Significance
More than 1 million people worldwide suffer from the debilitating neurological disorder multiple sclerosis (MS). The initiation of MS is associated with sustained inflammation and an autoimmune T-cell response targeting the central nervous system. The activities of Th1 and Th17 effector T cells, which are the main pathogenic drivers of MS, are balanced by regulatory T cells, which dampen inflammation and mitigate disease. Our study describes an important role for the surface receptor Toso in balancing these T-cell subsets and controlling inflammation. Using an animal model of MS, we have developed a preclinical treatment strategy in which Toso-Fc fusion protein ameliorates the inflammatory symptoms of experimental autoimmune encephalomyelitis, an MS-like disease.
Keywords: inflammation, Th1/Th17 cells
Abstract
The ability to mount a strong immune response against pathogens is crucial for mammalian survival. However, excessive and uncontrolled immune reactions can lead to autoimmunity. Unraveling how the reactive versus tolerogenic state is controlled might point toward novel therapeutic strategies to treat autoimmune diseases. The surface receptor Toso/Faim3 has been linked to apoptosis, IgM binding, and innate immune responses. In this study, we used Toso-deficient mice to investigate the importance of Toso in tolerance and autoimmunity. We found that Toso−/− mice do not develop severe experimental autoimmune encephalomyelitis (EAE), a mouse model for the human disease multiple sclerosis. Toso−/− dendritic cells were less sensitive to Toll-like receptor stimulation and induced significantly lower levels of disease-associated inflammatory T-cell responses. Consistent with this observation, the transfer of Toso−/− dendritic cells did not induce autoimmune diabetes, indicating their tolerogenic potential. In Toso−/− mice subjected to EAE induction, we found increased numbers of regulatory T cells and decreased encephalitogenic cellular infiltrates in the brain. Finally, inhibition of Toso activity in vivo at either an early or late stage of EAE induction prevented further disease progression. Taken together, our data identify Toso as a unique regulator of inflammatory autoimmune responses and an attractive target for therapeutic intervention.
More than 5% of the populations of Western countries suffer from inflammatory autoimmune diseases (1). In all cases, a hyperactivated immune system is responsible for the initiation of autoimmunity. In the periphery, inflammatory T cells such as IL-17–producing Th (Th17) and IFN-γ–producing Th1 cells are controlled by suppressive regulatory T (Treg) cells (2). Numeric or functional imbalance of these various T-cell populations can result in autoimmunity or immunodeficiency. How the immune system limits self-reactive inflammatory responses in healthy individuals, and how these mechanisms fail in patients, is still under intensive investigation.
The transmembrane receptor Toso belongs to the Ig superfamily, and its cytoplasmic domain shows homology to Fas-activated serine/threonine kinase (3). Toso has been implicated in the regulation of CD95 (Fas/Apo1)- and TNF receptor (TNFR)-dependent T-cell apoptosis, and is highly overexpressed in apoptosis-resistant B-cell lymphomas (3–6). Toso also functions as an Fc receptor for IgM, and so may be important for B-cell development (7–10). Recently, Toso expression was detected on granulocytes and monocytes and Toso was linked to the homeostasis and activation of the innate immune system (11–13). However, the precise physiological relevance of Toso’s multifaceted functionality is still unknown.
In this study, we investigated the impact of loss of Toso on inflammatory autoimmune responses. Toso-deficient (Toso−/−) mice were less susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). Disease resistance was dependent on Toso’s function in dendritic cells (DCs). DCs from Toso−/− mice initiated less intense inflammatory CD4+ and CD8+ T-cell responses that were associated with reduced immunopathology. Toso−/− DCs induced more Tregs than controls. Finally, interference with Toso activity in vivo significantly decreased the burden of EAE disease following induction. Our findings indicate that Toso is a crucial mediator of inflammatory autoimmune responses in vivo.
Results
EAE Is Significantly Reduced in Toso−/− Mice and Is Not Dependent on T-Cell Differentiation and Function.
