Significance
The carotid body chemosensory reflex is a principal regulator of breathing and blood pressure. Humans and experimental animals display marked interindividual variation in the carotid body chemosensory reflex; however, the underlying mechanisms are not known. Here, we demonstrate differences in carotid body O2 sensing to be mediated by inherent variations in carbon monoxide-sensitive hydrogen sulfide signaling in three distinct rat strains. Hyposensitivity of the carotid body to hypoxia was associated with higher CO and lower H2S levels, poor ventilatory adaptation to hypobaric hypoxia, and pulmonary edema. Hypersensitivity of the carotid body to low O2 was accompanied by reduced CO, greater H2S generation, and hypertension.
Keywords: high-altitude hypoxia, gasotransmitters, sympathetic tone, cystathionine-γ-lyase
Abstract
Oxygen (O2) sensing by the carotid body and its chemosensory reflex is critical for homeostatic regulation of breathing and blood pressure. Humans and animals exhibit substantial interindividual variation in this chemosensory reflex response, with profound effects on cardiorespiratory functions. However, the underlying mechanisms are not known. Here, we report that inherent variations in carotid body O2 sensing by carbon monoxide (CO)-sensitive hydrogen sulfide (H2S) signaling contribute to reflex variation in three genetically distinct rat strains. Compared with Sprague-Dawley (SD) rats, Brown-Norway (BN) rats exhibit impaired carotid body O2 sensing and develop pulmonary edema as a consequence of poor ventilatory adaptation to hypobaric hypoxia. Spontaneous Hypertensive (SH) rat carotid bodies display inherent hypersensitivity to hypoxia and develop hypertension. BN rat carotid bodies have naturally higher CO and lower H2S levels than SD rat, whereas SH carotid bodies have reduced CO and greater H2S generation. Higher CO levels in BN rats were associated with higher substrate affinity of the enzyme heme oxygenase 2, whereas SH rats present lower substrate affinity and, thus, reduced CO generation. Reducing CO levels in BN rat carotid bodies increased H2S generation, restoring O2 sensing and preventing hypoxia-induced pulmonary edema. Increasing CO levels in SH carotid bodies reduced H2S generation, preventing hypersensitivity to hypoxia and controlling hypertension in SH rats.
Oxygen, an essential substrate for generating ATP, is vital for sustaining much of life on earth. A low availability of oxygen directs vertebrates’ complex respiratory and cardiovascular systems to maintain optimal oxygenation of tissues by increasing ventilation and blood pressure. Interestingly, ventilatory responses to hypoxia are not uniform but, instead, exhibit substantial variation among humans (1). These varied ventilatory responses to hypoxia result in dire physiological consequences: a diminished hypoxic ventilatory response can result in poor adaptation to low O2 environments (2) and high-altitude pulmonary edema (3–5), whereas a heightened response is associated with essential hypertension (6). Similar variations in hypoxic response have also been documented in different strains of rodents (7–10). In comparison with Sprague-Dawley (SD) rats, Brown-Norway (BN) rats display a markedly reduced ventilatory response to hypoxia (8, 9), whereas Spontaneous Hypertensive (SH) rats exhibit an augmented response (11). SH rats also present enhanced sympathetic nerve activity and hypertension (7). Despite the physiological significance, the mechanisms underlying interindividual variation in systemic responses to hypoxia are not known.
The carotid body is the key sensor of arterial blood oxygen, and its chemosensory reflex is a critical regulator of breathing, sympathetic tone, and blood pressure (12, 13). Differing responses in ventilation and sympathetic nerve activity to ambient oxygen levels may reflect inherent variations in the O2 sensing ability of the carotid body. Although a number of hypotheses have been proposed to explain carotid body-mediated O2 sensing (12, 13), emerging evidence suggests that the gasotransmitter hydrogen sulfide (H2S) is required for O2 sensing by the carotid body (14–17). Hypoxia results in increased H2S generation in the carotid body. Glomus cells, the primary O2 sensing cells in the carotid body, express cystathionine-γ-lyase (CSE), an H2S catalyzing enzyme (15, 16). Homozygous CSE-null mice display diminished H2S generation and a severely impaired response to hypoxia (15, 16). We hypothesized that variations in the chemosensory reflex are a result of differences in H2S signaling in the carotid body. We tested this possibility by examining the carotid body response to hypoxia in SD, BN, and SH rats and further assessed the physiological consequences of variations in carotid body O2 sensing on ventilatory adaptations to hypoxia and blood pressure.
Results
Variability in Carotid Body O2 Sensing.
