Significance
Polypeptide N-acetylgalactosaminyltransferase-like protein 5 (GALNTL5) belongs to the pp-GalNAc-T family, but its in vivo activity has not yet been identified. To investigate the functions of GALNTL5, we attempted to establish Galntl5-deficient mice and found that the heterozygous mutation of Galntl5 causes infertility in male mice because of immotile sperm. In these mice, glycolytic enzymes required for sperm motility were decreased, their protein loading into acrosomes was disrupted, and aberrant localization of the ubiquitin–proteasome system was observed. We found a patient diagnosed with asthenozoospermia, poor sperm motility, who had a mutation of the GALNTL5 gene in sperm and blood cells. Our data suggest that GALNTL5 is an essential functional molecule for sperm development, and the GALNTL5 mutation may cause human asthenozoospermia.
Abstract
For normal fertilization in mammals, it is important that functionally mature sperm are motile and have a fully formed acrosome. The glycosyltransferase-like gene, human polypeptide N-acetylgalactosaminyltransferase-like protein 5 (GALNTL5), belongs to the polypeptide N-acetylgalactosamine-transferase (pp-GalNAc-T) gene family because of its conserved glycosyltransferase domains, but it uniquely truncates the C-terminal domain and is expressed exclusively in human testis. However, glycosyltransferase activity of the human GALNTL5 protein has not been identified by in vitro assay thus far. Using mouse Galntl5 ortholog, we have examined whether GALNTL5 is a functional molecule in spermatogenesis. It was observed that mouse GALNTL5 localizes in the cytoplasm of round spermatids in the region around the acrosome of elongating spermatids, and finally in the neck region of spermatozoa. We attempted to establish Galntl5-deficient mutant mice to investigate the role of Galntl5 in spermiogenesis and found that the heterozygous mutation affected male fertility due to immotile sperm, which is diagnosed as asthenozoospermia, an infertility syndrome in humans. Furthermore, the heterozygous mutation of Galntl5 attenuated glycolytic enzymes required for motility, disrupted protein loading into acrosomes, and caused aberrant localization of the ubiquitin–proteasome system. By comparing the protein compositions of sperm from infertile males, we found a deletion mutation of the exon of human GALNTL5 gene in a patient with asthenozoospermia. This strongly suggests that the genetic mutation of human GALNTL5 results in male infertility with the reduction of sperm motility and that GALNTL5 is a functional molecule essential for mammalian sperm formation.
O-glycosylation begins by the addition of N-acetylgalactosamine to the serine or threonine residues in the target protein. This first step occurs in the Golgi apparatus, and is mediated by UDP-GalNAc: polypeptide N-acetylgalactosaminyltransferases (pp-GalNAc-T; EC 2.4.1.41), which transfer GalNAc from the nucleotide sugar to the acceptor residues (1). Polypeptide N-acetylgalactosaminyltransferase-like protein 5 [GALNTL5, also described as pp-GalNac-T19 (2) or GalNac-T20 (3); Refseq accession no.: NP_660335.2] is classified as a member of the pp-GalNAc-T family because GALNTL5 possesses highly conserved catalytic domains of pp-GalNAc-T, whereas it uniquely lacks the conserved lectin domain at the C terminus. Thus far, 20 distinct pp-GalNAc-T genes have been identified in the human genome (2, 4–6). The in vitro enzymatic activities as a glycosyltransferase have been confirmed for 14 members of this family using acceptor peptide substrates (2, 7), but not identified for the other 6 members, including GALNTL5. During the preparation of this paper, it was reported that the transferase activity of GALNTL5 (GalNAc-T20) could not be detected using in vitro assays (3). The in vivo functions of these isoforms are poorly understood because of the absence of specific enzymatic activity. Meanwhile, O-fucosyltransferase 1, a member of a fucosyltransferase family, exhibits chaperon activity specific to Notch folding in Drosophila (8). One possibility is that the isoforms lacking enzymatic activities may have functions other than characteristics of glycosyltransferases, despite having typical glycosyltransferase motifs.
Spermatogenesis is a complex process in which spermatogonial stem cells form spermatozoa through the proliferative phase (spermatogonia), the meiotic phase (spermatocytes), and the differentiation or spermiogenic phase (spermatids). Spermatids are connected by intercellular bridges, through which cytoplasmic constituents are shared among haploid spermatids (9). In the last spermiogenic phase, the round haploid spermatids differentiate into spermatozoa where acrosomes and tails unique and necessary for fertilization are developed. Spermatozoa are released through the seminiferous lumen into the epididymis, where they undergo further maturation and acquire motility. Sperm motility is an important factor in normal fertilization, whereas over 80% of sperm samples from infertile men demonstrate asthenozoospermia, poor sperm motility (10). Although defects of many potential genes are reported in mouse models exhibiting asthenozoospermia (11), it is rare that mutations in these genes are identified in human patients with asthenozoospermia.
To investigate the biochemical machineries and biological functions of glycosylation, we performed comprehensive identification of the mammalian glycosyltransferase genes using various approaches and confirmed their enzymatic activity in vitro using biochemical methods (12). During these studies, we identified a unique isoform of the human GALNTL5 gene restricted to the human testis. However, we could not confirm the glycosyltransferase activity of GALNTL5, including whether it is a functional molecule in spermatogenesis. Therefore, using the mouse Galntl5 gene, we attempted to elucidate the biological role of GALNTL5 in spermatogenesis and found that the heterozygous mutation of Galntl5 causes male infertility by reducing sperm motility, which highly resembles human asthenozoospermia. In reference to the aberrant protein compositions of sperm from the Galntl5 heterozygous mutant mice (Ht mice), we found a patient with asthenozoospermia carrying one heterozygous nucleotide deletion at the sixth exon of the human GALNTL5 gene. Together with these data, we speculate that the function of GALNTL5 is indispensable for mature sperm formation and that GALNTL5 might have a unique role in mammalian spermiogenesis.