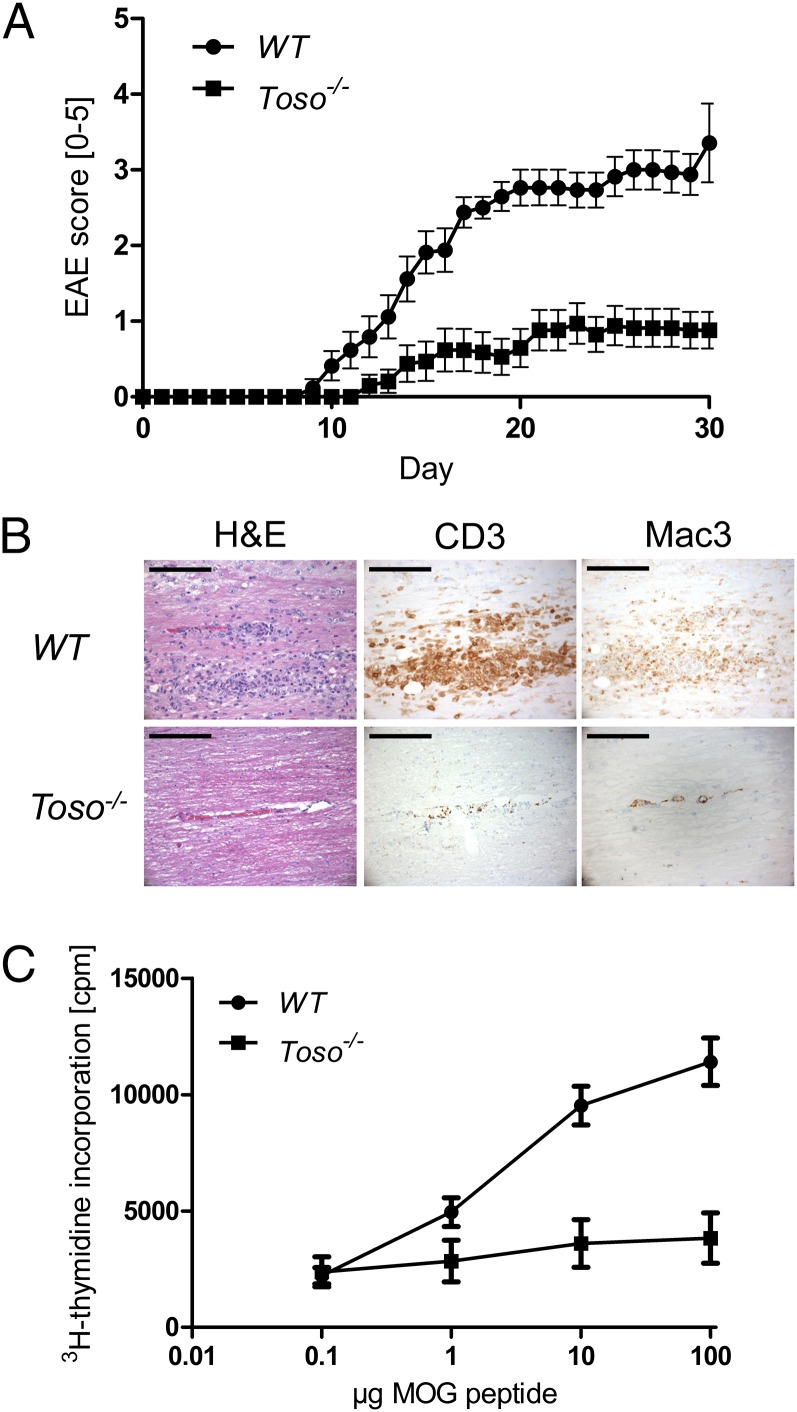
To clarify the role of Toso in autoimmune responses in vivo, we investigated whether Toso−/− mice were susceptible to EAE induction. WT mice developed severe clinical EAE symptoms. In contrast, EAE disease burden was significantly reduced by Toso deficiency such that Toso−/− mice were almost resistant to EAE development (Fig. 1A). Histological analysis revealed extensive leukocyte infiltration into the brain cord of WT mice, whereas leukocyte infiltration of Toso−/− brains was dramatically reduced (Fig. 1B, H&E). In the central nervous system (CNS) of WT mice, we detected perivascular-centered, diffuse, widespread T-cell infiltrates that were almost absent in the CNS of Toso−/− mice (Fig. 1B, CD3). The same pattern was observed for activated macrophages and microglia (Fig. 1B, Mac3), which are known to be crucial for EAE induction (14). However, the inflammatory T-cell infiltration of the leptomeninges was comparable to WT mice (Fig. S1). Taken together, these results indicate that Toso plays a key role in mediating autoimmune inflammation of EAE.
Fig. 1.
Toso−/− mice develop less severe EAE. (A) WT (n = 17) and Toso−/− (n = 17) mice were subjected to EAE. Data are the mean score ± SEM and are combined from four independent experiments. (B) Representative histopathological analyses of cross-sections of CNS from the mice in A at 20 d post-MOG injection. CNS sections were stained with H&E or immunostained to detect CD3+ T cells and macrophages (Mac3). Data are from one mouse per group. (Scale bar, 100 μm.) (C) Splenocytes isolated from WT and Toso−/− mice were pulsed with the indicated concentrations of MOG peptide. Cells were cultured for 72 h and pulsed for the last 8 h with [3H]thymidine. Data are the mean ± SEM of three biological replicates and are representative of two independent experiments.
