Significance
A mechanistic understanding of how calcium phosphate (CaP) minerals contribute to osteogenic commitment of stem cells and bone tissue formation is a necessary requirement for developing efficient CaP-based synthetic matrices to treat bone defects. This study unravels a previously unknown mechanism, phosphate-ATP-adenosine metabolic signaling, by which the CaP-rich mineral environment in bone tissues promotes osteogenic differentiation of human mesenchymal stem cells. In addition to a mechanical perspective on how biomaterials can influence stem cell differentiation through metabolic pathways, this discovery opens up new avenues for treating critical bone defects and bone metabolic disorders.
Keywords: bone metabolism, mineralized matrix, biomimetic material, phosphate signaling
Abstract
Synthetic matrices emulating the physicochemical properties of tissue-specific ECMs are being developed at a rapid pace to regulate stem cell fate. Biomaterials containing calcium phosphate (CaP) moieties have been shown to support osteogenic differentiation of stem and progenitor cells and bone tissue formation. By using a mineralized synthetic matrix mimicking a CaP-rich bone microenvironment, we examine a molecular mechanism through which CaP minerals induce osteogenesis of human mesenchymal stem cells with an emphasis on phosphate metabolism. Our studies show that extracellular phosphate uptake through solute carrier family 20 (phosphate transporter), member 1 (SLC20a1) supports osteogenic differentiation of human mesenchymal stem cells via adenosine, an ATP metabolite, which acts as an autocrine/paracrine signaling molecule through A2b adenosine receptor. Perturbation of SLC20a1 abrogates osteogenic differentiation by decreasing intramitochondrial phosphate and ATP synthesis. Collectively, this study offers the demonstration of a previously unknown mechanism for the beneficial role of CaP biomaterials in bone repair and the role of phosphate ions in bone physiology and regeneration. These findings also begin to shed light on the role of ATP metabolism in bone homeostasis, which may be exploited to treat bone metabolic diseases.
Harnessing the ability of adult stem cells to differentiate and contribute to tissue repair has enormous potential for wound healing, tissue regeneration, and restoration of organ functionality. However, controlling the fate of transplanted and/or endogenous progenitor cells to treat compromised tissues and organs remains a significant challenge (1, 2). Studies have shown that biomaterials recapitulating various physicochemical cues of the native tissue can be used to direct stem cell differentiation (3–9). Biomaterials-assisted transplantation of stem cells provides a promising approach to deliver cells to the targeted site and direct their differentiation to functional tissues. We and others have shown that biomaterials containing calcium phosphate (CaP) moieties, a major constituent of native bone tissue, can promote osteogenic differentiation of progenitor and stem cells and can facilitate in vivo bone tissue formation (10–20). However, to use CaP biomaterials efficiently for bone tissue repair, it is of paramount importance to understand the molecular mechanisms underlying the osteogenicity (osteogenic differentiation of progenitor cells in the absence of any exogenous chemical or biological osteogenic-inducing factors) and osteoinductivity (de novo bone growth in vivo even in locations where there is no vital bone) of a CaP mineral environment.