Results
Expression of the pp-GalNAc-T-Like Gene, GALNTL5, Lacking the Lectin Domain Is Restricted to Testis.
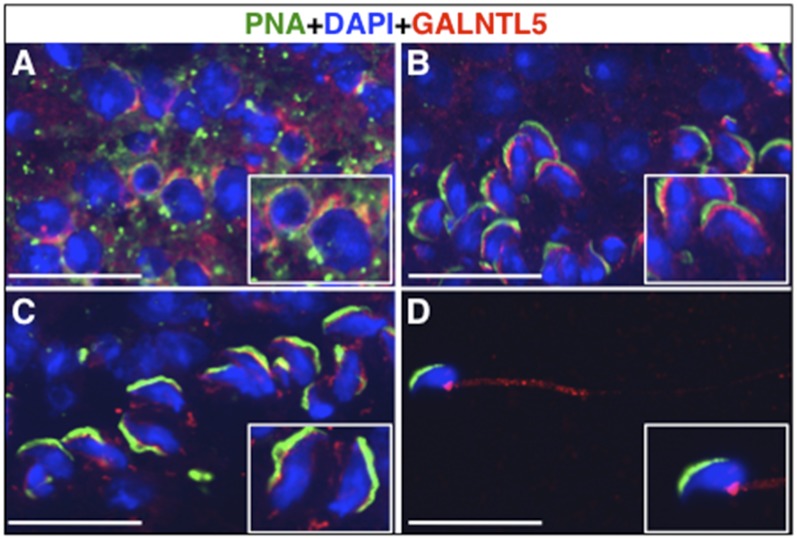
We previously cloned multiple human pp-GalNAc-T genes using in silico screening (12) and identified a pp-GalNAc-T-like gene that encodes human GALNTL5. GALNTL5 possesses the typical transmembrane domain and the catalytic unit containing two motifs, which are highly conserved in pp-GalNAc-T proteins (Fig. S1A). However, GALNTL5 characteristically lacks the typical lectin domain at the C terminus unlike GALNT3, which is also expressed mainly in testis (4, 13). The expression of GALNTL5 mRNA was mostly restricted to the human testis (Fig. S1B). We isolated the cDNA clone of mouse ortholog Galntl5 mRNA from the cDNA library of mouse testis. The National Center for Biotechnology Information (NCBI) database of Unigene also indicates that the expression of mouse Galntl5 is restricted in mouse testis. Through the in situ hybridization of the mouse Galntl5 cDNA with antisense RNA, we confirmed that Galntl5 mRNA is expressed mainly in the round and elongated spermatids during spermiogenesis, not in the outermost cells of the seminiferous tubules, which contain spermatogonia and somatic Sertoli cells (Fig. S1 C–E). Immunohistochemistry of mouse testis sections using anti-mouse GALNTL5 antibody and peanut agglutinin (PNA), which is a plant lectin protein used for counterstaining of acrosome vesicles, showed that GALNTL5 was distributed in the juxtanuclear space in the round spermatids, not in the acrosomal vesicles (Fig. 1A). In the elongating spermatids, GALNTL5 was strongly localized in the acroplaxome, the region between the developing acrosome and nucleus (14) (Fig. 1B). During differentiation, the signals were also weakly detected in the transient manchette containing microtubules (15) (Fig. 1 B and C). In epididymal spermatozoa, GALNTL5 was weakly detected in the midpiece, but was concentrated in the neck region around the head–tail coupling apparatus (Fig. 1D). This strongly suggests that mouse Galntl5 is involved in spermiogenesis.
Fig. 1.
Localization of mouse GALNTL5 protein during spermiogenesis. Sections of adult mouse testis were immunostained with anti-GALNTL5 antibodies (red). The acrosomal vesicles and nuclei were counterstained with PNA (green) and DAPI (blue), respectively. (A) Round spermatids. GALNTL5 was detected in the juxtanuclear space. (B) Step-10 spermatids. GALNTL5 was strongly detected in the region between the acrosome and the nucleus. (C) Step-14 spermatids. GALNTL5 was weakly observed in the transient manchette. (D) Immunostaining of epididymal spermatozoa with an anti-GALNTL5 antibody. GALNTL5 was weakly stained in the middle piece, but was concentrated in the head–tail coupling apparatus. Insets are close-ups of the spermatids at each step. (Scale bars, 20 μm.)
Establishment of Heterozygous Mutant Mice.
Although neither we nor another group (3) were able to detect glycosyltransferase activity of human GALNTL5 in an in vitro assay, we established a mouse model carrying mutated Galntl5, where the second exon including the translational start site was replaced with a neomycin-resistant gene, to investigate the function of GALNTL5 in spermiogenesis (Fig. S2A).
Seven chimeric male mice, selected by skin color, were completely infertile when mated with wild-type (WT, Galntl5+/+) females. Therefore, by breeding four chimeric females with WT C57BL/6J males, we obtained four heterozygous mutant (Ht, Galntl5+/−) males and two Ht females. Although vaginal plugs were observed in WT females mated with Ht males, Ht males had similar infertility to the chimeric males (Fig. 2A). The Ht females had normal fertility; therefore, we increased the size of the mouse colony by breeding Ht females and WT males. The amounts of GALNTL5 in the spermatozoa from Ht male mice were less than half that of WT mice (Fig. S2E).
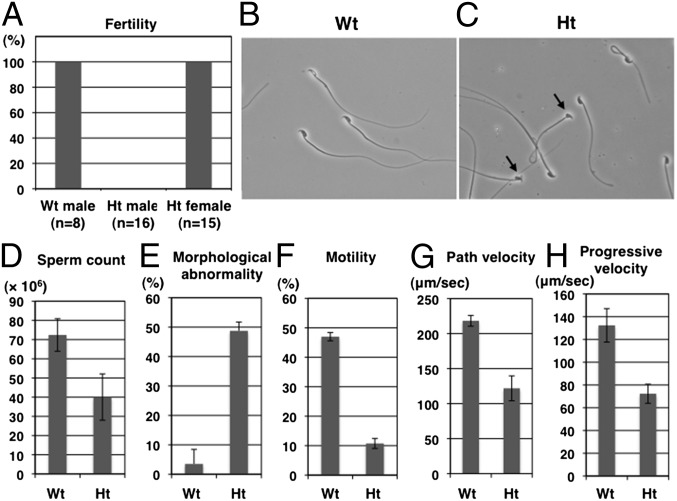
Fig. 2.