Antigen-specific T-cell expansion and differentiation are hallmarks of EAE. To evaluate MOG-specific T-cell responses in the absence of Toso, we isolated splenocytes from EAE-primed WT and Toso−/− mice and pulsed them with MOG peptide in vitro to measure proliferation. In contrast to the vigorous expansion of WT splenocytes, Toso−/− splenocytes showed almost no antigen-specific proliferation (Fig. 1C), indicating a lack of antigen-specific cells or a proliferation defect of Toso−/− cells.
EAE development depends on functional CD4+ T cells. Given our splenocyte data, we speculated that Toso might interfere directly with T-cell proliferation. To investigate this hypothesis, we isolated CD4+ T cells from WT and Toso-deficient mice and measured anti-CD3/CD28–driven expansion in vitro. However, no difference was detected (Fig. S2A). EAE also depends on naïve CD4+ T-cell differentiation into the Th17 and Th1 inflammatory subsets. To assess Toso’s potential role in this process, we isolated naïve CD4+CD62L+ T cells from WT and Toso−/− mice and induced them to differentiate in vitro into either Th1 or Th17 cells, as indicated by their secretion of signature cytokines (Th1 cells, IFN-γ; Th17 cells, IL-17). However, Toso deficiency did not alter naïve T-cell differentiation into inflammatory Th subsets (Fig. S2B). We also analyzed CD4+ T cells that were isolated from the CNS of EAE-primed WT and Toso−/− mice by intracellular flow cytometry for the expression of IFN-γ and IL-17. The proportion of IFN-γ–producing T cells in the CNS was slightly lower in MOG-injected Toso−/− mice than in MOG-injected WT animals, but there were no differences in the percentage of cells producing IL-17 alone or IL-17 plus IFN-γ (Fig. S1C). Taken together, Toso has no impact on Th cell differentiation in vitro or in vivo, and the EAE resistance observed in Toso−/− mice was not due to an intrinsic impairment of Th cell functions.
Toso Modulates DC Activation in Vitro.
Based on our results presented above, we speculated that Toso might be important for the function of splenic antigen-presenting DCs that are required to mount and maintain a T-cell response in vivo. To test this, we incubated WT or Toso−/− bone marrow-derived DCs (BMDCs) that had been activated with LPS and pulsed with MOG with either WT or Toso−/− CD4+ 2D2 (MOG-specific) T cells. In accordance with our earlier results, WT DCs triggered equivalent levels of antigen-specific proliferation of WT or Toso−/− T cells (Fig. 2A). However, incubation of Toso−/− DCs with WT or Toso−/− CD4+ 2D2 T cells led to a drastically reduced proliferative response in both cases. These results indicate that Toso is critical for mounting a DC-dependent T-cell response.
Fig. 2.
Toso modulates DC activation and function. (A) Toso−/− and WT BMDCs (3 × 104) were activated for 16 h with LPS and pulsed with MOG peptide (light gray; dark gray: nonpulsed). MOG-loaded Toso−/− and WT BMDCs were incubated with VCT-labeled Toso−/− or WT 2D2 CD4+ T cells (1 × 105). Proliferation was measured 72 h later by flow cytometry. (B) Toso−/− and WT BMDCs were activated for 16 h with the indicated TLR ligands and assessed for their expression of CD80, CD86, and MHCII. WT BMDCs, lower dark line; Toso−/− BMDCs, upper light line. Data are gated on live CD4+ T cells (A) or on live CD11c+ cells (B) and are representative of five independent experiments.
Upon activation, immature DCs begin to mature and up-regulate costimulatory molecules important for T-cell priming. To examine whether Toso plays a role in this process, we stimulated WT and Toso−/− BMDCs with various Toll-like receptor (TLR) ligands, including LPS, R848, and CpG, and monitored surface up-regulation of CD80, CD86, and MHCII by flow cytometry. Interestingly, even before TLR ligand stimulation, Toso−/− immature DCs exhibited lower surface levels of CD80, CD86, and MHCII (Fig. 2B). These levels were further reduced upon TLR ligation by LPS, R848, or CpG (Fig. 2B), implying that DC stimulation in the absence of Toso may block DC maturation and render these cells tolerogenic.
Toso-Deficient DCs Do Not Induce Inflammation in Vivo.
