Significance
Our studies demonstrate that the regulation of endothelial cellular senescence emerges as an additional mechanism underlying the antiatherogenic properties of liver X receptor (LXR) agents. Furthermore, our data suggest that combination therapy with LXR agents and metformin may provide a clinically useful therapeutic strategy to alleviate an LXR activation-mediated adverse effect on liver triglyceride metabolism. The beneficial effects of LXR activation appear to be reduced reactive oxygen species levels and increased endothelial NO synthase activity, both of which will lead to increased NO actions. Among the elderly population, atherosclerosis is a growing problem, leading to an increased risk of mortality by cardiovascular events. Adoption of combination therapy with LXR agents and widely prescribed metformin may be of clinical significance.
Keywords: T0901317, cholesterol efflux transporter
Abstract
Senescence of vascular endothelial cells leads to endothelial dysfunction and contributes to the progression of atherosclerosis. Liver X receptors (LXRs) are nuclear receptors whose activation protects against atherosclerosis by transcriptional regulation of genes important in promoting cholesterol efflux and inhibiting inflammation. Here we found that LXR activation with specific ligands reduced the increase in senescence-associated (SA) β-gal activity, a senescence marker, and reversed the decrease in telomerase activity, a replicative senescence marker, in human endothelial cells under high glucose. This effect of LXR activation was associated with reduced reactive oxygen species and increased endothelial NO synthase activity. A series of experiments that used siRNAs indicated that LXRβ mediates the prevention of endothelial cellular senescence, and that sterol regulatory element binding protein-1, which was up-regulated as a direct LXRβ target gene, may act as a brake of endothelial cellular senescence. Although oral administration of the LXR ligand led to severe fatty liver in diabetic rats, concomitant therapy with metformin avoided the development of hepatic steatosis. However, the preventive effect of the LXR ligand on SA β-gal–stained cells in diabetic aortic endothelium was preserved even if metformin was coadministered. Taken together, our studies demonstrate that an additional mechanism, such as the regulation of endothelial cellular senescence, is related to the antiatherogenic properties of LXRs, and concomitant treatment with metformin may provide a clinically useful therapeutic strategy to alleviate an LXR activation-mediated adverse effects on liver triglyceride metabolism.
Nuclear receptors are ligand-activated transcription factors that play an important role in the regulation of cellular metabolic function such as lipid and glucose metabolism (1). Dysregulation of these processes causes development of metabolic diseases such as hyperlipidemia, diabetes, and cardiovascular disease. In humans, 48 different types of nuclear receptors have been identified. These include the receptor for a metabolite of vitamin A, retinoic acid, retinoic acid receptor (RAR); the vitamin D receptor (VDR); the fatty acid receptor, peroxisome proliferator-activated receptor γ (PPARγ); the oxysterol receptor, liver X receptor (LXR); and their obligate heterodimeric partner, the retinoid X receptor (RXR) (2, 3). LXRs act as potent transcriptional switches for the coordinated regulation of genes involved in the control of hepatic lipid and cholesterol metabolism, and have a crucial role in reverse cholesterol transport, thereby stimulating of cholesterol efflux from the peripheral tissue to the liver (4). Several studies have reported that synthetic LXR ligands inhibit the development of atherosclerosis in animal models (5). In addition, LXR is a key modulator of systemic inflammation (6). Thus, activation of LXR has been considered as a prime drug target for the treatment of the macrovascular disease atherosclerosis (7). However, most LXR agonists, while lowering cholesterol, have the concomitant induction of lipogenic genes that leads to hypertriglyceridemia and liver steatosis, which have limited further clinical development.
Endothelial cellular senescence is an important contributor to the pathogenesis of age-associated vascular disorders. The senescent phenotype of endothelial cells can be transformed from antiatherosclerotic to proatherosclerotic (8). Previous studies have demonstrated that endothelial cells in atherosclerotic lesions display features of cellular senescence, including senescence-associated (SA) β-gal staining and telomere shortening (9, 10). We have also shown that senescent endothelial cells are observed in human atherosclerotic lesions but not in nonatherosclerotic lesions (11). Furthermore, endothelial cellular senescence is considered as an important cause of diabetes-associated vascular aging. Hyperglycemia is well established to accelerate senescence in endothelial cells (12). There is also in vivo evidence for the occurrence of vascular endothelial cellular senescence in diabetic vasculopathy (13). Accordingly, prevention of high glucose-associated endothelial cellular senescence may be a potential target to arrest the development of atherosclerosis in diabetes.
