Significance
Type 1 diabetes is due to immune-mediated pancreatic β-cell destruction. Lysine deacetylase inhibitors (KDACi) protect β-cells from inflammatory destruction in vitro and are promising immunomodulators. The orally active and clinically well-tolerated KDACi vorinostat and givinostat reverted diabetes in a mouse model of type 1 diabetes and counteracted inflammatory target cell damage. Importantly, these effects were achieved with doses that are safe and effective in human inflammatory diseases. Of note, the mechanism of action was compatible with transcription factor—rather than global chromatin—hyperacetylation, causing inhibition of transcription factor binding and reduction of proinflammatory gene expression in leukocytes and β-cells. These data provide a rationale for clinical trials of safety and efficacy of KDACi in patients with Type 1 diabetes.
Keywords: inflammation, histone deacetylase, posttranslational modification, epigenetics, autoimmunity
Abstract
Type 1 diabetes is due to destruction of pancreatic β-cells. Lysine deacetylase inhibitors (KDACi) protect β-cells from inflammatory destruction in vitro and are promising immunomodulators. Here we demonstrate that the clinically well-tolerated KDACi vorinostat and givinostat revert diabetes in the nonobese diabetic (NOD) mouse model of type 1 diabetes and counteract inflammatory target cell damage by a mechanism of action consistent with transcription factor—rather than global chromatin—hyperacetylation. Weaning NOD mice received low doses of vorinostat and givinostat in their drinking water until 100–120 d of age. Diabetes incidence was reduced by 38% and 45%, respectively, there was a 15% increase in the percentage of islets without infiltration, and pancreatic insulin content increased by 200%. Vorinostat treatment increased the frequency of functional regulatory T-cell subsets and their transcription factors Gata3 and FoxP3 in parallel to a decrease in inflammatory dendritic cell subsets and their cytokines IL-6, IL-12, and TNF-α. KDACi also inhibited LPS-induced Cox-2 expression in peritoneal macrophages from C57BL/6 and NOD mice. In insulin-producing β-cells, givinostat did not upregulate expression of the anti-inflammatory genes Socs1-3 or sirtuin-1 but reduced levels of IL-1β + IFN-γ–induced proinflammatory Il1a, Il1b, Tnfα, Fas, Cxcl2, and reduced cytokine-induced ERK phosphorylation. Further, NF-κB genomic iNos promoter binding was reduced by 50%, and NF-κB-dependent mRNA expression was blocked. These effects were associated with NF-κB subunit p65 hyperacetylation. Taken together, these data provide a rationale for clinical trials of safety and efficacy of KDACi in patients with autoimmune disease such as type 1 diabetes.
Although there is progress in controlling autoimmune diseases, therapies are limited by the risks and adverse effects of conventional immunosuppression, and patients require life-long therapy. Novel well-tolerated oral drugs that restore T-cell regulation, reduce target organ inflammation, and inhibit inflammatory target cell damage would represent a major advance. Recently, small-molecule drugs that revert posttranslational protein acetylation by inhibiting lysine deacetylase (KDAC) activity have shown promise as oral immunomodulatory therapeutics. KDAC inhibitors (KDACi) (i) reduce dendritic cell (DC) driven differentiation of naïve T lymphocytes toward a T helper (Th)1 and Th17 phenotype (1); (ii) promote regulatory T-cell (Treg) suppressive phenotype and function (2); (iii) reduce proinflammatory cytokine production from human peripheral blood mononuclear cells and serum cytokine levels in mice (3); and (iv) protect target cells from inflammatory insults (4). Safety and tolerability is paramount; thus, a phase I/II study of the KDACi givinostat in children with systemic-onset juvenile idiopathic arthritis (SOJIA) revealed the benefit and safety of 1.5 mg/kg for 12 wk yielding twice daily peak blood levels of 125–200 nM (5). Importantly the anti-inflammatory properties of KDACi are exerted at ∼100-fold lower concentrations than those needed to reduce tumor size in vitro and in animals (3).
Type 1 diabetes is a prototypic autoimmune disease (6). Proinflammatory cytokines such as IL-1β, TNF-α, and IFN-γ impair the function and viability of pancreatic β-cells via NF-κB, STAT1, and MAPK signaling and endoplasmic reticulum stress and mitochondrial death pathways (6–8). Anticytokine strategies improve β-cell secretory function and glycated hemoglobin in patients with type 2 diabetes (9). Insulin-producing β-cells express all classical KDACs (10), and the KDACi vorinostat and givinostat preserve islet insulin release and prevent cytokine-induced cell death in vitro and NO production in vivo (4, 10, 11). In addition, givinostat reduces diabetes in the Kilham virus animal model of type 1 diabetes without affecting virus-specific adaptive immunity (12). However, the efficacy of orally active, clinically tolerated KDACi in preventing disease in an autoimmune model of diabetes has not been investigated, and the underlying cellular and molecular protective mechanisms are poorly understood. Therefore, we administered vorinostat and givinostat in the drinking water from weaning until 100–120 d of age to nonobese diabetic (NOD) mice spontaneously developing autoimmune diabetes and determined the incidence of diabetes and parameters of immune function, as well as the molecular mechanism of action of low concentrations of these KDACi in the β-cell.
