Significance
The lesion-deficit experimental design has been a cornerstone approach in revealing the neural bases of many behaviors. Often, however, studies only qualitatively assess target lesions and, in doing so, fail to fully exploit the information available that can more specifically link subregions of damage to behavioral outcomes. Here, by developing a lesion-mapping system, we provide strong evidence that, in contrast to the prevailing view, normal expression of conditioned taste aversion does not depend on the conventionally defined gustatory cortex. Instead, this approach revealed a strong association between deficits in conditioned taste aversion expression and a subregion encompassing the posterior gustatory cortex and the surrounding insular cortex considered to be visceroceptive.
Abstract
Gustatory cortex (GC), an assemblage of taste-responsive neurons in insular cortex, is widely regarded as integral to conditioned taste aversion (CTA) retention, a link that has been primarily established using lesion approaches in rats. In contrast to this prevailing view, we found that even the most complete bilateral damage to GC produced by ibotenic acid was insufficient to disrupt postsurgical expression of a presurgical CTA; nor were such lesions sufficient to disrupt postsurgical acquisition and initial expression of a second CTA. However, some rats with lesions were significantly impaired on these tests. Further examination of all conditioned rats with lesions, regardless of the lesion topography, revealed a significant positive association between damage in the posterior portion of GC and especially within adjacent posterior regions of insular cortex. Accordingly, we developed a high-resolution lesion-mapping program that permitted the overlay of the individual lesion maps from rats with CTA impairments to produce a groupwise aggregate lesion map. Comparison of this map with one derived from the unimpaired counterparts indicated a specific lesion “hot spot” associated with CTA deficits that included the most posterior end of GC and overlying granular layer and encompassed an area provisionally referred to in the literature as visceral cortex. Thus, the detailed mapping of the lesion in behaviorally defined subgroups of rats allowed us to exploit the variability in performance to uncloak an important potential component of the functional topography of insular cortex; such an approach could have general applicability to other brain structure–function endeavors as well.
With its primary receptors situated at the front end of the alimentary tract, the gustatory system is integrally involved in guiding food selection, promoting and discouraging intake, and evoking preparatory physiological reflexes (1). To best serve these functions, taste signals must confer with both the contemporary physiological milieu (e.g., satiety, malaise) and neurally stored representations of the associated effects of that particular taste stimulus [e.g., associative history with visceral malaise, as in conditioned taste aversion (CTA)]. However, the neural circuits underlying these critical integrative processes remain to be fully elucidated. In this regard, gustatory cortex (GC), an assemblage of taste-responsive neurons in the anterior dysgranular and agranular layers of insular cortex, is of particular interest (2–7). Receiving convergent input from both the thalamic and limbic taste pathways, GC consists of neurons that may potentially respond to various features of the taste stimulus, including chemosensory and hedonic alike, situated in close proximity to one another (5, 8–13). Additionally, viscerosensory signals are received in the adjoining region of granular insular cortex (GI) just dorsal and posterior to GC (5, 6, 11, 14). Extensive and reciprocating projections are found both within the subdivisions of GC proper and among GC and its neighboring sensory fields in insular cortex, making it an ideal candidate region for multimodal sensory integration and associative processing of taste stimuli (15–17).
Indeed, several studies have already demonstrated that lesions to GC severely attenuate CTA retention and, albeit less consistently, CTA acquisition (e.g., refs. 2 and 18–30). However, much of the foundational research on the role of the GC in CTA has been limited to ablation and electrolytic lesion techniques, which are known to cause damage beyond the intended target. Pharmacological studies that produce reversible modulation of neuronal activity have buttressed the conclusion that GC is critically involved in taste aversion learning and memory (e.g., refs. 23 and 31–34), but the precise cortical site of action is often difficult to discern. In contrast to previous reports, a recent study in our laboratory (35) found no deficits in the postsurgical expression of a presurgically trained CTA following ibotenic acid (IBO) lesions that produced bilateral damage well centered in the conventionally defined GC. Given the GC and the immediately adjacent brain areas are purported sites of multisensory convergence and integrative processing, slight variations in lesion placement or extent, drug infusion spread, and/or inclusion criteria based on histology could significantly impact not only the degree, but the type of deficit observed. To date, little attention has been paid to behaviorally characterizing the functional topography of the GC and the surrounding insular cortex.
Here, we took a more comprehensive approach to systematically assess whether GC is requisite for CTA expression. Expanding upon our previous study (35), we included groups that had lesions targeted to the anterior GC or the posterior GC or both (all of GC) to assess the effects of the neural damage on the retention of a presurgically acquired CTA. We also tested the postsurgical acquisition and retention of a second CTA to a different taste stimulus. In addition to an analysis that included behavioral data only from those rats with histologically confirmed complete bilateral damage of the GC (i.e., lesion-based criterion), we compared the degree of individual behavioral impairment on each of the CTA tests with the extent of the neural damage in various subregions of GC and surrounding areas. Finally, we developed a high-resolution lesion-mapping program that permitted the overlay of the individual lesion maps belonging to a subset of subjects that displayed significantly impaired behavior to produce a corresponding groupwise aggregate lesion map. Comparison of this groupwise map to one that was generated from the subset of subjects that were not impaired revealed a specific lesion “hot spot” or area of damage best associated with CTA impairment.
Results
Extensive Lesion Damage to GC Fails to Disrupt Retention of a Presurgically Conditioned CTA.
