Significance
This paper describes a unique approach to target and kill cancer cells in the bloodstream, in which the extensive surface area of circulating leukocytes is used to display the cancer-specific TNF-related apoptosis inducing ligand (TRAIL) and E-selectin adhesion receptor to the surrounding fluid. The approach is inspired by the cytotoxic activity of natural killer cells and is quite effective at killing cancer cells both in vitro with human blood samples and in mouse blood circulation. The mechanism is surprising and unexpected in that this repurposing of leukocytes in flowing blood is more effective than directly targeting the cancer cells with liposomes or soluble protein.
Keywords: drug delivery, nanomedicine
Abstract
Metastasis through the bloodstream contributes to poor prognosis in many types of cancer. Mounting evidence implicates selectin-based adhesive interactions between cancer cells and the blood vessel wall as facilitating this process, in a manner similar to leukocyte trafficking during inflammation. Here, we describe a unique approach to target and kill colon and prostate cancer cells in the blood that causes circulating leukocytes to present the cancer-specific TNF-related apoptosis inducing ligand (TRAIL) on their surface along with E-selectin adhesion receptor. This approach, demonstrated in vitro with human blood and also in mice, mimics the cytotoxic activity of natural killer cells and increases the surface area available for delivery of the receptor-mediated signal. The resulting “unnatural killer cells” hold promise as an effective means to neutralize circulating tumor cells that enter blood with the potential to form new metastases.
Over 90% of cancer-related deaths are due to cancer metastasis, the spread of cancer cells from a primary tumor to anatomically distant organs (1). In many types of cancer, cancer cells from the primary tumor can intravasate into the peripheral circulation as circulating tumor cells (CTCs) (2, 3). These CTCs can then interact with the receptor-bearing endothelial cell wall under flow in other organs, in a manner similar to leukocyte extravasation during inflammation and lymphocyte homing to lymphatic tissues (4). Recent studies have shown that CTCs from many types of primary tumors express sialylated carbohydrate ligands similar to leukocytes, which mediate interactions with selectins on the endothelium (5, 6). Selectins possess rapid, force-dependent binding kinetics, which can trigger the rolling adhesion of CTCs along the blood vessel wall (7, 8). CTCs can subsequently transition from rolling to firm adhesion, allowing for transendothelial migration into tissues and eventual formation of micrometastases (9). Surgery and radiation have proven effective at treating primary tumors that do not invade the basement membrane; however, the difficulty of detecting distant micrometastases has made the majority of metastatic cancer treatments unsuccessful.
The development of technologies to directly target CTCs in vivo holds promise in reducing both the metastatic load and the formation of new tumors. Although studies have reported that as many as 1 × 106 cancer cells detach from the primary tumor (per gram) of patients per day (10, 11), CTCs are difficult to detect due to their sparse concentrations in the bloodstream, as low as 1–100 cells per mL (3, 12). Additionally, there are ∼1 × 106 leukocytes or 1 × 109 erythrocytes per single CTC in blood (13). Despite the difference in numbers, both leukocytes and CTCs share similar characteristics in terms of their migration within the bloodstream. Highly deformable erythrocytes experience a drift velocity away from the vessel wall and collect in the center region, displacing less deformable leukocytes and CTCs to the near-wall region in a mechanism termed margination (14). Such margination phenomena can effectively surround CTCs within the circulating leukocyte population, thus making leukocytes a potentially attractive carrier of treatments to CTCs by exploiting their numerous adhesion receptors.
The utilization of leukocytes to treat CTCs directly within the bloodstream has not previously been explored. Here, we describe a therapeutic approach to target and kill circulating cancer cells in the bloodstream by functionalizing leukocytes with the apoptosis-inducing ligand TRAIL, and the adhesion receptor E-selectin directly within blood under shear flow. The functionalization of leukocytes under flow, effectively creating a form of “unnatural killer cells” within the bloodstream, is shown to be highly effective at treating circulating cancer cells in flowing human blood in vitro, and in the peripheral circulation of mice in vivo.
Results
E-Selectin /TRAIL Liposomes Adhesively Interact and Induce Apoptotic Cancer-Cell Death Under Shear Flow.
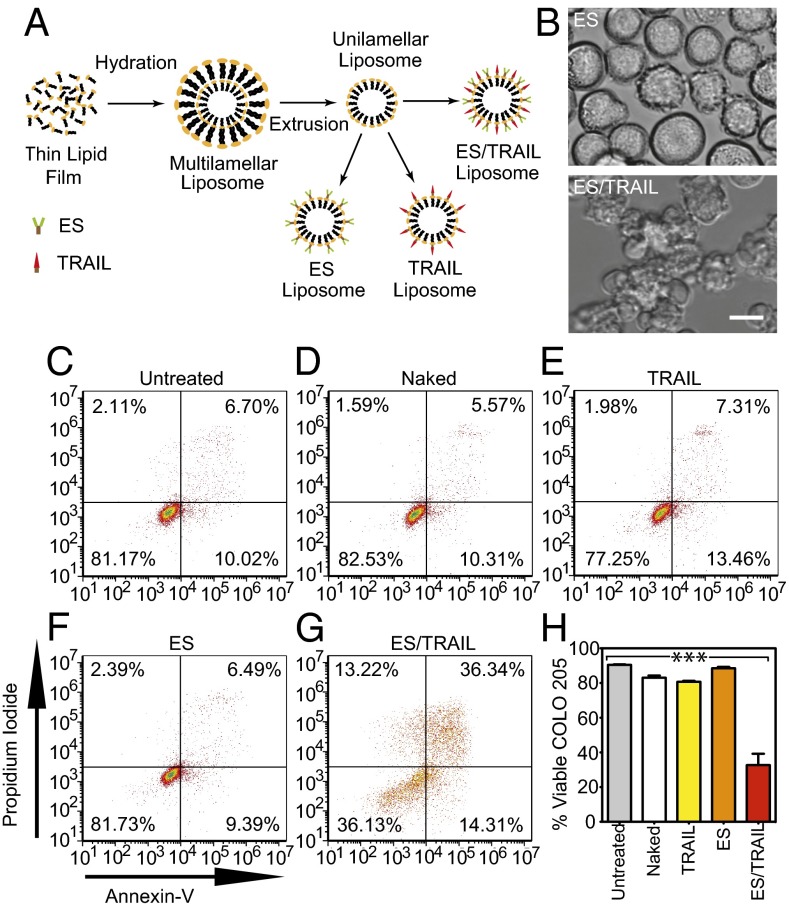
Many types of circulating tumor cells (CTCs) and cancer cell lines derived from colon, breast, prostate, and pancreas are known to display glycosylated ligands that allow them to adhesively interact with E-selectin (ES) under physiological shear flow (15). This interaction has been proposed to explain why some cancers home to tissue-specific capillary beds such as the bone marrow and liver (16, 17). In an attempt to target and kill cancer cells of this form, nanoscale liposomes conjugated with a mixture of recombinant human ES protein and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) were developed (Fig. 1A, Fig. S1, and Table S1). TRAIL binds to death receptors 4 and 5 on the surface of cancer cells to induce apoptosis through the intrinsic and extrinsic pathways (18, 19). ES/TRAIL liposomes consisting of a 10% weight ratio of (1,2-dioleoyl-sn-glycero-3-{[N-(5-amino- 1-carboxypentyl) iminodiacetic acid] succinyl}(nickel salt) (DOGS-Ni-NTA), used to conjugate ES and TRAIL to the liposome surface, were found to be most effective at inducing apoptotic cell death in a colorectal adenocarcinoma (COLO 205) cell line under static conditions (Fig. S2), as determined using an Annexin-V apoptosis assay.