The osteogenicity and osteoinductivity of CaP minerals have been attributed to different factors, such as the ability of CaP to modulate extracellular calcium (Ca2+) and phosphate  ions and the adsorption and release of osteoinductive growth factors like bone morphogenic proteins (BMPs) (18, 21–24). This is further supported by findings that exposure of osteoblasts and progenitor cells to Ca2+- or
ions and the adsorption and release of osteoinductive growth factors like bone morphogenic proteins (BMPs) (18, 21–24). This is further supported by findings that exposure of osteoblasts and progenitor cells to Ca2+- or 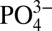 -rich medium promotes their osteogenic differentiation (25–27). Additionally, it has been shown that among various CaP materials, the ones that dissociate easily to Ca2+ and
-rich medium promotes their osteogenic differentiation (25–27). Additionally, it has been shown that among various CaP materials, the ones that dissociate easily to Ca2+ and  contribute to better bone healing (13, 21). Despite the large number of studies demonstrating the potential role of CaP minerals and Ca2+ and
contribute to better bone healing (13, 21). Despite the large number of studies demonstrating the potential role of CaP minerals and Ca2+ and 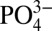 on osteogenic differentiation of osteoblasts and progenitor cells, the molecular mechanism through which these ions regulate osteogenic commitment of stem cells remains largely unknown. Recent studies have shown that influx of extracellular Ca2+ through L-type calcium channels promotes osteogenic differentiation of osteoprogenitor cells (28). However, very little is known about the mechanism through which
on osteogenic differentiation of osteoblasts and progenitor cells, the molecular mechanism through which these ions regulate osteogenic commitment of stem cells remains largely unknown. Recent studies have shown that influx of extracellular Ca2+ through L-type calcium channels promotes osteogenic differentiation of osteoprogenitor cells (28). However, very little is known about the mechanism through which  supports osteogenesis. During skeletal growth and bone remodeling,
supports osteogenesis. During skeletal growth and bone remodeling, 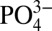 plays an important role in apatite formation (29, 30). In addition to osteoblasts and progenitor cells, studies have shown that exposure to
plays an important role in apatite formation (29, 30). In addition to osteoblasts and progenitor cells, studies have shown that exposure to  alters the cell phenotype of nonskeletal tissues, such as human vascular smooth muscle cells, into osteogenic-like cells (31, 32). Central to phosphate metabolism is solute carrier family 20 (phosphate transporter), member 1 (SLC20a1, or PiT-1), a sodium-phosphate symporter that transports
alters the cell phenotype of nonskeletal tissues, such as human vascular smooth muscle cells, into osteogenic-like cells (31, 32). Central to phosphate metabolism is solute carrier family 20 (phosphate transporter), member 1 (SLC20a1, or PiT-1), a sodium-phosphate symporter that transports  ions from the extracellular milieu into the cytoplasm and plays a key role in mineralization of both vascular smooth muscle cells and osteoblasts (33, 34).
ions from the extracellular milieu into the cytoplasm and plays a key role in mineralization of both vascular smooth muscle cells and osteoblasts (33, 34).
Here, we unravel a previously unknown mechanism, centered on phosphate metabolism, through which the CaP-rich mineral environment promotes osteogenic differentiation of human mesenchymal stem cells (hMSCs) by using an engineered matrix containing CaP moieties. Our studies show that the extracellular 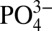 plays an important role in promoting osteogenic differentiation of hMSCs by regulating intramitochondrial phosphate content and ATP synthesis. ATP is then secreted and metabolized into adenosine, which promotes osteogenic differentiation of hMSCs via A2b adenosine receptors.
plays an important role in promoting osteogenic differentiation of hMSCs by regulating intramitochondrial phosphate content and ATP synthesis. ATP is then secreted and metabolized into adenosine, which promotes osteogenic differentiation of hMSCs via A2b adenosine receptors.
Results
Biomineralized Matrix-Induced Osteogenic Differentiation of Stem Cells Uses SLC20a1.
