Significance
Darkness and brightness are very different perceptually. To understand the neural basis for the perceptual difference, we studied populations of neurons in macaque primary visual cortex (V1) when a visual field alternated between black and white. Darkness but not brightness evoked sustained nerve-impulse spiking in V1 neurons. The sustained response to a white field in the local field potential, which is the sum of membrane currents in neurons near the recording microelectrode, was larger than for black. Taken together, these results imply that bright fields evoked strong intracortical synaptic inhibition, and this inhibition is the basis for cortical brightness adaptation. Such cortical brightness adaptation can explain many perceptual phenomena: interocular speeding up of dark adaptation, tonic interocular suppression, and interocular masking.
Keywords: light adaptation, cortical inhibition, laminar pattern
Abstract
Darkness and brightness are very different perceptually. To understand the neural basis for the visual difference, we studied the dynamical states of populations of neurons in macaque primary visual cortex when a spatially uniform area (8° × 8°) of the visual field alternated between black and white. Darkness evoked sustained nerve-impulse spiking in primary visual cortex neurons, but bright stimuli evoked only a transient response. A peak in the local field potential (LFP) γ band (30–80 Hz) occurred during darkness; white-induced LFP fluctuations were of lower amplitude, peaking at 25 Hz. However, the sustained response to white in the evoked LFP was larger than for black. Together with the results on spiking, the LFP results imply that, throughout the stimulus period, bright fields evoked strong net sustained inhibition. Such cortical brightness adaptation can explain many perceptual phenomena: interocular speeding up of dark adaptation, tonic interocular suppression, and interocular masking.
Light adaptation is a vitally important visual function for enabling a stable perception of the visual world when background luminance levels can be as different as night and day. Previous psychophysical studies suggested that light adaptation was caused mainly by gain control mechanisms in the retina (1–3) that have been well studied (4). However, some psychophysical results suggested that there might be also a cortical contribution to light adaptation (5), but the nature of the cortical contribution is much less well understood. Here, we report our studies of cortical adaptation to brightness and darkness in macaque primary visual cortex (V1) and the implications for visual perception.
We asked the following question: how does macaque V1 cortex respond to large dark and bright regions like those that would comprise the background of a visual scene during the night or the day, respectively? The experiments reported here focused on two cortical layers, 4C and 2/3. The layers of V1 are distinct stages of processing of visual signals (6, 7). The input layer 4C is the first cortical stage where the cortex could distinguish between blackness and whiteness (8). Layer 2/3 comprise one of the main visual outputs of V1 to extrastriate visual cortex (9). To obtain a comprehensive view of the response to black and white in cortical layers 4C and 2/3, we used measurements of population activity: multiunit spike rate, termed multiunit activity (MUA), and local field potential (LFP) (10–12).
Cortical brightness adaptation was evident in the qualitatively different dynamics of neural population activity in layers 4C and 2/3 when the monkeys viewed black and white regions. Both black and white large-area stimuli evoked transient excitatory responses in MUA, but in response to a white region, there was a slowly developing but much stronger inhibition of spike activity. Such suppression of sustained spiking in cortical neurons by white backgrounds would increase the signal/noise ratio of targets on white backgrounds. Such cortical brightness adaptation is likely the explanation for many previously observed perceptual phenomena such as tonic interocular suppression, dichoptic effects in light and dark adaptation, and interocular masking (5, 13–16).
Results
We studied V1 cortical population activity in response to square-wave stimuli alternating between black and white (Fig. 1A) presented monocularly. The stimuli were black and white squares that subtended 8° × 8° visual angle and that covered the receptive field of the recording site. The receptive field of the MUA of each recording site was mapped with reverse correlation as described previously (11) and all receptive fields were more than 2° from the boundary of the large squares used as stimuli in these experiments. The luminances in the bright, white field (107.3 cd/m2) and in the dark, black field (11.1 cd/m2) were chosen to have approximately equal contrast with the background luminance of the display screen (luminance 59.1 cd/ m2) that subtended 20° × 15° at the animal’s viewing distance (for more details, see Materials and Methods). Thus, the bright, white square stimulus was 9.667× more luminous (i.e., approximately 1 log unit more than the dark, black stimulus).
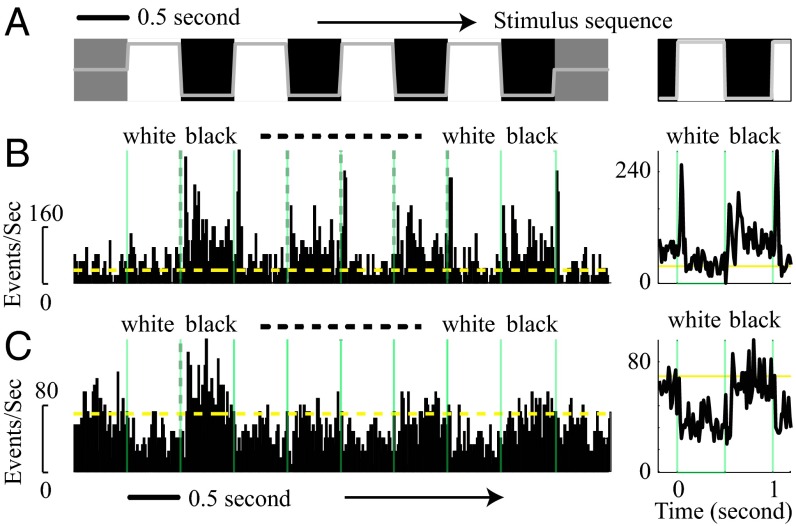
Fig. 1.