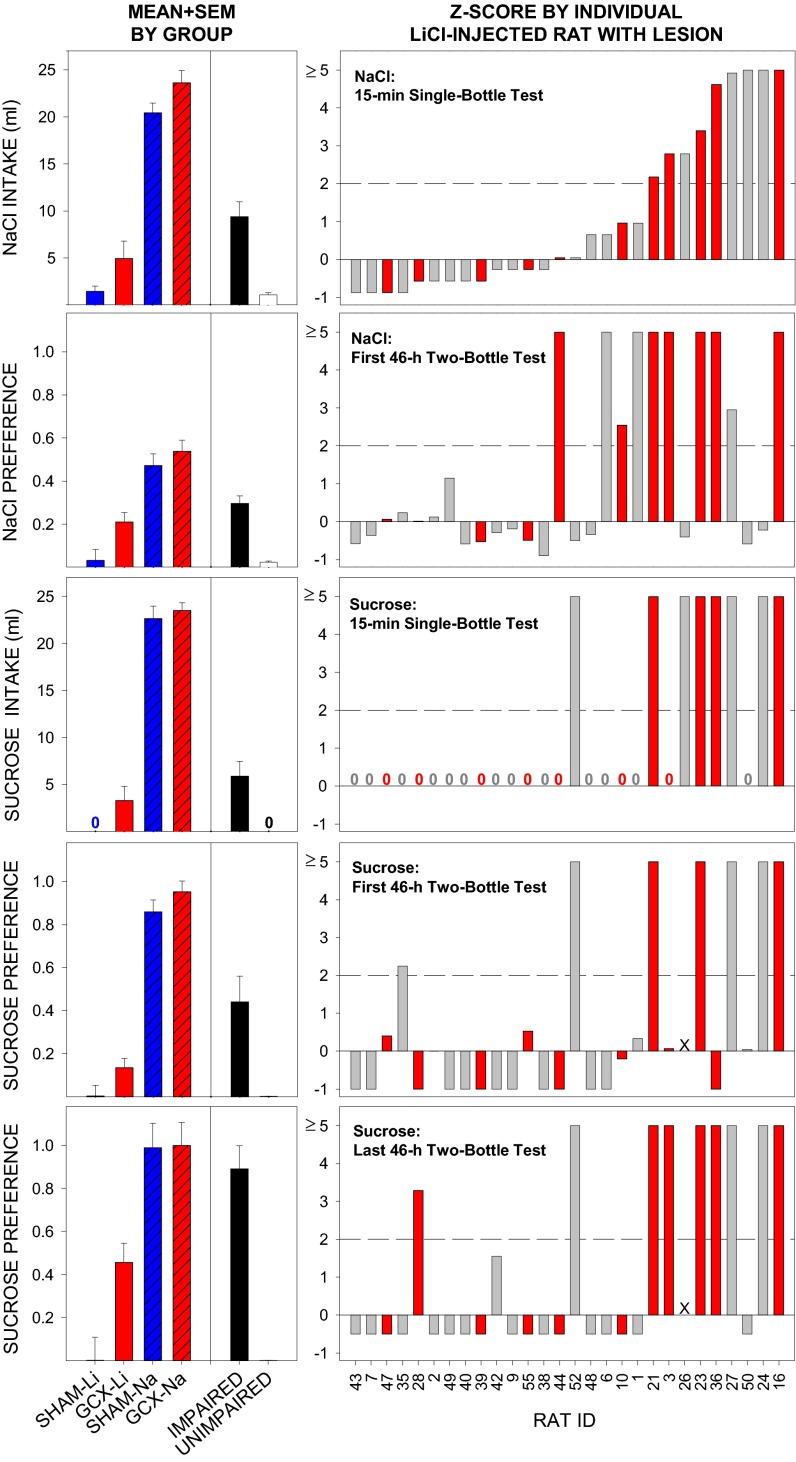
Retention of a presurgically conditioned taste aversion (see Fig. S1 for acquisition data) to 0.1 M NaCl [conditioned stimulus (CS)] was assessed postsurgically in a 15-min single-bottle test and across 16 46-h two-bottle preference tests. Only rats with at least 50% of the GC destroyed bilaterally (referred to as GCX), regardless of targeted placement group were included in this initial analysis. GCX rats (87.9 ± 4.1% GC destroyed) that had previously received CS-LiCl pairings exhibited markedly suppressed intake of the CS in the single-bottle test relative to the unconditioned control rats with (GCX-NaCl, 92.7 ± 1.6% GC destroyed) or without (SHAM-NaCl) GC lesions (Fig. 1). The CS intake of the GCX-LiCl rats was similar to that of the SHAM-LiCl rats. Additionally, the GCX-LiCl rats definitely exhibited avoidance of the CS [versus deionized water (DW)] in the first 46-h two-bottle test relative to their respective NaCl-injected controls but at levels slightly higher than that of the SHAM-LiCl rats (Fig. 1). With repeated two-bottle testing, LiCl-injected rats gradually increased intake of the CS. Although this shift in preference appeared to progress more rapidly in the GCX-LiCl–injected rats, this effect was not statistically significant relative to SHAM-LiCl rats (Fig. S1). Taken together, these results show that neurotoxin-induced lesions that virtually obliterated the entire GC, as conventionally defined (4–6, 10), failed to disrupt retention of a NaCl taste aversion that was conditioned before the insult.
Fig. 1.