Recently, we developed a mineralized matrix containing CaP minerals by using the principles of biomineralization (35). This mineralized matrix recapitulates different static and dynamic physicochemical cues of native bone ECM, including its composite structure, CaP-rich environment, and dynamic dissolution/formation of matrix-bound CaP minerals (which establish equilibrium with the surrounding milieu) (11, 35). This mineralized matrix possesses osteogenicity, osteoconductivity, and osteoinductivity (10, 11). Analyses of the mineralized matrix with SEM showed the presence of irregularly shaped spherulites (Fig. S1A, Right). Elemental analysis revealed that these minerals are mainly CaP with a Ca/P ratio of ∼1.43; this is close to the Ca/P ratio observed in other bioactive ceramics, such as β-tricalcium phosphate (1.5) and hydroxyapatite (1.67) (36). No such minerals were observed in corresponding nonmineralized matrices (Fig. S1A, Left). The presence of CaP minerals in mineralized matrices was further confirmed by X-ray diffraction analyses, which demonstrate peaks (20° ≈ 26°, 31°) corresponding to the diffraction spacing present in hydroxyapatite [PDF-4-010-6312, based on PDF4+ (International Centre for Diffraction Data)] (Fig. S1B). Measurement of Ca2+ and  contents of the mineralized hydrogels indicated that they contain 70.1 ± 1.9 mg and 105.8 ± 3.98 mg of Ca2+ and
contents of the mineralized hydrogels indicated that they contain 70.1 ± 1.9 mg and 105.8 ± 3.98 mg of Ca2+ and  per dry weight, respectively (Fig. S2A). As expected the CaP components of the mineralized matrix underwent dissolution to Ca2+ and
per dry weight, respectively (Fig. S2A). As expected the CaP components of the mineralized matrix underwent dissolution to Ca2+ and  when exposed to a medium devoid of these ions (Fig. S2B).
when exposed to a medium devoid of these ions (Fig. S2B).
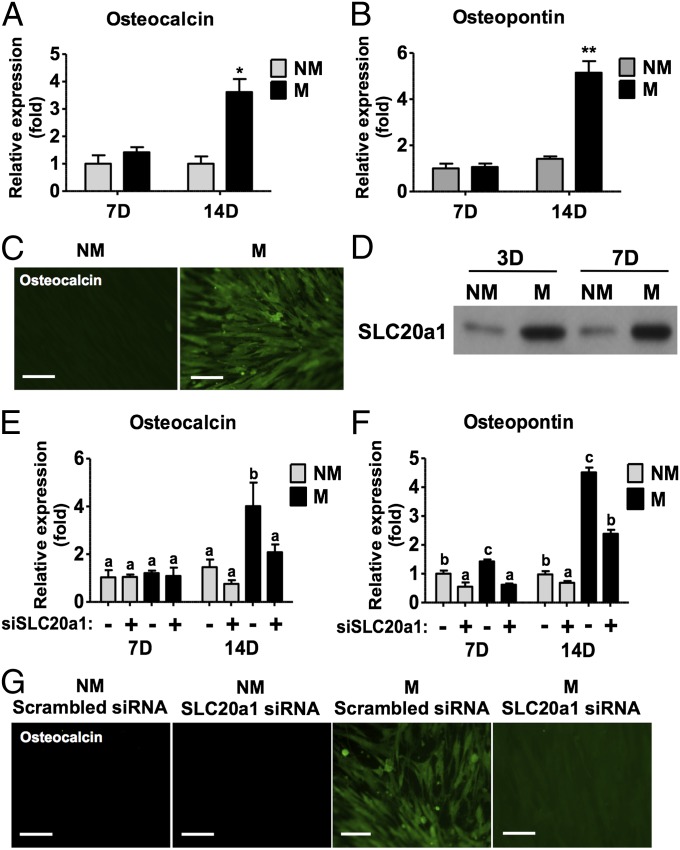
The hMSCs cultured on these mineralized matrices in growth medium, lacking any osteogenic-inducing soluble factors, consistently up-regulated the osteogenic markers, osteopontin (OPN) and osteocalcin (OCN) (Fig. 1 A–C). In addition to osteogenic markers, hMSCs cultured on these matrices showed up-regulation of sodium-phosphate symporter SLC20a1 (Fig. 1D and Fig. S3A). Interestingly, knockdown of SLC20a1 with siRNA (Fig. S3B) resulted in the down-regulation of OCN and OPN gene expression (Fig. 1 E and F) and decreased immunofluorescent staining for OCN (Fig. 1G).
Fig. 1.