Given the pleiotropic effects of activation of nuclear receptors with their specific ligands on lipid and glucose homeostasis, cardiac energy balance, and regulation of inflammatory gene expression, new drugs targeting nuclear receptors are emerging as promising therapeutic agents for the treatment of diabetes, obesity, atherosclerosis, and cardiovascular disease. However, very little is known about the precise role of nuclear receptors in the regulation of endothelial cellular senescence. The present study was thus designed to examine the effects of a number of nuclear receptor ligands—capable of being clinically used in various medical treatments and that are highly influential on lipid and glucose metabolism—on cellular senescence of the vascular endothelium.
Results
First of all, specific ligands for eight representative nuclear receptors were given to human umbilical venous endothelial cells (HUVECs) cultured for 72 h under a high glucose condition (22 mM), and their effects on high glucose-induced endothelial cellular senescence were examined by using SA β-gal as a quantitative indicator of senescence (Fig. 1A). The VDR agonist calcitriol (i.e., vitamin D3), the farnesoid X receptor (FXR) agonist GW4064, the RAR agonist all-trans-retinoic acid (ATRA), the PPARα agonist Wy-14643, the PPARδ agonist GW501516, the PPARγ agonist rosiglitazone, and the RXR agonist bexarotene did not prevent the increase in SA β-gal activity that was induced by high glucose conditions. In contrast, the LXR-activating ligands T0901317 and GW3965 significantly reduced SA β-gal activity under high glucose. The reducing effect of T0901317 on the high glucose-induced increase in SA β-gal activity was concentration-dependent, and its maximal effect was achieved with 100 nM (Fig. 1B). Telomerase activity was significantly decreased in a high glucose environment, and T0901317 significantly prevented the decrease in telomere length (Fig. 1C), suggesting that LXR activation can suppress a process termed replicative senescence, a limitation in the number of times that somatic cells can divide.
Fig. 1.
LXR-activating ligands inhibit cellular senescence in HUVECs. (A) Effects of nuclear receptor-activating ligands on SA β-gal activity under high glucose (HG) conditions for 3 d. Each ligand was given at a concentration of 100 nM simultaneously with exposure to high glucose. (B) Concentration-dependent inhibition of high glucose-induced increases in SA β-gal activity by T0901317. (C) Recovery effect of T0901317 (100 nM) on telomerase activity under high glucose exposure for 24 h. Telomerase activity was measured by the telomere repeat application protocol assay [*P < 0.05 and **P < 0.01 vs. normal glucose (NG); #P < 0.05 and ##P < 0.01 vs. high glucose without ligand treatment].
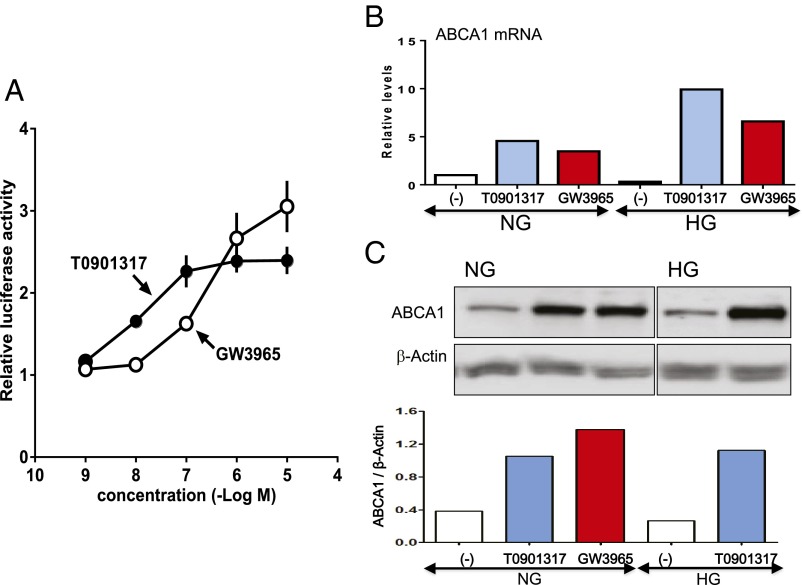
Because the effect of LXR activation on cholesterol excretion results from the ability to regulate expression of members of the ATP-binding cassette (ABC) family of membrane transporters (6), we next conducted a luciferase reporter assay to examine the effects of LXR-activating ligands on expression of the ATP-binding cassette transporter 1 (ABCA1) gene. HEK293T cells were transfected with the firefly luciferase gene linked to a promoter sequence of the LXR gene. As indicated by an increase in the relative luciferase activity, both T0901317 and GW3965 were capable of activating reporter gene transfection in a concentration-dependent manner (Fig. 2A). In HUVECs, we observed that T0901317 and GW3965 strikingly up-regulated the ABCA1 gene not only under normal but also high glucose conditions (Fig. 2B). On Western blots, expression of the ABCA1 protein was evidently increased by T0901317 and GW3965 in HUVECs under normal glucose conditions. Even in a hyperglycemic environment, we found the up-regulation of the ABCA1 protein in HUVECs after stimulation of LXR (Fig. 2C and Fig. S1). These findings demonstrate the presence of the target molecules for LXRs in vascular endothelial cells.