Population responses (MUA) to black and white stimuli. (A) White and black large squares (8° visual angle) were alternating on the screen every 0.5 s for 4–8 s. The luminance of black squares was 11.1 cd/m2 (lowest level of the gray line), and that for white squares was 107.3 cd/m2 (the highest level of the gray line). The background luminance was 59.1 cd/m2. (B) MUA of a recording site from layer 4C to black/white alternating stimuli is shown (Left). (Right) MUA was averaged from different stimulus cycles for black and white. To avoid response adaptation, the first cycle of MUA to black and white stimuli was excluded for cycle average responses. Green vertical lines show the time for stimuli switching. The yellow horizontal line shows the spontaneous activity for the site. (C) MUA of a recording site from layer 2/3 to black/white alternating stimuli.
The data were from 111 recording sites within layer 4C and 188 sites within layer 2/3 from five macaque monkeys. The inclusion criterion for a site was that the site’s spike rate at the peak time (either to black or to white stimuli) had to be significantly above the noise, meaning larger than 2× the SD of fluctuations around the baseline (for details, see Materials and Methods). Approximately 80% of all recorded sites were included in this study. For all included sites, both multiunit spike activity (MUA shown in Figs. 1–3) and the LFP (shown in Figs. 4 and 5) were measured and analyzed to obtain a comprehensive picture of V1 population activity in response to dark and bright stimuli.
Fig. 3.
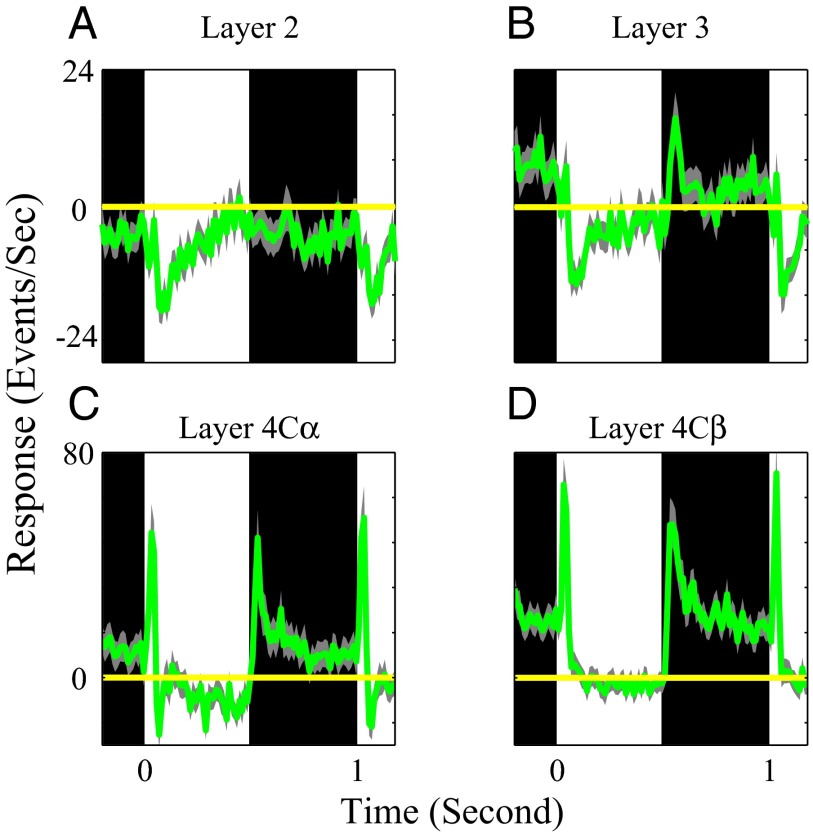
Population average-response dynamics of MUA to black and white stimuli in different cortical layers. Population-averaged time course of MUA from all recording sites in layer 2 (n = 93), layer 3 (n = 95), layer 4Cα (n = 40), and layer 4Cβ (n = 71) are shown in A–D. In A–D, the period for black and white stimuli are represented by black and white regions. Yellow horizontal lines are averaged spontaneous responses. The light gray curves represent 1 SEM for each population average MUA.
Fig. 4.
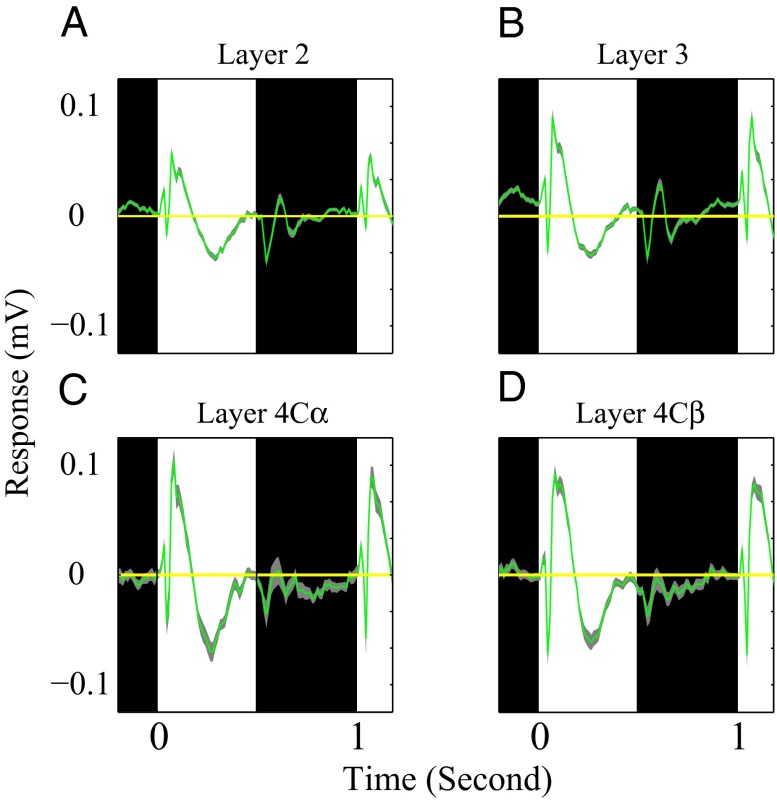
Average response dynamics of the LFP to black and white stimuli in different cortical layers. Population-averaged time course of LFP from all recording sites in layer 2 (n = 93), layer 3 (n = 95), layer 4Cα (n = 40), and layer 4Cβ (n = 71) are shown in A–D. In A–D, the period for black and white stimuli are represented by black and white regions. Yellow horizontal lines are averaged spontaneous LFP. The light gray curves represent 1 SEM for each population average LFP.