Fig. 1.
ES/TRAIL liposomes adhesively interact with and kill cancer cells under uniform shear flow. (A) Synthesis of ES, TRAIL, and ES/TRAIL unilamellar liposomes using a thin film hydration method. Briefly, lipids in chloroform were dried overnight to form a thin lipid film. Lipids were then hydrated and subjected to freeze–thaw cycles to form multilamellar liposomes, which were extruded through membranes to form unilamellar liposomes. ES, TRAIL, or a combination of ES and TRAIL was then conjugated to Ni-NTA on the liposome surface. To assess the ability of ES/TRAIL liposomes to target and kill cancer cells under flow, ES/TRAIL liposomes were added to a suspension of COLO 205 cancer cells and exposed to shear flow in a cone-and-plate viscometer at a shear rate of 188 s−1 for 2 h. Cells were then removed, washed, placed into culture for 24 h, and assessed for cell viability. (B) COLO 205 morphology after treatment with ES (Upper) and ES/TRAIL (Lower) liposomes under shear flow. (Scale bar, 20 µm.) (C–G) Representative propidium iodide/Annexin-V flow cytometry plots of unsheared cancer cells (C) and cells sheared with naked (D), TRAIL-bound (E), ES-bound (F), and ES/TRAIL-bound liposomes (G) under shear flow. Cells were classified into four categories based on dye uptake: viable cells [negative for Annexin-V and propidium iodide (PI)], early apoptotic cells (positive for Annexin-V only), late apoptotic cells (positive for Annexin-V and PI), and necrotic cells (positive for PI only). (H) Percent of viable cells after treatment for each group. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. ***P < 0.0001 (one-way ANOVA with Tukey posttest).
In the postcapillary venules where selectin-mediated adhesion and cell extravasation into tissues typically occur, moderate shear rates can initiate flowing cell interactions with the endothelial cell wall (20, 21). To recreate these physical forces in vitro, a cone-and-plate shear assay was developed to probe the interactions of cancer cells and ES/TRAIL liposomes under venular shear rates. After exposure to shear flow (shear rate: 188 s−1) for 2 h, COLO 205 cells exposed to ES liposomes displayed their normal morphology whereas substantial membrane blebbing was observed in samples exposed to ES/TRAIL liposomes, characteristic of cells undergoing apoptosis (Fig. 1B). Annexin-V assay revealed that exposure to shear flow for 2 h induced minimal COLO 205 cell apoptosis in untreated controls (Fig. 1C), in addition to treatment with liposomes in the absence of conjugated protein (Fig. 1D) or conjugated solely with ES (Fig. 1E) or TRAIL (Fig. 1F). However, a combination of ES/TRAIL conjugated to the liposome surface induced a significant decrease in COLO 205 viability following exposure to shear flow (Fig. 1 G and H).
To investigate the adhesive characteristics of ES/TRAIL liposomes to cancer cells under flow, COLO 205 cells were exposed to ES/TRAIL liposomes consisting of fluorescent cholesterol and exposed to shear flow as in previous in vitro shear assays. Flow cytometry revealed that >99.9% of the COLO 205 cell population was adhered to ES/TRAIL liposomes after exposure to shear flow (Fig. S3 A and B). Fluorescent micrographs and brightfield overlay images clearly displayed ES/TRAIL liposomes adhered to the surface of COLO 205 cells (Fig. S3 C and D). These data suggest that the presence of the ES adhesion receptor enhances the effect of TRAIL by promoting tighter contacts with the cancer-cell membrane.
ES/TRAIL Liposomes Functionalize Leukocytes in Whole Blood Under Shear Flow in Vitro.