Male mouse sterility caused by heterozygous mutation of Galntl5. (A) Fertility of wild-type (WT), heterozygous mutant (Ht) males, and Ht females. (B and C) Morphological phenotypes of epididymal spermatozoa from WT and Ht mice. Arrows indicate deformed or ventral flexuous sperm from Ht mice. (D–H) Sperm counts, morphological abnormality, motility, path velocity, and progressive velocity of sperm from WT and Ht mice. Error bars show the SEMs of four mice.
Heterozygous Mutation of Galntl5 Results in Immotile Sperm.
To elucidate the reason for male infertility in Ht mice, we performed morphological and biochemical studies of epididymal sperm. The sperm count in Ht mice slightly decreased compared with that of WT mice (Fig. 2D). However, the ratio of sperm with morphological abnormalities to the total sperm was fivefold or higher in Ht mice than in WT mice (Fig. 2 B, C, and E). Notably, flagella activity was lacking and therefore sperm motility was lost in Ht mice (Fig. 2F). In Ht mice, the motility parameters of path velocity and progressive velocity were decreased to about half of those in WT mice (Fig. 2 G and H). Direct injection of elongated spermatids from Ht males into WT oocytes resulted in production of embryos that reached full term (Table 1). The birth rates per number of embryos transferred were not significantly different, indicating that spermatids from Ht mice had the normal haploid set of chromosomes, and that defects in the germ cells of Ht mice are attributable to the postmeiotic biochemical modifications essential for spermiogenesis. These data suggest that the cause of sterility in Ht mice is the immotile sperm, and that the sperm phenotype observed in Ht mice closely resembles human asthenozoospermic sperm.
Table 1.
Development of embryos generated by injection with elongated spermatids from Ht or WT males
| Male | Female | No. embryos transferred | No. embryos implanted (%) | No. pups born (%) |
| Ht | WT | 76 | 45 (59) | 23 (30) |
| WT | WT | 43 | 27 (63) | 14 (33) |
There were no statistical differences in the rates of implantation and normal birth between the experimental groups (P > 0.05, Fisher's exact test).
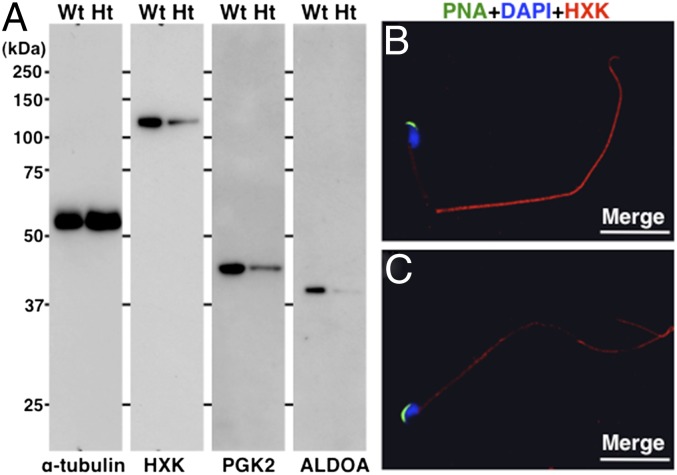
Immunostaining of sperm mitochondria from Ht mice showed that the sperm were alive. To assess the biochemical factors behind the lack of sperm motility, we compared the protein contents of spermatozoa from Ht and WT mice (Fig. S3). The proteins that had considerably low concentrations in sperm from Ht mice were glycolytic enzymes required for sperm motility (16, 17). The three lower levels of isozymes present in sperm from Ht mice were confirmed using antibodies (Fig. 3). The contents of these proteins in the testis were consistent in both genotypes (Fig. S4). These data support the theory that heterozygous mutation of Galntl5 depresses sperm motility by reducing the levels of glycolytic enzymes that normal mature sperm possess.
Fig. 3.
Comparison of glycolytic isozymes present in epididymal spermatozoa. (A) Sperm lysates were separated by SDS/PAGE and blotted with antihexokinase (HXK), antiphosphoglycerate kinase 2 (PGK2), and anti–fructose-bisphosphate aldolase A (ALDOA) antibodies. The levels of three isozymes were lower in the sperm from Ht mice. α-Tubulin served as the control. Protein standards are shown on the Left. (B) An epididymal spermatozoon from a WT mouse. The acrosome was stained with PNA (green). Localization of HXK was visualized with an anti-rabbit IgG immunofluorescent secondary antibody (red). The nucleus was stained with DAPI (blue). (C) An epididymal spermatozoon from a Ht mouse. The anti-HXK signal was weak and sparse in the tail region. (White scale bars, 20 μm.)
Lack of Acrosomal Components in Sperm from Ht Mice.