Fig. 5.
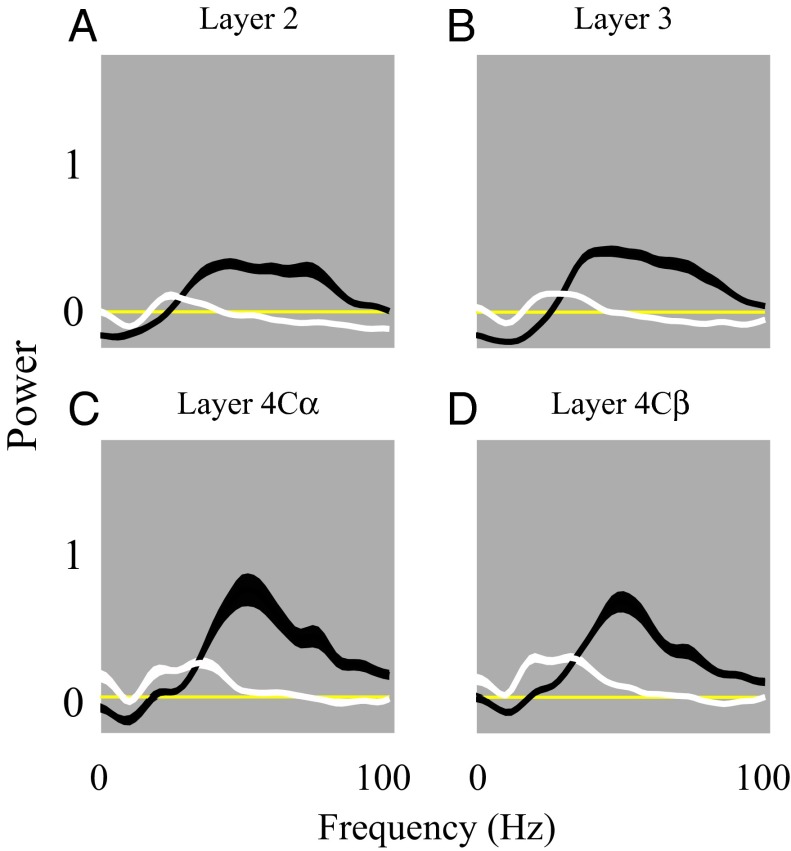
Average power spectra (LFP) to black and white stimuli in different cortical layers. Population-averaged power spectra of LFP from all recording sites in layer 2 (n = 93), layer 3 (n = 95), layer 4Cα (n = 40), layer 4Cβ (n = 71) depicted in plots A–D. In A–D, the black and white curves represent the visually induced power spectra of the LFP to black and white stimuli, respectively.
At most recording sites in V1, the spike rate responses to dark and bright stimuli of large area were very different. For example, in Fig. 1B from a site in layer 4C, the white-evoked MUA was significantly lower than black-evoked MUA during the sustained period of the response (defined as 100–500 ms after stimulus onset). For another example recording site, in layer 2/3 (Fig. 1C), again the sustained response to white was smaller than to black and even dipped below the level of baseline activity. A firing rate below baseline clearly suggests sustained inhibition evoked by the white area stimulus. The example data from individual sites in Fig. 1 are representative of the population data shown in Figs. 2 and 3.
Fig. 2.
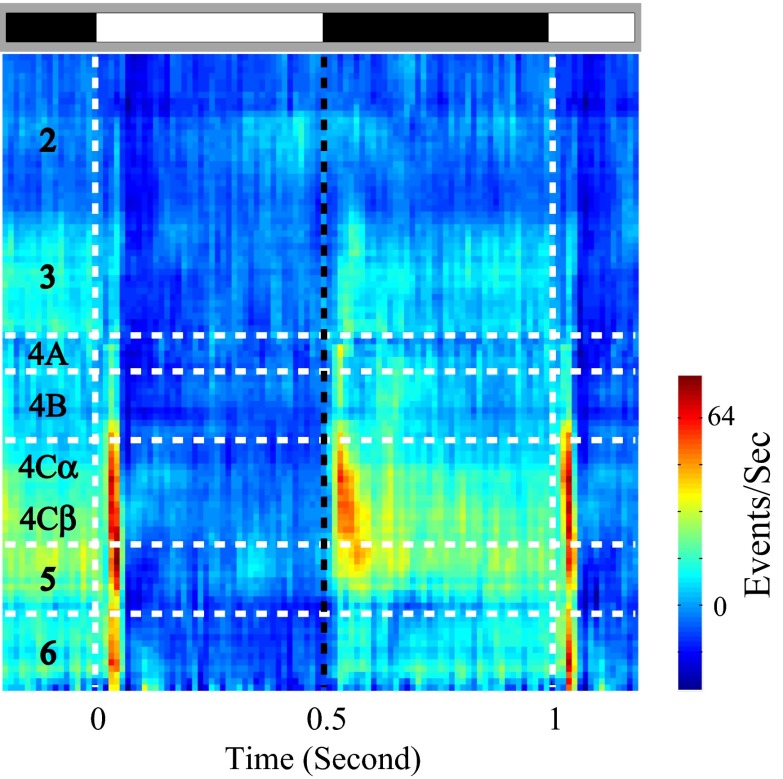
Laminar pattern of population responses (MUA) to black and white stimuli. Cycle-averaged MUA to black/white stimuli at different cortical depths (y axis) is represented by the color. At each cortical depth, the MUA is estimated by the averaged activity of the recording sites within ±0.05 mm around the cortical depth. Alternating black and white bars on gray background represent periods corresponding to black and white stimuli.