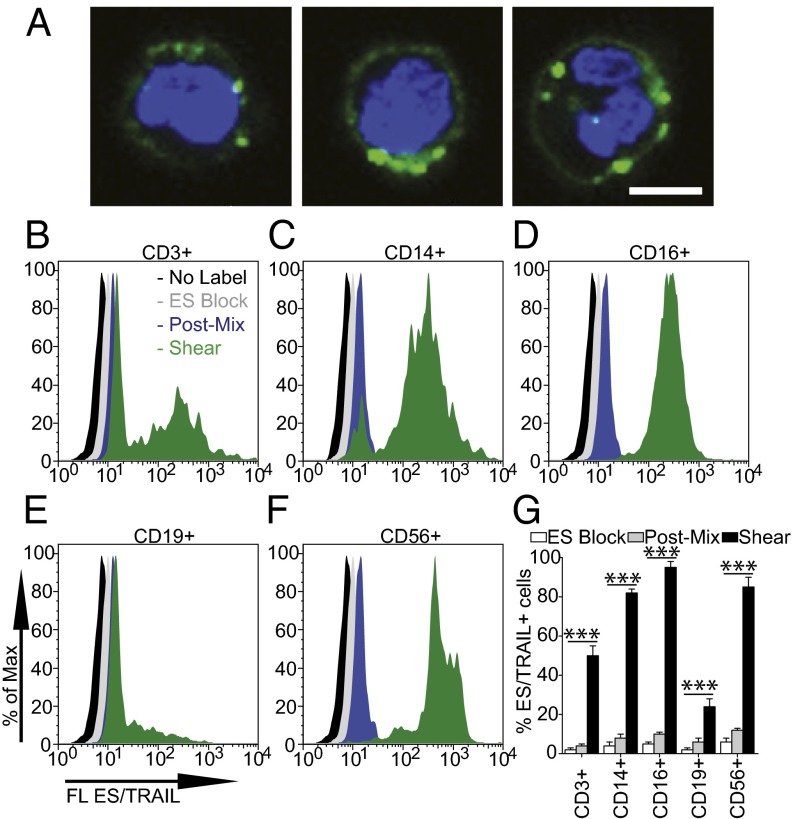
In addition to CTCs, circulating leukocytes also possess ligands for ES, which are necessary in the inflammatory response and lymphocyte homing to lymphatic tissues (1, 22). To assess the potential to functionalize leukocytes with ES/TRAIL to target and kill CTCs, we treated human blood with fluorescent ES/TRAIL liposomes under shear flow in a cone-and-plate viscometer. Upon exposure to shear (shear rate: 188 s−1), ES/TRAIL liposomes readily bind to leukocytes via selectin ligands on the leukocyte surface (Fig. 2A).
Fig. 2.
ES/TRAIL liposomes adhere to multiple leukocyte subpopulations after exposure to shear flow in whole blood. (A) Confocal images of ES/TRAIL liposomes (green) bound to human leukocytes (blue, cell nuclei) after exposure to shear flow in whole blood in a cone-and-plate viscometer at 188 s−1 for 30 min. Leukocytes have nuclear morphology characteristic of monocytes (Left), lymphocytes (Center), and neutrophils (Right). (Scale bar, 5 μm.) (B–G) To assess the adhesion of ES/TRAIL liposomes to leukocyte subpopulations, fluorescent ES/TRAIL liposomes were added to human blood and exposed to shear flow in a cone-and-plate viscometer at a shear rate of 188 s−1 for 30 min. Leukocytes were isolated from blood using a Polymorphs density gradient and labeled with CD3, CD14, CD16, CD19, and CD56, which is typically expressed on T lymphocytes, monocytes, neutrophils, B-lymphocytes, and natural killer cells, respectively. Expression of fluorescent ES/TRAIL (FL ES/TRAIL) liposomes on the surface of leukocytes that are CD3+ (B), CD14+ (C), CD16+ (D), CD19+ (E), and CD56+ (F), determined using flow cytometry. Expression of CD3, CD14, CD16, CD19, and CD56 on the leukocyte surface was determined using isotype controls. No label, unsheared cells that were not treated with fluorescent ES/TRAIL liposomes; ES Block, cells treated with fluorescent ES/TRAIL liposomes that were pretreated with an ES functional blocking antibody; Post-Mix, cells labeled with fluorescent ES/TRAIL liposomes immediately after mixing liposomes in whole blood. (G) Percent of CD3+, CD14+, CD16+, CD19+, and CD56+ leukocytes adhered to ES/TRAIL liposomes. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. ***P < 0.0001 (one-way ANOVA with Tukey posttest).
To quantify leukocyte subpopulations that adhere to ES/TRAIL liposomes under flow, leukocytes were separated from whole blood and analyzed for both leukocyte marker expression and adherent ES/TRAIL liposomes using flow cytometry. Functionalized leukocytes were labeled with CD3, CD14, CD16, CD19, and CD56 antibodies, as such markers are commonly expressed on most T lymphocytes, monocytes, neutrophils, B-lymphocytes, and natural killer (NK) cells, respectively (23). Minimal adhesion of ES/TRAIL liposomes to leukocytes in blood was observed in the presence of a functional blocking ES antibody (Fig. 2 B–G). Minimal ES/TRAIL liposome adhesion was also observed immediately after treatment with whole blood. However, after exposure to shear flow, flow cytometry analysis revealed that leukocyte subpopulations positive for CD3 (Fig. 2B), CD14 (Fig. 2C), CD16 (Fig. 2D), CD19 (Fig. 2E), and CD56 (Fig. 2F) adhered to ES/TRAIL liposomes to varying degrees (Fig. 2G). Adhesion was also observed on populations of lymphocytes, which suggests that some cytotoxic patrolling of the lymphatic system may also occur in vivo. Leukocyte subpopulations can vary in their E-selectin ligand expression (24) and thus could explain the variations in the number of bound ES/TRAIL liposomes.
ES/TRAIL Functionalization Does Not Induce Significant Leukocyte or Endothelial Cell Death.
To assess the effects of ES/TRAIL functionalization on leukocyte viability, mononuclear leukocytes isolated from human blood were treated with ES/TRAIL liposomes under both static and shear-flow conditions. Annexin-V assays revealed no significant differences in leukocyte viability when leukocytes were incubated with ES (Fig. S4B) or ES/TRAIL (Fig. S4C) liposomes under static conditions for 24 h, compared with untreated leukocyte controls (Fig. S4 A and D). Upon functionalization with liposomes under shear flow for 2 h (shear rate, 188 s−1), no significant decreases were found in ES (Fig. S4F) or ES/TRAIL (Fig. S4G) functionalized leukocytes, compared with untreated leukocyte controls (Fig. S4 E and H). These data suggest that ES/TRAIL liposomes can functionalize leukocytes under flow to target and kill cancer cells, while exerting negligible cytotoxic effects on leukocytes. It is important to note that individual subpopulations of leukocytes have shown apoptotic effects in the presence of TRAIL (25); however, this study focused on the overall effects of ES/TRAIL liposomes on blood-borne leukocytes.
To assess the effects of ES/TRAIL functionalization on endothelial cell viability, human umbilical vein endothelial cells (HUVECs) were treated with ES/TRAIL liposomes in human blood under shear-flow conditions in vitro. Treatment with ES/TRAIL liposomes or an equivalent concentration of soluble TRAIL in human blood under shear flow for 4 h induced no significant differences in HUVEC viability, compared with untreated HUVECs exposed to shear in human blood (Fig. S5). These data suggest that ES/TRAIL-functionalized leukocytes exert negligible toxic effects on human endothelial cells under blood-flow conditions.