Fig. 2.
LXR-activating ligands up-regulate expression of ABCA1, a major cellular cholesterol efflux transporter, in HUVECs. (A) HEK293T cells were cotransfected with LXR expression vectors and luciferase reporter plasmid together with pCMX–β-gal. Eight hours after transfection, the cells were treated with increasing concentrations of T0901317 or GW3965 for 24 h. The luciferase activity in each sample was normalized by using β-gal and expressed as fold induction relative to that of vehicle-treated cells. (B) The mRNA levels of ABCA1 in HUVECs under normal glucose (NG) and high glucose (HG) conditions were quantified by real-time PCR. Data were expressed as a fold increase vs. control under normal glucose normalized to β-actin. (C) Western blots of ABCA1 in HUVECs under normal and high glucose conditions. β-Actin served as loading control. The mRNA and protein levels of ABCA1 were evaluated 72 h after the addition of T0901317 or GW3965 at a concentration of 100 nM under normal and high glucose conditions.
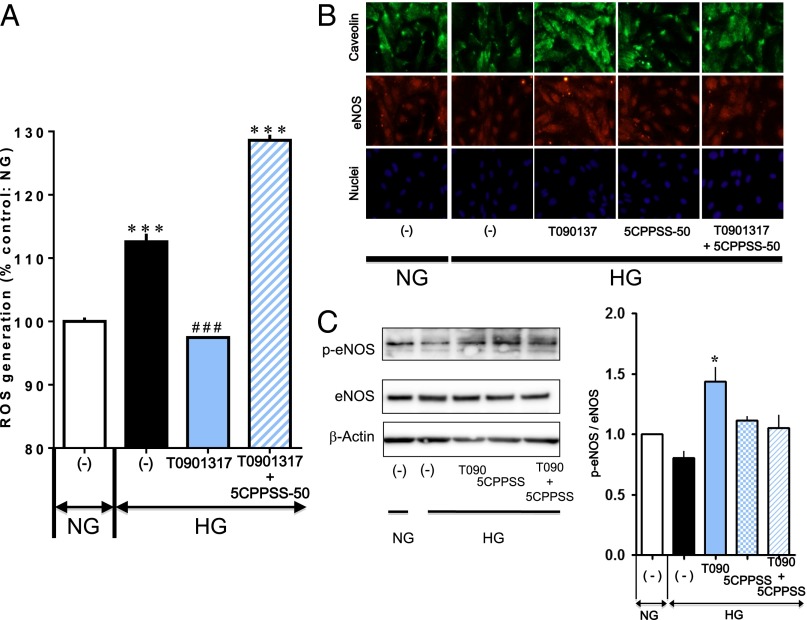
Increased reactive oxygen species (ROS) and decreased endothelial NO synthase (eNOS) under high glucose play a critical role in endothelial cellular senescence (8, 11, 14). When intracellular ROS were visualized using the fluorescence dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), cells exposed to high glucose had a significant increase in intracellular fluorescence (Fig. 3A). The LXR agonist T0901317 prevented this increase in ROS-induced intracellular fluorescence under high glucose conditions, which was completely blocked by the LXR antagonist 5CPPSS-50. T0901317 also showed increases in eNOS expression and phosphorylation, and caveolin-1 expression, which were damped under high glucose (Fig. 3 B and C). Such effects were inhibited in the presence of 5CPPSS-50. These observations indicate that LXR-mediated prevention of endothelial cell senescence in a hyperglycemic environment may involve the resolution of unbalance of NO and ROS.
Fig. 3.
LXR activation inhibits ROS generation and up-regulates expression of caveolin and eNOS in HUVECs. (A) Effects of 100 nM T0901317 in the absence and presence of 100 nM 5CPPSS-50 on ROS generation in HUVECs exposed to high glucose (HG) for 3 d. The cells were stained with fluorescent probe CM-H2DCFDA, and ROS were detected by flow cytometry [***P < 0.001 vs. normal glucose (NG); ###P < 0.001 vs. high glucose without ligand treatment]. (B) Immunofluorescent images showing increased expression of caveolin and eNOS by activation of LXR with 100 nM T0901317 in HUVECs exposed to high glucose for 3 d, which faded out in the presence of 100 nM 5CPPSS-50. (Lower) Nuclei were stained with Hoechst. No apparent difference in fluorescent signals from nuclei among groups was noted. T0901317 and 5CPSS-50 were added simultaneously with exposure to high glucose. (C) Western blots showing the effects of T0901317(T090) and 5CPSS-50(5CPSS) on eNOS expression and phosphorylation in HUVECs exposed to high glucose. β-Actin served as loading control (*P < 0.05 vs. high glucose alone).
