Significance
We present early evidence linking a high prevalence of caries to a reliance on highly cariogenic wild plant foods in Pleistocene hunter-gatherers from North Africa. This evidence predates other high caries populations and the first signs of food production by several thousand years. We infer that increased reliance on wild plants rich in fermentable carbohydrates caused an early shift toward a disease-associated oral microbiota. Systematic harvesting and processing of wild food resources supported a more sedentary lifestyle during the Iberomaurusian than previously recognized. This research challenges commonly held assumptions that high rates of caries are indicative of agricultural societies.
Abstract
Dental caries is an infectious disease that causes tooth decay. The high prevalence of dental caries in recent humans is attributed to more frequent consumption of plant foods rich in fermentable carbohydrates in food-producing societies. The transition from hunting and gathering to food production is associated with a change in the composition of the oral microbiota and broadly coincides with the estimated timing of a demographic expansion in Streptococcus mutans, a causative agent of human dental caries. Here we present evidence linking a high prevalence of caries to reliance on highly cariogenic wild plant foods in Pleistocene hunter-gatherers from North Africa, predating other high caries populations and the first signs of food production by several thousand years. Archaeological deposits at Grotte des Pigeons in Morocco document extensive evidence for human occupation during the Middle Stone Age and Later Stone Age (Iberomaurusian), and incorporate numerous human burials representing the earliest known cemetery in the Maghreb. Macrobotanical remains from occupational deposits dated between 15,000 and 13,700 cal B.P. provide evidence for systematic harvesting and processing of edible wild plants, including acorns and pine nuts. Analysis of oral pathology reveals an exceptionally high prevalence of caries (51.2% of teeth in adult dentitions), comparable to modern industrialized populations with a diet high in refined sugars and processed cereals. We infer that increased reliance on wild plants rich in fermentable carbohydrates and changes in food processing caused an early shift toward a disease-associated oral microbiota in this population.
Dental caries involves progressive dissolution of the mineral component of dental tissues by organic acids produced during fermentation of food debris by bacteria in dental plaque. The development of carious lesions requires infection by disease-associated oral bacteria, as well as a suitable oral environment for their survival and proliferation (1). Examination of fossil and archeological human dentitions provides direct evidence for the prevalence of dental disease in the past. High caries rates are associated with sedentary food-producing societies that rely on foods rich in fermentable carbohydrates as staples (2–4). Frequencies of carious lesions in archaeological populations range from 2.2–48.1% of teeth for agricultural populations, but only 0–14.3% for hunter-gatherers (4). Analysis of bacterial DNA from ancient calculus deposits provides direct evidence of the disease environment, with recent research pointing toward a shift toward a more disease-associated oral microbiota following the onset of food production (5).
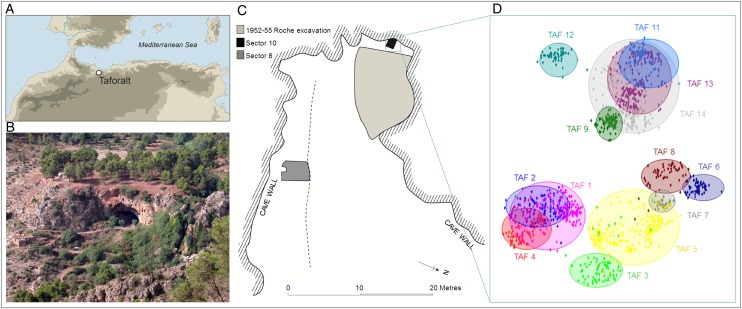
Grotte des Pigeons at Taforalt (Fig. 1) was a key location for ritual and economic activities during the Middle Stone Age and Later Stone Age (Iberomaurusian) (6, 7). Archaeological deposits document a marked intensification in the use of the cave during the Later Stone Age, with the rapid accumulation of thick ashy midden layers, known as the Grey Series, between 15,000 and 12,600 cal B.P. (8). The extreme dryness of the deposits has favored the preservation of organic material, including animal and human bone and charred plant remains. The Grey Series deposits incorporate numerous closely spaced burials of adults, children, and infants in a spatially demarcated area toward the rear of the cave (7, 9, 10). The large number of burials and exceptional preservation of charred macrobotanical remains in contemporaneous occupational deposits provides an opportunity to evaluate both oral health and the role of wild plants in the diet of a preagricultural population.
Fig. 1.
Grotte des Pigeons at Taforalt. (A) Location of the site. (B) View of the cave. (C) Plan showing Sector 8 and Sector 10 and the hypothesized area of the Iberomaurusian necropolis excavated by Roche during 1954 and 1955. (D) Plot of x-y coordinates of skeletal elements assigned to 13 partially articulated skeletons excavated from Sector 10 from 2005 to 2013. The approximate location of the primary depositional context of each skeleton is circled and numbered in the same color.