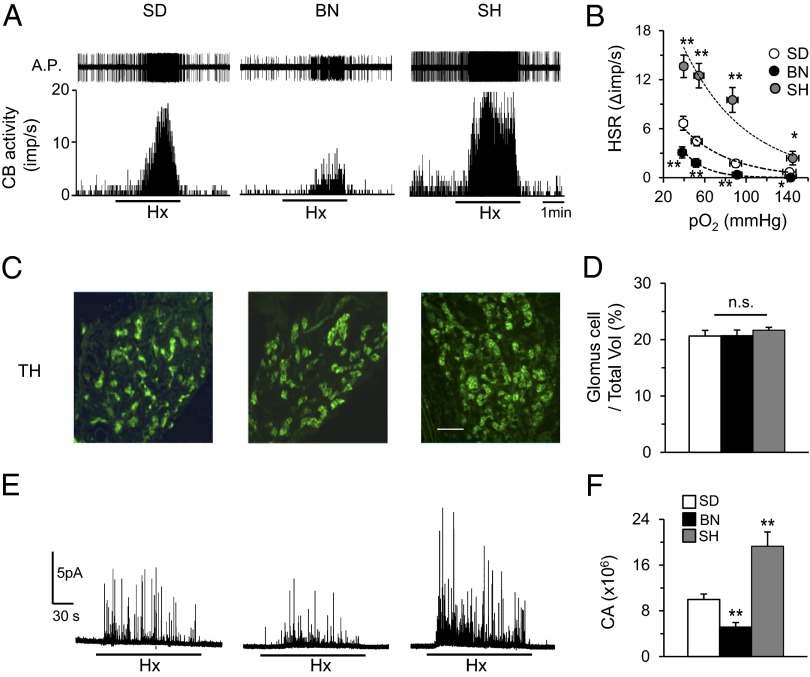
The carotid body hypoxic response was assessed by measuring sensory activity from ex vivo preparations to exclude influences from circulating vasoactive substances in intact rats. We examined the carotid body response to hypoxia in 5–6-wk-old SD, BN, and SH rats because SH rats develop hypertension beyond this age, potentially confounding results. The body weights of BN and SH rats were less than those of SD rats (Table S1). BN carotid bodies exhibited lower baseline activity and a severely impaired response to hypoxia. In contrast, carotid bodies from SH rats showed elevated baseline activity and an augmented hypoxic response (Fig. 1 A and B).
Fig. 1.
Variability in carotid body O2 sensing. (A) Sensory response of isolated carotid bodies to hypoxia (Hx; PO2 ∼36 mm Hg; at black bar) in SD, BN, and SH rats. Action potentials (A.P.) are from the sinus nerve. Integrated carotid body sensory activity (CB activity) is presented as impulses per second (imp/s). (B) Hypoxic sensory response (HSR) to graded hypoxia from SD, BN, and SH rats, measured as the difference in response between baseline and hypoxia (Δimp/s). Data are mean ± SEM from 15–20 fibers from seven to nine rats each. (C) Tyrosine hydroxylase (TH), a marker of glomus cells expression in carotid bodies from SD, BN, and SH rats. Horizontal bar represents 50 μm. (D) Average (mean ± SEM) data of ratio of number of glomus cell/total volume of the carotid bodies from n = 4 rats each. (E) Glomus cell CA secretion in hypoxic conditions (Hx; PO2 ∼30 mm Hg; at black bar) in SD, BN, and SH rats. (F) Average (mean ± SEM) data of CA secretion from n = 15–20 cells from each strain. *P < 0.05; **P < 0.01; n.s. P > 0.05 (i.e., not significant). See also Fig. S1 and Table S1.
Sensory activity of BN carotid bodies treated with NaCN, a potent carotid body stimulant, was comparable to SD, indicating that the diminished BN hypoxic response was not a result of reduced excitability of sensory nerve endings (Fig. S1 A and B). Sensory response to CO2, another physiological stimulus, was also comparable among all three strains (Fig. S1 C and D).
To determine whether variations in the number of glomus cells, the primary O2 sensing cells, account for the differences in carotid body O2 sensing, carotid body sections were stained for tyrosine hydroxylase, a marker of glomus cells (18) (Fig. 1C). Morphometric analysis revealed no significant difference in the ratio of number of glomus cells to the total volume of carotid body tissue among the three strains (Fig. 1D).
Hypoxia induces catecholamine (CA) secretion from glomus cells (15). Hypoxia-evoked CA secretion from individual cells was monitored by carbon-fiber amperometry. Consistent with the sensory nerve responses of the carotid body, the glomus cell hypoxic sensitivity was attenuated in BN and augmented in SH relative to SD (Fig. 1 E and F). However, glomus cell CA secretion was comparable across all three strains when treated with 40 mM KCl, a nonselective depolarizing stimulus (Fig. S1 E and F). These findings demonstrate inherent variation in O2 sensing by the carotid body, where BN and SH rats are hypo- and hypersensitive, respectively, to hypoxia relative to SD rats.
Altered H2S Generation in the Carotid Body.
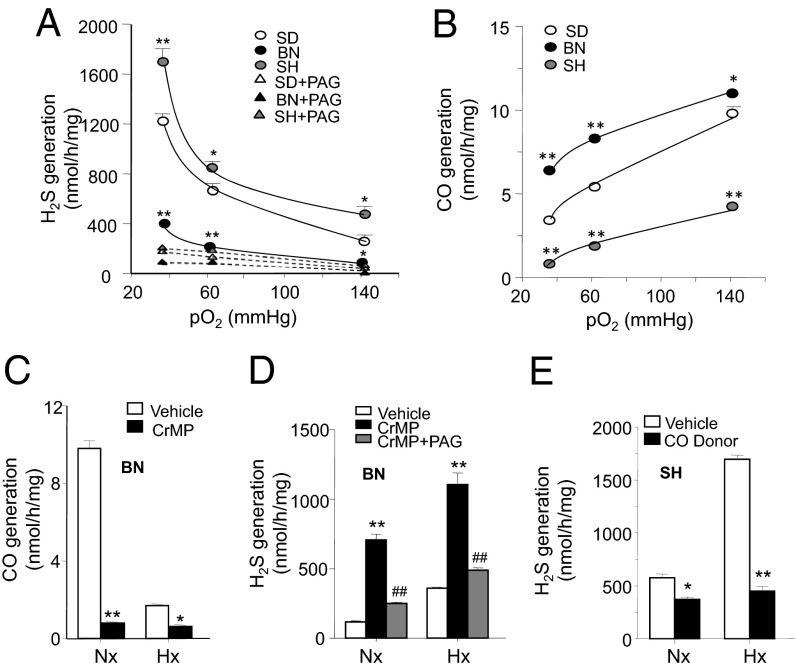
We then tested the hypothesis that either altered H2S signaling and/or generation in the carotid body contribute to strain-dependent variations in O2 sensing. Treatment with NaHS, an H2S donor, produced comparable sensory excitation in all three strains (Fig. S2 A and B), suggesting unaltered H2S signaling. However, measurements of H2S levels in normoxia and hypoxia revealed striking differences among the three strains. Although BN rats exhibited lower basal and hypoxia-evoked H2S generation compared with SD rats, SH rats showed higher generation of H2S under both conditions (Fig. 2A). l-propargylglycine (l-PAG), a CSE-selective inhibitor (19–21), blocked H2S generation in all three strains, suggesting that CSE is the primary H2S catalyzing enzyme in the carotid body (Fig. 2A). Immunocytochemistry of carotid body tissue showed no apparent differences in CSE-like immunoreactivity among the three strains (Fig. S3), suggesting that differential CSE expression does not contribute to strain-dependent differences in H2S generation.
