mice from SC into EE at PD110 (late EE) and imaged V1 activities after MD as before. After 3–7 mo of EE rearing, 7-d MD in animals up to PD320 again caused a significant OD shift: The ODI decreased significantly from 0.25 ± 0.02 (n = 6, PD222–PD344) to 0.07 ± 0.04 after MD (n = 5, PD212–PD320; P < 0.01, B t test) (Fig. 2A). The OD shift of the late EE mice was, however, not clearly mediated by a change in the response strength of either eye in V1; although deprived eye responses in V1 were slightly reduced and open-eye responses were slightly increased, neither change was significant [contralateral (deprived) eye: 1.71 ± 0.11; with MD: 1.46 ± 0.17; P > 0.05, t test; ipsilateral (open) eye: 1.01, n = 6; with MD: 1.28 ± 0.10, n = 5; P > 0.05, t test] (Fig. 2B). Furthermore, there was no significant correlation between the time in EE (between 102 and 210 d) and the ODI (P = 0.396, r = −0.496, Pearson correlation).
Because adult male mice from different cages would seriously fight in the EE cages when they are put together, we had to use female mice in the late EE experiments. For the other EE groups, male as well as female mice were used, whereas SC mice were all male. To exclude that the observed enhanced plasticity after EE-rearing compared with the SC paradigm was because of a sex and not an environmental difference, we additionally analyzed a group of adult female SC mice. Adult female SC mice (>PD110) also did not show an OD shift to the open eye after MD (SI Results, Data S1: No Sex Difference), which was previously shown for male SC mice of this age range (6). The prolonged sensitive phase for OD plasticity in our EE mice is, therefore, because of the EE rearing and not a sex difference of the experimental animals.
Inhibitory Circuits Are Modified After EE.
To test whether the prolonged sensitive phase for OD plasticity in our EE mice was mediated by a reduced GABAergic inhibition in V1, which was suggested for rat visual cortex (18, 19, 21), we used two different paradigms. First, we measured intracortical inhibition directly by in vitro patch-clamp electrophysiology in V1 slices from adult (>PD130) EE and SC mice, and second, we boosted GABAergic inhibition in vivo by injecting diazepam (22, 23) to test whether diazepam would prevent OD plasticity after MD.
Juvenile level of inhibition in adult EE mice.
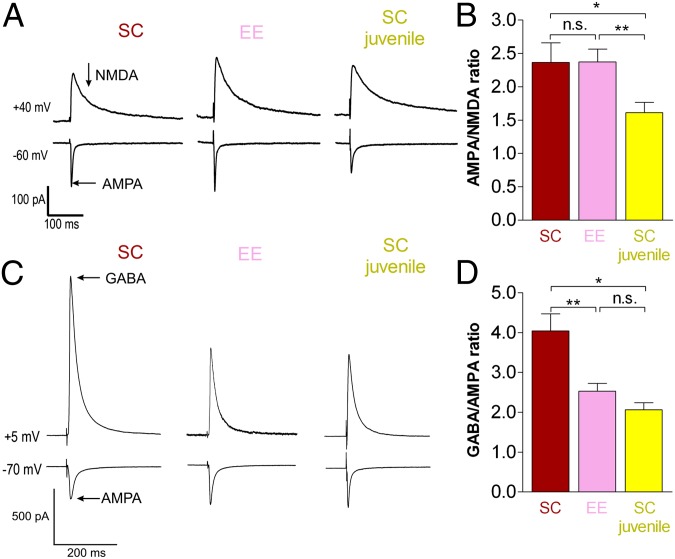
To conclusively test the involvement of inhibitory or excitatory circuits in the preservation of OD plasticity in adult EE mice, we measured the ratio of AMPA receptor (AMPAR) excitatory postsynaptic currents (EPSCs) and late NMDA receptor (NMDAR) EPSCs (AMPA/NMDA ratio) and the ratio of GABA receptor inhibitory postsynaptic currents (IPSCs) and AMPAR EPSCs (GABA/AMPA ratio). A concentric bipolar stimulating electrode was placed in layer IV, and synaptic events were recorded from layer II/III pyramidal cells. There was no significant difference between the AMPA/NMDA ratio in adult SC [2.37 ± 0.29; n = 5 mice (m), 21 cells (c)] and EE mice (2.37 ± 0.19; m/c = 9/17; P > 0.05, t test) (Fig. 3 A and B). However, the ratio of GABA IPSCs and AMPA EPSCs was significantly reduced in adult EE compared with SC mice: the GABA/AMPA ratio of adult EE mice was 2.53 ± 0.20 (m/c = 3/16) and thus, significantly lower than the 4.04 ± 0.43 of adult SC mice (m/c = 3/12; P < 0.01, t test) (Fig. 3 C and D). These data suggest that EE influenced the relative levels of intracortical inhibition. Moreover, the GABA/AMPA ratio of adult EE mice was not significantly different from values in juvenile SC mice (PD20–PD30; 2.07 ± 0.17; m/c = 3/18; P > 0.05, t test) (Fig. 3 C and D), indicating a juvenile level of inhibition in the adult EE mice. Finally, in SC mice, we measured a significant developmental increase in both the AMPA/NMDA (PD20–PD30: 1.61 ± 0.15; m/c = 4/13; P < 0.05, t test) (Fig. 3 A and B) and GABA/AMPA ratios (PD20–PD30: 2.07 ± 0.17; m/c = 3/18; P < 0.05, t test) (Fig. 3 C and D). Because the adult AMPA/NMDA ratio was similar in EE and SC mice, our data suggest that EE had no effect on the developmental changes in excitatory neurotransmission. In contrast, GABA/AMPA ratios increased significantly from juvenile to adult SC mice, consistent with an increase in the GABAergic tone (24), whereas this increase did not happen in EE mice.
Fig. 3.
EE reduced GABA/AMPA but not AMPA/NMDA ratio in V1 of adult EE mice to a juvenile level. (A) Representative traces of averaged 30 EPSCs of AMPAR component recorded at −60 mV and late component of NMDARs recorded at +40 mV (arrow marks time point; AMPAR EPSC was back to baseline, and the current is mediated solely by NMDARs). (B) The AMPA/NMDA ratio was not different between adult SC and adult EE mice, and it was reduced in juvenile SC mice. (C) Representative traces of averaged 30 IPSCs of GABA receptor (GABAR) component recorded at +5 mV and 30 EPSCs of AMPAR component recorded at −70 mV (arrows mark GABA and AMPA EPSCs peaks). (D) The GABA/AMPA ratio was significantly reduced in adult EE mice and indistinguishable from juvenile SC mice. *P < 0.05; **P < 0.01.
Diazepam reduced OD shift in EE mice.
For the in vivo measurements, we first determined a dosage of diazepam that reliably prevented an OD shift in SC mice, and then we used the same dosage in a separate group of EE mice. In SC mice, 1 mg diazepam per kg mouse, injected i.p. daily during the MD period, prevented an OD shift: ODI values after 7 d of MD (0.25 ± 0.02, n = 5, PD81–PD91) were not significantly different from values of age-matched PD90 mice without MD (6) (P > 0.05, B t test) and significantly higher than in untreated PD90 mice with MD (P < 0.001, B t test) (Fig. S1). In contrast, in EE mice, diazepam reduced but did not completely abolish the OD shift after 7-d MD. In addition, the effect of diazepam was quite variable in individual animals: ODI values ranged from −0.05 to corresponding to a very strong OD shift up to 0.26 to corresponding to no OD plasticity (mean ODI: 0.11 ± 0.03, n = 9, PD162–PD190) (Fig. S1). Nevertheless, compared with EE mice without MD (ODI: 0.22 ± 0.02, n = 9, PD130–PD261), there was still a significant OD shift in the diazepam-treated mice (P < 0.05, B t test) (Fig. S1). Thus, diazepam administration partly prevented the OD shift in adult EE mice. The diazepam dosage that we have used was adjusted such that it allowed normal activity and exploring behavior of the treated mice (SI Materials and Methods).
Number of parvalbumin-positive interneurons and PNNs was similar in EE and SC mice.
To examine whether a change in the number of parvalbumin-positive (PV+) inhibitory neurons or PNNs could contribute to the prolonged sensitive phase for OD plasticity in EE mice, we used triple immunofluorescence staining for PV, PNNs, and DAPI to visualize all cell nuclei and cortical layers. The number of PV+ cells in V1 of EE mice was not significantly different from values of SC mice (6,396 ± 278 vs. 5,683 ± 563 cells/mm3, n = 4; P > 0.05, t test) (Fig. S2A). Similarly, the number of PNN+ cells was also not significantly different between EE and SC mice (6,408 ± 131 vs. 6,167 ± 727 PNNs/mm3, n = 4 mice; P > 0.05, t test) (Fig. S2B).