Methods
New excavations of the Iberomaurusian sequence at Taforalt began in 2003 (Fig. 1 and SI Methods). Trenches were located on the south side of the cave (Sector 8) and in a recess at the back of the cave (Sector 10). Human bone samples from Sector 10 were directly dated by accelerator mass spectrometry using ultrafiltration (11) and calibrated by OxCal 4.1. Oral pathology (12), tooth wear (13, 14), and tooth loss (15) were recorded in partial or complete jaws from 52 adults from the Iberomaurusian necropolis. Adults were classified as young (n = 6), middle (n = 16), old (n = 3), or undetermined (n = 27) using skeletal indicators (16). Macrobotanical remains were collected from 100 soil samples (349.9 L) from four stratigraphic levels in Sector 8 (Fig. S1). The flot (light fraction) was passed through a column of sieves with a mesh size of 4, 2, 1, and 0.5 mm. Plant fossils were identified using a binocular microscope (8–80× magnification).
Results
The recently excavated Sector 10 (Fig. 1) and the burial deposits excavated by Roche in the 1950s form part of a contiguous and spatially demarcated collective burial area, with numerous closely spaced and intercutting burials. The distribution of articulated and disarticulated bones indicates intensive use and reuse of the area, with earlier burials disturbed or truncated by subsequent burials. Thirteen partially articulated skeletons have been recovered from Sector 10, together with disarticulated bones from additional individuals, including an adult skull (Fig. 1). Seven human bone samples from sector 10 yielded age estimations between 15,077 cal B.P. and 13,892 cal B.P. (Table S1), corresponding to the base of the Grey Series deposits in Sector 8. The skeletons excavated by Roche have not been directly dated, but were recovered from a greater depth of Grey Series deposits than those surviving in Sector 10. Burials situated toward the front of the cave and those higher within the deposits are likely to be progressively younger, and hence contemporary with higher levels in the Grey Series deposits recorded in Sector 8. Burials recovered during recent excavations are primary inhumations of complete bodies (7). The burials excavated in the 1950s include some bones or partial skeletons showing evidence of deliberate post mortem modification, including cut marks and extensive use of ochre (17, 18). This finding suggests a diversification in the treatment of bodies and skeletal parts during the period in which the cemetery was in use (7).
The Iberomaurusian occupants of Taforalt experienced impaired dental function from early adulthood because of a combination of poor oral health, heavy tooth wear, and cultural modification of the anterior dentition (Fig. 2, Table 1, and Table S2). Evulsion of the upper central incisors was recorded in more than 90.0% of adults from Taforalt (19). This intervention is characteristic of Iberomaurusian populations (20); it has a marked effect on dental and craniofacial architecture and limits the masticatory use of the anterior teeth (21). The prevalence of oral pathologies is exceptionally high (Table 1). More than half of surviving teeth (51.2%) exhibited carious lesions and only three of 52 adults showed no evidence of caries. Alveolar resorption affected 58.9% of observed teeth, resulting in exposure of the root surfaces. Root caries were present in 42.9% of individuals and 16.0% of carious teeth. The prevalence of root caries, caries forming at the mesial and distal contact points between teeth, and gross caries, often associated with a complete loss of structural integrity of the tooth, increased with age (Table 1).
Fig. 2.
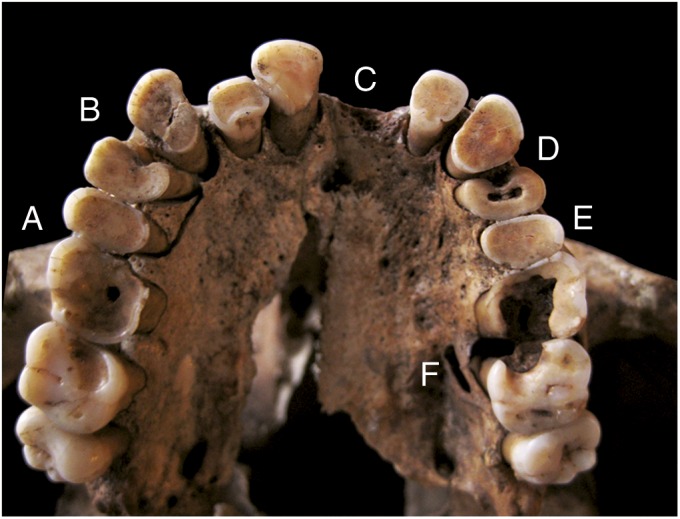
Oral pathology on the maxilla of Taforalt XI C1: (A) heavy tooth wear, (B) contact caries, (C) evulsion, (D) attrition caries, (E) gross caries, (F) abscess.
Table 1.