Mineralized matrix containing CaP minerals promotes osteogenic differentiation of hMSCs. OCN (A) and OPN (B) gene expression after 7 d (7d) and 14 d (14d) of culture on mineralized (M) and nonmineralized (NM) matrices. (C) OCN immunofluorescent staining (green) after 14 d of culture on M and NM matrices. (D) SLC20a1 protein expression after 3 and 7 d of culture. OCN (E) and OPN (F) gene expression after 7 and 14 d of culture with and without SLC20a1 knockdown. (G) OCN immunofluorescent staining (green) after 14 d of culture. The plus symbol (+) denotes SLC20a1 siRNA, and the minus symbol (−) denotes corresponding scrambled siRNA. (Scale bars: 100 μm.) Data are represented as the mean ± SD (Student t test or one-way ANOVA followed by Bonferroni post hoc test; *P < 0.05; **P < 0.01). Groups with different letters (a–c) are significant, P < 0.05; n = 3.
Inorganic Phosphate-Regulated Osteogenic Differentiation of hMSCs Uses SLC20a1.
Because SLC20a1 transports  and the biomineralized matrix contributes to the extracellular
and the biomineralized matrix contributes to the extracellular  , the role of extracellular
, the role of extracellular  on osteogenic commitment of hMSCs was further validated by culturing them in medium supplemented with varying amounts of
on osteogenic commitment of hMSCs was further validated by culturing them in medium supplemented with varying amounts of  . Similar to mineralized matrices, the hMSCs cultured in high
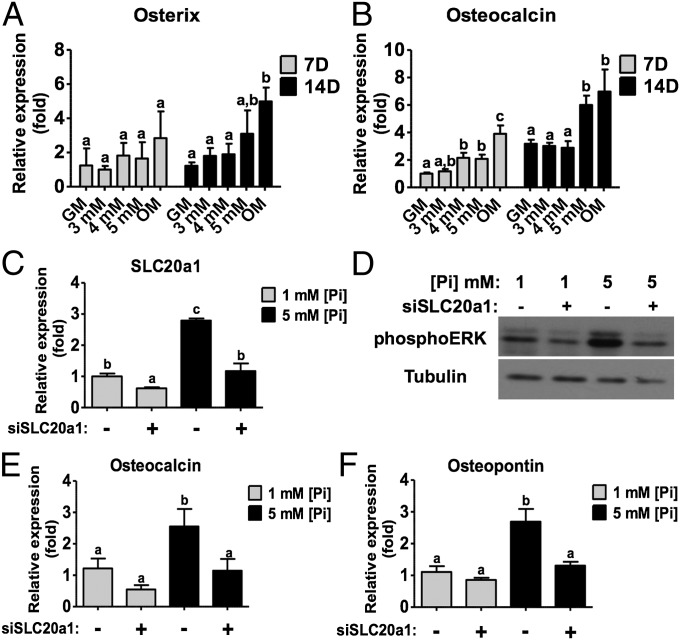
. Similar to mineralized matrices, the hMSCs cultured in high  (5 mM) medium showed up-regulation of various osteogenic markers, such as osterix, OCN and type I collagen, compared with control cultures (Fig. 2 A and B and Fig. S3C). The gene expression of SLC20a1 was up-regulated in hMSCs cultured in 5 mM
(5 mM) medium showed up-regulation of various osteogenic markers, such as osterix, OCN and type I collagen, compared with control cultures (Fig. 2 A and B and Fig. S3C). The gene expression of SLC20a1 was up-regulated in hMSCs cultured in 5 mM  medium and down-regulated upon SLC20a1 knockdown (Fig. 2C). Western blot analysis and image quantification demonstrated that SLC20a1 knockdown down-regulated extracellular signal-regulated kinases 1/2 (ERK1/2) activity, an important mitogen-activated protein kinase involved in osteogenic commitment during phosphate induction (Fig. 2D and Fig. S3D). Akin to mineralized matrices, the knockdown of SLC20a1 annulled the
medium and down-regulated upon SLC20a1 knockdown (Fig. 2C). Western blot analysis and image quantification demonstrated that SLC20a1 knockdown down-regulated extracellular signal-regulated kinases 1/2 (ERK1/2) activity, an important mitogen-activated protein kinase involved in osteogenic commitment during phosphate induction (Fig. 2D and Fig. S3D). Akin to mineralized matrices, the knockdown of SLC20a1 annulled the  -mediated osteogenesis of hMSCs (Fig. 2 E and F).