EE Protected from Stroke Induced Impairments of Cortical Plasticity.
As we have previously shown, a photothrombotically (PT) induced small stroke lesion in primary somatosensory cortex (S1) prevented OD plasticity in V1 of adult SC-raised mice (25). To test whether EE can protect mice from these lesion-induced impairments of visual plasticity, we raised another group of mice in EE and then exposed them to the same stroke lesion in S1 as before. PT lesions were located in the left S1 and measured, on average, 0.6 ± 0.10 mm in the mediolateral and 0.6 ± 0.08 mm in the anterioposterior directions. The lesion center was situated 1.0 ± 0.12 mm anterior to the anterior border of V1, 2.0 ± 0.21 mm lateral to the midline, and 1.2 ± 0.12 mm posterior to the Bregma. Lesion size (diameter, depth, and volume) and location (distance from V1 and distance from midline) correlated with neither the ODI nor the spatial frequency threshold of the optomotor reflex after MD (for all: P < 0.05, Pearson correlation).
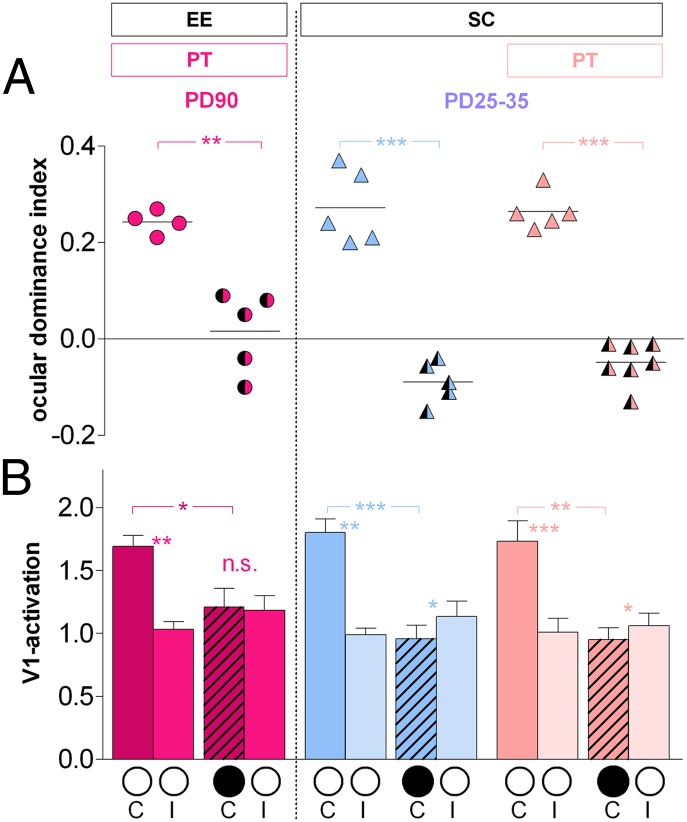
Seven days of MD in adult PT mice raised in EE induced a significant OD shift to the open eye, whereas there was no OD shift in lesioned SC mice of the same age (25). In enriched mice with a stroke lesion, the ODI decreased significantly from 0.24 ± 0.01 (n = 4) to 0.02 ± 0.04 after 7 d of MD (n = 5; P < 0.01, B t test) (Fig. 4A). Furthermore, the OD shift in EE mice with PT was mediated by a significant reduction of deprived eye responses in V1 after MD (from 1.69 ± 0.09, n = 4 to 1.21 ± 0.15, n = 5 after MD; P < 0.05, t test) (Fig. 4B), whereas open-eye responses did not change (1.04 ± 0.06, n = 4; with MD: 1.19 ± 0.12, n = 5; P > 0.05, t test). After MD in EE mice, both eyes activated V1 more equally strong, and V1 activation was no longer significantly different (P > 0.05, t test). Thus, EE housing reliably prevented the loss of OD plasticity after a PT lesion in S1.
Fig. 4.
EE and being young protected from stroke-induced impairments of cortical plasticity. Data are displayed as in Fig. 2. (A) Optically imaged OD indices and (B) V1 activation after stimulation of the contra- and ipsilateral eye in adult EE and juvenile SC mice without (control) and with a PT lesion in S1. PD90 mice were deprived for 7 d, and PD25–P35 mice were deprived for 4 d. *P < 0.05; **P < 0.01; ***P < 0.001.
Being Young Protected from Stroke-Induced Impairments of Cortical Plasticity.