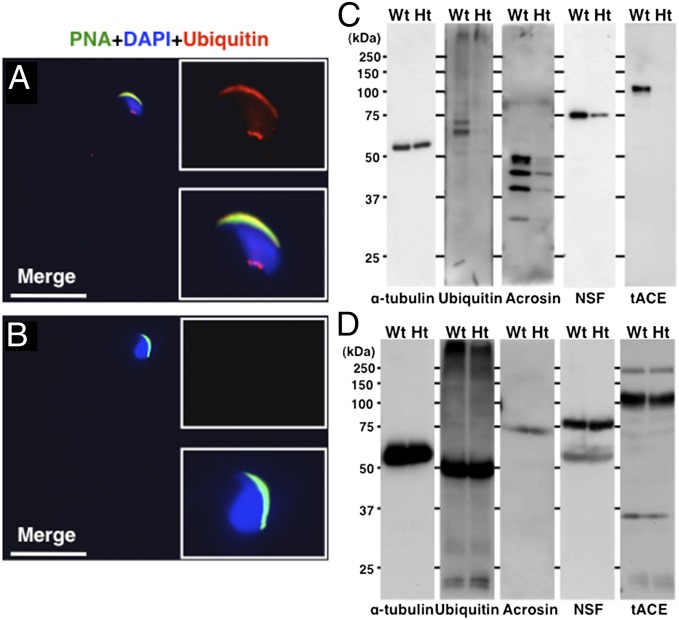
To find other abnormalities in the epididymal spermatozoa caused by heterozygous mutation of Galntl5, the acrosomal components were further investigated. In rat testis, ubiquitin signals accumulated on the outer acrosomal membrane of late-step spermatids (18). In the spermatozoa of WT mice, the ubiquitin signals were also detected on the outer acrosomal membrane and the neck region (Fig. 4A). However, the ubiquitin signals were lost from both the acrosomes and the neck of sperm from Ht mice (Fig. 4B).
Fig. 4.
Detection of acrosomal protein components in epididymal spermatozoa and testes. Immunofluorescence analysis with an antiubiquitin antibody in spermatozoa from WT (A) and Ht mice (B). Signals appear in the acrosomal membrane and in a spot in the neck region of spermatozoa in WT mice. No signals were detected in the spermatozoa from Ht mice. Insets are close-ups of a sperm head. The acrosomes and nuclei were stained with PNA and DAPI, respectively. (White scale bars, 20 μm.) (C) Epididymal sperm lysates were Western blotted with antiubiquitin, antiacrosin, anti-NSF, and anti-tACE antibodies. α-Tubulin was used as the control. (D) Testis lysates were Western blotted with anti–α-tubulin, antiubiquitin, antiacrosin, anti-NSF, and anti-tACE antibodies. The amount of each protein in the testis was equal between WT and Ht mice.
In the acrosomal proteins in spermatozoa, acrosin (19) and N-ethylmaleimide-sensitive factor (NSF), which function in the acrosomal reaction (20, 21), were rarely observed in the acrosome and head of sperm from Ht mice (Fig. S5 A–D). In sperm from WT mice, testicular angiotensin-converting enzyme (tACE), which is responsible for fertility (22–24), was located on the outer membrane of the acrosome, whereas it was ectopically localized on the plasma membrane of sperm from Ht mice (Fig. S5 E and F).
Western blot analyses confirmed that the levels of acrosin, NSF, tACE, and ubiquitinated proteins were remarkably lower in the mature epididymal sperm in Ht mice compared with those in WT mice (Fig. 4C). In contrast, the amounts of these proteins in the testis were consistent in both genotypes, suggesting that the protein expression is normal in the testis during spermatogenesis in Ht mice (Fig. 4D). These results support the theory that GALNTL5 contributes to the loadings of acrosomal proteins during spermiogenesis.
Aberrant Localization of the Components of the Ubiquitin–Proteasome System in Spermatozoa of Ht Mice.
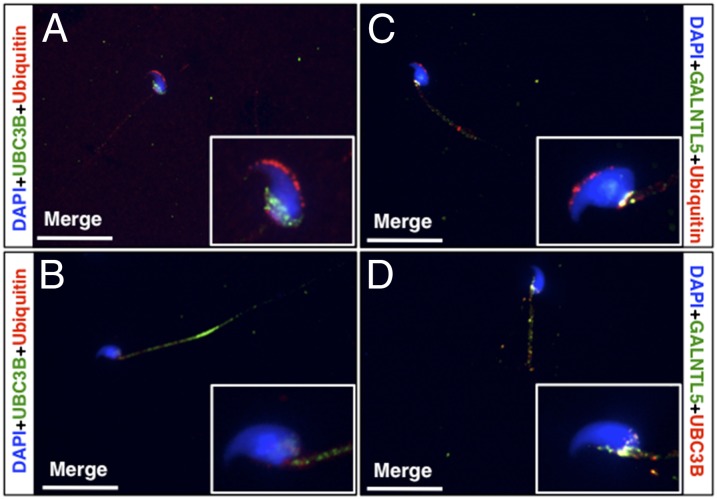
It has been reported that the components of the ubiquitin–proteasome system accumulate in the head–tail coupling apparatus in the neck region of rat epididymal sperm (18, 25). We confirmed that the ubiquitin signal was localized in both the neck region and acrosome of epididymal sperm of WT mice (Figs. 4A and 5A). In sperm of Ht mice, however, the signal was hardly detected in the neck region or acrosome (Figs. 4B and 5B).
Fig. 5.
Distribution of ubiquitin–proteasome proteins in epididymal spermatozoa. (A) UBC3B (green) was observed exclusively in the caudal region of sperm head in WT mice. Ubiquitin (red) was noted not only in the acrosome, but also around the head–tail coupling apparatus of the spermatozoa. (B) In sperm of Ht mice, UBC3B was distributed extensively in the tail. Ubiquitin was not seen around the head–tail coupling apparatus or the acrosome. (C) Ubiquitin signals are seen in the acrosome. Colocalization (yellow) of GALNTL5 and ubiquitin was detected only in the head–tail coupling apparatus region in sperm from WT mice. (D) Yellow signals represent GALNTL5 and UBC3B colocalization in the caudal region around the head–tail coupling apparatus in sperm from WT mice. (White scale bars, 20 μm.)
Immunofluorescence staining with the antibody to the E2 ubiquitin conjugating enzyme UBC3B showed that in the sperm of WT mice, UBC3B was restricted to the caudal region of the head around the head–tail coupling apparatus (Fig. 5A). In the sperm from Ht mice, however, the signals were distributed in the tail (Fig. 5B). Interestingly, colocalization of GALNTL5 with ubiquitin and UBC3B was observed frequently around the head–tail coupling apparatus of the sperm of WT mice (Fig. 5 C and D). These data suggest that GALNTL5 may be involved in the accumulation of ubiquitin–proteasome systems around the head–tail coupling apparatus region.