Fig. 2.
Variability in H2S and CO generation in the carotid body. (A) Effects of graded hypoxia on H2S generation in the carotid bodies from SD, BN, and SH rats in the absence and presence of l-PAG (10 µM), a CSE inhibitor. (B) Effects of graded hypoxia on CO generation in the carotid bodies from SD, BN, and SH rats. (C and D) Effect of CrMP (10 µM), an HO inhibitor, on CO generation (C) and H2S generation alone or in combination with l-PAG (10 µM) (D) in BN carotid body under normoxia (Nx) or hypoxia (Hx). (E) Effect of CORM-2 (20 µM), a CO donor, on H2S generation in SH rat carotid body under Nx and Hx (PO2 ∼30 mm Hg). Data are mean ± SEM from n = 5 rats. *P < 0.05; **P < 0.01. (See also Figs. S3 and S4).
Contribution of Carbon Monoxide to Altered H2S generation.
Carbon monoxide (CO) is a physiological inhibitor of CSE-generated H2S in the carotid body (16). Carotid body CO measurements revealed important differences across the three strains. Compared with SD, BN and SH carotid bodies exhibited higher and lower CO levels, respectively, under both normoxia and hypoxia (Fig. 2B). Heme oxygenase 2 (HO-2) is the primary CO-generating enzyme in the carotid body (22). Treating BN rats with Cr(III) meso porphyrin (CrMP), an HO inhibitor, markedly decreased CO generation in the carotid body with a concomitant increase in H2S generation in both normoxic and hypoxic conditions (Fig. 2 C and D). The effects of HO inhibition on H2S generation were prevented by inhibiting CSE with l-PAG (Fig. 2 D). However, CSE inhibition had no effect on CO generation (Fig. S4). Conversely, SH carotid bodies treated with CORM-2 ([Ru(CO)3 Cl2]2), a CO donor, decreased H2S generation in both normoxia and hypoxia (Fig. 2E). These data are consistent with the notion that variations in CO levels determine differences in H2S formation among the three rat strains.
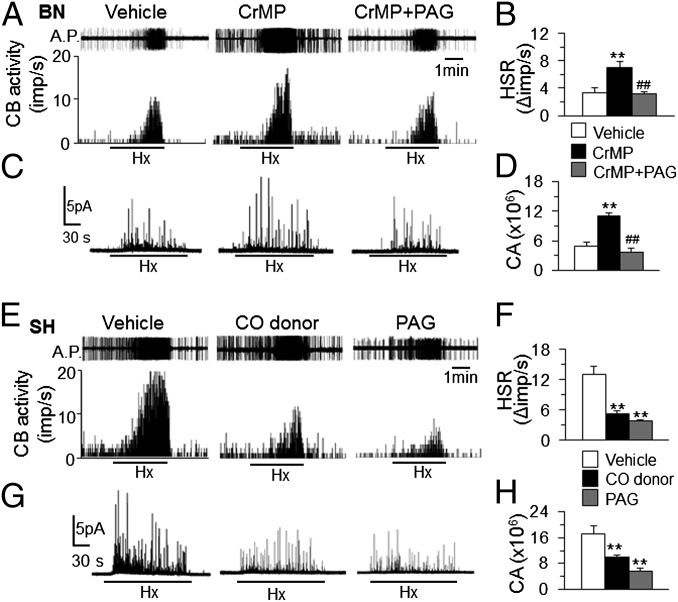
We then examined whether correcting H2S generation normalizes O2 sensing in BN and SH carotid bodies. The HO inhibitor, which increased H2S generation, markedly improved the BN carotid body and glomus cell response to hypoxia, effects that were prevented by CSE inhibition by l-PAG (Fig. 3 A–D). Conversely, treating SH carotid bodies with a CO donor or a CSE inhibitor, which both decreased H2S generation, prevented heightened sensitivity to hypoxia (Fig. 3 E–H).
Fig. 3.
Carbon monoxide contributes to variability in H2S generation and carotid body O2 sensing. (A–D) Effects of HO inhibition (CrMP) alone or in combination with CSE inhibition (l-PAG) on BN carotid body sensory (A and B) and glomus cell secretory (C and D) responses to Hx. (E–H) Effect of CO donor or l-PAG on SH carotid body sensory (E and F) and glomus cell secretory (G and H) responses to Hx. Action potentials (A.P.) are from the sinus nerve. Integrated carotid body sensory activity (CB activity) is presented as impulses per second (imp/s). Hx = PO2 ∼30 mm Hg. Hypoxic sensory response (HSR) measured as the difference in response between baseline and Hx (Δimp/s). See SI Materials and Methods for concentrations and route of administration of drugs for carotid body sensory activity and glomus cell responses. Average (mean ± SEM) data from n = 6–8 rats or 10–15 cells. **P < 0.01; ##P < 0.01.