Apoptotic Effects of ES/TRAIL Therapy Are Enhanced in Human Blood Under Flow.
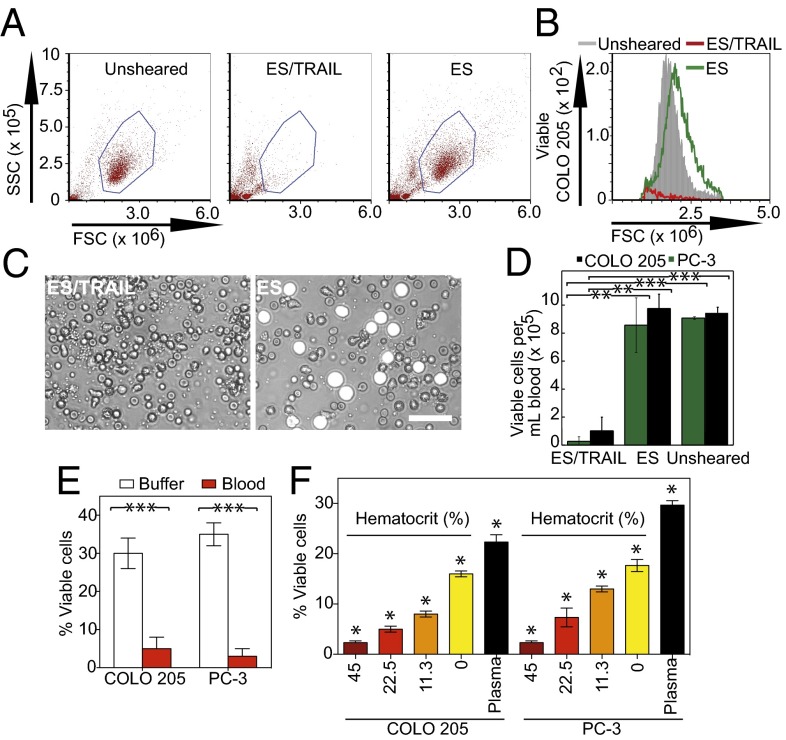
Clinically, CTCs are sparsely distributed in the complex milieu of whole blood, at concentrations as low as 1–100 cells per mL (3, 12). To examine whether ES/TRAIL liposomes would effectively target cancer cells in the presence of blood cells and serum under flow conditions, we fluorescently labeled colorectal COLO 205 and prostate PC-3 cancer-cell lines and spiked them into human peripheral blood. Surprisingly, under identical shear-flow conditions, ES/TRAIL therapy was even more effective at killing cancer cells in the presence of human blood (Fig. 3 A–D), compared with shearing COLO 205 or PC-3 cells alone in buffer (Fig. 3E), with <5% of the fluorescent, viable cancer-cell populations remaining after ES/TRAIL treatment (Fig. 3E). These results suggest that ES/TRAIL therapy is effective at targeting circulating cancer cells derived from multiple organs in human blood.
Fig. 3.
ES/TRAIL liposome therapeutic effects are enhanced in human blood under flow in vitro. (A) Flow cytometry of COLO 205 cancer cells after treatment with ES/TRAIL or ES liposomes in blood under shear flow in a cone-and-plate viscometer at 188 s−1 for 2 h. Unsheared, viable untreated cancer cell control. (B) Representative flow cytometry histogram showing the number of viable cancer cells collected. (C) Representative micrographs of COLO 205 cells (white) in blood when treated with ES/TRAIL (Left) and ES only (Right) liposomes in blood under shear flow. (Scale bar, 50 μm.) (D) Number of viable COLO 205 and PC-3 cells per volume of blood after treatment with ES/TRAIL or ES liposomes in blood under shear flow. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. **P < 0.001, ***P < 0.0001 (unpaired t test). (E) Comparison of fraction of COLO 205 and PC-3 cells that remained viable after treatment with ES/TRAIL liposomes in buffer versus blood. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. ***P < 0.0001 (unpaired t test). (F) Fraction of COLO 205 and PC-3 cells that remained viable after treatment with ES/TRAIL liposomes in blood with varying percentages of normal hematocrit. Hematocrit was varied whereas other blood components remained constant, based on a normal hematocrit of 45%. Plasma indicates removal of all blood cells. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. *P < 0.05 (one-way ANOVA with Tukey posttest).
To evaluate the impact of blood cells on the efficacy of ES/TRAIL treatment, fluorescent COLO 205 and PC-3 cells were spiked in human blood of varying hematocrit percentages. All additional blood-cell components were maintained whereas the volume of removed erythrocytes was replaced with plasma from the same blood donor. Interestingly, the apoptotic effects were hematocrit-dependent, as higher hematocrit significantly decreased the number of viable COLO 205 and PC-3 cells after ES/TRAIL treatment (Fig. 3F). The enhanced apoptotic effect suggests that blood-cell collisions under flow can promote the apoptotic effects of ES/TRAIL liposomes and agrees with our intuitive understanding of blood rheology, as the presence of erythrocytes is known to create erratic cell paths with frequent cross-streamline displacements (26) and increase the lifetime of colliding doublets to promote aggregate formation (27).