If the major effect of EE is to preserve a younger brain into adulthood and if a younger brain is less susceptible to stroke-induced impairments of cortical plasticity, then OD plasticity after a stroke lesion in S1 should also be preserved in juvenile mice. Therefore, we next tested the effect of a stroke lesion in S1 on OD plasticity in 4-wk-old mice. Indeed, juvenile mice continued to display a clear OD plasticity even after a PT lesion in S1. The PT lesions were again located in S1 of the left hemisphere and 0.6 ± 0.19 mm anterior to the anterior border of V1, and they measured 0.6 ± 0.11 mm in the mediolateral and 0.8 ± 0.09 mm in the anterioposterior directions. Lesions centers were located, on average, 1.7 ± 0.25 mm lateral to the midline and 1.6 ± 0.19 mm posterior to the Bregma. In 4-wk-old mice, 4-d MD induced a significant OD shift, even in the presence of a PT lesion in S1: The ODIs decreased from 0.26 ± 0.02 (n = 5) to −0.05 ± 0.02 after MD (n = 7; P < 0.001, B t test) (Fig. 4A). One mouse even had the PT lesion slightly extending into V1 and still displayed an ODI of −0.01 after MD, indicating a clear OD shift to the open eye. The ODIs of the juvenile PT mice were indistinguishable from sham-treated control animals after MD (P > 0.05, B t test), in which the ODIs decreased significantly from 0.27 ± 0.03 (n = 5) to −0.09 ± 0.02 after MD (n = 5; P < 0.001, B t test). In both control and PT mice, the OD shift was mediated by a significant reduction of deprived eye responses in V1 (control, no MD: 1.80 ± 0.11, n = 5; with MD: 0.96 ± 0.11, n = 5; P < 0.001, t test; PT, no MD: 1.73 ± 0.16, n = 5; with MD: 0.95 ± 0.09, n = 7; P < 0.01, t test), whereas open-eye responses remained unchanged (control, no MD: 0.99 ± 0.05, n = 5; with MD: 1.13 ± 0.12, n = 5; PT, no MD: 1.01 ± 0.11, n = 5; with MD: 1.06 ± 0.10, n = 7; for both, P > 0.05, t test) (Fig. 4B).
Basic Visual Abilities, Enhanced Optomotor Reflex After MD, and Cortical Maps Were Similar in EE- and SC-Raised Mice.
We also determined the highest spatial frequency (visual acuity) and lowest contrast (contrast sensitivity) gratings that elicited an optomotor response in animals of all experimental groups using the virtual reality optomotor setup (26). Neither EE nor late EE had an effect on any of the measured parameters (SI Results, Data S2: Basic Visual Abilities of EE Mice Were Indistinguishable from SC-Raised Mice and Fig. S3). After MD, both visual acuity and contrast sensitivity values of the open eye increased significantly in all groups, and values were indistinguishable from each other. Diazepam-treatment had also no measurable effect on the analyzed parameters (SI Results, Data S3: Experience-Dependent Enhancements of Vision After MD Were Similar in Adult EE and SC Mice). Using intrinsic signal optical imaging, we also analyzed the amplitude and layout of V1 maps of both EE- and SC-raised mice. Although the quality of retinotopic maps was indistinguishable between the groups, V1 activation after visual stimulation was lower in adult EE compared with SC mice (SI Results, Data S4: Lower Magnitude of Visual Responses in V1 of EE Mice).
EE Partially Preserved Enhancement of Vision After MD in PT Mice.
In PT-lesioned adult EE mice—unlike SC-raised mice—visual acuity values of the open eye increased significantly from 0.38 ± 0.002 cycles per degree (c/d) on day 0 to 0.42 ± 0.006 c/d after 7-d MD (n = 8; P < 0.05, B t test). These results correspond to an increase of 10 ± 2% on baseline. Although this increase was lower than in nonlesioned EE mice (20 ± 1%; PD220; F1,12 = 14.302; P < 0.05, ANOVA), it was nevertheless present, and visual acuity values were different from lesioned EE mice without MD (day 0: 0.38 ± 0.002 c/d; day 7: 0.38 ± 0.002 c/d, n = 8; F1,14 = 47.58; P < 0.001, ANOVA) (Fig. S3). Likewise, contrast sensitivity values of the open eye increased after MD in PT mice raised in EE (at least P < 0.05 at the measured frequencies, B t test). Because we have previously shown that the increase in both visual acuity and contrast sensitivity of the open eye after MD was completely abolished in SC mice with a PT lesion (25), EE, thus, at least partially preserved the experience-dependent enhancement of the optomotor reflex of the open eye. Enhancement of vision after MD was not preserved in juvenile mice after a PT lesion but restored after ibuprofen treatment (SI Results, Data S5: Enhancement of Vision After MD Was Not Preserved in Juvenile Mice After a PT Lesion But Restored After Ibuprofen Treatment and Fig. S4).