Results
Oral Administration of KDACi Reduces Autoimmune Diabetes Incidence and Severity, Restores Regulatory T Cells in NOD Mice, and Moderates Inflammatory DC Numbers and Activity.
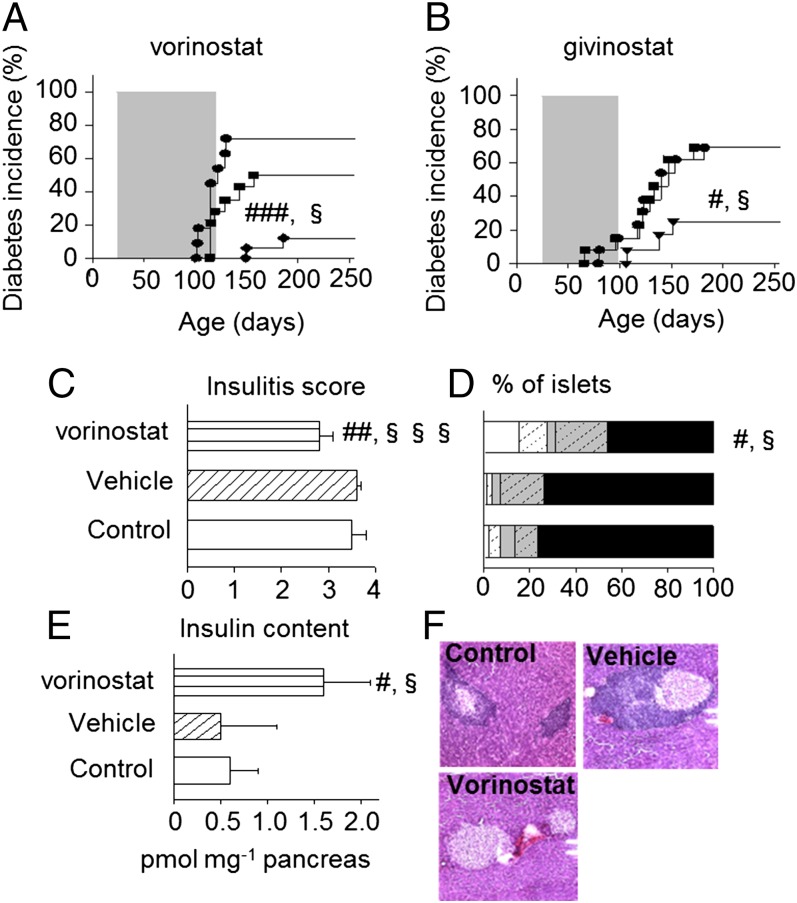
Daily administration of vorinostat (50 mg/kg) or givinostat (1 mg/kg) for 80 or 100 d, respectively, to weaning female NOD mice via the drinking water provided long-term protection against diabetes, even after discontinuation of treatment (Fig. 1). Vorinostat treatment reduced cumulative diabetes incidence to 12% compared with 50% of vehicle-treated (P ≤ 0.05) and 72% of untreated controls at 250 d of age (P ≤ 0.001; Fig. 1A). Accordingly, givinostat-treated animals had an incidence of 25% in contrast to 69% in the control groups (P ≤ 0.05; Fig. 1B). Further, disease onset was delayed (168 ± 25 vs. 128 ± 17 and 116 ± 10 d in vorinostat- vs. vehicle- and untreated controls, P ≤ 0.05 and P ≤ 0.001, respectively), and severity was decreased (273 ± 10 vs. 400 ± 75 mg/dL glucose in vorinostat- vs. untreated controls, P ≤ 0.05). KDACi-treated mice had normal weight curves (Fig. S1A). Vorinostat treatment reduced overall mean insulitis score (Fig. 1C) corresponding to a statistically significant increase in the percentage of islets without infiltration (16 ± 4% vs. 1 ± 2% and 2 ± 1% grade 0 islets in vehicle- and untreated controls; P ≤ 0.05; Fig. 1 D and F). Accordingly, vorinostat treatment increased pancreatic insulin content (Fig. 1E). KDACi did not prevent diabetes recurrence after syngeneic islet grafting in diabetic NOD mice (Table S1).
Fig. 1.
KDACi inhibits diabetes incidence in diabetes-prone NOD mice associated with a reduction in insulitis score and improvement in pancreatic insulin content. (A) Diabetes incidence is shown for untreated (n = 11; ●), vehicle-treated (n = 14; ■) or vorinostat-treated (50 mg/kg, via drinking water; n = 17; ◆) mice. (B) Diabetes incidence is shown for untreated (n = 13; ●), vehicle-treated (n = 13; ■), or givinostat-treated (1 mg/kg, via drinking water; n = 12; ▼) mice. All mice were monitored for the development of spontaneous disease until more than 250 d of age. #vs. control; §vs. vehicle. One symbol, P ≤ 0.05; three symbols, P ≤ 0.001. Pancreata from 250-d-old untreated (Control), vehicle- or vorinostat-treated mice were stained with H&E to determine insulitis severity (C, D, and F). Pancreatic islets were scored for the presence of mononuclear infiltration: 0, no insulitis; 1, peri-insulitis; 2, islets with lymphocyte infiltration in less than 50% of the area; 3, islets with lymphocyte infiltration in more than 50% of the area; 4, islets completely destroyed. (D) For each score (0: white; 1: white hatched; 2: gray; 3: gray hatched; 4: black), the percentage of infiltrated of the total number of islets was calculated. (E) Pancreatic insulin content was determined as described in Methods. Data in C and E are means + SEM from four to eight mice per group (C–E). #vs. control; §vs. vehicle. One symbol: P ≤ 0.05; two symbols: P ≤ 0.01; three symbols: P ≤ 0.001. (F) Representative staining.