Left panels, left of the black vertical line: Mean + SEM intake or preference score for the LiCl- and NaCl-injected rats that met the lesion-based criterion (≥50% bilateral damage to GC: GCX-Li, n = 11; GCX-Na, n = 8) and the sham-operated controls (SHAM-Li, n = 8; SHAM-Na, n = 7) across CTA measures (vertical panels). Although both GCX groups drank slightly more NaCl on the 15-min single-bottle retention test [effect of Lesion: F(1,30) = 5.28, P = 0.03), both GCX-Li and SHAM-Li rats consumed significantly less than did their NaCl-injected counterparts, as indicated by a significant effect of US [F(1,30) = 166.26, P ≤ 0.00001] that did not further interact with Lesion [US × Lesion: F(1,30) = 0.01, P = 0.91]. Similar patterns were found on the first 46-h NaCl two-bottle test [Lesion: F(1,30) = 5.96, P = 0.02; US: F(1,30) = 58.33, P ≤ 0.00001; US × Lesion: F(1,30) = 1.24, P = 0.27], 15-min single-bottle sucrose test, and first 46-h sucrose-two bottle test [Lesion: F(1,30) = 5.03, P = 0.03; US: F(1,30) = 282.37, P ≤ 0.00001; US × Lesion: F(1,30) = 0.16, P = 0.69]. Because none of the rats in the SHAM-Li group consumed any sucrose on the 15-min single-bottle test (hence no bar, 0), a series of nonparametric Mann–Whitney U tests were conducted (SHAM-Li versus GCX-Li: U = 60.0, P = 0.19; SHAM-Li versus SHAM-Na: U = 0, P ≤ 0.003; GCX-Li versus GCX-Na: U = 0, P ≤ 0.003; Bonferroni-corrected). By the final 46-h sucrose two-bottle test, however, the preference for sucrose had increased in the GCX-Li group, but not in the SHAM-Li group [Lesion: F(1,30) = 4.94, P = 0.03; US: F(1,30) = 53.74, P < 0.00001; Lesion × US: F(1,30) = 4.48, P = 0.04]. Post hoc independent t tests (all Bonferroni-corrected for multiple comparisons) following up on the Lesion × US interaction showed that SHAM-Na and GCX-Na rats had a higher preference for sucrose than SHAM-Li rats (values of P < 0.00006). Even though there was no difference between SHAM-Li and GCX-Li rats (P = 0.18), preference for sucrose in the GCX-Li group had increased somewhat and was no longer statistically different from the SHAM-Na group (P = 0.12). Left panels, right of the black vertical line: Mean + SEM intake or preference score for groups of rats with lesions, irrespective of lesion size or placement that were considered IMPAIRED (≥2 SDs from mean of the SHAM-Li group) or UNIMPAIRED for each CTA test. (Right) Standardized degree of impairment (Z score relative to mean and SD of SHAM-Li group) on each major CTA test plotted as a function of individual rat with a lesion. Rats that met the GCX criterion are colored in red. The y-axis scales are capped at 5.0 for presentation purposes. Numerical digit 0 is displayed above the x axis when the Z score of a given rat equaled zero. Rat 26 was excluded from the first and last 46-h sucrose two-bottle tests (due to illness, X = no data). Pearson’s correlation tests (all Bonferroni-corrected for multiple comparisons) indicated that degree of impairment on the 15-min NaCl single-bottle test was significantly positively correlated with degree of impairment on the 15-min sucrose single-bottle test (r = 0.71, P = 0.002, n = 28), and the last 46-h two-bottle preference test with sucrose (r = 0.69, P = 0.002, n = 27). Impairment on the first 46-h two-bottle test with NaCl was significantly positively correlated with impairment on the 15-min single-bottle retention test for sucrose (r = 0.53, P = 0.05, n = 28).
Extensive Damage to GC Does Not Attenuate Postsurgical Acquisition and Immediate Retention, but Decreases the Durability, of a CTA.
The GCX-LiCl group also demonstrated competent acquisition of a postsurgically trained taste aversion to a new CS, 0.1 M sucrose as measured in the 15-min single-bottle test (Fig. 1; acquisition data are shown in Fig. S2). However, whereas the SHAM-LiCl rats maintained strong avoidance of the CS across repeated 46-h two-bottle tests, GCX-LiCl rats increased their consumption of the CS across testing (Fig. 1 and Fig. S3). Thus, surgical damage to essentially the entire GC did not interfere with the postsurgical acquisition of a new CTA, but hindered, albeit only moderately, the maintenance of the aversion as revealed by increased CS consumption relative to DW in the two-bottle tests.
Impaired Retention of Presurgically and Postsurgically Acquired CTAs Is Associated with Damage to the Posterior GC and Surrounding Regions.
Despite the fact that, as a group, rats with the most extensive damage to GC showed no retention deficits on the single-bottle tests and only moderate impairment on the two-bottle tests, closer inspection of the performances of individual rats, including those that fulfilled the GCX criterion and those that did not, revealed that (i) indeed some rats showed robust impairments; and (ii) many of those rats were consistently impaired across all CTA tests (Fig. 1). Additionally, given that subsets of our lesion surgeries were targeted to the anterior GC or posterior GC (or both), correlational analyses were performed comparing the extent of damage to various subregions of GC as well as to those regions immediately surrounding GC with the degree of impairment on each CTA test (Table S1). Consistent with our analyses including only those rats with the most extensive damage to GC (GCX), the degree of damage to GC as a whole was not associated with CTA deficits (all r values ≤ 0.49), with the exception of a modest correlation observed between damage to the GC proper and retarded retention on the first 46-h two-bottle test with NaCl (r = 0.54). Interestingly, the most impressive positive correlations observed were between the performance on the tests for both presurgically and postsurgically acquired CTAs and damage to the area immediately adjacent to posterior GC (PERI-POST) (r values = 0.54–0.80, depending on test). Conversely, damage to the anterior GC or any particular subregion within or around anterior GC was not associated with any retention deficits. Altogether (see Table S1 for all correlations), the pattern of these associations suggest that damage to regions in and around the posterior GC, but not the GC alone (or anterior GC), may in fact be the site(s) critically involved in CTA retention and acquisition.
Rats Exhibiting CTA Impairments Have More Damage to Posterior GC and Adjacent Insular Cortex Regions Compared with Unimpaired Rats with Lesions.