Discussion
Raising mice from 7 d before birth in an EE preserved OD plasticity in V1 into late adulthood, which depended on modified inhibitory but not excitatory synaptic transmission. Transferring older SC-raised mice that were already beyond their sensitive phase for OD plasticity into EE cages restored OD plasticity up to an age of at least 320 d. In addition, mice were protected from stroke-induced impairments of OD plasticity when they were either raised in EE or just 4 wk old, indicating that one of the major effects of EE is to preserve a younger brain into adulthood. This conclusion is supported by three findings: first, preservation of a juvenile level of inhibition in V1 of adult EE mice (Fig. 3); second, cortical changes, which more resemble juvenile OD plasticity (Fig. 2); and third, preserving cortical plasticity after thrombotic lesioning (Fig. 4). Similar to previous results in adult and aging rats (18–21), we show that EE can restore OD plasticity in mice. In addition, we started EE housing before birth and compared it with the effects of EE housing late in life. Interestingly, EE rearing from before birth into adulthood caused very pronounced OD shifts of a size previously only observed in 4-wk-old SC-raised mice: After 7 d MD of the previously stronger, contralateral eye, ODIs became mostly negative, indicating a dominance of the previously weaker, ipsilateral eye. In addition, the OD shift of the adult EE-raised mice was mediated by a reduction in deprived eye responses in V1, which is another hallmark of juvenile OD plasticity (9, 10, 27, 28), whereas OD plasticity in adult SC-raised mice is predominantly mediated by an increase in open-eye responses in V1 (4, 9, 27) and absent beyond PD110 (6). In contrast to the very strong OD shifts of our EE mice, the OD shifts documented previously in adult and old rats after EE housing (18, 20) were not as prominent as in SC rats during the critical period (29–32). This difference is most likely because of the shorter time of EE housing (2–3 wk) and the later onset (EE housing started when rats were already adults) compared with our mice. Thus, our study documents the maximal possible effect caused by EE housing: to preserve a juvenile-like V1 into adulthood. EE raising also extended the sensitive phase for OD plasticity in mice until at least PD196 (the oldest mouse analyzed), raising the question of whether this phase will ever close or is postponed to a later age. OD plasticity was also restored in mice raised in an SC and transferred to EE housing at PD110, an age where OD plasticity was absent in SC mice (6). Although OD plasticity could be induced up to an age of 320 d, OD shifts were mediated by a combination of both increased V1 activity after open-eye stimulation and reductions of V1 activity after closed-eye stimulation. These observations indicate that the molecular machinery necessary for juvenile OD plasticity cannot be completely restored once the sensitive phase for OD plasticity is closed (i.e., because of SC rearing beyond a critical age). This interpretation is supported by the results of the rat EE studies showing a less-pronounced plasticity compared with critical period animals (18, 20). It is interesting, in this context, that some of our late EE mice even spend more time in EE compared with the mice raised from 7 d before birth in EE. Nevertheless, their OD shifts were less strong, indicating that either juvenile mice are more susceptible to the plasticity-promoting effects of EE or the more-intense maternal care in EE makes the difference (17, 33).