In addition to their important role in initiating immune responses against foreign antigens, DCs are central regulators of peripheral tolerance to self-antigens (15). Tolerogenic DCs are characterized by their decreased potential to stimulate inflammatory T-cell responses and their low surface levels of CD80, CD86, and MHCII (15–17). To investigate whether Toso−/− DCs can mount an autoimmune T-cell response in vivo, we took advantage of the RIP-GP mouse model (18). RIP-GP mice express the GP antigen on their pancreatic islet cells. Upon transfer of CpG-activated antigen-pulsed WT BMDCs into RIP-GP mice, GP-expressing pancreatic islet cells are attacked by GP-specific T cells. This immune response results in islet cell depletion, induction of diabetes, and subsequent death of the mice (19). We transferred WT or Toso−/− BMDCs into RIP-GP mice and monitored mouse survival and damage to pancreatic islets. Whereas almost all control animals receiving WT BMDCs became hyperglycemic and reached the humane end point, not a single animal that had received Toso−/− BMDCs displayed hyperglycemia (Fig. 3A). Histological analysis of the pancreas revealed abundant T-cell infiltrates in RIP-GP mice that had received WT BMDCs (Fig. 3B). Although T-cell infiltrates were also obvious in mice that had received Toso−/− BMDCs, this infiltration was far less pronounced and correlated with the lower blood glucose levels observed in these animals (Fig. 3C). Taken together, these results show that Toso−/− DCs cannot mount disease-initiating inflammatory effector T-cell responses, and indicate that Toso might play an important role in regulating the inflammatory vs. the tolerogenic state of DCs.
Fig. 3.
Toso−/− DCs are unable to induce diabetes in RIP-GP mice. Activated antigen-pulsed DCs were transferred into RIP-GP mice (n = 10 per group). (A) Kaplan–Meier analysis of mouse survival over a 30-d observation period. *P ≤ 0.05. (B) Histological cross-sections of the pancreas were taken on day 8 and immunostained to detect CD3+ T cells (brown infiltrates, CD3 staining). Results are representative of five pancreatic sections per group. (Scale bar, 100 μm.) (C) Blood glucose levels were determined every other day. Data are the mean score ± SEM (n = 10).
Toso−/− DCs Preferentially Induce Tregs in Vitro and in Vivo.
To further examine our hypothesis that Toso−/− DCs attenuate inflammatory T-cell responses, we cultured MOG antigen-pulsed WT or Toso−/− BMDCs with WT 2D2 CD4+ T cells and determined surface expression levels of the T-cell activation markers CD25, CD44, and ICOS by flow cytometry. Up-regulation of CD25, CD44, and ICOS on MOG-stimulated WT CD4+ 2D2 T cells was lower when these T cells were stimulated by Toso−/− DCs compared with WT DCs (Fig. 4A). Interestingly, we found a significantly higher proportion of FoxP3-expressing Treg cells among CD4+CD25+ T cells in cultures exposed to Toso−/− BMDCs compared with their numbers in cultures exposed to WT BMDCs (Fig. 4B). This could be further enhanced by addition of TGF-β (Treg skewing condition) to the DC/T-cell culture (Fig. S3A). These findings suggested that Toso−/− DCs are weak inducers of inflammatory T-cell responses but strong inducers of Tregs. In addition, Toso−/− DCs expressed more PD-L1 on their cell surfaces compared with controls (Fig. 4C). PD-L1 has been shown to induce FoxP3 expression in T cells (20), potentially explaining the increased Treg numbers in cultures of T cells exposed to Toso−/− DCs. Interestingly, T-cell intrinsic differentiation to Tregs and also their suppressive capacity showed no differences between WT and Toso−/− genotypes (Fig. S3 B and C). Considering this, we conclude that Toso does not regulate Treg functionality but rather Treg numbers. Further on, regulation of Treg numbers is not a T-cell intrinsic effect but is mediated by Toso−/− DCs.
Fig. 4.
Toso deficiency in DCs increases Treg induction in vitro and in vivo. (A–C) BMDCs from WT or Toso−/− mice were activated with LPS and cocultured with naïve CD62LhighCD4+ T cells as described in Fig. 2. (A) Flow cytometric determination of T-cell expression levels of CD25, CD44, and ICOS. T cells stimulated with WT DCs, tinted line; T cells stimulated with Toso−/− DCs, dark line. Data are representative of three independent experiments. (B) FoxP3 expression by the CD25+CD62LhighCD4+ T-cell subpopulations in A. Data are the mean ± SEM (n = 5). (C) Flow cytometric determination of PD-L1 expression by the DCs in A. Data are the mean ± SEM (n = 5). For A–C, data were acquired by gating on live CD4+ T cells (A and B) or on live CD11c+ cells (C). (D and E) WT and Toso−/− mice (n = 5 per group) were subjected to EAE induction and killed on day 14 postinduction. Samples of CNS were subjected to gradient centrifugation to isolate CNS-infiltrating leukocytes. (D) Percentage of peripheral CD4+ T cells expressing FoxP3 and CD25. (E) Total numbers of CNS-infiltrating cells in WT and Toso−/− mice. For D and E, data are mean ± SEM (n = 5 per group; *P < 0.05).