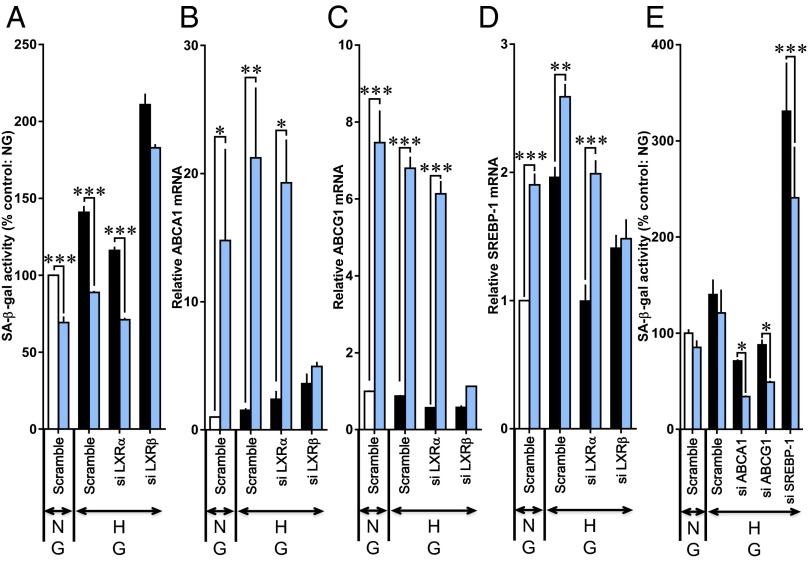
LXRs consist of LXRα (NR1H3) and LXRβ (NR1H2) (15). LXRβ is ubiquitously expressed, whereas LXRα expression is highest in the liver, kidney, intestine, and adrenal ligand. When the knockdown of LXRβ gene expression was performed in HUVECs by using siRNAs, the effect of T0901317 to reduce SA β-gal activity under high glucose conditions was less pronounced (Fig. 4A). However, T0901317 was fully effective in reducing high glucose-induced SA β-gal activity after transfection of LXRα siRNAs. The T0901317-stimulated up-regulation of mRNA levels of ABC family members of membrane transporters (ABCA1 and ABCG1) and sterol regulatory element binding protein-1 (SREBP-1), direct LXR target genes, were prevented by LXRβ siRNAs but not by LXRα siRNAs (Fig. 4 B–D). These results suggest that LXRβ is functionally important in vascular endothelial cells and mediates the prevention of endothelial cellular senescence. We further investigated whether ABCA1, ABCG1, and SREBP-1 are a critical molecule that is involved in the LXR-mediated prevention of endothelial cellular senescence. T0901317 significantly reduced SA β-gal activity under high glucose regardless of whether ABCA1 or ABCG1 siRNAs were applied (Fig. 4E). Transfection of SREBP-1 siRNAs greatly augmented high glucose-stimulated SA β-gal activity, suggesting that SREBP-1 may act as a brake on endothelial cellular senescence. However, T0901317 was significantly effective in reducing SA β-gal activity even in the presence of SREBP-1 siRNAs (Fig. 4E).
Fig. 4.
LXRβ mediates cellular senescence in HUVECs. The cells were transfected with scrambled, LXRα, LXRβ, ABCA1, ABCG1, or SREBP-1 siRNAs for 72 h. T0901317 (blue bars) was given simultaneously with exposure to normal glucose (NG) or high glucose (HG). (A–D) Influence of LXRα and LXRβ siRNAs on changes in (A) SA β-gal activity, (B) ABCA1 mRNA, (C) ABCG1 mRNA, and (D) SREBP-1 mRNA. (E) Influences of ABCA1, ABCG1, and SREBP-1 siRNAs on changes in SA β-gal activity (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. respective value untreated with T0901317).
Because LXRs are critical regulators of overall hepatic lipogenesis, LXR agonists, through induction of expression of lipogenic genes such as ABCA1, results in hypertriglyceridemia and liver steatosis (16). Oral administration of T0901317 (20 mg/kg) for 7 d did lead to severe fatty liver in streptozotocin (STZ)-induced diabetic rats, in which STZ was injected 7 d before oral administration (Fig. 5A). However, when metformin (50 mg/kg), widely given to patients with type 2 diabetes, was concomitantly used, hepatic steatosis did not apparently occur upon treatment with T0901317. Plasma glucose levels were 428.3 ± 21.9 mg/dL, 403.0 ± 43.9 mg/dL, 391.2 ± 39.0 mg/dL, and 352.2 ± 39.9 mg/dL in STZ, STZ plus metformin, STZ plus T0901317, and STZ plus metformin and T0901317 rats (no significant differences). In HUVECs, metformin was without effect on the increase in SA β-gal activity under high glucose (Fig. 5B). Furthermore, the preventive effect of T0901317 on high glucose-induced SA β-gal activity was unaffected by the presence of metformin. In diabetic rats, significant SA β-gal–stained cells in the aortic endothelium were detected (Fig. 5 C and D). Diabetic rats treated with T0901317 exhibited a significantly decreased ratio of SA β-gal–stained cells regardless of whether metformin was coadministered.