What are the mechanisms underlying this extended sensitive phase for OD plasticity? In rats, restored OD plasticity after EE housing was accompanied by reduced levels of the inhibitory neurotransmitter GABA (18, 19). Our patch-clamp recordings in adult EE mice deliver direct physiological proof of the hypothesized reduction of intracortical inhibition: EE rearing not only reduced GABA/AMPA ratio, but in addition, the GABA/AMPA ratio was indistinguishable from values in 4-wk-old SC-raised mice. Furthermore, the AMPA/NMDA ratio was not affected by EE. When inhibition in EE rats was increased by applying diazepam, restored OD plasticity was completely blocked (18, 19). Diazepam applications with a dosage that reliably blocked OD shifts in adult SC mice did, however, just partly abolish OD plasticity in our EE mice. This result suggests that mechanisms other than reduced inhibition are involved. Alternatively, EE housing may change the susceptibility to diazepam (34), and therefore, the applied dosage of diazepam was too low to effectively block OD plasticity. In fact, EE housing from birth might lower intracortical inhibition more than just putting the animals in EE for 2–3 wk, as in the rat experiments (18, 19). Taken together, our results show that raising mice in an EE preserved a juvenile inhibitory tone into adulthood without affecting excitatory transmission. The prominent role of reduced intracortical inhibition for promoting OD plasticity in EE mice does not rule out the involvement of additional mechanisms. It has been shown that, for example, neuromodulatory systems are affected by EE housing (35) and modulate OD plasticity (36–39). Brain-derived neurotrophic factor is increased after EE housing (18, 19, 40) and can reactivate OD plasticity (36). Likewise, insulin-like growth factor 1 plays an important role in mediating EE effects, possibly acting by modulating intracortical inhibitory circuitry (41). EE also alters the chromatin status of the brain (42), and epigenetic modifications, like the acetylation of histones, have been shown to influence OD plasticity in the adult visual cortex (43, 44).
The conclusion that EE raising preserves a juvenile V1 is further supported by our stroke experiments: Both mice raised in EE and 4-wk-old mice preserved OD plasticity after a PT lesion in S1. Thus, EE-raised adult mice reacted to a plasticity-compromising event like critical period animals and continued to show plasticity, despite the S1 lesion. It is, therefore, tempting to speculate that EE may not only help to restore plasticity after a lesion, but additionally, protect from or attenuate deficits. This conclusion is corroborated by our optomotor results. Raising mice in EE partially preserved the enhancement of the optomotor reflex of the open eye after a PT lesion, whereas the same lesion completely prevented this increase in SC mice (25). Because anti-inflammatory treatment with ibuprofen rescued the enhancement after PT to control levels (25), the present results indicate that EE housing may reduce inflammation levels in the brain. Ruscher et al. (45), indeed, found that in rats, EE housing reduced the increased inflammation level after a stroke. In addition, physical exercise has been shown to be neuroprotective after stroke: Voluntary training on a running wheel or a treadmill for 2–3 wk before a stroke induced by middle cerebral artery occlusion reduced cerebral infarct size and sensory motor deficits in rodents (46, 47).
To further analyze signatures of altered inhibitory circuits in V1 of EE mice, we quantified PV+ inhibitory interneurons that are thought to play a crucial role in OD plasticity (1). Although a reduced number of interneurons labeled for the GABA-synthesizing enzyme glutamic acid decarboxylase 67 was observed in V1 of rats after 2–3 wk of EE housing (18, 20), the number of PV+ interneurons was not different between our SC and EE mice. A change in GABAergic inhibition in our enriched mice could, nevertheless, be mediated by other GABAergic interneurons. The degradation of PNNs can restore OD plasticity in adult rats (48), and EE housing was accompanied by a reduced PNN density in rat visual cortex (19, 20). We did, however, not observe any difference in PNNs between SC and EE mice. The different results might be caused by differing experimental designs; whereas our mice were born and raised in EE for at least 5 mo, the rats were housed in EE just for 2–3 wk when they were already adults. We can only speculate that the rather abrupt change in housing conditions of the rats may have triggered a change in PNN density.
We have previously shown that a small PT lesion in S1 abolished OD plasticity in V1 of adult mice (25), showing that there must be some long-range influence from outside V1 on OD plasticity in V1 and that activity in the major thalamocortical afferents to V1 is not sufficient for OD shifts to happen. Our present results further indicate that the importance of long-range influences increases with age; although in both EE-raised and 4-wk-old mice, plasticity was preserved after a PT lesion in S1 and activity changes in the major thalamoortical afferents were obviously sufficient to induce an OD shift after MD, long-range influences from outside V1 get increasingly important for plasticity in an older V1.
Interestingly, in the optical imaging experiments, V1 activation after visual stimulation was lower in EE compared with SC mice. A recent study using simultaneous recordings of local field potentials in awake, freely moving mice and quantifying the degree of linear and nonlinear correlation between the local field potentials in the two regions as a measure of synchronization might offer an explanation (49). It was shown that EE rearing decreased the level of coupling between the electrical activities of the secondary motor cortex and V1 compared with SC mice. A decreased coupling of V1 with other cortical areas might contribute to a decreased stimulus-driven activation of V1 neurons.