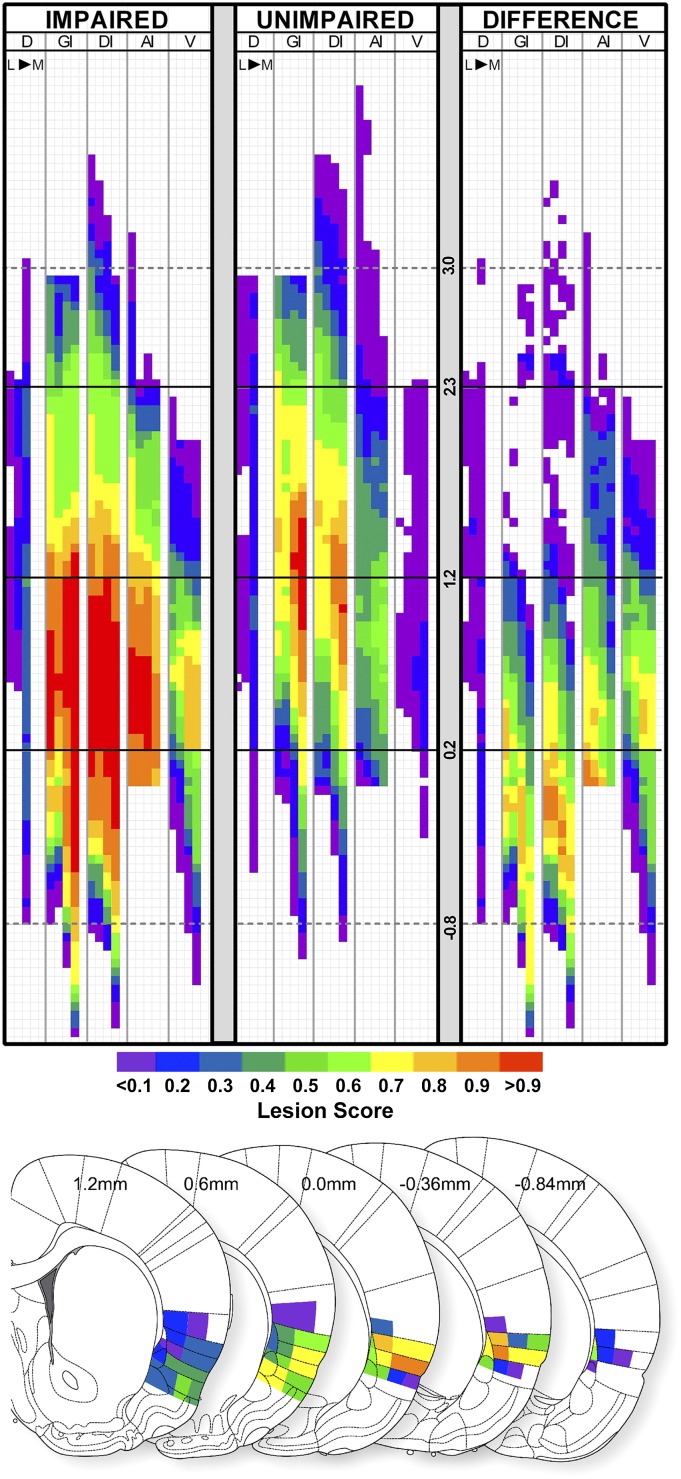
Having identified groups of rats with lesions that were impaired for each test, we used these designations to further investigate which specific areas of GC were more commonly damaged in the subset of rats that were IMPAIRED versus those rats that were without impairment. To accomplish this, we developed and validated (Table S2) a high–spatial-resolution lesion-mapping program (Fig. 2 and Figs. S4 and S5) that permitted the overlay of lesion maps of individual rats belonging to a defined subset (e.g., those designated as impaired, i.e., ≥2 SDs from SHAM-LiCl mean on a particular CTA test; Materials and Methods). Furthermore, this aggregate lesion profile of IMPAIRED rats was compared with that of UNIMPAIRED rats, revealing a lesion hot spot or area with greater lesion damage associated with behavioral deficit (Fig. 3). IMPAIRED rats (identified without regard to lesion size or placement) on the 15-min NaCl single-bottle retention test had more damage to the posterior GC, as well as the overlying granular layer, relative to UNIMPAIRED rats with lesions (Fig. 3). This associated damage extended ventrally and posteriorly from the posterior GC and medially including the claustrum, and was especially localized to the dysgranular layer around −0.35 mm (posterior to bregma). Importantly, even though these rats were considered impaired relative to SHAM-LiCl–injected rats, all of these rats, with the exception of one, were still 2 SDs away from the mean of the SHAM-NaCl group mean on this 15-min single-bottle test, suggesting that, even for this IMPAIRED group, the CTA was not entirely abolished. A similar lesion hot spot profile was indicated for the rats that were deemed IMPAIRED on the first 46-h of the NaCl versus DW two-bottle test (Fig. S7A).
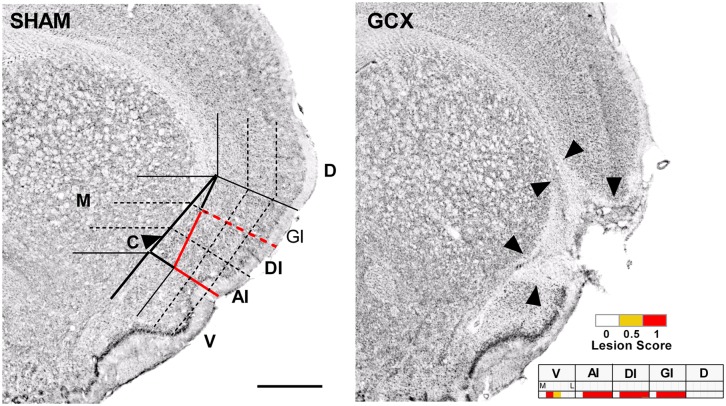
Fig. 2.
(Left) Example of the grid system displayed over the +1.2 mm coronal section of a representative SHAM section. See SI Materials and Methods for more detail on the definition of GC used and demarcation of subregions. GC is outlined in a solid red line. Abbreviations: AI, agranular insular cortex (dorsal to the rhinal fissure); C, claustrum (outlined column just medial to AI, GI, and DI); D, dorsal to insular cortex; DI, dysgranular insular cortex; GI, granular insular cortex; M, medial to claustrum; V, ventral to rhinal fissure; based on ref. 42. (Scale bar: 1.0 mm.) (Right) Representative GC lesion, the borders of which are noted by black arrowheads. (Inset) The corresponding mapping grid for this +1.2-mm section with lesion. Within each particular cell or subregion of the grid, if less than one-half the area was destroyed, then a lesion score of 0 was assigned (white). If at least one-half of the neuronal tissue of the grid cell was destroyed, then a lesion score of 0.5 was assigned (yellow). If the neuronal tissue in the entire grid cell was destroyed, then a lesion score of 1 was assigned (red).
Fig. 3.