Number of observed sockets affected by postmortem and antemortem tooth loss and abscesses, and teeth affected by alveolar resorption and caries
| Pathology | Young adult |
Middle adult |
Old adult |
Undetermined age |
Total |
|||||
| n | % | n | % | n | % | n | % | n | % | |
| Observable sockets | 167 | 287 | 86 | 401 | 941 | |||||
| Postmortem tooth loss* | 25 | 15.0 | 36 | 12.5 | 9 | 10.5 | 104 | 25.9 | 174 | 18.5 |
| Postmortem tooth loss* | 10 | 6.0 | 23 | 8.0 | 22 | 25.6 | 40 | 10.0 | 95 | 10.1 |
| Postcanine sockets | 107 | 181 | 55 | 256 | 599 | |||||
| Postcanine antemortem tooth loss | 1 | 0.9 | 6 | 3.3 | 16 | 29.1 | 22 | 8.6 | 45 | 7.5 |
| Abscesses* | 2 | 1.2 | 9 | 3.1 | 17 | 19.8 | 23 | 5.7 | 51 | 5.4 |
| Observed teeth | 132 | 228 | 55 | 257 | 672 | |||||
| Alveolar resorption† | 75 | 56.8 | 155 | 68.0 | 28 | 50.9 | 138 | 53.7 | 396 | 58.9 |
| Carious teeth† | 44 | 33.3 | 125 | 54.8 | 29 | 52.7 | 146 | 56.8 | 344 | 51.2 |
| Caries type‡ | ||||||||||
| Crown caries | 40 | 90.9 | 123 | 98.4 | 29 | 100.0 | 140 | 54.5 | 332 | 96.5 |
| Attrition caries | 38 | 86.4 | 112 | 89.6 | 27 | 93.1 | 129 | 50.2 | 306 | 89.9 |
| Molar fissure caries§ | 4 | 3.7 | 11 | 6.1 | 0 | 0.0 | 18 | 7.0 | 33 | 5.5 |
| Buccal caries | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Lingual caries | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 0.3 |
| Mesial caries | 0 | 0.0 | 8 | 6.4 | 1 | 3.5 | 10 | 3.9 | 19 | 5.5 |
| Distal caries | 1 | 2.3 | 7 | 5.6 | 1 | 3.5 | 2 | 0.8 | 11 | 3.2 |
| Pit caries | 0 | 0.0 | 6 | 4.8 | 0 | 0.0 | 4 | 1.6 | 10 | 2.9 |
| Rim caries | 0 | 0.0 | 8 | 6.4 | 1 | 3.5 | 3 | 1.2 | 12 | 3.5 |
| Gross caries | 0 | 0.0 | 9 | 7.2 | 8 | 27.6 | 11 | 7.5 | 28 | 8.1 |
| Root caries | 4 | 9.1 | 13 | 10.4 | 4 | 13.7 | 34 | 13.2 | 55 | 16.0 |
As percent of observed sockets.
As percent of observed teeth.
As percent of carious teeth.
As percent of postcanine carious teeth.
Charred macrobotanical remains extracted from contemporaneous occupation layers in Sector 8 (Fig. S1) provide direct evidence of plant foods available and proxy dietary information. Twenty-two taxa were identified (Table 2 and Fig. S2). Acorns from the Holm oak (Quercus ilex L.) and pine nuts from the Maritime pine (Pinus pinaster Aiton) were the most abundant edible plants. Other edible plants include juniper (Juniperus phoenicea L.), Terebinth pistachio (Pistacia terebinthus L.), wild pulses (Lens cf. nigricans, Lathryus sp., Vicia sp.), and wild oats (Avena sp.). These plants provide a broad range of nutrients and their representation reveals a preference for plant foods rich in carbohydrates and fats (Table S3). The majority of taxa ripen in autumn with a few ripening in spring or early summer, implying a human presence at the site from at least late spring to autumn. The abundance of acorns and pine nuts points to the deliberate selection of storable seeds with a high nutritional value that could be used as a staple food for most of the year.
Table 2.