Taken together, our results show that EE not only preserved V1 with a juvenile level of inhibition into adulthood, but also rejuvenated V1 after raising mice in standard cages. In addition, EE raising protected adult mice from stroke-induced impairments of cortical plasticity, offering a promising, nonpharmacological tool for both preserving and restoring the plasticity of neuronal circuits.
Materials and Methods
C57BL/6J mice were housed in an animal room with a 12-h light/dark cycle, with food and water available ad libitum. The indicated age of mice is at the day of the optical imaging experiment. All experimental procedures were approved by the local government under registration numbers 33.9–42502-04–10/0326 (Niedersachsen) and 02–003/08 (Thüringen). For EE housing, commercially available cages (Marlau) (50) were used. The right eye was deprived of vision for 4 d in PD25–PD35 mice and 7 d in all older mice (5). PT lesions were done in the left somatosensory cortex using the Rose Bengal technique (51). For anti-inflammatory treatment, mice received daily i.p. injections of ibuprofen starting directly after MD. To increase GABAergic inhibition, EE mice were treated with diazepam during the MD period. The spatial frequency and contrast sensitivity thresholds of the optomotor reflex were determined using an optomotor system (26). Mouse visual cortical responses were recorded and analyzed using the imaging method developed by Kalatsky and Stryker (52). The ratio of AMPA/NMDA and GABA/AMPA receptor-mediated currents in SC and EE mice was measured by means of patch-clamp recordings. Details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank K. Lehmann for providing some of the SC mice data, K.-F. Schmidt and S. Stodieck for help with some of the experiments, M. Schink for excellent animal care, and J. Staiger and R. Wagener for their expert help in immunofluorescence analyses. This work was supported by the Federal Ministry of Education and Research, Germany, Grants 01GQ0921 (to F.G., J.P.-F., and E.K.) and 01GQ0810 (to S.L.); grants from the Deutsche Forschungsgemeinschaft through the Collaborative Research Center 889 “Cellular Mechanisms of Sensory Processing” (to O.M.S., Project B3 and to S.L., Project B5); an Alexander von Humboldt Research Fellowship for Postdoctoral Researchers (to J.P.-F.); and the European Neuroscience Campus network of the European Commission (P.D.F. and O.M.S.). The European Neuroscience Institute Göttingen is jointly funded by the Max Planck Society and University Medicine Göttingen.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313385111/-/DCSupplemental.
References
- 1.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 3.Dräger UC. Observations on monocular deprivation in mice. J Neurophysiol. 1978;41(1):28–42. doi: 10.1152/jn.1978.41.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Sawtell NB, et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38(6):977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 5.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16(10):3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann K, Löwel S. Age-dependent ocular dominance plasticity in adult mice. PLoS One. 2008;3(9):e3120. doi: 10.1371/journal.pone.0003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dräger UC. Receptive fields of single cells and topography in mouse visual cortex. J Comp Neurol. 1975;160(3):269–290. doi: 10.1002/cne.901600302. [DOI] [PubMed] [Google Scholar]
- 8.Cang J, Kalatsky VA, Löwel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci. 2005;22(5):685–691. doi: 10.1017/S0952523805225178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9(1):127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- 10.Heimel JA, Hartman RJ, Hermans JM, Levelt CN. Screening mouse vision with intrinsic signal optical imaging. Eur J Neurosci. 2007;25(3):795–804. doi: 10.1111/j.1460-9568.2007.05333.x. [DOI] [PubMed] [Google Scholar]
- 11.Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 12.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 13.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 14.Landi S, et al. Retinal functional development is sensitive to environmental enrichment: A role for BDNF. FASEB J. 2007;21(1):130–139. doi: 10.1096/fj.06-6083com. [DOI] [PubMed] [Google Scholar]
- 15.Cancedda L, et al. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24(20):4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prusky GT, Reidel C, Douglas RM. Environmental enrichment from birth enhances visual acuity but not place learning in mice. Behav Brain Res. 2000;114(1–2):11–15. doi: 10.1016/s0166-4328(00)00186-8. [DOI] [PubMed] [Google Scholar]