To validate our hypothesis in vivo, we first characterized T-cell maturation of Toso−/− mice in the thymus. As expected, CD4+ and CD8+ T-cell compartments were not altered by Toso deficiency (Fig. S4). Within the CD4+ T cells, we found a similar amount of naturally occurring FoxP3+ Tregs (nTregs). To see whether these Treg proportions change during inflammatory conditions, we induced EAE in WT and Toso−/− mice by injecting MOG-peptide emulsion and killed the mice 14 d later. Consistent with our in vitro findings, we observed in vivo an increase in CD4+CD25+FoxP3+-expressing cells of our MOG-injected Toso−/− mice compared with MOG-injected WT animals (Fig. 4D). In line with this, quantification of CD4+ cells confirmed that overall CNS infiltration in MOG-injected Toso−/− mice was significantly lower than in controls (Fig. 4E). Taken together, we conclude that Toso deficiency shifts the balance from an inflammatory to a tolerogenic T-cell response and decreases the overall abundance of inflammatory cells in the brain. Toso is crucial for the proinflammatory function of DCs and suppresses Treg cell induction during EAE.
Blocking of Toso Action Interferes with EAE Development at Multiple Stages of the Disease.
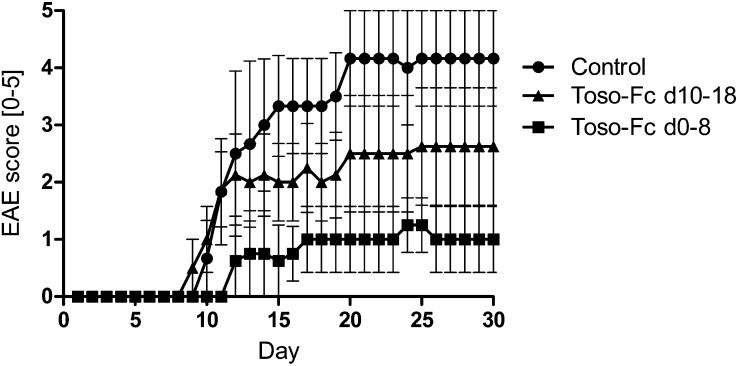
The blocking of signaling pathways by receptor-Fc fusion proteins has proven to be a powerful tool for manipulating disease-associated immune responses (21). For example, Etanercept is a fusion protein in which the TNF receptor is linked to the Fc portion of an IgG1 antibody. Etanercept acts as a decoy receptor that binds TNF (22), and so has been used therapeutically to treat human inflammatory autoimmune diseases such as rheumatoid arthritis (23). To translate our knowledge of the importance of Toso in inflammatory responses into a preclinical treatment application, we generated a Toso-Fc fusion protein consisting of the human Toso extracellular domain fused to the Fc portion of an IgG1 antibody that harbors a series of mutations preventing the activation of the complement system (SI Materials and Methods). We hypothesized that treatment of WT mice with this Toso-Fc fusion protein (Toso-Fc) might protect against EAE. We induced EAE, and treated these animals with Toso-Fc either during the disease-priming phase (days 0–8) or during the effector phase (days 10–18). Strikingly, treatment with Toso-Fc dramatically reduced disease severity and slowed disease progression in both cases. Mice that were injected every other day with Toso-Fc during the priming phase developed only a mild form of EAE, and the disease did not progress significantly after the treatment was stopped on day 8 (Fig. 5). Importantly, mice that already showed symptoms of EAE before Toso-Fc injection on day 10 also responded to the treatment in that disease progression was prevented (Fig. 5). Thus, we are confident that Toso-Fc treatment at any stage of EAE development can slow disease progression. This important result shows great promise for the therapeutic potential of our findings, and highlights the key role of Toso in inflammatory diseases.
Fig. 5.
Soluble Toso-Fc fusion protein blocks EAE disease progression. EAE was induced in WT mice that were then injected every other day with either 50 µg Toso-Fc fusion protein or PBS (control) (n = 4 per group). Toso-Fc was administered either between days 0 and 8 (Toso-Fc d0-8) or between days 10 and 18 (Toso-Fc d10-18). Disease severity was scored daily. Data are the mean score ± SEM and are representative of two independent experiments.
Discussion
Currently, the treatment of inflammatory autoimmune diseases is a major medical challenge. Multiple sclerosis (MS) is especially worrying because of its prevalence in young adults. For this group, MS is the most common cause of a nonreversible disability, as well as a severe social and occupational health problem (24). Effective treatment of autoimmune diseases requires knowledge of how they develop, the subject of much ongoing debate. In this study, we provide evidence that the transmembrane receptor Toso is crucial for the development of EAE, a mouse model of MS.