Macro botanical remains from occupational levels in Sector 8
| Plant remains | Common name | Yellow Series | Grey Series layers |
Total | ||
| 28–29 | 24–27 | 0–23 | ||||
| Avena sp., seed | Wild oat | 13 | 13 | |||
| cf. Bromus sp., seed | Brome | 2 | 2 | 4 | ||
| Cariophyllaceae, seed | Pink | 1 | 1 | |||
| Chenopodiaceae, seed | Goosefoot | 1 | 1 | |||
| Cistaceae, seed | Rock rose | 1 | 1 | |||
| Ephedra sp., bract | Ephedra | 3 | 3 | 6 | ||
| Fabaceae, seed | Wild pulse | 1 | 2 | 3 | 6 | |
| Fumaria sp., seed | Fumitory | 1 | 2 | 3 | ||
| Galium sp., seed | Bedstraw | 9 | 11 | 42 | 62 | |
| Juniperus phoenicea L., seed | Juniper | 5 | 109 | 114 | ||
| Lens cf. nigricans (M. Bieb.) Godr., seed | Wild lentil | 1 | 2 | 3 | ||
| Pinus pinaster Ait., seed | Maritime pine | 2 | 2 | 20 | 24 | |
| Pinus pinaster Ait., seed scale | Maritime pine | 28 | 31 | 232 | 291 | |
| Pistacia terebinthus L., seed | Terebinth pistachio | 64 | 64 | |||
| Poaceae, seed | Grass | 9 | 2 | 11 | ||
| Quercus ilex L., cupule | Holm oak | 5 | 5 | |||
| Quercus sp., cotyledon | Oak | 14 | 14 | |||
| Quercus sp., cupule | Oak | 101 | 76 | 204 | 381 | |
| Quercus sp., abscission scar | Oak | 1 | 3 | 2 | 73 | 79 |
| Quercus sp., pericarp | Oak | 16 | 16 | |||
| Rosaceae, seed | Rose | 1 | 1 | |||
| Rosaceae-type, seed | Rose-type | 1 | 1 | 2 | ||
| Rosaceae-type, fruit | Rose-type | 3 | 3 | |||
| Rubiaceae, seed | Bedstraw | 1 | 1 | |||
| Sambucus nigra/ebulus L., seed | Elderberry | 1 | 1 | |||
| Small seeded legume, seed | Wild pulse | 1 | 1 | 1 | 3 | |
| Stipa tenacissima L., rhizome | Alfa/ esparto | 10 | 3 | 61 | 74 | |
| Tetraclinis articulata (Vahl) Masters, leaf | Arar | 4 | 4 | |||
| Vicia/ Lathyrus sp., seed | Wild pulse | 1 | 3 | 4 | 15 | 23 |
| Woody legume, seed | Woody legume | 21 | 21 | |||
| Indeterminate seed | 3 | 7 | 17 | 27 | ||
| Indeterminate fruit | 3 | 3 | ||||
| Indeterminate nut | 8 | 8 | ||||
| Total number plant remains | 20 | 189 | 120 | 941 | 1270 | |
| Volume of sediment in liters | 67.1 | 68.1 | 23.8 | 186.7 | 345.7 | |
| Item density per liter sediment | 0.29 | 2.77 | 5.04 | 5.04 | 3.67 | |
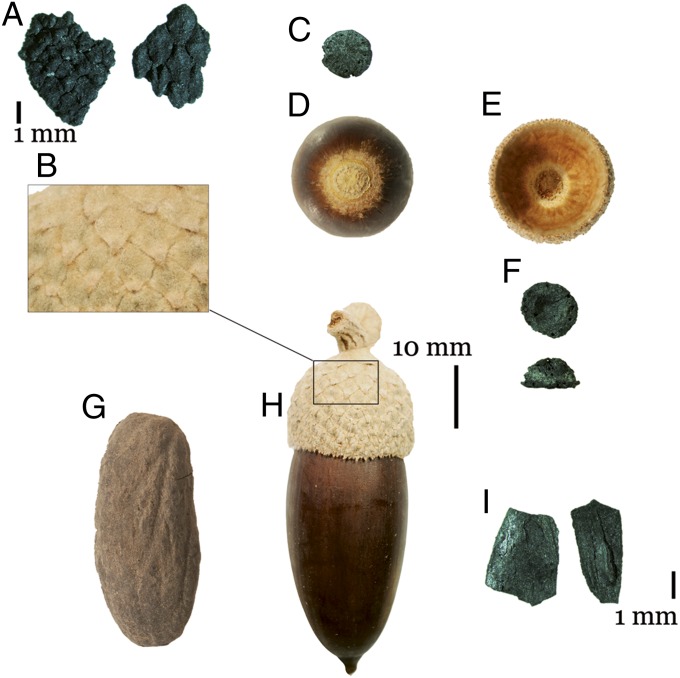
Species identifications imply preferential exploitation of sweet Holm oak acorns, which have low tannin content and can be eaten without extensive processing (22). Acorn remains comprise mainly unpalatable parts that would have been discarded before consumption of the seed, such as bases of the acorn shell (abscission scar), shells (pericarp), and cup (cupule) fragments (Fig. 3). The rarity of charred seeds indicates that acorns were consumed raw or underwent an initial processing stage that did not involve the use of fire. The high number of cups points to systematic gathering and storage of unripe acorns, as cups detach naturally once the acorn is ripe. Ethnographic records describe the beating of trees to enable collection of green acorns, as ripe acorns are more prone to be eaten by ground-feeding competitors or infested by insects or fungi (22). Acorns could have been dried and stored, and subsequently peeled and pounded to produce flour or boiled and eaten whole (23).
Fig. 3.