-mediated osteogenesis of hMSCs (Fig. 2 E and F).
Fig. 2.
Elevated levels of inorganic phosphate in culture medium promote osteogenic differentiation of hMSCs through SLC20a1. Gene expressions of osterix (A) and OCN (B) after 7 d and 14 d of culture in growth medium containing varying amounts of  ions. (C) SLC20a1 gene expression after 7 d of culture in normal (control; 1 mM) and high-phosphate (5 mM) medium with and without SLC20a1 knockdown. (D) Activation of ERK1/2 kinase after 1 d of SLC20a1 knockdown. OCN (E) and OPN (F) gene expression after 14 d of culture in low- and high-phosphate medium with and without SLC20a1 knockdown. GM, growth medium (1 mM
ions. (C) SLC20a1 gene expression after 7 d of culture in normal (control; 1 mM) and high-phosphate (5 mM) medium with and without SLC20a1 knockdown. (D) Activation of ERK1/2 kinase after 1 d of SLC20a1 knockdown. OCN (E) and OPN (F) gene expression after 14 d of culture in low- and high-phosphate medium with and without SLC20a1 knockdown. GM, growth medium (1 mM  ); OM, osteogenic medium; [Pi], concentration of
); OM, osteogenic medium; [Pi], concentration of  ; 3 mM, 3 mM
; 3 mM, 3 mM  ; 4 mM, 4 mM
; 4 mM, 4 mM  ; 5 mM, 5 mM
; 5 mM, 5 mM  . The plus (+) symbol denotes SLC20a1 siRNA and the minus (−) symbol denotes scrambled siRNA. Data are represented as the mean ± SD (one-way ANOVA, followed by Bonferroni post hoc test). Groups with different letters (a–c) are significant, P < 0.05; n = 3.
. The plus (+) symbol denotes SLC20a1 siRNA and the minus (−) symbol denotes scrambled siRNA. Data are represented as the mean ± SD (one-way ANOVA, followed by Bonferroni post hoc test). Groups with different letters (a–c) are significant, P < 0.05; n = 3.
Increase of Intracellular ATP on Mineralized Matrices Is Dependent on SLC20a1.
We observed a significant increase in intracellular  of hMSCs cultured on mineralized matrices as measured by the phosphate assay, but this increase was attenuated upon the knockdown of SLC20a1 (Fig. S4A). In addition to intracellular phosphate, intramitochondrial phosphate was increased on mineralized matrices but was down-regulated after partial loss of SLC20a1 (Fig. 3A). Because an obvious function of inorganic
of hMSCs cultured on mineralized matrices as measured by the phosphate assay, but this increase was attenuated upon the knockdown of SLC20a1 (Fig. S4A). In addition to intracellular phosphate, intramitochondrial phosphate was increased on mineralized matrices but was down-regulated after partial loss of SLC20a1 (Fig. 3A). Because an obvious function of inorganic  is to act as a substrate for ATP synthesis in the electron transport chain of the mitochondria, we next examined ATP production. A significant increase in intracellular ATP was observed for cells cultured on mineralized matrices, as evidenced by a luminescent assay, and this increase was abrogated with the partial loss of SLC20a1 (Fig. 3B). This finding was corroborated by the increase of fluorescence intensity of quinacrine staining for intravesicular ATP on mineralized matrices and the decrease in intensity upon SLC20a1 knockdown (Fig. 3C). To examine further whether
is to act as a substrate for ATP synthesis in the electron transport chain of the mitochondria, we next examined ATP production. A significant increase in intracellular ATP was observed for cells cultured on mineralized matrices, as evidenced by a luminescent assay, and this increase was abrogated with the partial loss of SLC20a1 (Fig. 3B). This finding was corroborated by the increase of fluorescence intensity of quinacrine staining for intravesicular ATP on mineralized matrices and the decrease in intensity upon SLC20a1 knockdown (Fig. 3C). To examine further whether  is directly involved in ATP synthesis, we cultured hMSCs in medium containing 5 mM