Our in vivo findings demonstrate that Toso−/− mice are less susceptible to EAE induction and show lower levels of inflammatory cell infiltrates in the CNS. Although Th17 cells are considered the main drivers of EAE (25–27), mice in which Th1 cell responses are impaired do show EAE resistance, supporting the theory that both inflammatory Th subsets are needed to induce the disease (28). In our study, we could not detect any influence of Toso on the differentiation of Th1 or Th17 cells in vitro or in vivo. Several groups have reported that functional Th17 cells are important for the recruitment of inflammatory macrophages to the CNS that sustain neuroinflammation (25, 29, 30). Although we found a reduced degree of macrophage infiltration into the CNS in sections of Toso-deficient brains, our in vitro work established that it is not the T-cell compartment but rather antigen-presenting DCs that are responsible for the reduced EAE disease burden in Toso−/− mice. When Toso−/− DCs were incubated in vitro with MOG-specific WT T cells, much weaker T-cell proliferation and costimulatory molecule expression were observed than when WT DCs were used to present MOG. Phenotypically, Toso−/− DCs retain a more immature state, with lower levels of MHCII, CD80, and CD86 expression compared with mature inflammatory DCs (31). These results suggest that Toso plays a critical driving role in the DCs that underpin autoimmune inflammation. This hypothesis was confirmed by our DC transfer experiments in RIP-GP mice (19). Toso−/− DCs were unable to induce diabetes, but did not completely abrogate T-cell activation. However, T-cell infiltration into the pancreas was reduced compared with mice that received WT DCs. These data strongly support a role for Toso in regulating the proinflammatory capacity of DCs.
Several studies have linked immature DCs to tolerance induction (15, 17, 31–33), and an interesting recent report has shown that the main physiological function of WT DCs during EAE induction is to induce naïve T cells to differentiate into Tregs (34). Accordingly, depletion of WT DCs in this model did not ameliorate EAE but instead exacerbated disease progression due to Treg deficiency (34). These results implied that although WT DCs can still prime inflammatory T cells, their main function is to induce Tregs and thereby control the balance between inflammation and tolerance. In this light, it will be interesting to define the molecules that regulate the tolerogenic function of WT DCs. In our study, we characterized Toso as a proinflammatory protein, and showed that loss of Toso induces a tolerogenic phenotype in DCs. Toso−/− DCs can both restrain the expansion of inflammatory T cells and induce Tregs in vitro and in vivo during EAE induction. Toso had no influence on the T-cell intrinsic differentiation process to Tregs, nor did Toso-deficient Tregs exhibit an altered activity compared with controls. We suggest that Treg induction in Toso−/− mice during EAE is solely dependent on DCs. We also demonstrated that Toso−/− DCs express increased amounts of PD-L1 on the cell surface compared with WT DCs. PD-L1 interactions have been shown to suppress autoreactive T cells, and are implicated in the induction of FoxP3-expressing induced Tregs (iTregs) (20, 35, 36). The up-regulation of PD-L1 on Toso−/− DCs might explain the higher numbers of iTregs found in Toso−/− mice upon EAE induction. Interestingly, the numbers of nTregs were not altered by Toso deficiency, which implies that Toso just restrains iTregs in response to an inflammatory stimulus.
Based on our finding that Toso has a crucial function in immune tolerance, we developed a preclinical strategy for treating autoimmune disease. Our recombinant Toso-Fc fusion protein successfully interfered with EAE induction in mice. Most importantly, Toso-Fc stopped disease progression in animals that were already symptomatic. Treatment strategies based on receptor-Fc fusion proteins have proven to be powerful alternatives to immunosuppressive drugs in the clinic (21). Conceptually, a therapeutic receptor-Fc fusion protein competes with the inflammatory receptor for binding to the natural ligand, preventing the initiation of inflammatory signaling. The success of this approach depends on creating a receptor-Fc fusion protein that has high affinity for the natural ligand and can accumulate to high concentrations in a patient’s blood. Several therapeutic receptor-Fc fusion proteins, including Etanercept (TNFR-Fc), Alefacept (LFA3-Fc), Rilonacept (IL1R-Fc), and Abatacept (CTLA4-Fc), are on the market, and many more have entered clinical trials (22, 37–39). Around the globe, patients suffering from inflammatory autoimmune diseases such as MS, rheumatoid arthritis, psoriasis, and gout have benefited significantly from therapeutics based on receptor-Fc fusion proteins. Our preclinical experiments with our Toso-Fc fusion protein are only the first step in the development of a such targeted therapeutic. Many open questions remain, not the least of which is the nature of the Toso ligand. Future studies will be needed to unravel the molecular mechanism underlying Toso’s function in the inflammation–tolerance balance. It is also evident that, based on Toso’s diverse roles, the generation of conditional Toso-deficient mutants will be an asset.