Population Spike Activity (MUA) to Dark and Light Backgrounds.
The higher sustained spike-firing rate response to dark stimuli was evident in MUA recorded in all V1 layers averaged across many recording sites (Figs. 2 and 3). On average, in layer 4C, the MUA in response to white only increased transiently and then relaxed to baseline levels during the sustained period of stimulation. Black responses were more sustained than white responses in layer 4C. In other layers also, the responses to black were less suppressed than white responses. For example, in layers 2 and 3, the MUA was generally weak in response to both black and white stimuli of large area but stimulation by white stimuli evoked a net suppression of MUA below baseline levels as in the example site of Fig. 1C (Figs. 2 and 3). Interestingly, sustained dark and light MUA responses in layer 2 were both suppressed below the baseline, and the black–white difference was less than that in layer 3. Note, the scales for Fig. 3 C and D are two times larger than those for Fig. 3 A and B. Layer 4B neurons responded like neurons in layer 2 and 3 to large area white and black stimuli (Fig. 2). These MUA population responses reflect the net spiking rate of all neurons that are recorded by the microelectrode. In previous work, we found that MUA and single-unit activity were highly correlated (8, 17).
The different V1 dynamics during the dark and light periods were not caused by unequal LGN inputs. The black and white transient MUA responses were approximately of equal amplitude in input layer 4C, indicating that the lateral geniculate nucleus (LGN) inputs were the same strengths for the dark and bright (Figs. 2 and 3 and detailed population data in Figs. S1 and S2; P > 0.05 for related sample t test). The equality of transient responses to darkness and brightness is consistent with the observations of Creutzfeldt et al. (18), who reported that stimuli of negative and positive contrasts elicited spike rate responses with similar response peaks, as well as similar response dynamics in “OFF” and “ON” macaque LGN cells, respectively (also see ref. 19). Our further analysis of the dark/bright asymmetry in the visually evoked LFP in layer 4C (Fig. 4) indicates that intracortical mechanisms are the cause of the cortical brightness adaptation observed in V1.
After the transient excitatory response, MUA in layer 4C in response to large bright stimuli relaxed back to the spontaneous level (population-averaged MUA responses in Figs. 2 and 3 and responses at individual sites in Figs. S1 and S2). However, the excitatory responses to dark stimuli remained significantly above the spontaneous firing rate throughout the stimulation period (Figs. 2 and 3). The fact that white responses relaxed back to the spontaneous level during stimulation is consistent with the hypothesis that cortical brightness adaptation is a result of cortical inhibition evoked by the bright field stimuli, a hypothesis supported by evidence from the LFP recordings presented below.
Cortical Brightness Adaptation in the Visually Evoked LFP: Cortical Inhibition Evoked by White.
The importance of brightness-evoked cortical inhibition is supported by the finding of different visually evoked LFPs (Fig. 4) during the bright and dark stimulus periods. Fig. 4 displays the population average of the visually evoked LFP in response to bright and dark stimuli. The average white-evoked LFP was a large-amplitude signal that persisted throughout the period of stimulation by the bright field. The amplitude of the average white-evoked LFP was, in fact, much larger than the black-evoked LFP (Fig. 4) in the input layer 4C and throughout V1. The evoked LFPs were complex in waveform (10), suggesting that the LFP reflected both excitatory and inhibitory synaptic activity, as has been suggested as an explanation for similarly complex waveforms of visually evoked EEG potentials (20). The large white-evoked LFP in layer 4C indicates that there was strong feed-forward ON input from the LGN and, therefore, that the weak white-evoked MUA response (Figs. 2 and 3) was not a consequence of lack of ON input to the cortex.
The association of a large white-evoked LFP with a weak or negative white-evoked MUA suggests that intracortical inhibition, evoked by the white area stimulus, suppressed white-evoked spike activity (Figs. 1–3). Strong inhibition caused by white-evoked responses was present already in the input layer 4C and also is evident in layer 2/3 activity (Figs. 2–4). The suppression of sustained spiking in cortical neurons by white backgrounds can be interpreted as cortical brightness adaptation. Such adaptation increases the signal/noise ratio of targets on white backgrounds by suppressing background spike activity. Cortical brightness adaptation is a likely explanation for many previously observed perceptual phenomena, such as tonic interocular suppression and interocular masking (13–15).
LFP Power Spectra: Different Dynamical States Induced by Black and White.
Further evidence for different dynamical states in brightness and darkness comes from our analysis of the power spectra of LFP fluctuations induced by visual stimulation. Power in the LFP between 1–100 Hz during the sustained period was higher for black stimuli than for white (Fig. 5). The peak frequencies of LFP power induced by black and white stimuli were also substantially different. Whereas black-induced LFP power peaked in the γ band around 50 Hz, white-induced LFP power peaked around 25 Hz (Fig. 5). We interpret this qualitative black/white difference in LFP power spectra as a consequence of the processing of black-evoked signals through balanced excitatory–inhibitory cortical circuitry and the processing of white-evoked signals in an inhibition-dominated cortical network (Discussion). The power spectral analysis also revealed that visual stimulation had different effects in different frequency bands. γ-Band activity was elevated above baseline by black, but very-low-frequency fluctuations in the δ and α bands were suppressed (Fig. 5). The relative suppression of very-low frequencies was not observed during the period of white stimulation because of the large amplitudes of visually evoked LFPs (Fig. 4).
Discussion
Cortical Brightness Adaptation.
Our results provide neurophysiological evidence that V1 cortex adapts its dynamical state to the brightness of a large background stimulus. Light adaptation is a vitally important visual function for enabling illumination-invariant perception of the visual world. Many light-adaptation mechanisms are embedded within the retina as gain controls for retinal neural responses (4). Most previous psychophysical work has been interpreted to mean that all or almost all light adaptation is retinal (1–3), although there are psychophysical results supporting a cortical contribution to light adaptation (5, 16).