Modern acorn (Quercus ilex) and archaeological specimens from Taforalt: (A) scales, archaeological; (B) scales, modern; (C) abscission scar, archaeological; (D) abscission scar, modern; (E) cupule, internal side, modern; (F) cupule, internal and lateral view, archaeological; (G) seed, archaeological; (H) acorn, modern; (I) Shell or pericarp, archaeological.
Similarly to acorns, macrobotanical evidence of pine nuts in Sector 8 is composed primarily of food debris. Remains comprise relatively few seeds but a large number of seed scales, an inedible part of the cone that contains the seed. Pine nuts are typically gathered unripe and stored within their cones. When required, the cones are laid out in the sun or placed over a fire to open the scales and release the seeds for consumption (24). The nuts can be eaten unprocessed as a snack or pounded and boiled to make porridge (24). In P. pinaster, nuts are covered by a thin shell (approximately 0.1 mm) and there is no need to unshell the seed before eating.
Charred aerial root (rhizome) fragments of esparto (alfa) grass (Stipa tenacissima L.) are common throughout the Grey Series samples. Leaves from esparto grass are a traditional material for basketry and rhizome fragments are a common by-product of this process. Charred rhizome fragments have been recorded at other prehistoric sites from the Iberian Peninsula and Morocco (25). The presence of these fragments implies that whole esparto plants were uprooted and carried to the site, where the leaves were used to produce basketry and unwanted rhizome fragments discarded into the fire. Baskets produced from esparto grass could have been used for collection, processing, storage, and cooking of seasonal foods, such as acorns.
Discussion
The late Iberomaurusian inhabitants of Taforalt were complex hunter-gatherers as demonstrated by the formation of rich cultural deposits, elaborate burials within a demarcated area, the potential use of grindstones in food preparation, and abundant evidence of systematic harvesting and processing of wild food resources, including acorns, pine nuts, and molluscs (26). The presence of rhizome fragments of esparto grass suggests that they were equipped with baskets and other plant fiber-made tools that could be used to collect, store, and process food plants. Taforalt was clearly a key location for ritual and economic activities but evidence for year-round use of the site is so far inconclusive. The more rapid formation of cultural deposits after 15,000 cal B.P. points toward an intensification of activity involving more prolonged occupation periods or a larger population or both. The cemetery includes complete primary burials, indicating that death occurred within the vicinity of the site (7), but there is some evidence for deliberate secondary deposition of skeletal elements with post mortem modifications involving removal of soft tissues (12, 17). These elements may be selected parts of individuals who died elsewhere that were carried back for burial, suggesting that at least part of the community traveled away from the site on a seasonal basis, or may simply reflect an elaboration of funerary behavior over time.
Macrobotanical evidence suggests that the site was occupied from at least late spring to autumn, but both pine nuts and acorns could have been stored, enabling occupation through the winter. The recovery of high quantities of cupules and seed scales indicates that both acorns and pine nuts were systematically collected. Acorns could have been stored unripe and unprocessed (with cupule and pericarp) and then shelled before cooking, whereas pine nuts were probably stored inside the cones. Reliance on edible acorns as a staple food could account for the high caries prevalence at Taforalt because frequent consumption of fermentable carbohydrates is a key factor in the initiation and progression of this disease (2). Exploitation of a variety of acorns as a source of carbohydrates is well documented in the ethnographic record, both historically and in recent times (27). In the Iberian Peninsula, the consumption of sweet acorns from the Holm Oak as a raw food or processed to make flour is attested archaeologically and historically (22). Storage of acorns can lead to an increase in sweetness (23). Processing and cooking of starchy foods, such as acorns, to improve digestibility causes increased food stickiness and reduced food clearance rates within the oral cavity, providing an ideal environment for acid-tolerant bacteria (28). Carbohydrates from other identified food plants, including wild pulses and wild oats, may have contributed to the high caries prevalence at Taforalt. Land snails were intensively collected and consumed during the Iberomaurusian (26), and although these are not known to be cariogenic, abrasive particles present within the snails may contribute to tooth wear (29) and, hence, influence the location of carious lesions.
The rarity of charred acorn seeds throughout the Grey Series implies that acorns were not directly exposed to fire during preparation. Huge quantities of burnt and heat-shattered rock are found throughout the Grey Series deposits and could be the result of deliberate heating of stones for cooking purposes. Heated stones can be used to boil fresh or dried acorns in water-filled vessels made from basketry or animal skin (23). Alternatively patties made from ground acorns could have been cooked directly on heated stones. The high rate of tooth wear observed at Taforalt and the predominance of attritional and root caries are consistent with use of grindstones in food preparation. Incorporation of abrasive particles from grindstones into ground foods contributes to rapid attrition of the occlusal surface, leading to continuous tooth eruption to maintain functional dental occlusion and the initiation of caries on worn occlusal surfaces and exposed roots (2, 30). Ochre-stained stones (palettes and mortars) were recovered from recently excavated burial deposits, where they were placed within or directly above individual burials. Previous excavations have recovered grindstones in association with Iberomaurusian burials at Taforalt and Afalou (9, 31). The presence of these grindstones demonstrates knowledge of grinding or pounding activities that could have been used in the preparation of acorns, and their inclusion in funerary deposits suggests that they were accorded a high value.