(Upper) Lesion maps showing the groupwise lesion score in each grid cell. Lesion scores represent the mean score in a given cell of all rats in the IMPAIRED group (Left, n = 9) versus the UNIMPAIRED group (Right, n = 19) on the 15-min 0.1 M NaCl single-bottle test (presurgically acquired CTA). The difference between the average lesion scores in each grid cell between groups (IMPAIRED minus UNIMPAIRED) is indicated in the DIFFERENCE map (rightmost panel). The solid black horizontal lines demarcate the anterior border of GC (2.3 mm), the center of GC (1.2 mm) and the posterior border GC (0.2 mm), relative to bregma. The dashed gray horizontal lines indicate the 3.0 mm and −0.8 mm AP levels for additional reference points. (Lower) The DIFFERENCE lesion score (represented in color scale) in each subregion projected back onto the nearest corresponding atlas plate across the AP extent of the lesion hot spot (atlas plates used with permission; ref. 42). Abbreviations: AI, agranular insular cortex (dorsal to the rhinal fissure); D, dorsal to insular cortex; DI, dysgranular insular cortex; GI, granular insular cortex; L, lateral; M, medial; V, ventral to rhinal fissure.
For the most part, a similar cohort of rats was impaired on the various measures of the postsurgical CTA expression, revealing a comparable posterior GC and insular cortex lesion hot spot (Figs. S6 and S7). Notably, associated damage likewise extended into the dysgranular and granular layers of insular cortex posterior to GC (especially robust around −0.35 mm posterior to bregma). Nevertheless, the “impairment” in these rats with more damage to the more posterior regions of the GC and surrounding areas should be considered partial, because all of the rats that were deemed impaired relative to the SHAM-LiCl group mean were still also ≥2 SDs from the mean of the SHAM-NaCl group mean on the 15-min single-bottle test with sucrose.
Discussion
In contrast to previous reports (e.g., refs. 2, 18–28, 30), we did not find evidence that GC, as traditionally defined, is necessary for the postsurgical retention of a presurgically learned CTA or expression of a CTA trained after the cortical damage was made. These findings are consistent with our previous study, in which smaller, centralized IBO lesions in GC likewise failed to impede the retention of a presurgically learned NaCl taste aversion (35). Even in the case here where the sucrose aversion appeared to partially dissipate with repeated two-bottle preference testing for the GCX-LiCl rats as a group, closer inspection of the intake of individual rats indicated that only a select few showed this shift, whereas others continued to avoid the CS. This variability in CTA retention provoked a more detailed examination of all of the LiCl-injected rats with lesions, including those that had damage that did not meet the GC lesion criterion, revealing a subset of rats with rather substantial CTA retention and/or expression deficits. Correlational analyses between CTA performance measures and lesion damage to the entire GC, subregions of GC, and the surrounding tissue indicated that impairment on the various CTA tests was best associated with damage to the posterior half of GC and posterior areas adjacent to the posterior GC. A lesion-mapping approach permitted the detection of shared regions of damage among IMPAIRED rats selected for the presence of a significant behavioral deficit (versus those without impairment). This unveiled an impressive association between impairments in CTA measures and a specific region of insular cortex, including the most posterior GC and overlying GI subdivision but also extending (i) ventrally, encompassing the subrhinal agranular insular cortex (AI); (ii) medially, encompassing the claustrum; and (iii) caudally, encompassing more posterior regions of insular cortex.
Much of the lesion hot spot identified here spans an area of insular cortex that includes what is thought to be the very posterior reaches of GC as well as an area that has been provisionally referred to in the literature as visceral cortex (cf. refs. 5, 6, and 14). However, the precise border of where GC ends and visceral cortex begins, if such a definitive boundary exists, is not well established. Whatever the case, it is likely that this area of insular cortex sustains collateral damage in cases in which the lesions were intended for, and the deficits were ascribed to, GC proper. In fact, widespread damage to areas consistent with this location have been reported in studies using ablation, electrolytic, and even neurotoxic approaches in studies indicating a role for GC in CTA (e.g., refs. 2, 18, 21, 22, 25, 27, 30, 36, 37). This, coupled with the fact that previous studies (24, 38) showed that neither damage to AI just caudal to GC nor to an area even more posterior in insular cortex than the hot spot identified here affected CTA, highlights the strong possibility that discrepancies across studies critically hinge upon the involvement of a specific area (or areas) of this cortical region. Especially considering that the lesion's impact on CTA expression was partial in the present study, it remains crucial to determine whether this relates more to the function of insular cortex in CTA (e.g., modulatory) and/or if the region identified here collaborates with some or all of nearby GC and/or brain sites outside of insular cortex that remained unscathed.
Although this area of insular cortex has been implicated in LiCl signal processing (39), the nature of the deficit observed here does not appear to be in processing of the LiCl-related signals alone because the same cortical locus was indicated for impaired retention of the presurgically and postsurgically trained taste aversion. Nor did this insular cortex hot spot appear to render the rats ageusic to NaCl and/or sucrose, because even the rats that were most impaired on the initial NaCl and sucrose CTA retention tests still exhibited some degree of suppressed consumption of the respective CSs (relative to sham-operated NaCl-injected controls), although perceived CS intensity may have been altered by such damage. Moreover, although GC lesions are thought to disrupt neophobia (e.g., refs. 21, 28, and 37), we did not find evidence of enhanced consumption of sucrose on the first exposure or thereafter in the Na-injected rats with lesions relative to the SHAM-Na group. Also, the deficits related to damage to the posterior site identified here may be somewhat specific to associations of taste with aversive, as opposed to nutritive, visceral signals (cf. ref. 40). All of this underscores the need for more systematic and finely tuned assessments of the functional topography of insular cortex with respect to taste-visceral integration, as well as other types of taste-guided behavior.