A Heterozygous Mutation of the Human GALNTL5 Gene in a Patient with Asthenozoospermia.
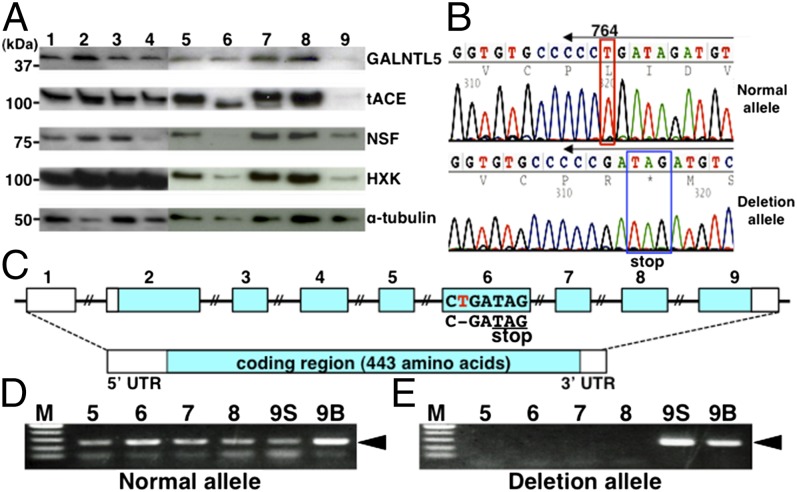
Based on the above results, to validate whether the mutation of human GALNTL5 affects male infertility, we investigated the protein compositions of human sperm cells from different individuals. The normal sperm samples collected from four healthy volunteers exhibited normal motility and morphology and contained steady amounts of the five proteins, including GALNTL5 (Fig. 6A, lanes 1–4 and Table S1). However, in the samples obtained from about 200 patients with male infertility whose diagnosis includes asthenozoospermia, the levels of certain proteins were diminished. In sample 9 with diagnosed asthenozoospermia (Fig. 6A, lane 9 and Table S1), GALNTL5 was barely detected. As observed in Ht mice, decline of GALNTL5 was accompanied by decreases of three proteins [tACE, NSF, and hexokinase (HXK)], but no reduction was observed for α-tubulin (Figs. 3A and 4C). In sample 9, tACE, NSF, and HXK were decreased and α-tubulin was moderate. These data suggest that clinically diagnosed asthenozoospermia in sample 9 might be caused by a decrease of GALNTL5.
Fig. 6.
Screening for mutant GALNTL5 in human male individuals. (A) Comparison of protein components in sperm from healthy volunteers (samples 1–4) and patients diagnosed with male infertility (samples 5–9). (B) DNA sequence chromatograms of the sixth exon in sample 9. In the normal allele, T at position 764 of the coding region retains a normal reading frame. In the deletion allele, the single nucleotide deletion produces a stop codon with a frame shift. Arrows above the sequences indicate PCR primer positions to detect each allele from genomic DNA samples. (C) GALNTL5 consists of nine exons, eight of which encode 443 amino acids. The deletion of T (red) in the sixth exon of GALNTL5 encoded on the genomic DNA in sample 9 consequently produces a new stop codon with a frame shift. (D and E) DNA fragments (black arrowheads) of ∼250 bp amplified with primers specifically detected each allele from sperm from samples 5–9 (lanes 5–8 and 9S) or from blood cells from sample 9 (lane 9B). The deletion alleles were detected in both the sperm DNA and blood cell DNA of sample 9 only.
Human GALNTL5 consists of nine exons distributed over a 62-kb region on chromosome 7 (Fig. 6C). To estimate the effect of the mutation of GALNTL5 on abnormal sperm, we designed flanking primers for each of the nine exons to amplify DNA fragments from abnormal sperm samples including samples 5–9 in Table S1. The direct sequencing of the PCR products from abnormal sperm identified many single nucleotide polymorphisms (SNPs) registered in the NCBI database of SNPs, including those that cause changes in amino acids. In the sperm of sample 9 (Fig. 6B), we found one nucleotide deletion allele in the sixth exon resulting in a new stop codon on the sixth exon causing a frame shift (Fig. 6 B and C). This deletion mutation found in the asthenozoospermic sperm that decreased amounts of GALNTL5 is not described in the NCBI database of SNPs. A normal allele was detected in both sperm and blood cells from this patient (Fig. 6D, lanes 9S and 9B). The mutation allele identified in the asthenozoospermic sperm was also observed in blood cells (Fig. 6E, lanes 9S and 9B), suggesting that the mutation on the GALNTL5 gene of sample 9 would be maternal inheritance because the heterozygous mutation of the mouse Galntl5 gene has no effect on female fertility (Fig. 2A). Given that this human asthenozoospermic sperm closely resembles the sperm phenotype of Ht mice, including the protein abnormality and a high rate of morphological abnormality, these data strongly suggest that the mutation of human GALNTL5 contributes to asthenozoospermia.
Discussion
We previously identified human GALNTL5 as a unique isoform in the pp-GalNAc-T gene family through in silico screening in the human genome, and we have since attempted to analyze it biochemically. However, we and another group (3) could not detect any glycosyltransferase activity of the GALNTL5 protein by in vitro experiments. To investigate the biological role of human GALNTL5 in testis, we observed localization of the mouse orthologous protein GALNTL5 in spermiogenesis and established Galntl5-deficient mice. The Ht male mice were infertile because of their immotile sperm, which corresponds to human asthenozoospermia. We also found that the heterozygous mutation of Galntl5 in mice impairs the amounts of glycolytic enzymes required for motility, protein loadings into acrosomes, and localization of the ubiquitin–proteasome system in the neck region in the spermatozoa, which consist of common cytoplasmic constituents shared among the haploid spermatids through the intercellular bridges (9, 26). We undertook the screening for patients with male infertility caused by mutation of the GALNTL5 gene and identified one patient whose asthenozoospermia was attributable to a heterozygous single nucleotide deletion of maternal inheritance in the exon of GALNTL5. These data suggest that GALNTL5, which shows no in vitro glycosyltransferase activity, is a functional molecule indispensable for mammalian sperm formation.