Fig. 5.
Combination treatment with T0901317 and metformin improves vascular endothelial senescence in STZ-induced diabetic rats without the development of hepatic steatosis. (A) Liver changes when diabetic rats were treated with T0901317 alone (20 mg/kg by mouth), metformin alone (50 mg/kg by mouth), and T0901317 plus metformin. (Scale bar: 10 mm.) (B) Effects of metformin and T0901317 plus metformin on the increase in SA β-gal activity in HUVECs under high glucose (HG) conditions for 3 d. T0901317 and metformin were given at a concentration of 100 nM simultaneously with exposure to high glucose [***P < 0.001 vs. normal glucose (NG); ###P < 0.001 vs. high glucose without drug treatment.] (C) SA β-gal–positive staining was observed in the intimal side of aortas of diabetic rats. T0901317 treatment eliminated its staining regardless of coadministration of metformin. (D) Relative ratio of the SA β-gal positively stained parts in the intimal side of aortas of diabetic rats untreated and treated with T0901317 alone or T0901317 plus metformin. Treatment with metformin (350 ± 34 mg/dL), T0901317 (321 ± 39 mg/dL), or metformin plus T0901317 (337 ± 40 mg/dL) did not significantly affect blood glucose levels in diabetic rats (389 ± 29 mg/dL).
We also examined the combined effect of T0901317 and metformin by using Zucker diabetic fatty rats (ZDFs), an insulin-resistant type 2 diabetes model. ZDFs receiving a high-fat diet developed fatty liver, which was moderated by administration of metformin/T0901317 but not T0901317 alone (Fig. S2A). Oil red O staining showed lipid accumulation in the aortic section from ZDFs fed a high-fat diet (Fig. S2B). In addition, consumption of a high-fat diet resulted in increased vascular cell adhesion molecule-1 (VCAM-1) expression in the aortas of ZDFs (Fig. S2C). Treatment with T0901317 effectively reduced lipid deposition and VCAM-1 expression in the aortas of ZDFs on a high-fat diet, regardless of coadministration of metformin. These beneficial effects were associated with the prevention of increased SA β-gal activity in aortas (Fig. S2D). However, it should be kept in mind that metabolic parameters summarized in Table S1 demonstrated that hyperglycemia was marked with the high-fat diet and amazingly improved by treatment with T0901317 alone and in combination with metformin.
Discussion
LXRs belong to a family of nuclear receptors and are activated by the oxidized cholesterol derivatives, namely the natural ligand oxysterols, which form heterodimers with the RXR to regulate transcription of target genes governing cholesterol, fatty acid, and glucose metabolism (17, 18). Furthermore, LXRs play an important role in the regulation of cytokine production and the antiinflammatory response (19, 20). A recent report also has shown that T0901317 serves as an inverse agonist of RAR-related orphan receptors α and γt, and suppresses differentiation of T-helper cells, that produce IL-17, related to chronic inflammation and autoimmune diseases (21). Because LXR-activating ligands promote reverse cholesterol transport and suppress inflammatory responses, LXR has had promise as a prime drug target for the treatment of atherosclerosis (7). Indeed, the LXR-activating ligand GW3965 has been shown to be effective at reducing plaque formation in the mouse model of atherosclerosis (22). However, LXRs are also critical regulators of overall hepatic lipogenesis, and LXR-activating ligands can lead to severe hepatic steatosis, which would limit their clinical use.
The present study reports that LXR-activating ligands can prevent endothelial cellular senescence, which was increased under high glucose conditions. A common feature of senescent endothelial cells is the presence of SA β-gal (23). This activity is a manifestation of an increase in lysosomal mass (24) and probably reflects the accumulation of autophagic vacuoles in the senescent cell containing nondegradable intracellular macromolecules and organelles. We showed that T0901317 and GW3965 significantly reduced SA β-gal activity in human endothelial cells exposed to high glucose. Furthermore, LXR activation reversed the decrease in telomerase activity, a replicative senescence marker, in these endothelial cells. Vascular endothelial cells with SA phenotypes are present in atherosclerotic lesions, and endothelial cellular senescence could contribute to atherogenesis (8, 11, 25). We thus suggest the involvement of reduced endothelial cellular senescence in LXR-mediated inhibition of the development of atherosclerosis. Specific ligands for other nuclear receptors did not prevent the increase in SA β-gal activity that was induced by high glucose conditions. Notably, it is interesting to note that endothelial cellular senescence was not inhibited by PPAR agonists that are currently used for the treatment of insulin-resistant diabetes and hyperlipidemia.