Lesion–behavior strategies have been instrumental in characterizing structure–function relationships across the entire CNS. Such approaches are continuously being refined to enhance target specificity. Perhaps equally important to the full characterization of these types of relationships is the comprehensive analysis of lesion placement and behavioral deficits. If only a conventional histologically based data-inclusion criterion were used, the conclusions of the present study would have been limited to simply showing that the GC is not involved in CTA expression. However, such an analysis would not have optimized the potential information available in the dataset regarding cortical involvement in CTA. The spatially detailed mapping program developed here offered additional and very meaningful information in this regard. This program allowed us to further explore correlations between behavioral deficits and the extent of damage to particular subregions of GC as well as to the surrounding area by generating aggregate maps for a chosen subset of individual rats (e.g., impaired on retention) and comparing the different lesion patterns between the two aggregate maps (e.g., impaired versus unimpaired). This strategy revealed, in fact, that a very specific region encompassing the posterior GC and the surrounding posterior cortical areas was related to CTA deficits. Thus, in a sense, this type of behaviorally guided analysis complements the conventional lesion-guided analysis, whereby the comparison of those rats with common behavioral deficits can be used to locate areas of common damage not seen in rats that exhibit normal behavioral phenotypes. In turn, the maps representing the loci of these associations can be used to more specifically target the hot spot, a step required to establish the selective necessity of the candidate brain site to the hypothesized function.
In summary, the present experiment indicates that the GC, as conventionally defined, is not necessary for CTA retention. Instead, detailed lesion mapping indicates a strong association between CTA deficits and posterior GC coupled with the adjacent posterior region of insular cortex. It would be instructive for future studies to target this proposed anatomical hot spot with smaller, more selective lesions to determine whether damage to this region alone is sufficient to lead to the impairment or whether other areas of GC or insular cortex in general must be conjointly affected. It also remains to be explicitly tested whether the deficits described here are specific to taste and/or visceral integration, sensory or visceral signal processing, memory, or response generation. Indeed, given the recent findings in the mouse model based on two-photon imaging of neuronal calcium responses to orally applied taste stimuli suggesting that GC has an explicit chemotopic organization (41), the specific lesion–behavior approaches adopted here could have considerable utility in testing the functional significance of this proposed topography. The approach could also be adapted for the determination of the locus of virally transduced expression of light-sensitive ion channels in optogenetic applications. In regions such as the GC and the neighboring areas of insular cortex where multiple types of input and sensory signals converge, are processed, and contribute to various behavioral outputs, bidirectional lesion analysis strategies (lesion-based and deficit-based), along with a battery of taste-dependent behavioral tasks each designed to test a different aspect of gustatory function, have great promise in advancing our understanding of the functional organization of the central gustatory system.
Materials and Methods
Subjects.
Naïve male Sprague-Dawley rats (n = 55; Charles River Laboratories), weighing 352 ± 21g upon arrival, were individually housed in polycarbonate shoebox cages in a climate-controlled colony room on a 12:12-h light:dark cycle with ad libitum access to standard chow (Labdiet 5001; PMI) and DW, except as noted below. All procedures complied with the National Institutes of Health guidelines for humane handling of animals and were approved by the Florida State University Animal Care and Use Committee. Rats were systematically divided into six groups combining lesion placement (or sham surgery, SHAM) and unconditioned stimulus (US) treatment based on 15-min DW intake and body weight on the day before the first trial of the presurgical CTA (see below): Anterior GC lesion, LiCl-injected (n = 10); Posterior GC lesion, LiCl-injected (n = 10); Anterior GC plus Posterior GC lesion, LiCl-injected (n = 10); Anterior GC plus Posterior GC lesion, NaCl-injected (n = 10); SHAM, LiCl-injected (n = 8), SHAM, NaCl-injected (n = 7).
Surgery.
Following acquisition of the presurgical CTA (see below), rats underwent stereotaxic surgery to produce lesions in GC (or sham surgery; SHAM). Surgeries were performed under isoflurane anesthesia (∼2–2.5%/min flow rate). After the rat was positioned in the stereotaxic frame (model 900; Kopf Instruments), a midline incision was made through the scalp, exposing bregma and lambda, which were then used to guide adjustments to the mouth bar to level the skull. Access holes were drilled in the skull overlying the intended targets. Then, using bregma as the zero reference, a glass micropipette tip (diameter, ∼40 µm), attached to a 1.0-μL Hamilton syringe containing IBO (20 mg/mL in PBS; Sigma-Aldrich) or just PBS (for SHAM surgeries) was positioned to the designated anteroposterior (AP) and mediolateral (ML) coordinates and then lowered to the designated dorsoventral (DV) coordinate. The following stereotaxic coordinates (42) and total IBO volumes were used for the different lesion placement groups: Anterior GC (AP, +1.5 mm; ML, ±5.2 mm; DV, −6.4 mm; 0.21 µL of IBO), Posterior GC (AP, +0.5 mm; ML, ±5.6 mm; DV, −6.6 mm; 0.18 μL of IBO), Anterior plus Posterior GC (all coordinates and infusion volumes listed above). IBO was administered across three equivolume infusions (0.07 or 0.06 μL) with each infusion separated by ∼2–7 min to allow for adequate diffusion. SHAM lesions were produced by infusing PBS in the same manner at both the anterior and posterior GC coordinates. All rats were administered analgesic (Ketorolac, 2 mg/kg, s.c.) and antibiotic (Penicillin G Procaine; 30,000 units in 0.1 mL, s.c.) on the day of surgery and for 3 d thereafter. One rat in the Anterior GC lesion group died during surgery.
CTA Stimuli and Training and Testing Procedures.