The GalNAc-Ts localize in the Golgi apparatus and initiate O-glycosylation (1). The transmembrane domain conserved in GALNTL5 initially made us assume that GALNTL5 may be present in the Golgi stack. However, immunohistochemical analysis of mouse testis sections revealed temporary localization of GALNTL5 in the juxtanuclear region, but not in the Golgi apparatus of the round spermatids (Fig. 1A). Considering this as well as the absence of glycosyltransferase activity of GALNTL5 in vitro, it is postulated that GALNTL5 may not function as a typical pp-GalNAc-T enzyme in spermiogenesis. It is noteworthy that GALNTL5 localizes temporarily in the acroplaxome and manchette, a transient microtubular/actin structure, during elongation of the spermatids and accumulates finally in the neck region around the head–tail coupling apparatus of mature spermatozoa (Fig. 1 B–D). A recent report described that the protein components involved in the vesicle cargo transport and ubiquitin–proteasome system alter the distribution continuously in the differentiating spermatids with the route similar to GALNTL5 (27). It supports the theory that GALNTL5 would participate in the transport of vesicle cargos and the ubiquitin–proteasome system to the region of the head–tail coupling apparatus for mature sperm formation. In fact, we observed impairment of protein loadings into acrosomes and aberrant localization of the ubiquitin–proteasome system in the spermatozoa of Ht mice (Figs. 4 and 5) and colocalization of GALNTL5 signals with signals of UBC3B and ubiquitin in the ubiquitin–proteasome system in the neck region of spermatozoa from WT mice (Fig. 5 C and D). These data further predict a possibility that GALNTL5, a gene containing a glycosyltransferase motif, would contribute to protein loading into the acrosomes and the accumulation of ubiquitin–proteasome components in the neck region as a component of vesicle cargo transport, which might be the alternative in vivo function specific to the formation of mature sperm. In future studies, identification of the factors interacting directly with GALNTL5 as well as the chronological behavior of GALNTL5 at each stage of spermatogenesis would permit elucidation of its precise role, including other functions such as chaperon activity in spermiogenesis.
Our data suggested that the lack of sperm motility in Ht mice is caused by the reduction of glycolytic enzymes (Fig. 3) (16, 17). We hypothesize that the aberrant distribution of ubiquitin–proteasome systems could not only result in reductions of glycolytic enzymes, but also acrosomal proteins and GALNTL5 protein to less than 50% in the spermatozoa of Ht mice (Fig. 4C and Fig. S2E). Because sperm phenotypes in both the mouse model and the human patient were very similar, with a lack of motility, protein abnormalities, and a high rate of morphological abnormalities despite the different mutation sites in each case (Figs. S2A and Fig. 6C), it is unlikely that the mutant genes inhibit the GALNTL5 function and prominently attenuate the GALNTL5 protein. As mentioned above, the ubiquitin–proteasome system must be under the control of GALNTL5 protein during spermiogenesis. In the heterozygous mutant, reduced expression of the GALNTL5 protein in differentiating spermatids could not restrict the proteolysis activity of these systems during spermiogenesis. Ubiquitin–proteasome systems once activated might lead to further degradation of the GALNTL5 protein, acrosomal proteins, and glycolytic enzymes in mature spermatozoa of heterozygous mutants. However, the role of ubiquitin–proteasome systems in protein degradation during spermiogenesis is still unknown. To elucidate the degradation mechanism in spermiogenesis from heterozygous mutants, it will be necessary to quantify the expression levels of proteins including GALNTL5, glycolytic enzymes, and acrosomal proteins at each step of differentiating spermatids in the testis from heterozygous mutants. We have confirmed that the spermatids from Ht mice can fertilize oocytes (Table 1) and that the intracytoplasmic sperm injection was effective in achieving fertilization for the patient with asthenozoospermia who provided the spermatozoa carrying the GALNTL5 mutation. These findings also support our contention that the GALNTL5 gene affects normal sperm development but does not interfere with male reproductive potential.
Materials and Methods
Identification of GALNTL5 cDNA.
Human GALNTL5 cDNA (Refseq accession no.: NP_660335.2) was identified using a BLAST search of human expressed sequence tags with cloned human pp-GalNAc-Ts as queries. The cDNA encoding the ORF of mouse Galntl5 was obtained by PCR using QUICK-Clone cDNA of mouse testis (Clontech Laboratories) as a template and primers 5′-ATGAAAGTGTCATAATTCAGGG-3′ and 5′-CTAGAAACGATTTTTTTTCCTTTTCCTCTCTGTGTTAAATGG-3′.
Quantitative Analysis of Human GALNTL5 Transcripts.
Quantitative real-time PCR was performed using TaqMan Universal PCR Master Mix and the ABI PRISM 7700 Sequence Detection system (Applied Biosystems) as described previously (28, 29). The PCR primers were 5′-GAAGCTTGGGCATCGAAA-3′ and 5′-GCGGGCTGGGTAATGTT-3′.
In Situ Hybridization Analyses.
Sense and antisense RNA probes were generated from a linearized mouse full-length Galntl5 cDNA, subcloned into pBluescript using digoxigenin-UTP (Roche Molecular Biochemicals), according to the manufacturer’s instructions. Section in situ hybridization of adult mouse testis was performed as described previously (30).
Generation of Ht Mice.