The way in which the cortex adapts to brightness and darkness may seem nonintuitive. Cortical brightness adaptation makes targets on a bright background more visible. Light, white backgrounds suppress V1-sustained spike firing, whereas, when V1 is driven by dark, black backgrounds, the spike rate remains elevated. The reduction in background-evoked firing rate during bright background stimulation will serve to reduce cortical “noise,” which is target-irrelevant activity, when a visual target is to be detected on the background. One can interpret the cortical brightness adaptation as a cortical compensation for the retinal gain controls that turn down the gain of retinal responses on bright, white backgrounds. The retina reduces the signal elicited by a target on a bright background, but the cortex reduces the noise against which the target must be discriminated on the same bright background.
There are several visual psychophysical experiments about interocular interactions between backgrounds and targets that can be explained by neuronal phenomena of cortical light adaptation we found. Dark adaptation in one eye is speeded by illumination of corresponding areas in the contralateral (nonadapted) eye (14, 15). There is tonic interocular suppression of grating detection in one eye caused by dark adaptation of the other eye that is released by illumination of the dark-adapted eye (13). The interocular effects are understood to mean that there are cortical influences on increment and grating detection because V1 is the first processing stage at which the signals from the two eyes are combined. Our neurophysiological results provide a neural mechanism for the tonic interocular suppressive effect of darkness–the V1 cells are firing spikes at a higher rate on dark backgrounds. When a dark-adapted eye is illuminated, the spike-firing rate in the V1 neuron population will drop to a much lower level, and therefore target-locked signals will be much more discriminable.
A perceptual demonstration of the cortical brightness adaptation we have been studying is presented in Fig. 6. Two fields are provided that can be cross-fused using the fixation points and nonius lines around the targets. The targets are two small (identical) blue squares that are above threshold in monocular view. We used color targets in this version of the demonstration only because the colored squares are salient perceptually; the demonstration works well also with achromatic targets. During cross-fusion, only the right eye sees the colored squares. When fused with the larger background circles in the left eye’s view, the blue square on the white circular background is more easily detectable than the square placed on the dark background. The enhanced visibility on the brighter background is the opposite of what would happen with a target and background presented to the same eye because of retinal light adaptation; the greater visibility is a consequence of cortical brightness adaptation.
Fig. 6.
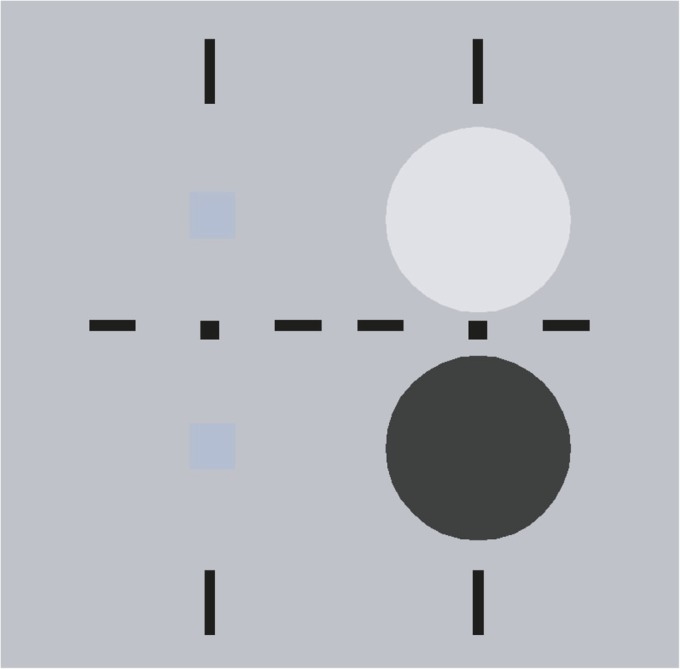
Perceptual demonstration of cortical light adaptation. The targets and backgrounds can be free-fused by cross-fusion. Targets (small blue squares) presented to the right eye are masked by dichoptic presentation of large light or dark backgrounds presented to the left eye. Most observers report seeing stronger interocular masking on the dark background than on the light (white) background (Discussion).
Cortical Locus of Brightness Adaptation.
The asymmetry between responses to black and white has been studied at multiple stages in the visual system: in the retina, LGN, and V1. Although several studies have shown black/white asymmetry in the retina (21–23) and LGN (24), we conclude that the differences in response dynamics between dark and bright in the experiments in this paper were mainly attributable to cortical mechanisms. If the dark/bright asymmetry in V1 were mainly caused by an asymmetry in the LGN input, then (i) visually evoked LFPs in V1 should be asymmetric in the same direction as MUAs, and (ii) the dynamics of visually evoked LFPs should be similar for black and white, only rescaled in amplitude. These two predictions are not consistent with our main results: larger MUA to dark but larger visually evoked LFP to bright stimuli (Figs. 1–4 and Fig. S1); different dynamics in the dark- and bright-evoked LFPs (Fig. 4); and absence of γ-band activity in the power spectrum of the LFP during the sustained phase of bright stimuli (Fig. 5). Therefore, we conclude that the dark/bright asymmetry in our experiments was mainly attributable to cortical mechanisms.
The change of LFP γ-band power and the shift of peak γ frequency between the black and white stimulus periods reinforces the idea that there are different dynamical cortical states during black and white stimulation. LFP γ-band activity is an important feature of cortical activity (12, 25, 26) that can be understood as the consequence of resonance in a balanced recurrent excitatory–inhibitory cortical network (6, 27–29). Many stimulus factors can change the LFP γ-band power and/or peak frequency. Whereas the input strength to visual cortex can modulate γ-band activity (25, 30), changes in γ-band activity often are indications of a change of cortical state (12, 26, 27, 31–33). The substantial changes of γ-band power and peak frequency in Fig. 5 reflect the different cortical states induced by dark and bright large-area stimuli. Our interpretation of the increased γ-band activity during the dark phase of the stimulus is that in darkness, there was a balanced increase in spike firing in both excitatory and inhibitory cells. On the other hand, the during the bright phase of the stimulus the inhibitory cells were activated more than the excitatory cells, leading to a reduction in γ-band activity at the same time that spike firing in excitatory cells was greatly reduced (as revealed in Figs. 2 and 3).