Despite the presence of givinostat in plasma from treated animals (∼3 nM; Table S2), we did not observe histone H3 hyperacetylation in pancreas or immune tissues [spleen and pancreatic draining lymph nodes (pLNs)] from treated mice (Fig. S1B). As a control for the histone acetylation assay, peritoneal macrophages isolated from prediabetic NOD mice preexposed for 1 h to givinostat (100 nM) or vorinostat (1 µM) demonstrated clear histone H3 hyperacetylation after 6 h of additional culture (Fig. S1B).
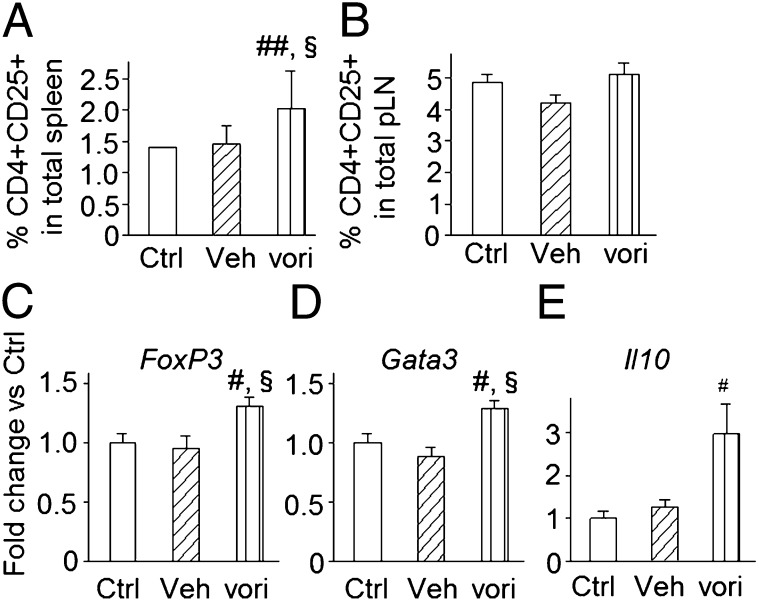
Lysine deacetylase targeting induces functional Tregs without disturbing T-cell effector activity (2, 13). Vorinostat treatment increased CD4+CD25+ Tregs in spleen but not in pLNs (Fig. 2 A and B). Gata3, FoxP3, and IL10 gene expression was increased in total spleen homogenate from vorinostat-treated mice (Fig. 2 C–E). FoxP3 and Gata3 are characteristic of naturally occurring Tregs and Th2 cells, respectively. We further demonstrated that CD4+CD25+ Tregs were also positive for FoxP3 and CD62L at the protein level (Fig. S2 A and B). Functionality of the putative Tregs was confirmed, as CD4+CD25+ T cells isolated from protected NOD mice under active KDACi treatment also inhibited autologous CD4+CD25− responder T-cell proliferation by an average of 53 ± 1% at a 1:1 ratio (Fig. S3A). In mixed lymphocyte reactions, vorinostat had no influence on T-cell proliferative response of CD4+CD25− effector T cells isolated from protected NOD mice after allogeneic stimulation; however, IFN-γ secretion was reduced (Fig. S3B). In line with the ability of KDACi to increase the percentage of T cells with a regulatory profile, absolute CD4+CD25+ T-cell numbers in splenic isolates from vorinostat-treated mice were also increased (25.3 ± 8.2 vs. 14.6 ± 1.5 and 18.6 ± 3.0 CD4+CD25+ T cells per thousand cells compared with vehicle- and untreated mice; P ≤ 0.05). Pharmacological targeting of KDACs has also been reported to trigger important changes in DC subsets and function, which are fundamental in initiating modulation of the host immune system (3, 14, 15). Vorinostat-treated NOD mice showed slightly decreased levels of CD11c+CD11b+ bona fide myeloid DCs in spleen and pLNs (Fig. S2C), and CD11c+ DCs from vorinostat-treated mice displayed a moderate decrease in spontaneous secretion of IL-6, IL-12p70, and TNF-α (Fig. S2D).
Fig. 2.
KDAC inhibition increases percentages of Treg subsets and their transcription factors. Frequency of CD4+CD25+ Tregs in spleen (A) and pancreatic draining lymph nodes (pLN) (B) from vorinostat- (vori), vehicle- (veh), and untreated (Ctrl) NOD mice at 250 d of age by fluorescence assisted cell sorting. (C–E) Expression of FoxP3, GATA3, and Il10 in spleen from vorinostat-, vehicle-, and untreated NOD mice (at 250 d of age). Data are presented as means + SEM. (A and B) Three to eight mice per group. (C–E) Three independent experiments (with two to four mice per group). #vs. control; §vs. vehicle. One symbol, P ≤ 0.05; two symbols, P ≤ 0.01.