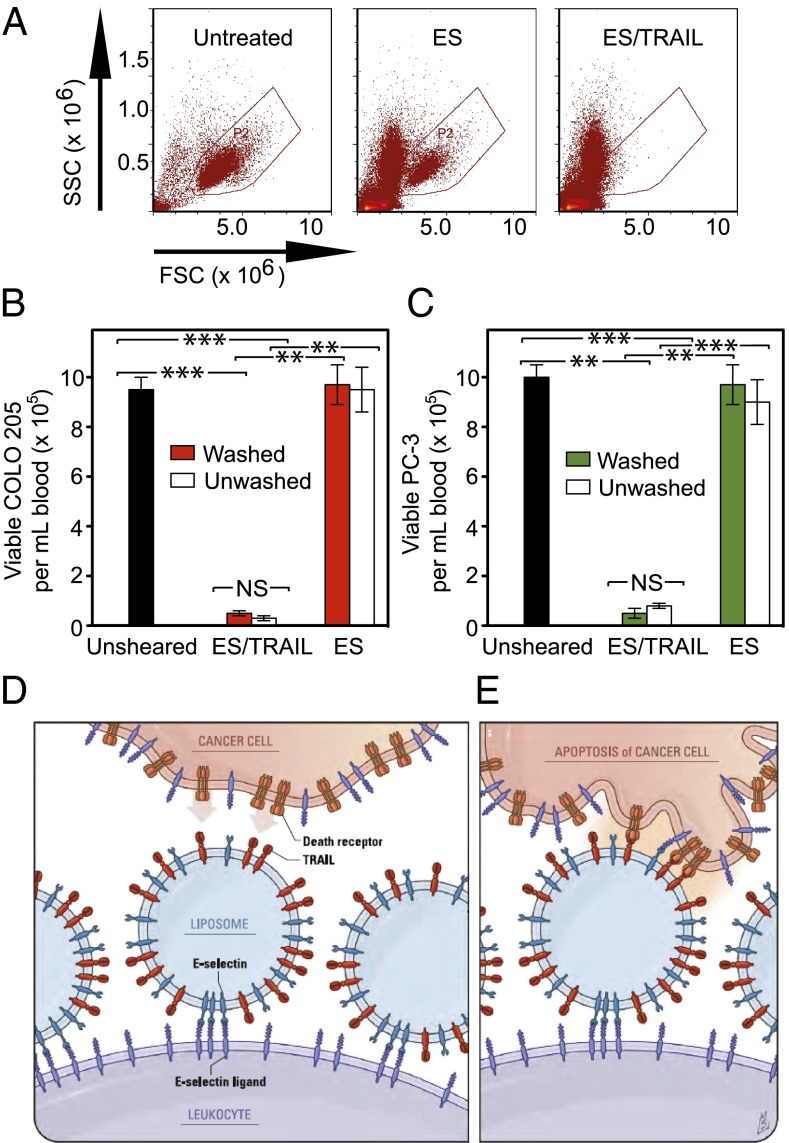
To assess the mechanism by which leukocytes act as a pervading carrier surface for functional TRAIL, blood was pretreated with ES/TRAIL liposomes under shear flow, with blood cells subsequently separated from unbound ES/TRAIL via centrifugation and replaced with fresh blood plasma. ES/TRAIL therapeutic effects under shear flow remained nearly identical, as COLO 205 and PC-3 cells, spiked into a suspension of blood with washed, pretreated blood cells, were killed at roughly the same rate as unwashed blood (Fig. 4 A–C). Thus, upon addition of the ES/TRAIL liposomes to cancer cell-spiked blood, liposomes attach to the surface of leukocytes and are available for inducing apoptosis in cancer cells that they come into contact with (Fig. 4 D and E). As an indicator of unbound ES/TRAIL liposomes and/or liposome fragments remaining in human blood after shearing pretreatment, the toxicity of supernatant collected from pretreated blood was tested in COLO 205 culture. An Annexin-V assay showed minimal COLO 205 cell death after treatment with a supernatant of human plasma and unbound ES/TRAIL, compared with cells treated with plasma supernatant alone (Fig. S6). These data suggest that ES/TRAIL liposomes readily bind to the surface of leukocytes, with minimal unbound liposomes remaining to target and kill cancer cells under flow.
Fig. 4.
ES/TRAIL liposomes functionalize leukocytes under shear flow in vitro to target and kill cancer cells. (A) Flow cytometry plots of COLO 205 cells in untreated samples (Left) and when treated in human blood with ES (Center) or ES/TRAIL (Right) functionalized leukocytes (but no unbound liposomes) under shear flow. (B and C) Number of viable COLO 205 (B) and PC-3 (C) cells per volume of blood after treatment with leukocytes functionalized with ES/TRAIL or ES liposomes, but with no unbound liposomes, in human blood (Washed), or after treatment with ES or ES/TRAIL liposomes in blood (Unwashed). n = 3 for all samples. Bars represent the mean ± SD in each treatment group. **P < 0.001, ***P < 0.0001 (unpaired t test). (D and E) Schematic of the two-step mechanism involving decoration of leukocytes with liposomes (D), which then contact circulating cancer cells and activate the death receptor (E).
ES/TRAIL Functionalized Leukocytes Reduce Number of Viable Cancer Cells in Mouse Circulation in Vivo.
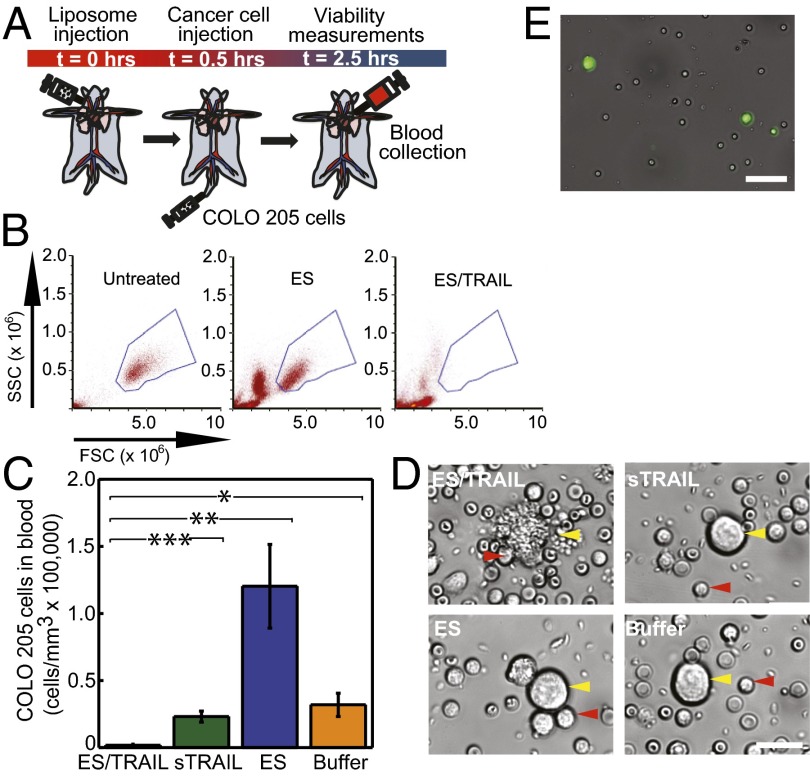
To assess and compare the cytotoxic effects in vivo with our previous results in vitro, ES/TRAIL liposomes were also tested for their ability to kill cancer cells flowing in the peripheral circulation of mice. Two million fluorescently labeled COLO 205 cells were injected into the tail vein of immunocompetent C57BL/6J mice, 30 min after injection of 120 μL of either ES/TRAIL liposomes, ES liposomes, or soluble TRAIL (Fig. 5A). Tail-vein injection was used to model leukocyte/CTC interactions in mouse circulation, as this technique has been an accepted and widely used model of lung metastasis (28–31) since the pioneering work of Fidler et al. (32–34). For these studies, the use of recombinant human E-selectin was continued because of its ability to bind both human COLO 205 cancer cells and mouse neutrophils, which were previously shown to have cross-reactivity and roll on E-selectin (35). Mice were killed 2.5 h after the initial injection, and cancer cells were recovered from the circulation via cardiac puncture. Cancer cells were placed back into culture for 2–3 h before the number of viable cells was quantified.