LXRs consist of two isoforms, including LXRα, which is expressed in several tissues such as liver, small intestine, and kidney, whereas LXRβ is more ubiquitously expressed; however, both isoforms are highly related, sharing 78% amino acid identity in their DNA-binding and ligand-binding domains (15, 26). Our experiments with the use of LXRα and LXRβ siRNAs indicated that LXRβ mediates the prevention of the senescence of human endothelial cells. In addition, endothelial expression of ABC family members of membrane transporters, ABCA1 and ABCG1, up-regulated by T0901317 appeared to result from activation of LXRβ. However, we found by using gene-specific siRNAs for ABCA1 and ABCG1 that these target genes of LXRs are not critically involved in the LXR-mediated prevention of endothelial cellular senescence. On the contrary, SREBP-1, which was also up-regulated as a direct LXRβ target gene, may act as a brake of endothelial cellular senescence, because transfection of SREBP-1 siRNAs greatly augmented high glucose-stimulated SA β-gal activity. However, we observed that T0901317 was significantly effective in reducing SA β-gal activity even in the presence of SREBP-1 siRNAs, suggesting an involvement of further mechanism(s) in the LXR-mediated prevention of endothelial cellular senescence. Our previous studies have demonstrated that endothelial cellular senescence caused by high glucose stimuli is associated with an increase in ROS and a decrease in eNOS-derived NO (8, 11, 14). In this study, activation of LXR reduced ROS generation and increased eNOS and cavelion-1 protein expression in human endothelial cells exposed to high glucose. Therefore, the beneficial effects of LXR-activating ligands on high glucose-induced endothelial cellular senescence may, at least in part, involve their actions on ROS and eNOS.
As predicted, oral administration of T0901317 for 7 d resulted in fatty liver in STZ-induced diabetic rats. It has been pointed out that fatty liver is associated with a decline in the NAD(+)-dependent protein acetylase sirtuin 3 (27–29). Sirtuins regulate cellular senescence and are generally considered as longevity factors (30). However, T0901317-induced fatty liver seems to occur independently of surtuin 3. Metformin, a popular oral drug for treating type 2 diabetes, is well known to activate the AMP-activated protein kinase (AMPK) in intact cells and in vivo (31). AMPK suppresses hepatic expression of the lipogenic gene SREBP-1c by inhibiting LXR-dependent SREBP-1c transcription (32–34). When metformin was concomitantly used, T0901317-induced hepatic steatosis was effectively avoided without affecting blood glucose levels in the type 1 diabetic rat model. On the contrary, the concurrent use of metformin did not hamper the inhibitory effect of T0901317 endothelial senescence in both in vitro and in vivo experiments.
To conclude, activation of LXRs can prevent endothelial cellular senescence accelerated by hyperglycemia. This beneficial effect on endothelial cellular senescence would be an additional mechanism by which LXRs inhibit the development of atherosclerosis. Considering the potential antiatherogenic properties of LXRs, we believe LXRs could be a good target for the development of therapy to limit atherosclerosis in diabetes. Metformin can contribute to a reduction in LXR-mediated hepatic steatosis. Therefore, combination treatment with metformin may hold the promise for the key to the clinical use of LXR-activating ligands.
Methods
Materials.
T0901317, GW3965, GW4064, and calcitriol were purchased from Cayman Chemical. ATRA and metformin were purchased from Sigma-Aldrich. 5CPPSS-50 was purchased from Wako Pure Chemical. Wy14643, GW501516, and rosiglitazone were purchased from Alexis Biochemicals. Bexarotene was purchased from Kishida and Toronto Research Chemicals.
Cell Culture.
HUVECs were purchased from Lonza and cultured in endothelial cell growth medium-2 until the start of the experiment. The cells were cultured in modified endothelial cell growth medium-2 that lacked insulin-like growth factor-1 but contained 2% (wt/vol) FBS during the experimental term. According to our previous study (11), five- to seven-passage subconfluent cells were used in the experiments. Cells were harvested at subconfluence and seeded into six-well plates.
SA β-Gal.