Brightness Adaptation in V1: Previous Results.
We found substantial differences in the dynamical state of the cortex in response to dark and bright stimuli: differences in the time courses and power spectra of V1 cortical-population activity MUA and the LFP. This finding is quite different from those in several previous studies that reported a general increase of response amplitude without changes of dynamics in V1 responses to small black vs. white stimuli. For instance, investigations of the visual cortex of human (20, 34, 35), monkey (8, 17), and cat (36, 37) reported an increase of response gain for small black targets but no change in response dynamics. Two possibilities might explain why our results on V1 dynamical states were not found in earlier experiments. (i) Black/white asymmetry was studied previously mostly with small black or white spots (0.1° to ∼0.3° squares). Such small stimuli only activate a small region in the visual cortex (11, 38, 39) and mainly activate local feed-forward and recurrent inputs. However, the stimuli used in the present study covered 8° × 8° and affected a much larger area of V1. Such large patches of visual stimulus will activate more long-range horizontal and feedback mechanisms (38). Local perturbations in two networks (the black-preferring and white-preferring cell networks) might only elicit a difference of response gain but unchanged response dynamics. Dynamic differences in such two networks are more likely to be observed when they are activated by large stimuli. (ii) Previous studies on black/white asymmetry of responses to small targets used rapid reverse correlation (each stimulus presented for only 20–50 ms). Neural responses were concentrated in the first 100 ms after stimulus onset and decayed back to baseline after 100 ms. In the present study, our visual stimuli lasted for 500 ms, enabling us to observe an initial transient response (in the first 100 ms), as well as a response in the sustained phase (100–500 ms) of stimulus presentation. In fact, the large difference in black/white dynamics and the large white-evoked LFP were found in the sustained period.
There have been previous studies of the spike rates of single cells in macaque visual cortex in response to dark and bright large-area stimuli. The earliest such study by Kayama et al. (40) reported that most (75%) single cells in V1 that responded to diffuse light were “photergic,” meaning excited by brighter stimuli. However, it is difficult to compare our results with those of Kayama et al. (40), because of differences in methods. Kayama et al. (40) used ganzfeld stimuli that were much larger than the stimuli we used and measured spike rates over much longer times. Later, Peng and Van Essen (41) studied a population of single cells in V1 and V2 cortex of awake monkeys and found that most of the neuronal population they studied had maximal spike rate for the darkest stimuli they used, in agreement with our MUA results (Figs. 1–3). More recently, Dai and Wang (42) [see also Geisler et al. (43)] reported a similar result from experiments on a population of single cells in V1 in anesthetized cats: cat V1 cells that responded to luminance variation had highest spike-firing rates for the lowest luminances. Both studies (41, 42) used visual stimuli of large area that were much larger than the cells’ classical receptive fields, as we did. What differentiates our results from those of these earlier studies was our focus on the different dynamical states of the cortex in response to dark (black) and bright (white). Comparing the response time course of evoked LFP with spike rate (in our case MUA), and analyzing the power spectrum of the LFP of the responses to black and white area stimuli, we found that there were two different cortical dynamical states in brightness and darkness, and this finding led us to the mechanism of cortical brightness adaptation as discussed below.
Mechanistic Scheme for Cortical Brightness Adaptation.
For the bright stimuli, we hypothesize that there is a strong cortical inhibition with a slow time course, consistent with a previous study in tree shrew V1 by Tucker and Fitzpatrick (44). The inhibition-by-white hypothesis is inferred from the lack of sustained white-evoked spike activity accompanied by a large, sustained white-evoked LFP. These two seemingly contradictory observations can be reconciled if bright, white stimuli generated strong inhibition in layer 4C that strongly suppressed sustained responses to white. An unavoidable implication of the inhibition hypothesis is that the large evoked LFP recorded in layer 4C was produced predominantly by inhibitory currents in V1 neurons (44). Similarly, we infer that the transient spike responses to white stimuli in layer 4C further elicited strong inhibition in layer 2/3, to explain the persistent inhibition of the MUA in layer 2/3 (Figs. 2 and 3). We further hypothesize that such a strong inhibitory component in response to the bright, white stimulus is mainly attributable to either horizontal or feedback circuits and that such interactions require signal integration over a large spatial extent in the cortex. This hypothesis could explain why small black/white spots did not elicit such significant inhibition in our previous studies (8, 17).
The large brightness-evoked LFP and the lower MUA response to brighter stimuli indicate that increased brightness produced a predominantly inhibitory signal. The smaller black-evoked LFP and relatively higher MUA during darkness are consistent with the hypothesis that dark backgrounds produce balanced inhibition and excitation in V1 cortex. The γ-band peaks for dark stimuli, evident in Fig. 5, can be understood as a consequence of recurrent networks with balanced excitation and inhibition (28, 45, 46). The larger γ-band power in the responses to black backgrounds could also be functionally important for visual perception (12, 26). γ-Band power is likely to be effective in evoking spikes and may be an additional reason (besides the lower mean inhibition during darkness) for the higher spike rates during darkness. γ-Band spiking induced by a dark background would be uncorrelated with the presentation of a target on the background and could contribute to the decreased detectability of a target on a dark background. Indeed some of the Eigengrau, the “dark–light,” the internal noise of the visual system that limits the absolute threshold of vision (4), may come from this γ-band activity in V1 cortex, which is much higher when the cortex is viewing a dark background.
Materials and Methods
Surgery and Preparation.