is directly involved in ATP synthesis, we cultured hMSCs in medium containing 5 mM  . Similar to cells on mineralized matrices, hMSCs cultured in 5 mM
. Similar to cells on mineralized matrices, hMSCs cultured in 5 mM  medium showed higher levels of intracellular and intramitochondrial phosphate compared with those cultured in 1 mM
medium showed higher levels of intracellular and intramitochondrial phosphate compared with those cultured in 1 mM 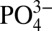 medium; this exogenous
medium; this exogenous  -assisted up-regulation was found to decrease upon SLC20a1 knockdown (Fig. 3D and Fig. S4B). Measurement of intracellular ATP by luminescent assay displayed a similar trend, where ATP levels increased in 5 mM
-assisted up-regulation was found to decrease upon SLC20a1 knockdown (Fig. 3D and Fig. S4B). Measurement of intracellular ATP by luminescent assay displayed a similar trend, where ATP levels increased in 5 mM  medium but were abolished with SLC20a1 knockdown (Fig. 3E). Additionally, quinacrine staining for intravesicular ATP demonstrated an increase in ATP fluorescent signals for hMSCs cultured in 5 mM
medium but were abolished with SLC20a1 knockdown (Fig. 3E). Additionally, quinacrine staining for intravesicular ATP demonstrated an increase in ATP fluorescent signals for hMSCs cultured in 5 mM 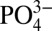 medium, which diminished after SLC20a1 knockdown (Fig. 3F).
medium, which diminished after SLC20a1 knockdown (Fig. 3F).
Fig. 3.
Mineralized matrices regulate intracellular  and ATP content through SLC20a1. (A–C) hMSCs cultured on M and NM matrices with and without SLC20a1 knockdown. (A) Intramitochondrial
and ATP content through SLC20a1. (A–C) hMSCs cultured on M and NM matrices with and without SLC20a1 knockdown. (A) Intramitochondrial  after 1 d of culture. (B) Intracellular ATP luminescent assay after 4 d of culture. (C) Quinacrine staining for ATP after 4 d of culture. (D–F) hMSCs cultured in normal (control; 1 mM) and high- (5 mM)
after 1 d of culture. (B) Intracellular ATP luminescent assay after 4 d of culture. (C) Quinacrine staining for ATP after 4 d of culture. (D–F) hMSCs cultured in normal (control; 1 mM) and high- (5 mM)  medium with and without SLC20a1 knockdown. (D) Intramitochondrial
medium with and without SLC20a1 knockdown. (D) Intramitochondrial 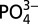 after 1 d of culture. (E) Intracellular ATP luminescent assay after 4 d of culture. (F) Intravesicular ATP staining with quinacrine after 4 d of culture. Pi, phosphate ion. The plus (+) symbol denotes SLC20a1 siRNA and the minus (−) symbol denotes scrambled siRNA Data are represented as the mean ± SD (one-way ANOVA, followed by Bonferroni post hoc test). Groups with different letters (a–c) are significant, P < 0.05; n = 3. (Scale bars: 200 μm.)
after 1 d of culture. (E) Intracellular ATP luminescent assay after 4 d of culture. (F) Intravesicular ATP staining with quinacrine after 4 d of culture. Pi, phosphate ion. The plus (+) symbol denotes SLC20a1 siRNA and the minus (−) symbol denotes scrambled siRNA Data are represented as the mean ± SD (one-way ANOVA, followed by Bonferroni post hoc test). Groups with different letters (a–c) are significant, P < 0.05; n = 3. (Scale bars: 200 μm.)