In summary, we have demonstrated that Toso has an important proinflammatory function that is rooted in its effects on DCs. Toso−/− mice show a significantly decreased EAE disease burden, and Toso−/− DCs are tolerogenic and weak stimulators of inflammatory T-cell responses. Importantly, we have developed an effective preclinical treatment strategy that reduced EAE disease progression in mice. Our results set the stage for potential therapeutic strategies aimed at mitigating autoimmune inflammation in humans.
Materials and Methods
Mice.
Toso−/− and RIP-GP mice, as well as P14 transgenic strains, were bred in-house and were described earlier (12, 19, 40). 2D2 and C57/BL6 mice were from The Jackson Laboratory.
EAE Induction.
Mice were s.c. immunized with MOG 35–55 peptide, and clinical signs of EAE were monitored daily as described previously (41). For treatment with Toso-Fc fusion protein during EAE induction, mice were treated i.p. with 50 µg Toso-Fc (additional information is available in SI Materials and Methods) or PBS (vehicle control) every other day.
Histology and Immunohistochemistry.
Brains were obtained from mice at 30 d post-MOG injection. Brains and pancreas were fixed, processed, and stained as previously described (41). For immunohistochemical staining, the following antibodies and dilutions were used: anti-CD3 (Dako; 1:200) and anti-Mac3 (BD Pharmingen; 1:100).
Lymphocyte Isolation and CD4+ T-Helper Cell Purification and Differentiation.
Lymphocytes from brain tissue and Th cells from spleen and lymph nodes were isolated according to standard protocols. Naïve CD4+CD26Lhigh T cells were purified and induced to differentiate into Th1, Th17, or Treg cells with subset-specific cytokine mixtures as described previously (41).
For DC-dependent Treg skewing experiments, DCs were pulsed for 3 h with 1 μg/mL lymphocytic choriomeningitis virus (LCMV) peptide gp61–80 (GLNGPDIYKGVYQFKSVEFD), washed twice with PBS, and cocultured with WT CD4+ Smarta T cells (10 T-cells:1 DC) in the presence of TGF-β (10 ng/mL), IL-2 (10 ng/mL), and anti–IFN-γ Ab (10 μg/mL) (BioLegend). Cells were stained 72 h later for CD4 and FoxP3 using the FoxP3 Staining Buffer Set (eBioscience) and analyzed using a Canto II (BD Biosciences).
Cell-Proliferation Assay.
Whole spleens were homogenized to generate single-cell suspensions, and erythrocytes were lysed using a red blood cell lysis buffer (Sigma). Splenocytes were washed in PBS, resuspended at a concentration of 1 × 106 cells per mL, and pulsed with 0.1–100 µg/mL MOG peptide. CD4+ T cells were purified by standard protocols and stimulated with plate-bound anti-CD3 antibodies at the indicated concentration. Splenocytes or T cells were incubated for 48 h and pulsed for the last 8 h with [3H]thymidine (1 μCi per well). Incorporation of [3H]thymidine was measured with a Matrix 96 Direct β Counter System (Canberra Packard).
Cytokine Production.
Cytokines were analyzed by intracellular staining. T cells were isolated directly from mouse brain, spinal cord, or spleen or harvested after culture in vitro. Cells were analyzed by flow cytometry as described previously (41).
BMDC Differentiation, Antigen-Specific Proliferation, and Treg Suppression Assay.
DCs were induced to differentiate from bone marrow stem cells by culture for 10 d in RPMI containing 40 ng/mL GM-CSF (PeproTech). On day 10, cells were harvested, washed twice in PBS, and stimulated for 16 h with 10 ng/mL LPS (Sigma), 10 ng/mL R848, or 10 nM CpG. DCs were then stained as described above or pulsed for 3 h with 1 µM MOG peptide, washed twice with PBS, and cocultured with WT CD4+ 2D2 T cells that had been labeled with 2.5 µM CellTrace Violet (VCT) (Invitrogen) according to the manufacturer’s instructions. Proliferation was measured 72 h later using a Canto II plate reader (BD Biosciences) and analyzed using FlowJo 7.5 software (Tree Star). For Treg suppression assays, we used 1 × 104 activated and MOG-pulsed WT BMDCs. In vitro differentiated iTregs (1 × 105) were coincubated with 1 × 105 VCT-labeled 2D2 CD4+ T cells. Proliferation was analyzed after 72 h by flow cytometry.
Surface Marker Staining.
BMDCs were cultured alone or in combination with T cells as described above. Cells were stained by standard protocols with the following antibodies (BioLegend): anti-CD25, anti-CD4, anti-MHCII, anti-CD80, anti-CD86, anti-ICOS, and anti–PD-L1.