Altered Kinetic Properties of HO-2.
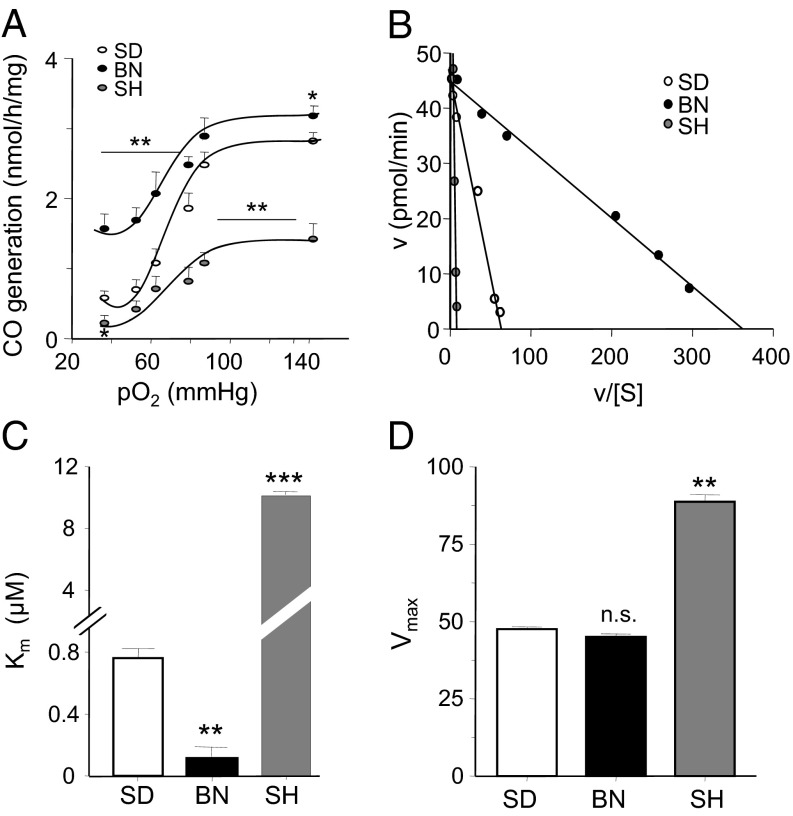
Strain-dependent variations in CO generation in the carotid body can be a result of either differential expression and/or altered kinetic properties of the enzyme HO-2. HO-2-like immunoreactivity in the carotid body was comparable in all three strains (Fig. S5), suggesting no apparent differences in its expression. Limited availability of carotid body tissue necessitated studying the kinetic properties of HO-2 in liver microsomal fractions. Western blot analysis showed most HO-2 to be in the liver microsomal fraction, whereas expression of HO-1, another CO generating enzyme, was undetectable (Fig. S6A). HO-2 activity is more temperature-sensitive than HO-1, and HO-2 activity decreases with higher temperatures (23). Increasing the temperature from 20 °C to 60 °C markedly inhibited CO generation (Fig. S6 B and C), further suggesting that HO-2 and not HO-1 was the predominant CO-generating enzyme in the liver microsomal fractions. Similar to in the carotid body, hypoxia progressively inhibited CO generation in the microsomal fractions, with high and low levels of CO in BN and in SH, respectively, under normoxic and hypoxic conditions relative to SD (Fig. 4A).
Fig. 4.
Variability in kinetic properties of HO-2. (A) Effect of graded hypoxia on CO generation in liver microsomal fractions in SD, BN, and SH rats. (B) Analysis of half-maximal initial rate of CO generation using Eadie-Hofstee plot. (C and D) Apparent Km for hemin (C) and Vmax (D) of HO-2-catalyzed CO generation in liver microsomes from SD, BN, and SH rats. Data represent mean ± SEM from 5 individual experiments. **P < 0.01; ***P < 0.001; n.s. P > 0.05 (i.e., not significant). See also Fig. S5.
HO-2 generates CO by degrading heme, with NADPH and cytochrome P450 reductase as cofactors (24). To determine the apparent Km, HO-2 activity was monitored as a function of increasing concentrations of hemin, an iron-containing porphyrin. The apparent Km and the maximal velocity of CO production were analyzed by the Eadie-Hofstee plot (25, 26) (Fig. 4B). SD, BN, and SH rats all had notably different Km values. The apparent Km for hemin was ∼6.6-fold lower in BN rats and ∼13-fold higher in SH compared with SD rats (Fig. 4C). Vmax was comparable between SD and BN strains but was ∼1.8-fold higher in SH rats (Fig. 4D).
Physiological Significance of Variations in O2 Sensing.
Restoration of chemosensory reflex and ventilatory adaptation by HO inhibition in BN rats.