A CTA (or unconditioned control) was established before surgery (referred to here as presurgical CTA); retention of this CTA was tested after surgery (see below). A second CTA (or unconditioned control) involving a different taste stimulus was both established and tested after the production of the GC lesion or sham surgery in the same rats (referred to as postsurgical CTA). Training procedures were essentially identical for both CTAs, with the exception that the taste CS for the presurgical CTA was 0.1 M NaCl and the CS for the postsurgical CTA was 0.1 M sucrose. For CTA acquisition, rats were first placed on a restricted water access schedule whereby DW was only available during a 15-min session in the morning and for a 30-min rehydration session in the afternoon (separated by ∼5 h) at the same times each day. Intake volumes were recorded (to the nearest 0.5 mL) for each session. After rats were acclimated to this schedule, the CS was presented to all rats during the 15-min morning session, in place of the usual DW. At the conclusion of the session, CS intake was recorded and rats were immediately injected with either 0.15 M LiCl (2.0 mEq/kg body weight, i.p.) to condition an aversion or an equivalent volume of saline as a control (0.15 M NaCl, i.p.). The afternoon water session was conducted as usual. On the following 2 d, rats were again presented with DW during both the morning and afternoon sessions, but on the third day, the CS was again presented in place of DW in the morning session and LiCl and saline were injected at the conclusion of the 15-min session (trial 2). Rats that failed to consume at least 1 mL of the CS during the 15-min session were administered ∼1 mL of the CS directly into the oral cavity via syringe to guarantee CS exposure before receiving the i.p. injection of LiCl or saline. Two types of tests were used to examine CTA retention. First, following reacclimation to the restricted water access schedule as described above, the CS was presented in place of DW for a single 15-min session, under the same conditions as the rats had originally experienced the CS in conditioning, except that no US was administered (single-bottle retention test). Then, two bottles were presented side-by-side on the home-cage for a two-bottle preference test to provide a more sensitive (e.g., in the absence of a conflicting need-state, water deprivation) long-term measure of the avoidance of the CS by offering the rats the opportunity to consume an alternative repletive stimulus (DW) at the same time. The bottles remained on the home cage ∼23 h/day. CS and DW intakes were recorded, the bottles were refilled during the remaining hour each day, and the bottle positions were switched at the beginning of each 23-h period to control for side biases. To additionally assess the durability of the effects of the presurgical CTA to NaCl and the postsurgical CTA to sucrose, the two-bottle preference tests were continued for 32 23-h and 16 23-h periods, respectively. The two-bottle preference testing of the presurgical CTA was divided into two phases of 16 consecutive tests each, separated by a period of 4 d. Preference scores were calculated [CS intake/(CS intake + DW intake)] for each 46-h block of testing. Retention testing of the presurgical CTA to NaCl began 14–37 d from the day of surgery. The acquisition phase of the postsurgical CTA to sucrose began 3 d after the final NaCl versus DW two-bottle preference test and tests for retention of the postsurgical CTA to sucrose began 3 d after the second and final sucrose CTA trial. One rat with a posterior GC lesion became ill during the final days of sucrose two-bottle testing; this rat was discontinued from the experiment and perfused for lesion histology. Behavioral measures obtained before its illness (up through and including the 15-min single-bottle sucrose test) were included in the data analyses.
Histology and Lesion Analyses.
Rats were given a lethal overdose of sodium pentobarbital (Euthasol, i.p.) and transcardially perfused with PBS, followed by a mixture of 4% paraformaldehyde and 0.8% methyl alcohol in PBS solution. Brains were removed from the skull, postfixed for at least 24 h in the same fixative, and transferred to the same fixative containing 10% glycerol for at least 72 h for cryoprotection. Serial coronal sections (50 µm thick) were made on a freezing microtome. Free-floating sections were postfixed for at least 30 min, rinsed in PBS and then DW, mounted on gelatin-coated slides, Nissl stained (with thionin), and coverslipped to visualize the cytoarchitecture in and around insular cortex and IBO-induced neuron death and gliosis. GC lesions were first confirmed and lesion volumes and ratios relative to total GC volume (for a given region or subregion) were calculated using Neurolucida software (MicroBrightField) along with a Leica microscope (model DMRB; McBain Instruments) (see SI Materials and Methods for a more detailed description of the GC definition and landmark demarcation). A lesion-mapping program was then developed and used to visualize the lesion in GC and surrounding areas for each rat with a higher spatial resolution as assessed under the DMRB Leica microscope (see SI Materials and Methods for description of the program and cross-validation with Neurolucida). Three rats were excluded from all analyses due to poor histological processing that rendered the tissue and lesion difficult to visualize (two GCX-Na rats and one anterior GC lesion rat).
Lesion-Based Data Analyses.
In the first analyses to assess the involvement of GC in CTA, we only included rats that had at least 50% damage to GC in each hemisphere. Eleven LiCl-injected and eight NaCl-injected rats met this criterion and were compared with SHAM controls. The behavioral outcomes for the different groups were analyzed with standard multifactorial ANOVAs. Simple one-way ANOVAs and/or post hoc t tests (Bonferroni-corrected) were conducted when appropriate. In one case in which there was no variability in the scores of a group, Mann–Whitney comparisons were conducted. Then, to further assess the relationship between the extent of damage within all of GC as well as within specific regions surrounding the GC, we divided the GC into six subregions; percentage of each zone destroyed was separately correlated with each rat’s degree of impairment. Performance of each rat was standardized against the mean and SD of the SHAM-LiCl group for each measure (z score). Z scores of ≥2.0 SD from the SHAM-LiCl mean were considered to indicate significant impairment. These Z scores and the extent of lesion damage for a given region were subjected to Pearson’s correlation tests. Values of P ≤ 0.05 were considered to indicate statistical significance in all analyses.
Behavioral-Deficit–Based Analyses and Lesion Mapping.