BMDC Transfer into RIP-GP Mice.
BMDCs were generated as described above, activated with 10 ng/mL LPS for 20 h, and pulsed for 16 h with 1 μM GP33, GP276, and GP61 peptides. Two million cells were i.v. injected per RIP-GP mouse. Blood glucose levels were determined every other day in tail blood using Accu-Chek III glucometers and Chemstrips (Roche).
Statistical Methods.
Where appropriate, all differences were evaluated using the two-tailed Student t test as calculated using Prism 5.0 software (GraphPad). Data are presented as the mean ± SEM. Differences with P values ≤0.05 were considered significant.
Study Approval.
All mouse procedures were approved by the University Health Network Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank all T.W.M. laboratory members for their support, M. Saunders and J. Thomsen for insightful scientific editing, and F. Wu and W. Zhou for technical assistance. This work was supported by grants by the Canadian Institutes of Health Research (to T.W.M.). A.B. was and D.B. currently is supported by a postdoctoral fellowship from the German Research Foundation, and D.B. was supported by Feodor-Lynen fellowships from The Alexander von Humboldt Foundation. P.A.L. is a Sofja Kovalevskaja Fellow of The Alexander von Humboldt Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323166111/-/DCSupplemental.
References
- 1.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 2.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Hitoshi Y, et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8(4):461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 4.Pallasch CP, et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood. 2008;112(10):4213–4219. doi: 10.1182/blood-2008-05-157255. [DOI] [PubMed] [Google Scholar]
- 5.Proto-Siqueira R, et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood. 2008;112(2):394–397. doi: 10.1182/blood-2007-11-124065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen XH, et al. Antiapoptotic function of Toso (Faim3) in death receptor signaling. Blood. 2012;119(7):1790–1791. doi: 10.1182/blood-2011-11-386839. [DOI] [PubMed] [Google Scholar]
- 7.Shima H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22(3):149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 8.Kubagawa H, et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med. 2009;206(12):2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouchida R, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci USA. 2012;109(40):E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SC, et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J Immunol. 2013;190(3):987–996. doi: 10.4049/jimmunol.1202227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigruener A, et al. E-LDL upregulates TOSO expression and enhances the survival of human macrophages. Biochem Biophys Res Commun. 2007;359(3):723–728. doi: 10.1016/j.bbrc.2007.05.169. [DOI] [PubMed] [Google Scholar]
- 12.Lang KS, et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA. 2013;110(7):2593–2598. doi: 10.1073/pnas.1222264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang KS, et al. Reply to Honjo et al.: Functional relevant expression of Toso on granulocytes. Proc Natl Acad Sci USA. 2013;110(28):E2542–E2543. doi: 10.1073/pnas.1306422110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172(4):1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13(8):566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192(9):1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garza KM, et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191(11):2021–2027. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dissanayake D, et al. Nuclear factor-κB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nat Med. 2011;17(12):1663–1667. doi: 10.1038/nm.2556. [DOI] [PubMed] [Google Scholar]
- 20.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C. Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology. Curr Opin Biotechnol. 2009;20(6):692–699. doi: 10.1016/j.copbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174(6):1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducharme E, Weinberg JM. Etanercept. Expert Opin Biol Ther. 2008;8(4):491–502. doi: 10.1517/14712598.8.4.491. [DOI] [PubMed] [Google Scholar]
- 24.Kantarci O, et al. Turkish Multiple Sclerosis Study Group (TUMSSG) Survival and predictors of disability in Turkish MS patients. Neurology. 1998;51(3):765–772. doi: 10.1212/wnl.51.3.765. [DOI] [PubMed] [Google Scholar]
- 25.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croxford AL, Kurschus FC, Waisman A. Mouse models for multiple sclerosis: Historical facts and future implications. Biochim Biophys Acta. 2011;1812(2):177–183. doi: 10.1016/j.bbadis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 30.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 31.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002;23(9):445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 32.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6(12):1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki S, et al. CD8+CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181(10):6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yogev N, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37(2):264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6(3):280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 36.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strober BE, Menon K. Alefacept for the treatment of psoriasis and other dermatologic diseases. Dermatol Ther. 2007;20(4):270–276. doi: 10.1111/j.1529-8019.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman HM, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: Results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58(8):2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 39.Posadas A, Lisse J, Sarkar S. Abatacept in the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2009;5(1):9–17. doi: 10.1586/1744666X.5.1.9. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi PS, et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 41.Brüstle A, et al. The NF-κB regulator MALT1 determines the encephalitogenic potential of Th17 cells. J Clin Invest. 2012;122(12):4698–4709. doi: 10.1172/JCI63528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.