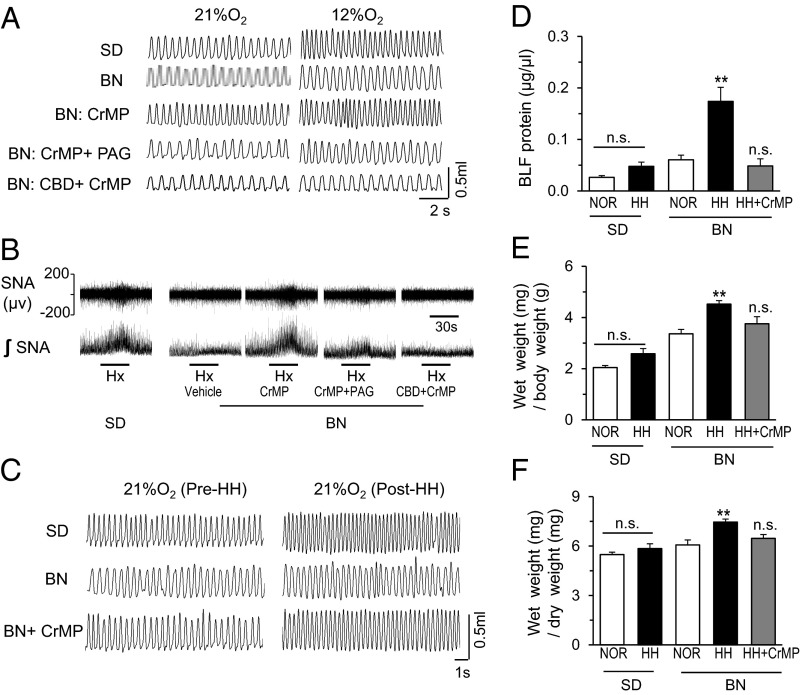
We examined whether an HO inhibitor, which improved the carotid body response to hypoxia, would restore the chemosensory reflex function in BN rats. Reflex function was assessed by monitoring the hypoxic ventilatory response (HVR) by plethysmography in conscious rats and splanchnic sympathetic nerve activity, another measure of carotid body reflex, in anesthetized rats. BN rats exhibited reduced HVR and a near absence of hypoxia-evoked sympathetic nerve activity compared with SD rats (Fig. 5 A and B). HO inhibition markedly improved both responses. The effects of HO inhibition were negated after treatment with l-PAG, a CSE inhibitor, or carotid body transection (Fig. 5 A and B; Fig. S7 A and B). HO inhibition had no effect on O2 consumption (VO2) and CO2 production (VCO2) in BN rats (Table S2).
Fig. 5.
HO inhibition in the carotid body restores chemosensory reflex and ventilatory adaptation in BN rats. (A and B) Representative examples of (A) ventilatory responses to 21% and 12% inspired O2 and (B) splanchnic sympathetic nerve responses to 12% inspired O2 (Hx; black bar; B) in SD and BN rats. BN rats were treated with an HO inhibitor (CrMP) alone, in combination with a CSE inhibitor (l-PAG), or after carotid body transection. (C) Representative examples of breathing before (pre) and after (post) exposure to HH (0.35 atmospheres) in SD and BN rats treated with either vehicle or CrMP. (D–F) Average data (mean ± SEM) of the effects of HH on protein levels in bronchoalveolar lavage fluid (D), wet weight/body weight (E), and wet weight/dry weight of lungs (F) in SD and BN rats treated with either vehicle or CrMP. n = 7–8 rats in each group. See SI Materials and Methods for concentrations and route of administration of drugs. *P < 0.05; **P < 0.01; n.s. P > 0.05 (i.e., not significant). See also Fig. S5 and Table S2.
High-altitude hypoxia leads to a carotid body-mediated increase in breathing, or ventilatory adaptation to hypoxia (VAH) (2). Insufficient VAH often results in pulmonary edema (3–5). BN rats exposed to hypobaric hypoxia (HH; 0.35 atmospheres; simulating 8,500 m altitude) for 16 h exhibited an absence of VAH compared with SD rats (Fig. 5C; Fig. S7C). Mean blood pressure was elevated in SD rats (control 86 ± 2 vs. HH 100 ± 3 mm Hg; P < 0.05) but was absent in BN rats (control 85 ± 3 vs. HH 88 ± 5 mm Hg; P > 0.05). Furthermore, HH-exposed BN rats developed pulmonary edema, characterized as elevated protein levels in the bronchoalveolar lavage fluid and an increased wet-to-dry lung weight ratio (Fig. 5 D–F). Treating BN rats with an HO inhibitor restored VAH and prevented pulmonary edema (Fig. 5 C–F; Fig. S7C).
Control of hypertension by CSE inhibition in SH rats.
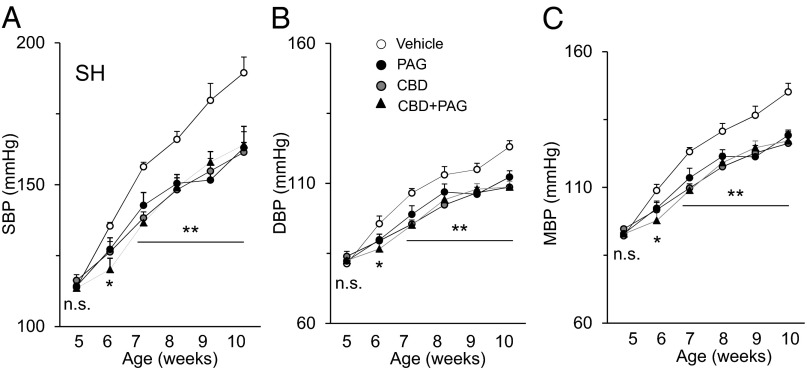
An exaggerated carotid body chemosensory reflex has been implicated in the development of essential hypertension in SH rats (7). We tested whether treating SH rats with a CSE inhibitor, which prevented carotid body hypersensitivity to hypoxia, would also reduce blood pressure. SH rats were treated with either vehicle (saline) or l-PAG every day, with blood pressures measured in 5-wk-old SH rats every week for 5 wk. Compared with vehicle-treated SH rats, l-PAG-treated rats presented a pronounced reduction in blood pressures (Fig. 6 A–C). Ablation of the carotid bodies from 5-wk-old SH rats also attenuated age-dependent hypertension to the same extent as l-PAG treatment. Treating carotid body-ablated rats with l-PAG caused no further decline in blood pressures (Fig. 6 A–C). These results underscore a mechanism through which elevated H2S signaling in the carotid body contributes to the progression of hypertension in SH rats.