- 17.Sale A, et al. Enriched environment and acceleration of visual system development. Neuropharmacology. 2004;47(5):649–660. doi: 10.1016/j.neuropharm.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Baroncelli L, et al. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp Neurol. 2010;226(1):100–109. doi: 10.1016/j.expneurol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Sale A, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10(6):679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 20.Scali M, Baroncelli L, Cenni MC, Sale A, Maffei L. A rich environmental experience reactivates visual cortex plasticity in aged rats. Exp Gerontol. 2012;47(4):337–341. doi: 10.1016/j.exger.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Baroncelli L, et al. Enriched experience and recovery from amblyopia in adult rats: Impact of motor, social and sensory components. Neuropharmacology. 2012;62(7):2388–2397. doi: 10.1016/j.neuropharm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Kanold PO, Kim YA, GrandPre T, Shatz CJ. Co-regulation of ocular dominance plasticity and NMDA receptor subunit expression in glutamic acid decarboxylase-65 knock-out mice. J Physiol. 2009;587(Pt 12):2857–2867. doi: 10.1113/jphysiol.2009.171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by alpha1 GABAA receptor-specific expression profiling. J Neurochem. 2004;88(5):1059–1067. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- 24.Baroncelli L, et al. Brain plasticity and disease: A matter of inhibition. Neural Plast. 2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greifzu F, et al. Global impairment and therapeutic restoration of visual plasticity mechanisms after a localized cortical stroke. Proc Natl Acad Sci USA. 2011;108(37):15450–15455. doi: 10.1073/pnas.1016458108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45(12):4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 27.Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28(41):10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44(6):917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Spolidoro M, Putignano E, Munafò C, Maffei L, Pizzorusso T. Inhibition of matrix metalloproteinases prevents the potentiation of nondeprived-eye responses after monocular deprivation in juvenile rats. Cereb Cortex. 2012;22(3):725–734. doi: 10.1093/cercor/bhr158. [DOI] [PubMed] [Google Scholar]
- 30.Restani L, et al. Functional masking of deprived eye responses by callosal input during ocular dominance plasticity. Neuron. 2009;64(5):707–718. doi: 10.1016/j.neuron.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Mandolesi G, et al. A role for retinal brain-derived neurotrophic factor in ocular dominance plasticity. Curr Biol. 2005;15(23):2119–2124. doi: 10.1016/j.cub.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 32.Caleo M, Lodovichi C, Maffei L. Effects of nerve growth factor on visual cortical plasticity require afferent electrical activity. Eur J Neurosci. 1999;11(8):2979–2984. doi: 10.1046/j.1460-9568.1999.00737.x. [DOI] [PubMed] [Google Scholar]
- 33.Guzzetta A, et al. Massage accelerates brain development and the maturation of visual function. J Neurosci. 2009;29(18):6042–6051. doi: 10.1523/JNEUROSCI.5548-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson J, Kelly JP. The effects of isolated and enriched housing conditions on baseline and drug-induced behavioural responses in the male rat. Behav Brain Res. 2012;234(2):175–183. doi: 10.1016/j.bbr.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Baroncelli L, et al. Nurturing brain plasticity: Impact of environmental enrichment. Cell Death Differ. 2010;17(7):1092–1103. doi: 10.1038/cdd.2009.193. [DOI] [PubMed] [Google Scholar]
- 36.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 37.Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7(6):1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 39.Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320(6058):172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 40.Ickes BR, et al. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164(1):45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- 41.Ciucci F, et al. Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS One. 2007;2(5):e475. doi: 10.1371/journal.pone.0000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer A, Sananbenesi F, Wang XY, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 43.Putignano E, et al. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53(5):747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31(12):2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 45.Ruscher K, et al. Enriched environment reduces apolipoprotein E (ApoE) in reactive astrocytes and attenuates inflammation of the peri-infarct tissue after experimental stroke. J Cereb Blood Flow Metab. 2009;29(11):1796–1805. doi: 10.1038/jcbfm.2009.96. [DOI] [PubMed] [Google Scholar]
- 46.Wang RY, Yang YR, Yu SM. Protective effects of treadmill training on infarction in rats. Brain Res. 2001;922(1):140–143. doi: 10.1016/s0006-8993(01)03154-7. [DOI] [PubMed] [Google Scholar]
- 47.Endres M, et al. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54(5):582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 48.Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 49.Di Garbo A, Mainardi M, Chillemi S, Maffei L, Caleo M. Environmental enrichment modulates cortico-cortical interactions in the mouse. PLoS One. 2011;6(9):e25285. doi: 10.1371/journal.pone.0025285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fares RP, Kouchi H, Bezin L. Standardized Environmental Enrichment for Rodents in Marlau Cage. New York: Protocol Exchange; 2012. [Google Scholar]
- 51.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17(5):497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- 52.Kalatsky VA, Stryker MP. New paradigm for optical imaging: Temporally encoded maps of intrinsic signal. Neuron. 2003;38(4):529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.