KDACi Reduces Human Islet Cell Death and Resets Aberrant Islet Cell and Leukocyte Proinflammatory Gene Expression.
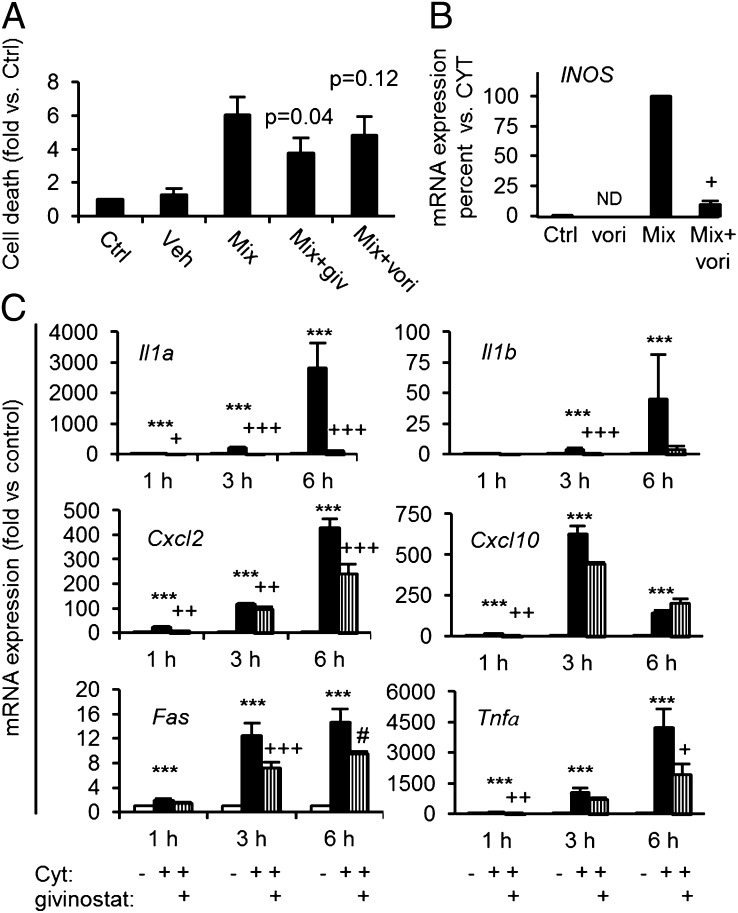
KDACi reduce expression and secretion of several proinflammatory cytokines from human and rodent leukocytes (3) and proinflammatory cytokine-induced death in rodent β-cells (11). It is unknown whether KDACi also reduce proinflammatory cytokine cell death in human islets. Both givinostat and vorinostat reduced cytokine-induced cell death, although the effect of vorinostat failed to reach statistical significance (Fig. 3A). Further, vorinostat reduced iNOS mRNA (Fig. 3B) in human islets.
Fig. 3.
KDAC inhibition reduces cytokine-induced cell death in human islets and resets aberrant cytokine-induced islet cell proinflammatory gene expression. (A) Human islets isolated from three donors were precultured for 1 h with the KDAC inhibitors givinostat (giv, 500 nM) or vorinostat (vori, 1 µM) before adding a combination (Mix) of cytokines (150 pg/mL IL-1β + 10 ng/mL IFN-γ + 50 ng/mL TNF-α). Islets were cultured with Mix for 6 d before determining cell death (Cell Death Detection ELISA; P values indicate comparisons with Mix). (B) IL-1β+ IFN-γ+ TNF-α (Mix) induced iNOS mRNA in isolated human islets with or without vorinostat (vori, 10 μM) preexposure (+P < 0.05 vs. Mix, n = 3). (C) INS-1 cells were cultured for 1, 3, or 6 h in the presence (filled bars, CYT) or absence [open bars, Control (Ctrl)] of IL-1β (150 pg/mL) + IFNγ (5 ng/mL). Givinostat (giv, 125 nM) was added 1 h before cytokine exposure (vertically hatched bars). Total RNA was isolated, cDNA was generated by reverse transcription, and expression of genes was quantified by real-time quantitative PCR. Data were normalized to the reference gene Hprt1. Data from four to six independent experiments are presented as mean fold + SEM compared with Ctrl condition with time points indicated. ***P < 0.001 vs. control; +++P < 0.05, ++P < 0.01, and +P < 0.001 vs. CYT; and #P = 0.06 vs. Cyt.
To investigate the β-cell protective mechanisms of action, we performed an explorative microarray study in isolated rat islets exposed to IL-1β and IFN-γ in the presence or absence of KDAC inhibition. Network analysis clustered around a reduction in inflammatory mediators and their signaling pathways (Fig. S4) including NF-κB and the classical MAPKs JNK, ERK, and p38. Real-time quantitative PCR (qPCR) validation verified that KDACi abrogated induction of Il1a, and Il1b mRNA expression (Fig. 3C) and reduced Cxcl2, Fas, and Tnfα mRNA levels (Fig. 3C), whereas Cxcl10 (IP-10) was unaffected (Fig. 3C). Of note, KDACi did not upregulate expression of the anti-inflammatory genes Socs1-3 or sirtuin-1 (Fig. S5).