Fig. 6.
CSE inhibition in the carotid body controls hypertension in SH rats. (A–C) Average (mean ± SEM) data of systolic (SBP), diastolic (DBP), and mean (MBP) blood pressures in SH rats over 5 wk. Arterial blood pressures were measured in 5-wk-old SH rats every week for 5 wk. Rats were treated every alternate day with vehicle (saline) or a CSE inhibitor (l-PAG; 30 mg/kg, i.p.) either alone or after carotid body transection. n = 6–7 rats in each group. *P < 0.05; **P < 0.01; n.s. P > 0.05 (i.e., not significant).
Discussion
Our results establish inherent variations in CO-regulated H2S as a fundamental mechanism contributing to the differences in carotid body O2 sensing in three distinct rat strains, with profound implications for cardiorespiratory functions. O2 sensing is severely impaired in BN and exaggerated in SH relative to SD rats. This variation in O2 sensing manifested itself at the cellular level in the individual glomus cells of the carotid body, as evidenced by the strains’ differential hypoxia-induced CA secretion. CA secretion itself is insufficient to explain differential O2 sensing, as there is a lack of correlation between the downstream chemoreceptor nerve excitation and CA secretion (12). Thus, differences in O2 sensing are not a result of generalized changes in the carotid body morphology or glomus cell excitability but, rather, the result of intrinsic differences in O2 sensing mechanisms. Before birth, carotid bodies are adapted to the low partial pressure of oxygen (PO2) of fetal blood and are relatively insensitive to hypoxia. After birth, the sensitivity of the carotid body to hypoxia is reset (27). Whether the observed variation in hypoxic sensitivity in these rat strains is a result of inadequate resetting of the carotid body in BN rats or an acquired hypersensitivity during development in SH rats may be a point of future study.
A major finding of this study is an easily exploitable system including native CO and H2S as major determinants of variations in carotid body O2 sensing. Although endothelial CO and H2S both yield local blood vessel relaxation (28, 29), the gasotransmitters exert diametrically opposed effects on carotid body activity: CO acts as a physiologic inhibitor, whereas H2S is excitatory. Impaired hypoxic sensitivity in BN rats was associated with reduced H2S and augmented basal levels of CO. Simply inhibiting CO generation was sufficient to increase H2S levels and improve hypoxic sensing by the BN carotid body. Conversely, the amplified hypoxic response in SH was associated with higher H2S and lower CO in the carotid body. Elevating CO levels in SH carotid bodies reduced both H2S generation and hypoxic sensitivity. Intriguingly, CSE inhibition had no effect on CO generation, suggesting that CO is an upstream signaling molecule that regulates O2 sensing by inhibiting H2S generation.
Our results identified a potential mechanism contributing to inherent variations in carotid body CO levels in the three rat strains. The SD, BN, and SH rat strains demonstrate strikingly different substrate affinities for HO-2, the major CO catalyzing enzyme in the carotid body. Higher substrate affinity appears responsible for higher CO levels in BN, whereas lower affinity explained reduced CO generation in SH rats. Although technical limitations required kinetic analysis of liver microsomal HO-2, we believe that the substrate affinities are similarly varied in the strains’ carotid bodies because hypoxic responses of the liver microsomes paralleled those of carotid bodies of each strain. Differences in substrate affinity among the three distinct strains could be a result of genetic variation in HO-2.
Variations in carotid body-mediated O2 sensing have important physiological consequences. Impaired O2 sensing in BN rats was associated with a near absence of chemosensory reflex, as evidenced by attenuated ventilatory and sympathetic nerve responses to hypoxia, and impaired VAH, an important physiological adaptation to high-altitude hypoxia. BN rats also developed pulmonary edema when challenged with HH, similar to humans with impaired hypoxic sensitivity (3–5). Although many factors may contribute to hypoxia-induced pulmonary edema, including differences in pulmonary vasculature and fluid reabsorption in the lung (30), treating BN rats with an HO inhibitor alone was sufficient to restore VAH and prevent HH-induced pulmonary edema. HO inhibition was ineffective in improving VAH in carotid body-ablated BN rats, evidence that HO inhibition is acting at the carotid body to restore its chemosensory reflex function. Inhibiting carotid body HO activity could be a therapeutic strategy for improving hypoxic sensitivity and preventing high-altitude pulmonary edema. It should be noted that with exposure to high-altitude hypoxia, a hypersensitive carotid body is also not without its significant pathophysiological sequelae, including heightened sympathetic activity, hypertension, and periodic breathing. Interestingly, individuals can adapt to prolonged hypoxia via blunted carotid body and ventilatory responses (2). Whether exposure to chronic hypoxia can elicit the same systemic adaptations in SH rats could be an important future study.