Cells were fixed for 10 min in 2% formaldehyde, 0.2% glutaraldehyde in PBS solution, and incubated for 12 h at 37 °C without CO2 with fresh β-gal staining solution: 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 2 mM MgCl2, pH 6.0. The cells were counterstained with DAPI (0.2 mg/mL in 10 mM NaCl) for 10 min to count the total cell number. The percentage of SA β-gal–positive cells was determined by counting the number of blue cells within a sample of 1,000 cells. SA β-gal activity was also measured by flow cytometry as described previously (14). After the experiment, cells were incubated with C12FDG (fluorogenic substrate 5-dodecanoyl-aminofluorescein di-β-d-galactopyranoside; 33 mM) at 37 °C for 30 min. Cells were trypsinized and analyzed by using a FACSCalibur flow cytometer (Becton Dickinson).
Human Telomere Length Assay.
Telomere length was measured by fluorescence in situ hybridization by using flow cytometry (14).
Luciferase Reporter Gene Assay.
Transfection of HEK293T cells were performed by using calcium phosphate coprecipitation. HEK293T cells can be stably transfected by this transfection protocol suitable for comparing the effect of LXRα and -β. Transfection mixtures contained 30 ng of human LXRα ligated into pBApo-CMV Neo DNA vector (Takara Bio) and 120 ng of pGL4.1-DR4-Luc for the LXR reporter assay with the addition of 30 ng of the pCMX β-gal expression vector and carrier DNA pUC18 to yield 600 ng of total DNA per well. After 8 h of transfection, cells were thoroughly washed with fresh medium and further incubated in the presence of the compounds at the indicated concentrations in medium containing 10% FBS for another 24 h. Luciferase and β-gal activities of cell lysates were analyzed by using a luminescence reader and a spectrophotometer. Luciferase activity was normalized relative to the activity of an internal β-gal control and expressed as the relative luciferase activity, which was determined in triplicate experiments.
RNA Extraction and Quantitative Real-Time PCR.
Total RNA was isolated from cells with use of RNAiso Plus (Takara Bio). Following treatment of the RNA samples with DNase (Invitrogen), first-strand cDNA was synthesized from 0.5 μg of total RNA by using an oligo(dT)20 RT primer and ReverTra Ace (Toyobo) according to the manufacturer’s instructions. Quantitative real-time PCR (SYBR green) analysis was performed by using a TP800 Thermal Cycler Dice real-time system (Takara Bio). The mRNA expression levels were normalized by the β-actin mRNA levels and calculated according to the delta–delta Ct method.
Western Blot Analysis.
Cells were harvested and lysed in 50 μL of RIPA buffer (25 mM Tris⋅HCl, 1% Nonidet P-40, 150 mM NaCl, 0.1% SDS, 1% sodium deoxycholate, 5 mM EDTA, pH 7.4) containing protease inhibitor mixture tablets (Roche Diagnostics) on ice. The lysates were centrifuged at 12,000 × g for 15 min at 4 °C, and the resulting supernatants were assayed for their protein concentrations (Bradford Assay; Bio-Rad). The supernatants (10 μg of protein) were separated by SDS/PAGE by using a 12% gel and transferred onto polyvinylidene fluoride membranes. After blocking with 5% powdered skim milk in TBS for 1 h, the membranes were probed with anti–ABCA-1 (supplied by Shinji Yokoyama, Chubu University, Kasugai, Japan) or anti-β-actin (Santa Cruz Biotechnology) in TBS containing 1% powdered skim milk and 0.05% Tween-20 overnight at 4 °C. The membranes were then washed and incubated with an alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology) in TBS as described earlier for 1 h at room temperature. The membranes were thoroughly washed, and the bound antibodies were detected by using CDP-Star (Applied Biosystems) as a substrate for alkaline phosphatase. The protein concentrations were determined by using an LAS-3000 Mini system (Fujifilm).
Flow Cytometric Analysis of ROS Generation.
Intracellular oxidant generation was detected with the fluorescent probe CM-H2DCFDA (Invitrogen). Cells were incubated with CM-H2DCFDA (10 mM) at 37 °C for 30 min, and flow cytometry was performed.
Immunofluorescence and Confocal Analysis.
Cells were fixed with a 4% formalin solution and exposed to the secondary antibody conjugated to high-quality fluorophores, including Alexa Fluor 488 and Alexa Fluor 647, after overnight with an anti–caveolin-1 antibody (BD Bioscience) or an anti-eNOS antibody (BD Biosciences). The nucleus was stained with Hoechst 33258 (Nacalai Tasque). Images were observed by using a Leica TCS-SP5 confocal system.
Transfection of siRNAs.
LXRα, LXRβ, ABCA1, ABCG1, SREBP-1, and scrambled siRNAs were purchased from Santa Cruz Biotechnology. siRNA (10 nM) was transfected by using Lipofectamine RNAiMAX (Invitrogen). After incubation for 72 h, the down-regulation of the target genes was confirmed by real-time PCR.
Generation of STZ Diabetic Animal Model.