We then investigated if KDAC inhibition reduced the expression of these inflammatory mediators in mouse leukocytes. Givinostat inhibited LPS-induced expression of Il1b, Il6, Tnfα, and Cox2 in peritoneal macrophages from NOD mice and non–diabetes-prone C57BL/6 mice (Fig. S6).
Givinostat Inhibits ERK Activation.
The microarray analysis of rat islets suggested that KDACi reduce cytokine-signaling via the classical MAPK ERK, JNK, and p38 pathways known to be important in cytokine-induced β-cell death (6). To validate this possibility, we assessed the effect of givinostat (125 nM) on cytokine-induced ERK, JNK, and p38 phosphorylation. Givinostat inhibited cytokine-induced ERK phosphorylation in INS-1 cells, but neither JNK nor p38 phosphorylation was affected (Fig. S7).
KDAC Activity Controls Cytokine-Induced NF-κB Subunit p65 Transcriptional Activity and DNA Binding in β-Cells by p65 Deacetylation.
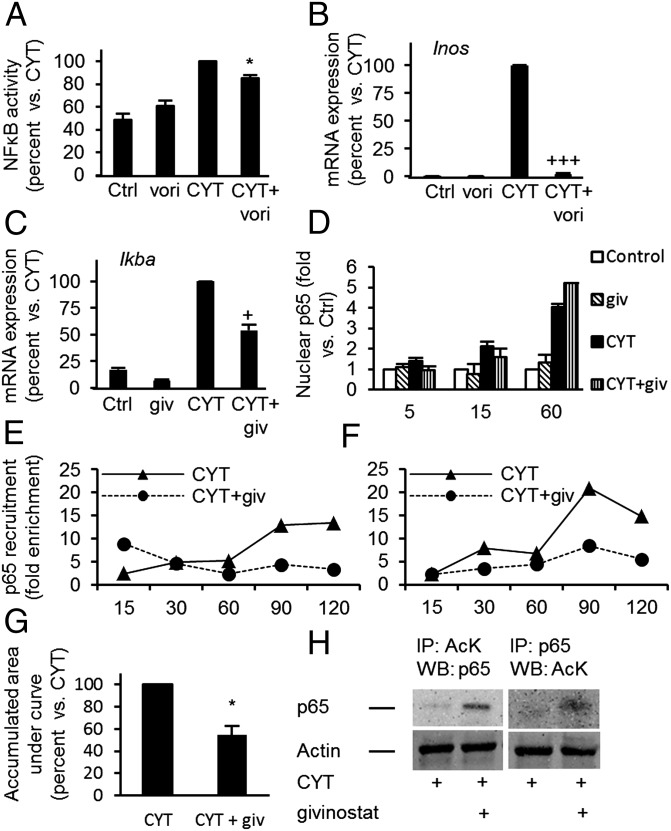
ERK activity is required for NF-κB activity in β-cells (16), and our microarray analysis and previous studies (17–19) suggested that NF-κB is a central target for KDACi. We have previously shown that cytokine-induced inhibitor IκBα degradation is independent of KDAC activity (4). We show here that givinostat inhibited cytokine-induced NF-κB activity (Fig. 4A) and expression of the NF-κB–dependent genes iNos in human and rodent islets (Figs. 3B and 4B) and IκBα in INS-1 cells (Fig. 4C). However, givinostat did not prevent nuclear accumulation of p65 (Fig. 4D). We therefore asked if givinostat reduced NF-κB binding to promoter elements using a ChIP assay followed by real-time qPCR for detection of NF-κB subunit p65 binding to the proximal and distal NF-κB sites in the native iNos promoter in genomic DNA from insulin-producing cells (20). Cytokine exposure increased recruitment of p65 to the iNos promoter (Fig. 4 E and F), and givinostat significantly reduced the binding by ∼50% (Fig. 4G). Reduction in iNos promoter binding was associated with p65 hyperacetylation (Fig. 4H). Taken together, these data suggest that KDAC activity in insulin-producing cells is important for cytokine-induced NF-κB transcriptional activity, but not nuclear translocation, by direct p65 deacetylation.
Fig. 4.
KDAC activity regulates cytokine-induced NF-κB subunit p65 binding to genomic DNA and cytokine-induced NF-κB activity, but not p65 nuclear accumulation. (A) IL-1β+ IFN-γ (CYT)–induced NF-κB activity was determined in INS-1 cells with or without givinostat (giv, 125 nM) preexposure (**P < 0.01 vs. CYT, n = 6). (B) Cyt induced iNos mRNA in isolated rat islets with or without vori (500 nM) preexposure (+++P < 0.001 vs. Cyt, n = 3). (C) IκBα expression in INS-1 cells preexposed to givinostat (giv, 125 nM) for 1 h, before addition of CYT for 30 min (n = 6). ***P < 0.001 vs. Ctrl and +P < 0.001 vs. CYT. (D) IL-1β+ IFN-γ (CYT)–induced p65 nuclear accumulation. INS-1 cells were cultured for indicated time periods in the presence or absence of CYT with or without givinostat (giv, 125 nM) preexposure (*P < 0.05 vs. CYT n = 3). (E and F) IL-1β+ IFN-γ (CYT)–induced p65 recruitment to genomic iNos promoter at proximal and distal p65 binding sites, respectively. INS-1 cells were cultured for indicated time periods with or without preexposure to givinostat (giv, 250 nM). Graphs show fold enrichment data from one representative out of four independent experiments. (G) Quantification of E and F: area under curves for the two binding sites were added and subtracted background signal (*P < 0.05 vs. CYT, n = 4). (H) Acetylation of p65 was determined after 90-min exposure to IL-1β + IFN-γ by cross-immunoprecipitation; representative blots from one of three independent experiments are shown. All data are presented as means + SEM. AcK, acetylated lysine.