Fig. 5.
ES/TRAIL functionalized leukocytes target and kill cancer cells in the circulation of mice in vivo. (A) Schematic of in vivo mouse experiment. (B) Flow cytometry of untreated COLO 205 cancer cells (Left) and those recovered from cardiac puncture from mice treated with ES (Center) and ES/TRAIL liposomes (Right). (C) Number of viable cancer cells recovered per volume of mouse blood for mice treated with ES/TRAIL liposomes, soluble TRAIL (sTRAIL), ES liposomes, and buffer injections. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. *P < 0.01, **P < 0.001, ***P < 0.0001 (one-way ANOVA with Tukey posttest). (D) Representative micrographs of COLO 205 cells removed from circulation in mice treated with ES/TRAIL liposomes (Upper Left), sTRAIL (Upper Right), ES liposomes (Lower Left), and buffer (Lower Right) injections. (Scale bar, 20 μm.) (E) Leukocytes functionalized with fluorescent ES/TRAIL liposomes (green) upon removal from mouse circulation 2.5 h after injection. (Scale bar, 50 μm.)
Using flow cytometry, we measured ∼130,000 cancer cells per mL of blood for mice injected with control ES liposomes, compared with <2,000 cancer cells per mL of blood surviving from ES/TRAIL-treated mice (Fig. 5 B and C). Mice injected with buffer or soluble TRAIL had intermediate numbers of cells (Fig. 5C) compared with ES and ES/TRAIL-treated samples, likely indicating that ES functionalized liposomes help to retain cancer cells in the circulation by blocking selectin-mediated interaction of the COLO 205 cells with the endothelium. Brightfield micrographs of COLO 205 cells exposed to buffer and soluble TRAIL showed characteristic cancer-cell morphology (Fig. 5D). Micrographs of COLO 205 cells exposed to ES revealed adherent leukocytes on the COLO 205 cell surface without significant morphological change whereas the cancer cells from ES/TRAIL-treated mice showed notable membrane blebbing of COLO 205 cells in the proximity of leukocytes (Fig. 5D). To assess the adhesion of ES/TRAIL liposomes to leukocytes in mouse circulation, mice were injected with ES/TRAIL liposomes containing fluorescent cholesterol and were allowed to circulate for 2.5 h. Brightfield overlay micrographs showed that leukocytes recovered from the mouse circulation were functionalized with ES/TRAIL liposomes (Fig. 5E), suggesting that ES/TRAIL remains functionalized to leukocytes, which can exert cytotoxic effects onto cancer cells in mouse circulation.
ES/TRAIL Treatment Reduces Number and Increases Apoptosis of Remaining Circulating COLO 205 Cells Lodged in Vivo in Mouse Lung.
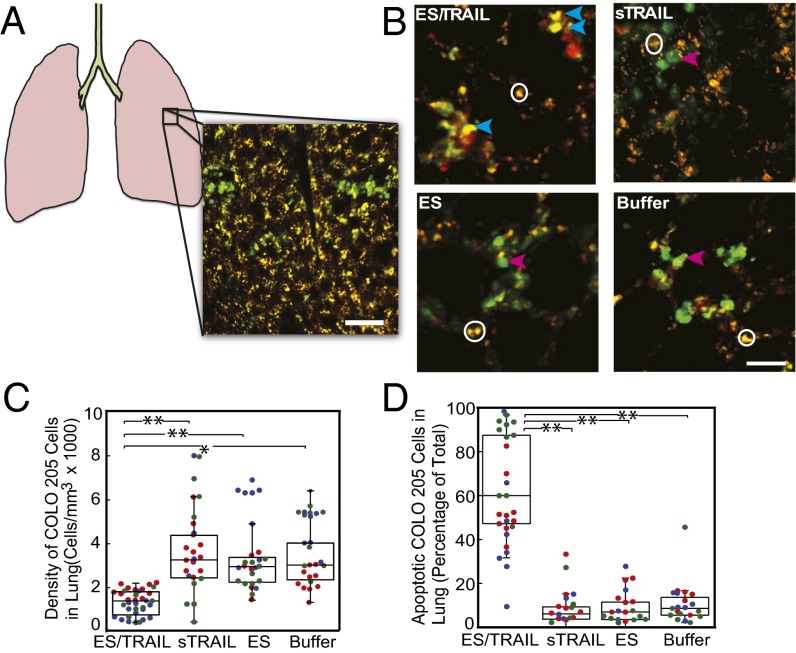
Previous studies have shown that tumor cells that enter the vasculature via tail-vein injection can lodge into lung vasculature within the first two hours (36). To assess the viability of the remaining circulating cancer cells lodged within mice, fluorescent COLO 205 cells lodged within the lungs of mice were identified using two-photon excited fluorescence microscopy (Fig. 6 A and B). Immediately following euthanasia, the lungs were resected, and two-photon images were acquired in three regions of the lung that were identical for each animal, to obtain an accurate estimate of cell counts (Fig. 6A). Roughly twice as many cells were found in the ES-, soluble TRAIL (sTRAIL)-, and buffer-treated animals as the ES/TRAIL-treated animals (Fig. 6C), suggesting that many COLO 205 cells had already died and degenerated. The cancer-cell density found in lung suggests that about 1.4 million cancer cells were lodged in the lung for buffer-treated animals (assuming a lung volume of ∼0.4 mL), representing the bulk of the two million COLO 205 cells injected.