All animal experiments were performed in accordance with the National Institute of Advanced Industrial Science and Technology (AIST) compliance guidelines for experiments after obtaining the approval of the institutional animal care and use committee for the animal experiments. A mouse Galntl5 genomic BAC clone, RP23-229N3, was purchased from Research Genetics. The targeting vector was constructed in which the second exon of Galntl5 was replaced with a neomycin-resistance gene. Plasmid (20 μg) was electroporated into E14 embryonic stem cells that had been cultured on neomycin-resistant fibroblasts. After double-drug selection with G418 (Gibco) and gancyclovir (Wako), individual colonies were analyzed by Southern blotting and PCR to identify homologous recombination. After digestion with SpeI, a 5′ probe detected 19.8-kbp fragments from the normal allele and 19.8- and 14.6-kbp fragments from the targeted allele. Embryonic stem cells and mouse tails were genotyped using PCR and genomic DNA extracted with a DNeasy Blood and Tissue kit (Qiagen). The wild-type allele was detected with the primers 6605A (5′-GAGACCTTAGCTTGAAACAAAACCAC-3′) and 5970S (5′-GATTTCCCAGGTTCATCTGCATCCG-3′) flanking the second exon of mouse Galntl5. The mutant allele was identified with primers NeoLeft25 (5′-TGCGCTGACAGCCGGAACAGGCGG-3′) in the neo gene and AvrHind2-2 (5′-TATACCACAATTGAATGGATATAGA-3′) in the third intron outside of the 3′ end of the 3.4-kbp homologous region.
Round Spermatid Injection.
Spermatogenic cells were collected from the testes of WT and Ht male mice, as described previously (31). Elongated spermatids were directly injected into the ooplasm of mature oocytes of Ht mice using a piezo-driven micromanipulator. Embryos at the four- to eight-cell stage after 48 h in culture were transferred into the oviducts of pseudopregnant females on day 0.5.
Sperm Motility Assay.
To assess sperm motility, sperm samples were loaded onto a microslide (0.1 × 2.0 mm; HTR 1099; VitroCom). Sperm motility parameters were measured in samples containing >300 sperm using a TOX IVOS automated system (Hamilton Thorne).
Antibodies.
Three oligopeptides were synthesized (LLKKRSLGKNAHQQTRH, LRWDNVFAYELDGPEG, and SKALSQHRRANQSALS), corresponding to amino acids deduced from the mouse Galntl5 mRNA sequences. Three oligopeptides (QQIIYGSEQIPKPHVIVKR, FKWDNVFSYEMDGPEG, and SKKQTGKPSTIISAMT), corresponding to amino acid regions of human, were synthesized. A cysteine residue was added to the N terminus of each oligopeptide for conjugation to the carrier. Guinea pigs for anti-mouse GALNTL5 and rabbits for anti-human GALNTL5 were immunized with a mixture of species-specific conjugated peptides, and the antiserum was purified with an affinity matrix column. For the final purification of the anti-human GALNTL5 antibody, a Protein A-Sepharose column was used (Medical and Biological Laboratories). Antibodies to the following proteins were purchased: α-tubulin (Sigma), HXK I, phosphoglycerate kinase 1/2 (PGK 1/2), aldolase A (ALDOA), NSF, ACE, ubiquitin, and acrosin (Santa Cruz).
Western Blotting.
Mouse sperm were collected from the cauda epididymis, suspended in PBS (pH 7.4), and counted with a Makler Counting Chamber (Sefi-Medical Instruments). Human sperm were collected from semen via several washings with PBS. Protein lysates of 5 × 106 sperm were prepared in RIPA lysis buffer (25 mM Tris⋅HCl, pH 7.6; 150 mM NaCl; 1% Nonidet P-40; 1% sodium deoxycholate; 0.1% SDS) using a vortexer. Mouse testes were homogenized in lysis buffer to produce crude lysates. Protein lysates were separated using SDS/PAGE and transferred onto Immune-Blot PVDF membrane (BIO-RAD) for the immunoblot analysis.
Mass Spectrometry.
Protein lysates of 5 × 105 sperm were electrophoresed on 10% (wt/vol) SDS-PAGE denaturing gel. The protein bands were visualized with Silver Staining kit (Invitrogen). The materials eluted from the gel were destained and then digested with trypsin. The peptide sequences were analyzed with mass spectrometry (Ultraflex; Bruker Daltoniks) and then analyzed with the MS-Fit search program (http://prospector.ucsf.edu/prospector/mshome.htm).
Immunocytochemistry.
Sperm from the cauda epididymis were mounted on coverslips as described (32). Sperm and testis frozen sections were incubated with secondary antibody conjugated with Alex Fluor 488 or 594 (Molecular Probes) and PNA conjugated with fluorescein isothiocyanate (EY Laboratories) for 30 min at room temperature. Coverslips were mounted on glass slides using Vectashield with DAPI (4′, 6-diamidino-2-phenylindole) for counterstaining the nucleus (Vector Laboratories). Images were observed using a fluorescence microscope (Olympus), captured with a digital camera (DP70; Olympus), and formatted with Photoshop Elements 4.0 software.
Human Subjects.
Informed consent was obtained from all subjects, with approval received from the ethics committee or institutional review board of Tokyo Dental College and the National Institute of Advanced Industrial Science and Technology.
PCR and DNA Sequencing Analysis of Individual Human Tissues.
Genomic DNA extractions were performed using DNeasy Blood and Tissue kit (Qiagen). For PCR amplification, 20 ng of genomic DNA was combined with exon 4 primers (5′-ACACAGTCTCAGGAAAGAGC-3′ and 5′-ATACTCAAGTGCCATCGGGG-3′) and with Takara LA Taq (Takara Bio). Thirty cycles (20 s at 94 °C, 30 s at 60 °C, and 1 min at 72 °C) were performed, and the products were purified from agarose gels for direct sequencing and subcloning into pGEM-T Easy Vector (Promega).
Supplementary Material
Acknowledgments
We thank N. Yaguchi and M. Okazaki for their technical assistance. This work was supported by the New Energy and Industrial Technology Development Organization, as a part of the Medical Glycomics Project.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310777111/-/DCSupplemental.