Starch grains have been recovered on grindstones from Mid-Upper Paleolithic sites in Europe and the Levant, indicating that processing of plant foods was practiced from at least ∼30 ka (32, 33). Starch residues preserved in dental calculus provide direct evidence that Neanderthals consumed cooked carbohydrate-rich foods (34, 35). Despite this, Paleolithic modern humans and Neanderthals have a low prevalence of caries (36). Caries are present in semisedentary Natufian hunter-gatherers from the Levant populations whose diet included wild cereals prepared using grindstones, but at much lower prevalence than at Taforalt (37). The lower rates of caries in earlier and contemporaneous human groups may indicate a lesser reliance on foods rich in fermentable carbohydrates, but may also reflect a difference in bacterial virulence or changes in the pattern and likelihood of transmission between infected and uninfected individuals. Reconstruction of the demographic history of Streptococcus mutans, the main causative agent of human dental caries today, indicates an exponential population expansion within a time frame that broadly coincides with the earliest evidence for plant cultivation and domestication ∼10 ka (38). Nevertheless the estimated confidence interval for this expansion (95% confidence interval 3,268–14,344 y ago) does not preclude its onset before the origins of food production and the Taforalt population falls at the upper end of this interval.
The presence of carious lesions in 94% of adult dentitions implies that most, if not all, adults at Taforalt were infected by cariogenic oral bacteria. The colonization, survival, and proliferation of oral bacteria are influenced by a complex interaction of behavioral and environmental factors, as well as bacterial virulence (2, 39). The risk of transmission (colonization) is increased by frequent close contact between infected and noninfected individuals, sharing of food and utensils, and behaviors leading to higher salivary levels of cariogenic bacteria in source individuals, such as poor oral hygiene or frequent snacking on carbohydrate-rich foods (1). Correlates of a more sedentary lifestyle and changes in resource exploitation could have contributed to a high risk of both horizontal and vertical transmission of cariogenic bacteria. Potential risk factors include increased population density and shared childcare, resulting in close contact of uninfected infants with a larger number of potentially infected adults and children. Virulence factors, including adherence ability, acid tolerance, and acidogenicity influence the survival and proliferation of oral bacteria and, hence, the magnitude of pH reduction and the extent of enamel demineralization following fermentation of carbohydrates in the oral cavity (39). The Iberomaurusian occupants of Taforalt may have harbored particularly virulent bacterial strains that spread rapidly among the population, although the specific causal agent has not been identified.
Taforalt presents multidisciplinary evidence linking a high prevalence of caries to a reliance on highly cariogenic wild plant foods, predating other high caries populations and the first signs of food production by several thousand years. Systematic exploitation of wild plant resources may have developed within the context of a semiarid and highly seasonal environment during the latter part of the Iberomaurusian (40). Consumption of carbohydrates among modern hunter-gatherers is highest in groups inhabiting semiarid regions and tropical grasslands (41). Storage of plant foods rich in fat and carbohydrate is a traditional strategy for coping with seasonal food stress (42, 43). Regardless of its origins, this subsistence strategy had profound consequences on oral health. The combination of high caries prevalence, rapid tooth wear, and periodontal disease, together with deliberate removal of the upper central incisors—typically by early adulthood—would have severely compromised use of the dentition for mastication. Periodontal disease has been linked to serious health complications, such as respiratory and cardiovascular disease. Although there is uncertainty of the directionality between oral pathology and systemic health (44), the high prevalence of oral pathology recorded at Taforalt can be interpreted as an indication of overall poor health status with increased disease load and mortality.
Supplementary Material
Acknowledgments
We thank Professor de Lumley and Amélie Vialet for access to collections; Steve Ward, Victoria Taylor, Ingrid Brack, and Cath Price for sample retrieval and extraction of biological remains; the excavation team and Marijke Van Der Veen, Joel Irish, Ali Freyne, and Chris Stringer for discussions; the reviewers and the editor for helpful comments on the manuscript; and the National Institute of Archaeological Science and Heritage (INSAP, Morocco) and the project Protars P32/09-CNRST (Morocco). This research was supported by the Leverhulme Trust (F/08 735/F) and the Calleva Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.G.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318176111/-/DCSupplemental.