Discussion
Here we show that low doses of the clinically tolerated KDACi vorinostat and givinostat administered orally to weaning NOD mice reduced diabetes incidence and delayed disease onset without apparent adverse effects. Remarkably, protection against autoimmune diabetes was maintained after treatment discontinuation and accompanied by reduced islet infiltration and increased pancreatic insulin content. KDACi administration increased functional Treg frequency as well as splenic IL-10 production and modestly suppressed inflammatory DC subset numbers and their production of IL-6, IL-12, and TNF-α compared with vehicle. These data indicate that KDACi administration counteracts diabetes immunopathogenesis and provides long-term homeostatic immune regulation primarily by enhancing Treg (CD4+CD25+FoxP3+) cell numbers (2) and secondarily via reduction of inflammatory DCs and their cytokines. The lack of detectable in vivo H3 hyperacetylation in pancreatic and immune tissues from protected animals together with lack of up-regulated protective genes suggests that hyperacetylation of proteins other than nuclear histones (e.g., transcription factors) is the main mechanism of action. In vitro, we found that KDAC inhibition prevented cytokine-induced ERK phosphorylation, caused p65 hyperacetylation associated with reduced NF-κB genomic iNos promoter binding, and inhibited cytokine induction of NF-κB–dependent genes iNOS and IκBa without affecting p65 nuclear translocation. We cannot, however, exclude the possibility that hyperacetylation of specific histone sites contributes to givinostat-mediated reduced NF-κB DNA binding.
Our findings have considerable translational potential because the in vitro and in vivo protection was obtained at KDACi doses compatible with concentrations obtained after oral dosing in humans (5, 21). Givinostat reduced diabetes incidence in vivo at a serum concentration of ∼3 nM of the active metabolite (Table S2). Numerous studies have investigated the safety and pharmacokinetics of givinostat in animal models and clinical trials (3). In a recent phase I human trial, 50–100 mg of givinostat per day, corresponding to a minimum concentration of the active givinostat metabolite of ∼5.6–8.3 nM, was safe (21), in agreement with studies where low-dose oral administration of givinostat to children with systemic onset juvenile idiopathic arthritis (22) or vorinostat to patients with graft vs. host disease (23) were effective and safe. Therefore, concentrations of orally active givinostat used in vivo and in vitro in our study are clinically feasible, safe, and effective. Although we did not measure vorinostat plasma levels in the treated NOD mice, the dose used was clinically relevant, because oral administration of 50 mg/kg of vorinostat in mice in other studies resulted in a plasma concentrations equal to those obtained by safe and tolerated doses in humans (24, 25).
A further argument in favor of the clinical relevance of our findings is the striking similarity in mechanism of action to that observed in human inflammatory disease. Thus, in graft vs. host disease, vorinostat treatment led to hyperacetylation of the STAT3 transcription factor and increased expression of FoxP3 mRNA and percent and total numbers of CD4+CD25+CD127− Tregs in peripheral blood mononuclear cells (PBMCs) in study patients compared with control or normal healthy patients (23, 26). Further, PBMCs from the study patients stimulated with the TLR agonist LPS ex vivo demonstrated reduced secretion of TNFα and IL-6 compared with controls or healthy volunteers (23).
IL-17 expression in splenocytes was not detectable after vorinostat treatment. The finding that KDAC inhibition selectively inhibits production of Th1- in addition to Th17-inducing cytokines such as IL-12, TNF-α, and IL-6 by DCs and LPS-stimulated macrophages from autoimmune diabetes-prone animals offers an attractive mechanism to control pathology and induce tolerogenic rather than effector immune responses in type 1 diabetes. In addition to NF-κB and STAT3, activation of other transcription factors such as GATA3 and FoxP3, essential regulators of Th2 and Treg development and function, respectively, are dependent on protein acetylation (2, 27). Accordingly, vorinostat increased splenic GATA3 and FoxP3 gene expression, as well as the frequency of CD4+CD25+FoxP3+ Tregs.
Cytokines activate the ERK1/2, JNK, and p38 cascades in insulin-producing cells and ERK regulates NF-κB activation (16, 28, 29). Cytokine-induced ERK phosphorylation was reduced by givinostat in INS-1 cells (Fig. S7). In H322 lung cancer cells, KDACi hyperacetylates the chaperone heat shock protein (Hsp90) disrupting binding to Hsp90 binding clients (e.g., Erb1) that are upstream mediators of ERK activation, thereby destabilizing and depleting upstream ERK activators (30). In parallel to reduced p65 DNA binding, KDACi-mediated Hsp90 chaperone deficiency may thus contribute to KDACi-mediated inhibition of NF-κB transcriptional activity in INS-1 cells. Further experiments are needed to clarify this.