The present study demonstrates exaggerated hypoxic sensitivity of the carotid body in SH rats even before the development of hypertension, providing evidence that this strain is inherently hypersensitive to hypoxia. Although the contributions of aortic bodies and carotid baroreceptors to the development of hypertension in SH rats remain uncertain, ablation of carotid sinus nerves alone lowered blood pressures in SH rats, a finding consistent with a recent report (31). As a result, surgical ablation of the carotid sinus nerves has been proposed as a potential therapeutic intervention for reducing blood pressure (32). However, ablation of the carotid sinus nerves would abolish systemic responses to hypoxia and may lead to the adverse physiological consequences seen in BN rats, such as the absence of VAH and increased susceptibility to high-altitude pulmonary edema. Our findings provide direct evidence that elevated H2S levels mediate the inherent hypersensitivity of the SH carotid body to hypoxia. Treating SH rats with a CSE inhibitor alone successfully controlled hypertension to the same extent as transection of carotid sinus nerves. Although endothelial cell-derived H2S acts as a vasodilator (28), systemic administration of a CSE inhibitor nevertheless lowered blood pressures in SH rats. Although the current study has not examined the vascular reactivity to H2S in SH rats, it is likely that the more potent effects of reduced carotid body H2S generation on blood pressure may be reflective of the relative importance of sympathetic tone over local control of vascular tone in determining blood pressure. Interestingly, CSE-null mice still develop age-dependent hypertension (28), suggesting an important balance between carotid body activity and endothelial CSE that requires further studies. These results suggest that reducing H2S levels in the carotid body by pharmacological inhibition of CSE may be a more viable and safer therapy than proposed carotid sinus nerve transection for treating hypertensive patients.
Materials and Methods
Preparation of Animals.
Experiments were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were performed on 5–6-wk-old male SD, BN, and SH rats unless otherwise noted. In experiments requiring sedation, rats were anesthetized with i.p. injections of urethane (1.2 g/kg; Sigma). Animals were allowed to breathe spontaneously. Core body temperature was monitored by a rectal thermistor probe and maintained at 37 ± 1 °C by a heating pad. Animals were killed by intracardiac injection (0.1 mL) of euthanasia solution (Beuthanasia-D Special; Schering-Plough).
Carotid Body Sensory Activity.
Sensory activity from carotid bodies was recorded in an ex vivo preparation, as previously described (16).
Measurements of Breathing Variables.
Ventilation was monitored by whole-body plethysmograph, and O2 consumption and CO2 production were determined by the open-circuit method in conscious rats at an ambient temperature of 25 ± 1 °C, as previously described (33). Sighs, sniffs, and movement-induced changes in breathing were excluded in the analysis.
Measurements of Splanchnic Nerve Activity.
The splanchnic nerve was isolated on the left side, using a retroperitoneal approach, cut above the celiac ganglion, and desheathed. The cut central end was placed on bipolar platinum–iridium electrodes for recording electrical activity, as described previously (34).
Measurements of Catecholamine Secretion from Glomus Cells.
Glomus cells were isolated from carotid bodies and monitored for catecholamine secretion by carbon fiber amperometry, as previously described (15). The number of catecholamine secretory events and quantity secreted per event were analyzed and expressed as total catecholamine molecules secreted.
Measurements of H2S.
H2S levels in the carotid body were determined as previously described (16). In experiments assessing the contribution of CSE to H2S production, the tissue homogenates were incubated with l-PAG (10 µM) for 30 min before initiation of the reaction.
Measurements of CO.
CO production was measured using a spectrophotometric procedure, as previously described (35, 36), with a few modifications. Mixtures containing 10 µg carotid body protein, NADPH (1 mM), hemin (25 µM), and NADPH Regenerating System Solution (BD Biosciences) were equilibrated to various PO2 values by flushing with differing combinations of O2 and N2 at 37 °C for 1 h in sealed tubes. CO generated in the reaction was trapped in a reaction mixture containing 25 µM leuco crystal violet, 200 µM palladate, and 4 µM iodate. CO concentrations were calculated from a standard curve relating CORM-2 concentration to absorbance of 620 nm light. To assess the effects of hemeoxygenase inhibition on CO production, the tissue homogenates were incubated with CrMP (10 µM) at 37 °C for 30 min before initiating the reaction.
Determination of Kinetic Properties of HO-2 Enzyme.
Anesthetized rats were transcardially perfused with ice-cold heparinized (heparin 120 IU/mL) saline. Livers were homogenized at 4 °C with 3 volumes 0.05 M Tris⋅HCl buffer at pH 7.4 with 10 mM EDTA and 20% (vol/vol) glycerol and centrifuged at 100,000 × g for 70 min at 4 °C. HO activity was monitored by measuring CO formation at different times (10–40 min) as a function of hemin concentration (from 0.01 to 100 µM). The apparent hemin binding affinity (Km) and maximum reaction velocity (Vmax) were derived from Eadie-Hofstee plot analysis (25, 26).
Exposure to HH.
Unrestrained rats were fed ad libitum and exposed to HH (0.35 atmospheres, simulating 8,500 m altitude) for 16 h. Breathing and blood pressures were monitored by plethysmography and tail cuff method, respectively, before HH and 1 h after concluding HH. Blood pressures measured by the tail–cuff method were 3–5 mm Hg lower than telemetry measurements. To circumvent the problems encountered with telemetry sensors during continuous measurements for 5 wk, we opted to measure blood pressures by the tail–cuff method.
Measurements of Pulmonary Edema.
An hour after concluding HH, the rats were anesthetized and intubated. Bronchoalveolar lavage fluid was collected by lavaging lungs twice with 2.5 mL sterile PBS each time and centrifuged at 1,500 × g for 10 min at 4 °C. Protein content was determined using a modified Bradford Protein Assay (Bio-Rad Laboratories). In another series of experiments, the lungs were removed and wet weight was determined immediately. The lung was then dried in a hot oven at 55 °C for 72 h to obtain a constant dry weight. The data were expressed as wet weight as well as the ratio of wet weight to dry weight.
Carotid Body Transection.
Carotid sinus was transected bilaterally under aseptic conditions in ketamine (70 mg/kg) + xylazine (8 mg/kg) anesthetized rats. Experiments were performed 1 wk after surgical recovery.