Fig. 6.
Decreased number and increased apoptosis in COLO 205 cells lodged in mouse lung after treatment with ES/TRAIL liposomes. (A) Schematic of mouse lung and example two-photon excited fluorescence (2PEF) image stack from mouse lung where Hoechst-labeled COLO 205 cells (green) are arrested in lung tissue (visible by autofluorescence, yellow). (Scale bar, 80 µm.) (B) The 2PEF images of Hoescht-labeled COLO 205 cells (green) with Alexa Flour 568-labeled Annexin-V apoptosis probe (red) for each experimental group. Red arrows point to apoptotic COLO 205 cells (red and green colocalized), and blue arrows indicate nonapoptotic COLO 205 cells (green only). White circles indicate regions of autofluorescence from lung tissue. (Scale bar, 30 µm.) (C) Density of COLO 205 cells lodged in the lung for each experimental group. (D) Percentage of lodged COLO 205 cells positive for Annexin-V probe for each experimental group. Individual data points represent data from one image stack, with points shown in the same color representing image stacks from the same animal. Superimposed box plots bound the 25th to 75th percentage of all data points and the whiskers extend 1.5 times the interquartile range beyond the boxes. The horizontal lines within the boxplot represent the median. n = 3 animals for each experimental group. *P < 0.01, **P < 0.0001 (one-way ANOVA with Tukey posttest).
We then evaluated the apoptotic effects of ES/TRAIL liposomes on cancer cells that have already lodged into the lungs of mice. After the injections of liposomes and COLO 205 at previously used time points (Fig. 5A), we injected a solution of Annexin-V tagged with a fluorescent Alexa 594 dye to assess for phosphatidylserine flipping on the COLO 205 cell membrane, characteristic of apoptosis. Mouse lungs were imaged using two-photon microscopy to determine whether Hoescht-labeled COLO 205 cells were also positive for Annexin-V labeling. In addition to the decreased density of cancer cells lodged in mouse lung (Fig. 6C), we also found a dramatic increase in apoptosis of the cancer cells (Fig. 6 B and D) in the ES/TRAIL liposome-treated mice compared with other groups. Soluble TRAIL protein injected into mice following the same protocol displayed minimal cytotoxic activity comparable with control, as expected due to its short circulation half-life (37). Mice injected with ES/TRAIL liposomes survived for over 2 wk with no loss in body weight (n = 3). These data suggest that ES/TRAIL treatment serves to decrease the number of remaining circulating COLO 205 cells lodged in mouse lung, while increasing the fraction of them that are apoptotic.
Discussion
Natural killer cells, activated by interleukin-2 or other factors, are induced to present TRAIL protein on their surface. These cells participate in immunosurveillance against micrometastases in the body and comprise 10–20% of peripheral blood mononuclear cells (38, 39). Although the liposome-coated leukocytes described here are not specifically programmed to actively invade tissues and seek out solid tumors, they do have frequent opportunities for incidental contact with CTCs in the bloodstream. Interestingly, infiltration of neutrophils and macrophages throughout the interior of solid tumor masses has been found in dynamic, self-seeding tumors, suggesting that some degree of homing of normally functioning leukocytes to solid tumors could be expected (40, 41). We find that TRAIL is most potent when in its natural state—tethered to the surface of leukocytes in shear flow—rather than freely soluble or on untethered liposomes in the absence of blood. Tethering nanoscale liposomes to the surface of peripheral blood leukocytes is also beneficial for increasing liposome circulation time, by avoiding renal clearance mechanisms.
So why do leukocytes coated with ES/TRAIL liposomes have much higher cytotoxic activity in shear flow, compared with isolated ES/TRAIL liposomes or soluble TRAIL protein? The answer may lie in the compressive force between surfaces. Two spherical particles colliding in linear shear flow will experience a compressive force between them, which scales as Fc ∼ μ*G*a*b, where μ is the fluid viscosity, G is the shear rate, and a and b are the radii of the smaller and larger sphere, respectively (42). Thus, a 10-μm-diameter leukocyte colliding with a cancer cell will experience 100 times the compressive force of a 100-nm liposome colliding with a cancer cell. Compressive forces act to flatten down any cell-surface glycocalyx composed of biologically inert macromolecules, thus allowing TRAIL to come within a reactive distance to the cancer cell death receptors and form bonds. The physics of force-induced flattening and penetration of cell glycocalyx to facilitate surface receptor binding to ligands on an opposing cell surface has been analyzed in the context of leukocyte adhesion to the vascular endothelium (43, 44).
Recombinant human TRAIL/Apo2L, also known as PRO1762 developed by Amgen/Genentech, has been the subject of numerous Phase 1, 1a, 2, and 3 clinical trials over the past decade, with minimal adverse effects reported (45, 46). There are many intracellular proteins, such as the inhibitors of apoptosis protein (IAPs) family members, that also confer TRAIL resistance to normal cells (47). Additionally, the dosages of TRAIL used in this current study ranged from 0.06–0.08 mg/kg, two orders of magnitude lower than the clinical dosages of 1–30 mg/kg used in human clinical trials. Although different types of cancer cells show different levels of sensitivity to TRAIL-induced apoptosis, it has been well documented that there is a wide range of agents known to sensitize cancer cells to TRAIL-mediated apoptosis, including conventional chemotherapeutics (camptothecin, cisplatin, doxorubicin, 5-fluorouracil, irinotecan, paclitaxel, gemcitabine), proteasome inhibitors, Bcl-2 inhibitors, IAP antagonists, histone deacetylase inhibitors, CD20 antibodies, irradiation, synthetic triterpenolds, Sorafenib, aspirin, and natural products such as curcumin and piperlongumine (48).