In conclusion, the orally active, clinically tolerated KDACi vorinostat and givinostat counteracted autoimmune disease and inflammatory cell damage in vivo as in vitro via effects on both the immune cells and target cells independent of chromatin remodeling, but associated with p65 hyperacetylation reducing p65 DNA binding and transcriptional activity. Thus, KDACi may be a unique and promising preventive therapy in individuals at risk for developing Type 1 diabetes, but more safety data on the use of low-dose KDACi in humans are needed before embarking on clinical trials in prediabetic individuals. Ongoing safety and efficacy studies with givinostat in patients with Polyarticular Juvenile Idiopathic Arthritis (NCT01557452 and NCT01261624) may provide such data.
Methods
KDACi.
Suberoylanilide hydroxamic acid (vorinostat) and ITF2357 (givinostat) were synthesized by Italfarmaco (Cinisello Balsamo, Italy). Purities were ≥99% as assessed by HPLC.
Experimental Animals and Drug Administration Protocol.
All animal experiments were approved by the Institutional Animal Care and Research Committee of the Katholieke Universiteit Leuven under Project 057/2009.
Prophylactic protocol.
Vorinostat or givinostat were solubilized in 1% 2-hydroxypropyl-β-cyclodextrin (Cyclodextrin Technologies Development) in sterile drinking water. NOD female littermates were randomly divided into three different treatment groups (untreated, vehicle control, and treatment), 11–17 mice per group. Vorinostat was administered at 50 mg/kg and givinostat at 1 mg/kg via drinking water. Treatment was given continuously from weaning until 120 and 100 d of age, respectively. For givinostat determination in blood samples, see SI Methods.
Peritoneal macrophage cultures.
Resident macrophages were isolated by peritoneal lavage from female 8- to 10-wk-old NOD and C57BL/6 mice and purity (>90%) was assessed by flow cytometry (31). Macrophages from each group were preincubated for 1 h with KDACi (100 nM givinostat or 1 µM vorinostat in medium with 0.01% DMSO) and stimulated for different time periods with LPS (100 µg/mL; Sigma). For details on immunostaining, cell viability, and mRNA analysis, see SI Methods.
Cell and Islet Culture and Experimentation.
The insulin-producing cell line INS-1 (32) was maintained as described previously (10). Primary rat islets were isolated and cultured in RPMI medium 1640 containing 11 mM glucose as previously described (10). Human islets were cultured in RPMI 1640 containing 5.6 mM glucose. Before experimentation, islets were left for at least 2 h to reduce handling stress. Mouse IL-1β (150–600 pg/mL) was from BD Pharmingen, and rat and human IFN-γ (2–5 and 10–100 ng/mL, respectively) and human TNF-α (50–100 ng/mL) were from R&D Systems. For details on cell viability, NO measurements, mRNA, and protein analysis, see SI Methods.
Cell death was assessed by a cell death detection ELISA as previously described (10).
For details on the microarray of isolated rat pancreatic islets, see SI Methods.
ChIP assay.
INS-1 cells were exposed to givinostat (250 nM) 1 h before addition of IL-1β (600 pg/mL) and IFN-γ (5 ng/mL). At the indicated time points protein/DNA cross-linking for 10 min was performed at room temperature before lysing cells. Samples were sonicated at high intensity for 10 min in cycles of 30-s pulses followed by 30 s without pulse (Diagenode Bioruptor), and p65 was immunoprecipitated as previously described (33) with 5 µg p65 antibody (#sc372-X; Santa Cruz Biotechnology). Immunoprecipitated chromatin was extracted by phenol-chloroform extraction, and the presence of specific DNA coprecipitated with p65 was determined by real-time PCR directed against a proximal and distal p65-binding site in the iNos promoter. For details, see SI Methods.
For details on the immunoprecipitation of acetylated p65, see SI Methods.
For details on in vitro immune phenotyping and functionality assays, assessment of diabetes, pancreatic histopathology with insulitis scoring, and insulin content determination, RNA isolation and real-time quantitative PCR analysis, protein isolation, and Western blotting, see SI Methods.
Statistics.
Values are expressed as means + SEM. Univariate ANOVA and Student t test were used to calculate significance levels between groups. Dunnett's, Bonferroni’s, and Tukey’s corrections were used to correct P values for multiple comparisons against a single group or against multiple groups, respectively. For analysis of insulitis and diabetes incidence data, Kaplan–Meier survival curves, the log-rank test, and the χ2 test were used.
Supplementary Material
Acknowledgments
We thank Wim Cockx, Jos Depovere, Molly Holte-Nelson, Josef Laureys, Dirk Valckx, and Gurmeet K. Singh for excellent technical assistance. This work was supported by the Flemish Research Foundation (C.M. and C.G.), Koning Boudewijn Stichting (C.M. and C.G.), National Institutes of Health Grant AI-15614 (to C.A.D.), JDRF Grant 26-2008-893 (to D.P.C. and T.M.-P.), the Novo Nordisk Foundation (D.P.C. and T.M.-P.), the Interleukin Foundation (T.M.-P.), and European Foundation for the Study of Diabetes/JDRF/Novo Nordisk European Programme in Type 1 Diabetes Research (D.P.C. and T.M.-P.). Human islets were provided through JDRF Award 31-2008-413 ECIT Islet for Basic Research program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320850111/-/DCSupplemental.