Acute experiments were performed on five adult Old World monkeys (Macaca fascicularis). After surgery, anesthesia was maintained with a continuous infusion of sufentanil citrate (6–12 µg⋅kg−1⋅h−1, i.v.), and the animal was paralyzed with vecuronium bromide (0.1 mg⋅kg−1⋅h−1, i.v.). Details are provided in SI Materials and Methods.
Electrophysiological Recordings and Data Acquisition.
Seven independently moveable electrodes (Thomas Recording) were used to record simultaneously from multiple cortical sites in V1. The seven electrodes were arranged in a straight line with each electrode separated from its neighbor by ∼300 µm. Details are provided in SI Materials and Methods.
Signal Processing for LFP and MUA.
The LFP is defined as the low-pass-filtered (300 Hz) continuous signal recorded by each microelectrode, and MUA is defined as follows: high-pass-filtered (1000 Hz) raw signal was half-wave-rectified in the negative direction; then, the mean and SD for the half-wave-rectified signal were estimated; MUA was finally defined as the number of events 3 SDs more negative than the mean.
Visual Stimuli.
The stimulus consisted of a sequence of alternating black and white squares (19) (8° × 8°) against a gray background (luminance: 59.1 cd/m2). The luminance of white and black squares was adjusted so the contrasts of the light increment (luminance: 107.3 cd/m2) and light decrement (luminance: 11.1 cd/m2) with the background were nearly equal. Each black or white square appeared for 500 ms, and the entire sequence lasted ∼4 or ∼8 s.
Cycle-Averaged Responses.
MUA or the LFP was aligned by the onset of the black or white stimulus for each cycle. For each recording site, MUA and the LFP to black or white stimuli at each time delay were calculated and averaged across stimulus cycles (Fig. 1 B and C, Right). To minimize the effect of adaptation, we excluded the first stimulus cycle in the analysis. We defined the cycle-averaged responses, Rblack(t) and Rwhite(t), as the mean MUA or LFP across different cycles. The spontaneous response for each recording site, Rspontaneous, is defined as the site’s average responses to a gray background (luminance: 59.1 cd/m2) before stimulus onset.
Signal/Noise Ratio.
We used the signal/noise ratio of the cycle-averaged MUA and LFP to determine whether or not a recording site had a stimulus-driven signal that was out of the noise. Signal/noise ratio for MUA (or LFP) was defined as the peak difference of the visual evoked MUA (or LFP) and spontaneous MUA (or LFP) divided by the SD of the spontaneous MUA (or LFP) (see details in SI Materials and Methods). We included a recording site in the population averages if any of the four signal/noise ratios were larger than 2.
Histology.
Recording sites were assigned to different layers of V1 based on the results of track reconstruction. Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Patrick Williams and Marianne Maertens for help collecting data and Michael Hawken, Siddhartha Joshi, and Anita Disney for advice about histology. This work was supported by National Key Basic Research Program of China 2014CB846101; National Natural Science Foundation of China Grant 31371110; the Fundamental Research Funds for the Central Universities; US National Institutes of Health Grants T32 EY-07158 and R01 EY-01472; US National Science Foundation Grant 0745253; and fellowships from the Swartz Foundation and the Robert Leet and Clara Guthrie Patterson Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314690111/-/DCSupplemental.
References
- 1.Battersby WS, Wagman IH. Neural limitations of visual excitability. IV. Spatial determinants of retrochiasmal interaction. Am J Physiol. 1962;203:359–365. doi: 10.1152/ajplegacy.1962.203.2.359. [DOI] [PubMed] [Google Scholar]
- 2.Whittle P, Challands PD. The effect of background luminance on the brightness of flashes. Vision Res. 1969;9(9):1095–1110. doi: 10.1016/0042-6989(69)90050-9. [DOI] [PubMed] [Google Scholar]
- 3.Blake R, Breitmeyer B, Green M. Contrast sensitivity and binocular brightness - dioptic and dichoptic luminance conditions. Percept Psychophys. 1980;27(2):180–181. [Google Scholar]
- 4.Shapley R, Enroth-Cugell C. Visual adaptation and retinal gain controls. In: Osborne N, Chader G, editors. Progress in Retinal Research. Vol 3. Oxford: Pergamon; 1984. pp. 263–346. [Google Scholar]
- 5.Wolfson SS, Graham N. Forty-four years of studying light adaptation using the probed-sinewave paradigm. J Vis. 2006;6(10):1026–1046. doi: 10.1167/6.10.3. [DOI] [PubMed] [Google Scholar]
- 6.Xing D, Yeh CI, Burns S, Shapley RM. Laminar analysis of visually evoked activity in the primary visual cortex. Proc Natl Acad Sci USA. 2012;109(34):13871–13876. doi: 10.1073/pnas.1201478109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: Diversity and laminar dependence. J Neurosci. 2002;22(13):5639–5651. doi: 10.1523/JNEUROSCI.22-13-05639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing D, Yeh CI, Shapley RM. Generation of black-dominant responses in V1 cortex. J Neurosci. 2010;30(40):13504–13512. doi: 10.1523/JNEUROSCI.2473-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- 10.Victor JD, Purpura K, Katz E, Mao B. Population encoding of spatial frequency, orientation, and color in macaque V1. J Neurophysiol. 1994;72(5):2151–2166. doi: 10.1152/jn.1994.72.5.2151. [DOI] [PubMed] [Google Scholar]
- 11.Xing D, Yeh CI, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci. 2009;29(37):11540–11549. doi: 10.1523/JNEUROSCI.2573-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gail A, Brinksmeyer HJ, Eckhorn R. Contour decouples gamma activity across texture representation in monkey striate cortex. Cereb Cortex. 2000;10(9):840–850. doi: 10.1093/cercor/10.9.840. [DOI] [PubMed] [Google Scholar]
- 13.Denny N, Frumkes TE, Barris MC, Eysteinsson T. Tonic interocular suppression and binocular summation in human vision. J Physiol. 1991;437:449–460. doi: 10.1113/jphysiol.1991.sp018605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansford TG, Baker HD. Dark adaptation: An interocular light-adaptation effect. Science. 1969;164(3885):1307–1309. doi: 10.1126/science.164.3885.1307. [DOI] [PubMed] [Google Scholar]