We generated diabetic rats (Sprague-Dawley rats; 26 wk old) using STZ (60 mg/kg, i.p.) (14). When T0901317 or metformin were used, they were administered orally at 20 mg/kg/d and 50 mg/kg/d for 7 d from 5 d after STZ injection, respectively. Blood glucose levels and body weights were measured daily. This animal research was conducted in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals and with approval of the Nagoya University Animal Care Committee.
Statistical Analysis.
Analysis was performed with Prism software (version 4; GraphPad) by using one-way ANOVA followed by Tukey multiple comparison or t test when appropriate. Data are expressed as mean ± SE (n = 3∼6). P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Toshio Fujimori for his technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322153111/-/DCSupplemental.
References
- 1.Hansen MK, Connolly TM. Nuclear receptors as drug targets in obesity, dyslipidemia and atherosclerosis. Curr Opin Investig Drugs. 2008;9(3):247–255. [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 3.Woods CG, Heuvel JP, Rusyn I. Genomic profiling in nuclear receptor-mediated toxicity. Toxicol Pathol. 2007;35(4):474–494. doi: 10.1080/01926230701311351. [DOI] [PubMed] [Google Scholar]
- 4.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 5.Parikh N, Frishman WH. Liver X receptors: A potential therapeutic target for modulating the atherosclerotic process. Cardiol Rev. 2010;18(6):269–274. doi: 10.1097/CRD.0b013e3181e8067a. [DOI] [PubMed] [Google Scholar]
- 6.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116(3):607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faulds MH, Zhao C, Dahlman-Wright K. Molecular biology and functional genomics of liver X receptors (LXR) in relationship to metabolic diseases. Curr Opin Pharmacol. 2010;10(6):692–697. doi: 10.1016/j.coph.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, et al. Nitric oxide and endothelial cellular senescence. Pharmacol Ther. 2008;120(3):333–339. doi: 10.1016/j.pharmthera.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The pathogenesis of atherosclerosis—an update. N Engl J Med. 1986;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 10.Bürrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb. 1991;11(6):1678–1689. [PubMed] [Google Scholar]
- 11.Hayashi T, et al. Endothelial cellular senescence is inhibited by nitric oxide: implications in atherosclerosis associated with menopause and diabetes. Proc Natl Acad Sci USA. 2006;103(45):17018–17023. doi: 10.1073/pnas.0607873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoi T, et al. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55(6):1660–1665. doi: 10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- 13.Brodsky SV, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004;94(3):377–384. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- 14.Matsui-Hirai H, et al. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: A relationship between telomeres and nitric oxide. J Pharmacol Exp Ther. 2011;337(3):591–599. doi: 10.1124/jpet.110.177584. [DOI] [PubMed] [Google Scholar]
- 15.Willy PJ, et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 16.Parks JS, Chung S, Shelness GS. Hepatic ABC transporters and triglyceride metabolism. Curr Opin Lipidol. 2012;23:196–200. doi: 10.1097/MOL.0b013e328352dd1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peet DJ, Janowski BA, Mangelsdorf DJ. The LXRs: A new class of oxysterol receptors. Curr Opin Genet Dev. 1998;8(5):571–575. doi: 10.1016/s0959-437x(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 18.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325(5936):100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph SB, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119(2):299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 21.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph SB, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA. 2002;99(11):7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998;241(2):309–315. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 24.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113(pt 20):3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 25.Erusalimsky JD. Vascular endothelial senescence: From mechanisms to pathophysiology. J Appl Physiol (1985) 2009;106(1):326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 27.Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendrick AA, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433(3):505–514. doi: 10.1042/BJ20100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury M, Jonscher KR, Friedman JE. Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany, NY Online) 2011;3(2):175–178. doi: 10.18632/aging.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyle JG, Salt IP, McKay GA. Metformin action on AMP-activated protein kinase: A translational research approach to understanding a potential new therapeutic target. Diabet Med. 2010;27(10):1097–1106. doi: 10.1111/j.1464-5491.2010.03098.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Craddock L, Hong S, Liu ZM. AMP-activated protein kinase suppresses LXR-dependent sterol regulatory element-binding protein-1c transcription in rat hepatoma McA-RH7777 cells. J Cell Biochem. 2009;106(3):414–426. doi: 10.1002/jcb.22024. [DOI] [PubMed] [Google Scholar]
- 33.Hwahng SH, Ki SH, Bae EJ, Kim HE, Kim SG. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-α-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology. 2009;49(6):1913–1925. doi: 10.1002/hep.22887. [DOI] [PubMed] [Google Scholar]
- 34.Yap F, Craddock L, Yang J. Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int J Biol Sci. 2011;7(5):645–650. doi: 10.7150/ijbs.7.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.