References
- 1.Law V, Seow WK, Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J. 2007;52(2):93–100, quiz 159. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 2.Hillson S. In: Technique and Application in Dental Anthropology. Irish JD, Nelson GC, editors. Cambridge, UK: Cambridge Univ Press; 2008. pp. 111–135. [Google Scholar]
- 3.Gibbons A. Evolutionary biology. An evolutionary theory of dentistry. Science. 2012;336(6084):973–975. doi: 10.1126/science.336.6084.973. [DOI] [PubMed] [Google Scholar]
- 4.Lanfranco LP, Eggers S. The usefulness of caries frequency, depth, and location in determining cariogenicity and past subsistence: A test on early and later agriculturalists from the Peruvian coast. Am J Phys Anthropol. 2010;143(1):75–91. doi: 10.1002/ajpa.21296. [DOI] [PubMed] [Google Scholar]
- 5.Adler CJ, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45(4):450–455, e1. doi: 10.1038/ng.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouzouggar A, et al. 82,000-year-old shell beads from North Africa and implications for the origins of modern human behavior. Proc Natl Acad Sci USA. 2007;104(24):9964–9969. doi: 10.1073/pnas.0703877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey L, Bello SM, Turner E, Bouzouggar A, Barton N. Iberomaurusian funerary behaviour: Evidence from Grotte des Pigeons, Taforalt, Morocco. J Hum Evol. 2012;62(2):261–273. doi: 10.1016/j.jhevol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Barton RNE, et al. Origins of the Iberomaurusian in NW Africa: New AMS radiocarbon dating of the Middle and Later Stone Age deposits at Taforalt Cave, Morocco. J Hum Evol. 2013;65(3):266–281. doi: 10.1016/j.jhevol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Roche J. L'Épipaléolithique Marocain [The Moroccan Epipalaeolithic] Portugal: Livraria Bertrand; 1963. French. [Google Scholar]
- 10.Ferembach D. La Nécropole Épipaléolithique de Taforalt (Maroc oriental): Étude des Squelettes Humains. [The Epipalaeolithic Necropolis of Taforalt (Eatern Morocco: Study of the Human Skeletons] Rabat: Édita-Casablanca; 1962. French. [Google Scholar]
- 11.Brock F, Higham T, Ditchfield P, Ramsey CB. Current pretreatment methods for AMS radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (ORAU) Radiocarbon. 2010;52(1):103–112. [Google Scholar]
- 12.Hillson S. Recording dental caries in archaeological human remains. Int J Osteoarchaeol. 2001;11(4):249–289. [Google Scholar]
- 13.Smith BH. Patterns of molar wear in hunger-gatherers and agriculturalists. Am J Phys Anthropol. 1984;63(1):39–56. doi: 10.1002/ajpa.1330630107. [DOI] [PubMed] [Google Scholar]
- 14.Scott EC. Dental wear scoring technique. Am J Phys Anthropol. 1979;51(3):213–217. [Google Scholar]
- 15.Ortner DJ, Putschar W. Identification of Pathological Conditions in Human Skeletal Remains. Washington, DC: Smithsonian Institution; 1981. [Google Scholar]
- 16.Buikstra JE, Ubelaker DH. Standards for Data Collection from Human Skeletal Remains: Proceedings of a Seminar at the Field Museum of Natural History. Fayetteville: Arkansas Archaeological Survey Press; 1994. [Google Scholar]
- 17.Mariotti V, Bonfiglioli B, Facchini F, Condemi S, Belcastro MG. Funerary practices of the Iberomaurusian population of Taforalt (Tafoughalt; Morocco, 11-12,000BP): New hypotheses based on a grave by grave skeletal inventory and evidence of deliberate human modification of the remains. J Hum Evol. 2009;56(4):340–354. doi: 10.1016/j.jhevol.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Belcastro MG, Condemi S, Mariotti V. Funerary practices of the Iberomaurusian population of Taforalt (Tafoughalt, Morocco, 11-12,000 BP): The case of Grave XII. J Hum Evol. 2010;58(6):522–532. doi: 10.1016/j.jhevol.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 19.De Groote I, Humphrey L. Evulsion practices among the Iberomaurusians. Bull Mém Soc Anthropol. 2011;23:S9–S10. [Google Scholar]
- 20.Humphrey LT, Bocaege E. Tooth evulsion in the Maghreb: Chronological and geographical patterns. Afr Archaeol Rev. 2008;25(1–2):109–123. [Google Scholar]
- 21.Hadjouis D. Hominids of upper Paleolithic of Afalou Bou Rhummel (Bedjaia, Algeria). New interpretation of the cranio-facial cinetics and dental mutilations effects. Cranial malformations, growth's perturbation, dental anomalies and illness. Anthropologie. 2002;106(3):337–375. [Google Scholar]