- 15.Makous W, Teller D, Boothe R. Binocular interaction in the dark. Vision Res. 1976;16(5):473–476. doi: 10.1016/0042-6989(76)90024-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Stevenson SB. Post-retinal processing of background luminance. Vision Res. 1999;39(24):4045–4051. doi: 10.1016/s0042-6989(99)00116-9. [DOI] [PubMed] [Google Scholar]
- 17.Yeh CI, Xing D, Shapley RM. “Black” responses dominate macaque primary visual cortex v1. J Neurosci. 2009;29(38):11753–11760. doi: 10.1523/JNEUROSCI.1991-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creutzfeldt OD, Lee BB, Elepfandt A. A quantitative study of chromatic organisation and receptive fields of cells in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1979;35(3):527–545. doi: 10.1007/BF00236770. [DOI] [PubMed] [Google Scholar]
- 19.Moore BD, 4th, Kiley CW, Sun C, Usrey WM. Rapid plasticity of visual responses in the adult lateral geniculate nucleus. Neuron. 2011;71(5):812–819. doi: 10.1016/j.neuron.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemon V, et al. Contrast-dependent responses in the human visual system: Childhood through adulthood. Int J Neurosci. 1995;80(1-4):181–201. doi: 10.3109/00207459508986100. [DOI] [PubMed] [Google Scholar]
- 21.Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002;22(7):2737–2747. doi: 10.1523/JNEUROSCI.22-07-02737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balasubramanian V, Sterling P. Receptive fields and functional architecture in the retina. J Physiol. 2009;587(Pt 12):2753–2767. doi: 10.1113/jphysiol.2009.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratliff CP, Borghuis BG, Kao YH, Sterling P, Balasubramanian V. Retina is structured to process an excess of darkness in natural scenes. Proc Natl Acad Sci USA. 2010;107(40):17368–17373. doi: 10.1073/pnas.1005846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J, Wang Y, Lashgari R, Swadlow HA, Alonso JM. Faster thalamocortical processing for dark than light visual targets. J Neurosci. 2011;31(48):17471–17479. doi: 10.1523/JNEUROSCI.2456-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67(5):885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray CM, Engel AK, König P, Singer W. Stimulus-dependent neuronal oscillations in cat visual cortex: Receptive field properties and feature dependence. Eur J Neurosci. 1990;2(7):607–619. doi: 10.1111/j.1460-9568.1990.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 27.Xing D, et al. Stochastic generation of gamma-band activity in primary visual cortex of awake and anesthetized monkeys. J Neurosci. 2012;32(40):13873–80a. doi: 10.1523/JNEUROSCI.5644-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang K, Shelley M, Henrie JA, Shapley R. LFP spectral peaks in V1 cortex: Network resonance and cortico-cortical feedback. J Comput Neurosci. 2010;29(3):495–507. doi: 10.1007/s10827-009-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia XX, Xing DJ, Kohn A. No consistent relationship between gamma power and peak frequency in macaque primary visual cortex. J Neurosci. 2013;33(1):17–25. doi: 10.1523/JNEUROSCI.1687-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henrie JA, Shapley R. LFP power spectra in V1 cortex: The graded effect of stimulus contrast. J Neurophysiol. 2005;94(1):479–490. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- 31.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 32.Chalk M, et al. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron. 2010;66(1):114–125. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosman CA, et al. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75(5):875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemon V, Gordon J. Luminance-contrast mechanisms in humans: Visual evoked potentials and a nonlinear model. Vision Res. 2006;46(24):4163–4180. doi: 10.1016/j.visres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Komban SJ, Alonso JM, Zaidi Q. Darks are processed faster than lights. J Neurosci. 2011;31(23):8654–8658. doi: 10.1523/JNEUROSCI.0504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin JZ, et al. On and off domains of geniculate afferents in cat primary visual cortex. Nat Neurosci. 2008;11(1):88–94. doi: 10.1038/nn2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin J, Wang Y, Swadlow HA, Alonso JM. Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nat Neurosci. 2011;14(2):232–238. doi: 10.1038/nn.2729. [DOI] [PubMed] [Google Scholar]
- 38.Angelucci A, et al. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22(19):8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing D, Shapley RM, Hawken MJ, Ringach DL. Effect of stimulus size on the dynamics of orientation selectivity in Macaque V1. J Neurophysiol. 2005;94(1):799–812. doi: 10.1152/jn.01139.2004. [DOI] [PubMed] [Google Scholar]
- 40.Kayama Y, Riso RR, Bartlett JR, Doty RW. Luxotonic responses of units in macaque striate cortex. J Neurophysiol. 1979;42(6):1495–1517. doi: 10.1152/jn.1979.42.6.1495. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Van Essen DC. Peaked encoding of relative luminance in macaque areas V1 and V2. J Neurophysiol. 2005;93(3):1620–1632. doi: 10.1152/jn.00793.2004. [DOI] [PubMed] [Google Scholar]
- 42.Dai J, Wang Y. Representation of surface luminance and contrast in primary visual cortex. Cereb Cortex. 2012;22(4):776–787. doi: 10.1093/cercor/bhr133. [DOI] [PubMed] [Google Scholar]
- 43.Geisler WS, Albrecht DG, Crane AM. Responses of neurons in primary visual cortex to transient changes in local contrast and luminance. J Neurosci. 2007;27(19):5063–5067. doi: 10.1523/JNEUROSCI.0835-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucker TR, Fitzpatrick D. Luminance-evoked inhibition in primary visual cortex: A transient veto of simultaneous and ongoing response. J Neurosci. 2006;26(52):13537–13547. doi: 10.1523/JNEUROSCI.3723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62(4):566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunel N, Wang XJ. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90(1):415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.