References
- 1.Röttger S, et al. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci. 1998;111(Pt 1):45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Peng C, et al. Identification of a novel human UDP-GalNAc transferase with unique catalytic activity and expression profile. Biochem Biophys Res Commun. 2010;402(4):680–686. doi: 10.1016/j.bbrc.2010.10.084. [DOI] [PubMed] [Google Scholar]
- 3.Raman J, Guan Y, Perrine CL, Gerken TA, Tabak LA. UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferases: Completion of the family tree. Glycobiology. 2012;22(6):768–777. doi: 10.1093/glycob/cwr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett EP, Hassan H, Clausen H. cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3. J Biol Chem. 1996;271(29):17006–17012. doi: 10.1074/jbc.271.29.17006. [DOI] [PubMed] [Google Scholar]
- 5.White T, et al. Purification and cDNA cloning of a human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J Biol Chem. 1995;270(41):24156–24165. doi: 10.1074/jbc.270.41.24156. [DOI] [PubMed] [Google Scholar]
- 6.Guo JM, et al. Molecular cloning and characterization of a novel member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, pp-GalNAc-T12. FEBS Lett. 2002;524(1-3):211–218. doi: 10.1016/s0014-5793(02)03007-7. [DOI] [PubMed] [Google Scholar]
- 7.Ten Hagen KG, Fritz TA, Tabak LA. All in the family: The UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13(1):1R–16R. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- 8.Okajima T, Xu A, Lei L, Irvine KD. Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science. 2005;307(5715):1599–1603. doi: 10.1126/science.1108995. [DOI] [PubMed] [Google Scholar]
- 9.Ventelä S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: Mechanisms of haploid gene product sharing. Mol Biol Cell. 2003;14(7):2768–2780. doi: 10.1091/mbc.E02-10-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curi SM, et al. Asthenozoospermia: Analysis of a large population. Arch Androl. 2003;49(5):343–349. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]
- 11.Matzuk MM, Lamb DJ. The biology of infertility: Research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narimatsu H. Construction of a human glycogene library and comprehensive functional analysis. Glycoconj J. 2004;21(1-2):17–24. doi: 10.1023/B:GLYC.0000043742.99482.01. [DOI] [PubMed] [Google Scholar]
- 13.Hagen FK, Van Wuyckhuyse B, Tabak LA. Purification, cloning, and expression of a bovine UDP-GalNAc: polypeptide N-acetyl-galactosaminyltransferase. J Biol Chem. 1993;268(25):18960–18965. [PubMed] [Google Scholar]
- 14.Kierszenbaum AL, Rivkin E, Tres LL. Acroplaxome, an F-actin-keratin-containing plate, anchors the acrosome to the nucleus during shaping of the spermatid head. Mol Biol Cell. 2003;14(11):4628–4640. doi: 10.1091/mbc.E03-04-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67(4):271–284. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Gerton GL, Moss SB. Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics. 2006;5(5):801–810. doi: 10.1074/mcp.M500322-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Danshina PV, et al. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod. 2010;82(1):136–145. doi: 10.1095/biolreprod.109.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haraguchi CM, et al. Ubiquitin signals in the developing acrosome during spermatogenesis of rat testis: An immunoelectron microscopic study. J Histochem Cytochem. 2004;52(11):1393–1403. doi: 10.1369/jhc.4A6275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo JM, et al. Characterization of a monoclonal antibody to human proacrosin and its use in acrosomal status evaluation. J Histochem Cytochem. 1991;39(3):273–282. doi: 10.1177/39.3.1704391. [DOI] [PubMed] [Google Scholar]
- 20.Michaut M, Tomes CN, De Blas G, Yunes R, Mayorga LS. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc Natl Acad Sci USA. 2000;97(18):9996–10001. doi: 10.1073/pnas.180206197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramalho-Santos J, Moreno RD, Wessel GM, Chan EK, Schatten G. Membrane trafficking machinery components associated with the mammalian acrosome during spermiogenesis. Exp Cell Res. 2001;267(1):45–60. doi: 10.1006/excr.2000.5119. [DOI] [PubMed] [Google Scholar]
- 22.Kessler SP, Rowe TM, Gomos JB, Kessler PM, Sen GC. Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J Biol Chem. 2000;275(34):26259–26264. doi: 10.1074/jbc.M004006200. [DOI] [PubMed] [Google Scholar]
- 23.Ramaraj P, Kessler SP, Colmenares C, Sen GC. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J Clin Invest. 1998;102(2):371–378. doi: 10.1172/JCI3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondoh G, et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat Med. 2005;11(2):160–166. doi: 10.1038/nm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivkin E, Kierszenbaum AL, Gil M, Tres LL. Rnf19a, a ubiquitin protein ligase, and Psmc3, a component of the 26S proteasome, tether to the acrosome membranes and the head-tail coupling apparatus during rat spermatid development. Dev Dyn. 2009;238(7):1851–1861. doi: 10.1002/dvdy.22004. [DOI] [PubMed] [Google Scholar]
- 26.Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337(6205):373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- 27.Kierszenbaum AL, Rivkin E, Tres LL. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis. 2011;1(3):221–230. doi: 10.4161/spmg.1.3.18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwai T, et al. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002;277(15):12802–12809. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- 29.Togayachi A, et al. Molecular cloning and characterization of UDP-GlcNAc:lactosylceramide beta 1,3-N-acetylglucosaminyltransferase (beta 3Gn-T5), an essential enzyme for the expression of HNK-1 and Lewis X epitopes on glycolipids. J Biol Chem. 2001;276(25):22032–22040. doi: 10.1074/jbc.M011369200. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara Y, et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci USA. 1994;91(25):12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogura A, Yanagimachi R. Round spermatid nuclei injected into hamster oocytes from pronuclei and participate in syngamy. Biol Reprod. 1993;48(2):219–225. doi: 10.1095/biolreprod48.2.219. [DOI] [PubMed] [Google Scholar]
- 32.Miki K, et al. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248(2):331–342. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.