- 22.Pereira-Sieso J. In: in Arqueología, Sociedad, Territorio y Paisaje. Estudios Sobre Prehistoria Reciente, Protohistoria y Transición al Mundo Romano [Archaeology, Society, Territory and Landscape. Studies about Recent Prehistory, Protohistory, and Transition to the Roman World] Bueno P, Gilman A, Martín-Morales C, Sánchez-Palencia FJ, editors. Madrid: CSIC; 2010. pp. 279–290. Spanish. [Google Scholar]
- 23.Mason S, Nesbitt M. In: From Foragers to Farmers. Fairbairn A, Weiss E, editors. Oxford, UK: Oxbow; 2009. pp. 71–85. [Google Scholar]
- 24.Lanner RM. The Pinon Pine: A Natural and Cultural History. Reno: Univ of Nevada Press; 1991. [Google Scholar]
- 25.Morales J, et al. The origins of agriculture in North-West Africa: Macro-botanical remains from Epipalaeolithic and Early Neolithic levels of Ifri Oudadane (Morocco) J Archaeol Sci. 2013;40(6):2659–2669. [Google Scholar]
- 26.Tayor VK, et al. The Epipalaeolithic (Iberomaurusian) at Grotte des Pigeons (Taforalt), Morocco: A preliminary study of the land Mollusca. Quat Int. 2011;244(1):5–14. [Google Scholar]
- 27.Mason S. In: in Food in Antiquity. Wilkins J, Harvey D, Dobson M, editors. Exeter, UK: Univ of Exeter Press; 1995. pp. 12–24. [Google Scholar]
- 28.Tayles N, Domett K, Halcrow S. Can dental caries be interpreted as evidence of farming? The Asian experience. Front Oral Biol. 2009;13:162–166. doi: 10.1159/000242411. [DOI] [PubMed] [Google Scholar]
- 29.Lucas PW, et al. Mechanisms and causes of wear in tooth enamel: Implications for hominin diets. J R Soc Interface. 2013;10(80):20120923. doi: 10.1098/rsif.2012.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinl A, et al. Caries frequency and distribution in an early medieval Avar population from Austria. Oral Dis. 2010;16(1):108–116. doi: 10.1111/j.1601-0825.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 31.Hachi S. ). Aux Origins des Arts Premiers en Afrique du Nord [Origins of the First Arts in North Africa] Alger: Centre National de Recherches Préhistoriques, Anthropologiques et Historiques; 2003. French. [Google Scholar]
- 32.Revedin A, et al. Thirty thousand-year-old evidence of plant food processing. Proc Natl Acad Sci USA. 2010;107(44):18815–18819. doi: 10.1073/pnas.1006993107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piperno DR, Weiss E, Holst I, Nadel D. Processing of wild cereal grains in the Upper Palaeolithic revealed by starch grain analysis. Nature. 2004;430(7000):670–673. doi: 10.1038/nature02734. [DOI] [PubMed] [Google Scholar]
- 34.Henry AG, Brooks AS, Piperno DR. Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium) Proc Natl Acad Sci USA. 2011;108(2):486–491. doi: 10.1073/pnas.1016868108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy K, et al. Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften. 2012;99(8):617–626. doi: 10.1007/s00114-012-0942-0. [DOI] [PubMed] [Google Scholar]
- 36.Sołtysiak A. Comment: low dental caries rate in Neandertals: The result of diet or the oral flora composition? Homo. 2012;63(2):110–113. doi: 10.1016/j.jchb.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Eshed V, Gopher A, Pinhasi R, Hershkovitz I. Paleopathology and the origin of agriculture in the Levant. Am J Phys Anthropol. 2010;143(1):121–133. doi: 10.1002/ajpa.21301. [DOI] [PubMed] [Google Scholar]
- 38.Cornejo OE, et al. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol Biol Evol. 2013;30(4):881–893. doi: 10.1093/molbev/mss278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banas JA. Virulence properties of Streptococcus mutans. Front Biosci. 2004;9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 40.Barton RNE, et al. In: Rethinking the Human Revolution: New Behavioural & Biological Perspectives on the Origins and Dispersal of Modern Humans. Mellars P, Stringer C, Bar-Yosef CO, Boyle K, editors. Cambridge, UK: Macdonald Institute for Archaeological Research Monograph Series; 2007. pp. 177–186. [Google Scholar]
- 41.Ströhle A, Hahn A. Diets of modern hunter-gatherers vary substantially in their carbohydrate content depending on ecoenvironments: Results from an ethnographic analysis. Nutr Res. 2011;31(6):429–435. doi: 10.1016/j.nutres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Speth JD. Early hominid subsistence strategies in seasonal habitats. J Archaeol Sci. 1987;14(1):13–29. [Google Scholar]
- 43.Hardy BL. Climatic variability and plant food distribution in Pleistocene Europe: Implications for Neanderthal diet and subsistence. Quat Sci Rev. 2010;29(5–6):662–679. [Google Scholar]
- 44.Garcia R, Dietrich T. Introduction to periodontal epidemiology. Periodontol 2000. 2012;58(1):7–9. doi: 10.1111/j.1600-0757.2011.00412.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.