Immunocytochemistry, immunoblotting, drug treatments, and data analysis methods are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) Grant HL90554 (to N.R.P.) and US Public Health Service Grant DA 000226 and Research Scientist Award DA 00074 (to S.H.S.). M.M.G. is supported by the NIH Medical Scientist Training Program Award T32 GM007309.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322172111/-/DCSupplemental.
References
- 1.Weil JV. Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol. 2003;135(2-3):239–246. doi: 10.1016/s1569-9048(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey JA, Forster HV. Mediation of Ventilatory Adaptations. Physiol Rev. 1982;62(1):262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- 3.Hackett PH, Roach RC, Schoene RB, Harrison GL, Mills WJ., Jr Abnormal control of ventilation in high-altitude pulmonary edema. J Appl Physiol (1985) 1988;64(3):1268–1272. doi: 10.1152/jappl.1988.64.3.1268. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzawa Y, et al. Blunted hypoxic ventilatory drive in subjects susceptible to high-altitude pulmonary edema. J Appl Physiol (1985) 1989;66(3):1152–1157. doi: 10.1152/jappl.1989.66.3.1152. [DOI] [PubMed] [Google Scholar]
- 5.Hohenhaus E, Paul A, McCullough RE, Kücherer H, Bärtsch P. Ventilatory and pulmonary vascular response to hypoxia and susceptibility to high altitude pulmonary oedema. Eur Respir J. 1995;8(11):1825–1833. doi: 10.1183/09031936.95.08111825. [DOI] [PubMed] [Google Scholar]
- 6.Trzebski A. Arterial chemoreceptor reflex and hypertension. Hypertension. 1992;19(6 Pt 1):562–566. doi: 10.1161/01.hyp.19.6.562. [DOI] [PubMed] [Google Scholar]
- 7.Tan ZY, et al. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106(3):536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol (1985) 2002;93(3):974–983. doi: 10.1152/japplphysiol.00019.2002. [DOI] [PubMed] [Google Scholar]
- 9.Strohl KP, et al. Ventilation and metabolism among rat strains. J Appl Physiol (1985) 1997;82(1):317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- 10.Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol. 1994;267(5 Pt 2):R1371–R1377. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- 11.Hayward LF, Castellanos M, Noah C. Cardiorespiratory variability following repeat acute hypoxia in the conscious SHR versus two normotensive rat strains. Auton Neurosci. 2012;171(1-2):58–65. doi: 10.1016/j.autneu.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Prabhakar NR. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr Physiol. 2012;2(1):141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhakar NR. Sensing hypoxia: Physiology, genetics and epigenetics. J Physiol. 2013;591(Pt 9):2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, et al. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010;12(10):1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- 15.Makarenko VV, et al. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am J Physiol Cell Physiol. 2012;303(9):C916–C923. doi: 10.1152/ajpcell.00100.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng YJ, et al. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107(23):10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telezhkin V, et al. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir Physiol Neurobiol. 2010;172(3):169–178. doi: 10.1016/j.resp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Nurse CA, Fearon IM. Carotid body chemoreceptors in dissociated cell culture. Microsc Res Tech. 2002;59(3):249–255. doi: 10.1002/jemt.10199. [DOI] [PubMed] [Google Scholar]
- 19.Abeles RH, Walsh CT. Acetylenic enzyme inactivators. Inactivation of gamma-cystathionase, in vitro and in vivo, by propargylglycine. J Am Chem Soc. 1973;95(18):6124–6125. doi: 10.1021/ja00799a053. [DOI] [PubMed] [Google Scholar]
- 20.Asimakopoulou A, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br J Pharmacol. 2013;169(4):922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washtien W, Abeles RH. Mechanism of inactivation of gamma-cystathionase by the acetylenic substrate analogue propargylglycine. Biochemistry. 1977;16(11):2485–2491. doi: 10.1021/bi00630a026. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: A role in carotid body chemoreception. Proc Natl Acad Sci USA. 1995;92(6):1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261(1):411–419. [PubMed] [Google Scholar]
- 24.Snyder SH, Barañano DE. Heme oxygenase: A font of multiple messengers. Neuropsychopharmacology. 2001;25(3):294–298. doi: 10.1016/S0893-133X(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 25.Eadie GS. The inhibition of cholinesterase by physostigmine and prostigmine. J Biol Chem. 1942;146:85–93. [Google Scholar]
- 26.Hofstee BH. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- 27.Roux JC, Brismar H, Aperia A, Lagercrantz H. Developmental changes in HIF transcription factor in carotid body: Relevance for O2 sensing by chemoreceptors. Pediatr Res. 2005;58(1):53–57. doi: 10.1203/01.PDR.0000163390.78239.EA. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009;2(68):re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherrer U, et al. High-altitude pulmonary edema: From exaggerated pulmonary hypertension to a defect in transepithelial sodium transport. Adv Exp Med Biol. 1999;474:93–107. doi: 10.1007/978-1-4615-4711-2_8. [DOI] [PubMed] [Google Scholar]
- 31.Abdala AP, et al. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590(Pt 17):4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paton JF, et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61(1):5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 33.Peng YJ, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577(Pt 2):705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng YJ, et al. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol (1985) 2012;112(1):187–196. doi: 10.1152/japplphysiol.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert JL, Wiens RE. Induced colorimetric method for carbon monoxide. Anal Chem. 1974;46(7):929–930. doi: 10.1021/ac60343a021. [DOI] [PubMed] [Google Scholar]
- 36.Ryter S, Kvam E, Tyrrell RM. Heme oxygenase activity determination by high-performance liquid chromatography. Methods Enzymol. 1999;300:322–336. doi: 10.1016/s0076-6879(99)00138-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.