Then, to examine which areas of the GC and their surrounding regions were specifically damaged in the rats with impaired behavior, compared with the rats that had lesions but showed no CTA deficit, the lesion topographies of IMPAIRED rats (Z scores of ≥2.0 relative to the mean and SD of the SHAM-LiCl rats) were compared with those of UNIMPAIRED rats for each CTA test with a high-resolution mapping program (using Microsoft Excel). Briefly, with this program, the area of interest (GC and surrounding insular cortex) was plotted in a 2D grid, where each row corresponded to a 50-μm coronal section of tissue and each column corresponded to a specific region of insular cortex or the surrounding area [e.g., AI, DI, GI, claustrum]. The columns were further broken down by medial-lateral subregions (and plotted in subcolumns) (Fig. 2 and Figs. S4 and S5). The lesion in each hemisphere for each rat was mapped onto this grid by scoring the extent of damage in each region and subregion on a ternary scale (0 = less than one-half of the subregion damaged; 0.5 = at least one-half of the subregion damaged; 1.0 = entire subregion damaged); the assigned score was recorded in the corresponding cell on the 2D grid. This was done for each coronal section with lesion and/or GC. The lesions in the left and right hemispheres of each rat were integrated on a third symmetry map such that each grid cell was given the lowest of the two lesion scores of the corresponding grid cells between the two hemispheres. These symmetry maps were further used to generate groupwise lesion maps by averaging the lesion scores in each particular grid cell from a selected group of symmetry maps. IMPAIRED and UNIMPAIRED status was separately assessed for each CTA test and groupwise lesion maps were accordingly generated for each test. A detailed description of the lesion-mapping procedure is given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Charles Badland for his assistance with figure production. This work was supported by funding from the National Institutes of Health Grants R01-DC-DC009821 (to A.C.S.), T32-DC-000044 (Florida State University), and F32-DC-013494 (to L.A.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315624111/-/DCSupplemental.
References
- 1.Spector AC, Glendinning JI. Linking peripheral taste processes to behavior. Curr Opin Neurobiol. 2009;19(4):370–377. doi: 10.1016/j.conb.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol. 1980;44(3):440–455. doi: 10.1152/jn.1980.44.3.440. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol. 1984;51(4):616–635. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- 4.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379(2):329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- 5.Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262(1):27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- 6.Hanamori T, Kunitake T, Kato K, Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol. 1998;79(5):2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa H, Ito S, Murayama N, Hasegawa K. Taste area in granular and dysgranular insular cortices in the rat identified by stimulation of the entire oral cavity. Neurosci Res. 1990;9(3):196–201. doi: 10.1016/0168-0102(90)90004-x. [DOI] [PubMed] [Google Scholar]
- 8.Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92(1):123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- 9.Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210(2):163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- 10.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986;379(2):342–352. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- 11.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311(1):1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 12.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21(12):4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes (Lond) 2009;33(Suppl 2):S34–S43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krushel LA, van der Kooy D. Visceral cortex: Integration of the mucosal senses with limbic information in the rat agranular insular cortex. J Comp Neurol. 1988;270(1):39–54, 62–63. doi: 10.1002/cne.902700105. [DOI] [PubMed] [Google Scholar]
- 15.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399(4):440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Fujita S, Adachi K, Koshikawa N, Kobayashi M. Spatiotemporal dynamics of excitation in rat insular cortex: Intrinsic corticocortical circuit regulates caudal-rostro excitatory propagation from the insular to frontal cortex. Neuroscience. 2010;165(1):278–292. doi: 10.1016/j.neuroscience.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 17.Maffei A, Haley M, Fontanini A. Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol. 2012;22(4):709–716. doi: 10.1016/j.conb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun JJ, Lasiter PS, Kiefer SW. The gustatory neocortex of the rat. Physiol Psychol. 1982;10:13–45. [Google Scholar]
- 19.Lasiter PS, Glanzman DL. Cortical substrates of taste aversion learning: Dorsal prepiriform (insular) lesions disrupt taste aversion learning. J Comp Physiol Psychol. 1982;96(3):376–392. doi: 10.1037/h0077894. [DOI] [PubMed] [Google Scholar]
- 20.Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion. II. Insular (gustatory) cortex. Brain Res. 1998;800(1):40–47. doi: 10.1016/s0006-8993(98)00492-2. [DOI] [PubMed] [Google Scholar]
- 21.Dunn LT, Everitt BJ. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav Neurosci. 1988;102(1):3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549(1):165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- 23.Naor C, Dudai Y. Transient impairment of cholinergic function in the rat insular cortex disrupts the encoding of taste in conditioned taste aversion. Behav Brain Res. 1996;79(1-2):61–67. doi: 10.1016/0166-4328(95)00262-6. [DOI] [PubMed] [Google Scholar]
- 24.Nerad L, Ramírez-Amaya V, Ormsby CE, Bermúdez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiol Learn Mem. 1996;66(1):44–50. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- 25.Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: Effects of conditioning method and timing of lesion. Brain Res. 1999;839(2):323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- 26.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;291(5512):2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 27.Fresquet N, Angst M-J, Sandner G. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose delivery. Behav Brain Res. 2004;153(2):357–365. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat. Eur J Neurosci. 2007;26(9):2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 29.Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122(5):1038–1050. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehberg J, Simon F. Involvement of the insular cortex in retention of conditioned taste aversion is not time dependent. Neurobiol Learn Mem. 2011;95(1):14–18. doi: 10.1016/j.nlm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Rosenblum K, et al. Modulation of protein tyrosine phosphorylation in rat insular cortex after conditioned taste aversion training. Proc Natl Acad Sci USA. 1995;92(4):1157–1161. doi: 10.1073/pnas.92.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17(13):5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5(3):209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 34.Gal-Ben-Ari S, Rosenblum K. Molecular mechanisms underlying memory consolidation of taste information in the cortex. Front Behav Neurosci. 2011;5:87. doi: 10.3389/fnbeh.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto K, Spector AC. Extensive lesions in the gustatory cortex, as conventionally defined in the rat, do not disrupt the retention of a presurgically conditioned taste aversion and do not impair unconditioned concentration-dependent licking to sucrose and quinine. Chem Senses. 2014;39(1):57–71. doi: 10.1093/chemse/bjt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark EW, Bernstein IL. Establishing aversive, but not safe, taste memories requires lateralized pontine-cortical connections. Behav Brain Res. 2009;197(2):356–363. doi: 10.1016/j.bbr.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin JY, Roman C, Reilly S. Taste-potentiated odor aversion learning in rats with lesions of the insular cortex. Brain Res. 2009;1297:135–142. doi: 10.1016/j.brainres.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24(1):71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- 39.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318(5850):655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 40.Touzani K, Sclafani A. Insular cortex lesions fail to block flavor and taste preference learning in rats. Eur J Neurosci. 2007;26(6):1692–1700. doi: 10.1111/j.1460-9568.2007.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333(6047):1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Ed. San Diego: Academic; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.