References
- 1.Bosisio D, et al. Blocking TH17-polarizing cytokines by histone deacetylase inhibitors in vitro and in vivo. J Leukoc Biol. 2008;84(6):1540–1548. doi: 10.1189/jlb.0708401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13(11):1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA, Fossati G, Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med. 2011;17(5-6):333–352. doi: 10.2119/molmed.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen L, et al. Inhibition of histone deacetylases prevents cytokine-induced toxicity in beta cells. Diabetologia. 2007;50(4):779–789. doi: 10.1007/s00125-006-0562-3. [DOI] [PubMed] [Google Scholar]
- 5.Vojinovic J, Damjanov N. HDAC inhibition in rheumatoid arthritis and juvenile idiopathic arthritis. Mol Med. 2011;17(5-6):397–403. doi: 10.2119/molmed.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donath MY, Størling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: From concept to clinical translation. Endocr Rev. 2008;29(3):334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- 7.Eldor R, et al. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006;103(13):5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas HE, Biden TJ. Bad news for beta-cell apoptosis. Diabetes. 2009;58(8):1725–1727. doi: 10.2337/db09-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 10.Lundh M, et al. Lysine deacetylases are produced in pancreatic beta cells and are differentially regulated by proinflammatory cytokines. Diabetologia. 2010;53(12):2569–2578. doi: 10.1007/s00125-010-1892-8. [DOI] [PubMed] [Google Scholar]
- 11.Christensen DP, et al. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol Med. 2011;17(5-6):378–390. doi: 10.2119/molmed.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara N, Alkanani AK, Dinarello CA, Zipris D. Histone deacetylase inhibitor suppresses virus-induced proinflammatory responses and type 1 diabetes. J Mol Med (Berl) 2013 doi: 10.1007/s00109-013-1078-1. [DOI] [PubMed] [Google Scholar]
- 13.Beier UH, et al. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31(5):1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brogdon JL, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109(3):1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 15.Song W, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25(1):161–168. doi: 10.1038/leu.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen L, et al. Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor kappaB-mediated gene expression in insulin-producing INS-1E cells. Diabetologia. 2005;48(12):2582–2590. doi: 10.1007/s00125-005-0039-9. [DOI] [PubMed] [Google Scholar]
- 17.Fabre C, et al. A novel effect of DNA methyltransferase and histone deacetylase inhibitors: NFkappaB inhibition in malignant myeloblasts. Cell Cycle. 2008;7(14):2139–2145. doi: 10.4161/cc.7.14.6268. [DOI] [PubMed] [Google Scholar]
- 18.Batra S, Sahu RP, Kandala PK, Srivastava SK. Benzyl isothiocyanate-mediated inhibition of histone deacetylase leads to NF-kappaB turnoff in human pancreatic carcinoma cells. Mol Cancer Ther. 2010;9(6):1596–1608. doi: 10.1158/1535-7163.MCT-09-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21(23):6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998;41(9):1101–1108. doi: 10.1007/s001250051036. [DOI] [PubMed] [Google Scholar]
- 21.Furlan A, et al. Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat) Mol Med. 2011;17(5-6):353–362. doi: 10.2119/molmed.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vojinovic J, et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(5):1452–1458. doi: 10.1002/art.30238. [DOI] [PubMed] [Google Scholar]
- 23.Choi SW, et al. Targeting histone deacetylases as a new strategy for graft versus host disease prevention. Blood. 2012;120:740. [Google Scholar]
- 24.Kelly WK, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novotny-Diermayr V, et al. SB939, a novel potent and orally active histone deacetylase inhibitor with high tumor exposure and efficacy in mouse models of colorectal cancer. Mol Cancer Ther. 2010;9(3):642–652. doi: 10.1158/1535-7163.MCT-09-0689. [DOI] [PubMed] [Google Scholar]
- 26.Choi SW, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: A phase 1/2 trial. Lancet Oncol. 2014;15(1):87–95. doi: 10.1016/S1470-2045(13)70512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamagata T, et al. Acetylation of GATA-3 affects T-cell survival and homing to secondary lymphoid organs. EMBO J. 2000;19(17):4676–4687. doi: 10.1093/emboj/19.17.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh N. Interleukin-1 beta-induced ceramide and diacylglycerol generation may lead to activation of the c-Jun NH2-terminal kinase and the transcription factor ATF2 in the insulin-producing cell line RINm5F. J Biol Chem. 1996;271(14):8307–8312. doi: 10.1074/jbc.271.14.8307. [DOI] [PubMed] [Google Scholar]
- 29.Larsen CM, et al. Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem. 1998;273(24):15294–15300. doi: 10.1074/jbc.273.24.15294. [DOI] [PubMed] [Google Scholar]
- 30.Yu X, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94(7):504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 31.Gysemans C, et al. Unaltered diabetes presentation in NOD mice lacking the vitamin D receptor. Diabetes. 2008;57(1):269–275. doi: 10.2337/db07-1095. [DOI] [PubMed] [Google Scholar]
- 32.Asfari M, et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130(1):167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen R, Grøntved L, Stunnenberg HG, Mandrup S. Peroxisome proliferator-activated receptor subtype- and cell-type-specific activation of genomic target genes upon adenoviral transgene delivery. Mol Cell Biol. 2006;26(15):5698–5714. doi: 10.1128/MCB.02266-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.