What remains to be seen is whether ES/TRAIL liposomes can successfully prevent the formation of metastatic tumors; future work should focus on addressing this question. Additionally, human hepatocytes have shown sensitivity to TRAIL (49) although ES/TRAIL liposome adhesion to the leukocyte surface could reduce TRAIL uptake by the reticulo-endothelial system in the liver. The present study, however, represents an important first step toward the targeting of CTCs in the bloodstream as a means to prevent cancer metastasis. Clinically, for instance, one could envision using these liposomes as a preventive measure upon diagnosis of highly metastatic hematogenous cancers such as those originating in breast, prostate, and lung.
Materials and Methods
All reagents and additional procedures used in this study, including cell culture, liposome synthesis, static and shear treatment assays in buffer and human blood, leukocyte isolation and functionalization with ES/TRAIL in human blood, mouse studies, circulating cancer cell analysis, two-photon imaging of mouse tissue, flow cytometry, and statistical analyses are described in SI Materials and Methods. All human subject protocols were approved by the Institutional Review Board for Human Participants of Cornell University. All animal procedures were approved by the Cornell University Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
The authors thank Dr. Razelle Kurzrock for discussion on TRAIL toxicity, Julie Kohn and Brooke Mason of the Reinhart-King Lab for endothelial cell protocols, and Jeff Mattison for blood work. The work described was supported by the Cornell Center on the Microenvironment and Metastasis through Award U54CA143876 from the National Cancer Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316312111/-/DCSupplemental.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123(9):1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 3.Maheswaran S, Haber DA. Circulating tumor cells: A window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20(1):96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald B, et al. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125(6):1298–1305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- 6.van Ginhoven TM, van den Berg JW, Dik WA, Ijzermans JNM, de Bruin RWF. Preoperative dietary restriction reduces hepatic tumor load by reduced E-selectin-mediated adhesion in mice. J Surg Oncol. 2010;102(4):348–353. doi: 10.1002/jso.21649. [DOI] [PubMed] [Google Scholar]
- 7.Gassmann P, Kang ML, Mees ST, Haier J. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell—endothelial cell interaction. BMC Cancer. 2010;10:177. doi: 10.1186/1471-2407-10-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köhler S, Ullrich S, Richter U, Schumacher U. E-/P-selectins and colon carcinoma metastasis: First in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br J Cancer. 2010;102(3):602–609. doi: 10.1038/sj.bjc.6605492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahn JJ, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22(6):475–483. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 10.Chang YS, et al. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97(26):14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35(3):512–516. [PubMed] [Google Scholar]
- 12.Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: Approaches to isolation and characterization. J Cell Biol. 2011;192(3):373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firrell JC, Lipowsky HH. Leukocyte margination and deformation in mesenteric venules of rat. Am J Physiol. 1989;256(6 Pt 2):H1667–H1674. doi: 10.1152/ajpheart.1989.256.6.H1667. [DOI] [PubMed] [Google Scholar]
- 15.Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Gout S, Tremblay PL, Huot J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin Exp Metastasis. 2008;25(4):335–344. doi: 10.1007/s10585-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 17.Barthel SR, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci USA. 2009;106(46):19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26(21):3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 19.Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 20.Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation. 2003;10(2):167–178. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:44. doi: 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 23.Doherty TA, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303(7):L577–L588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malý P, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86(4):643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 25.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith HL. The microrheology of red blood cell suspensions. J Gen Physiol. 1968;52(1):5–28. [PMC free article] [PubMed] [Google Scholar]
- 27.Goldsmith HL, Bell DN, Braovac S, Steinberg A, McIntosh F. Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophys J. 1995;69(4):1584–1595. doi: 10.1016/S0006-3495(95)80031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res. 2013;19(17):4706–4716. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucci P, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci USA. 2012;109(38):15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C-Y, Lin S-C, Su W-H, Ho C-M, Jou Y-S. Somatic LMCD1 mutations promoted cell migration and tumor metastasis in hepatocellular carcinoma. Oncogene. 2012;31(21):2640–2652. doi: 10.1038/onc.2011.440. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35(1):218–224. [PubMed] [Google Scholar]
- 33.Fidler IJ. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974;34(5):1074–1078. [PubMed] [Google Scholar]
- 34.Fidler IJ, Nicolson GL. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976;57(5):1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 35.Kato N, et al. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol. 2009;20(7):1565–1576. doi: 10.1681/ASN.2008090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidler IJ, Nicolson GL. Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients. J Natl Cancer Inst. 1977;58(6):1867–1872. doi: 10.1093/jnci/58.6.1867. [DOI] [PubMed] [Google Scholar]
- 37.Xiang H, Nguyen CB, Kelley SK, Dybdal N, Escandón E. Tissue distribution, stability, and pharmacokinetics of Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in human colon carcinoma COLO205 tumor-bearing nude mice. Drug Metab Dispos. 2004;32(11):1230–1238. doi: 10.1124/dmd.104.000323. [DOI] [PubMed] [Google Scholar]
- 38.Takeda K, et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105(5):2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- 39.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 40.Kim MY, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernal M, et al. Leukocyte infiltrate in gastrointestinal adenocarcinomas is strongly associated with tumor microsatellite instability but not with tumor immunogenicity. Cancer Immunol Immunother. 2011;60(6):869–882. doi: 10.1007/s00262-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankaran H, Neelamegham S. Hydrodynamic forces applied on intercellular bonds, soluble molecules, and cell-surface receptors. Biophys J. 2004;86(1 Pt 1):576–588. doi: 10.1016/S0006-3495(04)74136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Chien S, Weinbaum S. Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys J. 2001;80(3):1124–1140. doi: 10.1016/S0006-3495(01)76090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabri S, et al. Glycocalyx modulation is a physiological means of regulating cell adhesion. J Cell Sci. 2000;113(Pt 9):1589–1600. doi: 10.1242/jcs.113.9.1589. [DOI] [PubMed] [Google Scholar]
- 45.Subbiah V, et al. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol Cancer Ther. 2012;11(11):2541–2546. doi: 10.1158/1535-7163.MCT-12-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbst RS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28(17):2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XD, Nguyen T, Thomas WD, Sanders JE, Hersey P. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000;482(3):193–199. doi: 10.1016/s0014-5793(00)02042-1. [DOI] [PubMed] [Google Scholar]
- 48.Ashkenazi A, Herbst RS. To kill a tumor cell: The potential of proapoptotic receptor agonists. J Clin Invest. 2008;118(6):1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jo M, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6(5):564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.