Mineralized Matrices Promote Osteogenic Differentiation Through A2b Adenosine Receptor.
The function of ATP as a signaling molecule, in addition to being an energy source, has long been established (37). ATP can mediate osteogenic signaling through purinergic receptors (38, 39). To determine the role of extracellular ATP on osteogenic differentiation, we inhibited the transport of ATP to the extracellular milieu with the vesicular transport inhibitor N-ethyl maleimide (NEM). The addition of NEM significantly abrogated OCN and OPN gene expression (Fig. 4 A and B) and decreased OCN immunofluorescent intensity (Fig. 4C) on mineralized matrices, suggesting that inhibition of ATP transport negatively affects mineralized matrix-assisted osteogenesis of hMSCs. However, pharmacological inhibition of purinergic receptors with suramin did not abrogate osteogenic differentiation of hMSCs on mineralized matrices, as shown by OCN staining (Fig. S5A). Additionally, we were unable to detect any significant amount of ATP in the culture medium at a measurable threshold of 100 ng/mL (Fig. S5B). On the contrary, HPLC measurements showed a significant amount of adenosine in cell cultures involving mineralized matrices, and this presence of extracellular adenosine was abrogated with SLC20a1 knockdown (Fig. 4D). To validate the role of adenosine in the mineralized matrix-mediated osteogenesis of hMSCs further, we examined the role of two likely candidates of adenosine signaling: A1 and A2b adenosine receptors. Specific pharmacological inhibition of A1 and A2b adenosine receptors by 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) and 8-[4-[4-(4-Chlorophenzyl)piperazide -1-sulfonyl)phenyl]]-1-propylxanthine (PSB603) inhibitors, respectively, demonstrated that the PSB603 down-regulated the increase in OCN and OPN gene expression on mineralized matrices, whereas DPCPX had no effect (Fig. 4 E and F). The decrease of OCN in presence of PSB603 was also demonstrated by immunofluorescent staining for OCN (Fig. 4G).
Fig. 4.
Mineralized matrix-mediated osteogenic differentiation through A2b adenosine receptor. OCN (A) and OPN (B) gene expression of hMSCs after 3 wk of culture on NM matrices, M matrices, and M matrices with vesicle transport inhibitor NEM. The plus (+) and minus (−) symbols denote the presence and absence of NEM, respectively. (C) Immunofluorescent staining for OCN after 3 wk of culture on NM, M, and M in the presence of NEM. OCN (green) and nuclei (blue). CTL, control. (Scale bars: 200 μm.) (D) HPLC measurement of adenosine in culture medium after 7 d. The plus (+) symbol denotes SLC20a1 siRNA, and the minus (−) symbol denotes scrambled siRNA. OCN (E) and OPN (F) gene expressions of hMSCs cultured on M and NM for 3 wk with (+) and without (−) the presence of adenosine, A2b receptor antagonist (PSB603), or A1 receptor antagonist (DPCPX). (G) Immunofluorescent staining of OCN after 3 wk of culture on NM and M for 3 wk in the presence and absence of adenosine, PSB603, or DPCPX. OCN (green) and nuclei (blue). (Scale bars: 100 μm.) Data are represented as the mean ± SD (one-way ANOVA, followed by Bonferroni post hoc test). Groups with different letters (a–c) are significant, P < 0.05; n = 3.
We also examined the effect of adenosine on osteogenic differentiation of hMSCs by culturing the cells on nonmineralized matrices in growth medium containing exogenous adenosine. We chose nonmineralized matrices because they do not support osteogenic differentiation of hMSCs in growth medium despite having similar chemical composition of the polymer network, except for the CaP moieties (Fig. 1 A–C). Supplementation of adenosine in growth medium promoted osteogenic differentiation of hMSCs on nonmineralized matrices, as evidenced by the up-regulation of OCN and OPN (Fig. 4 E–G). Furthermore, the exogenous adenosine-mediated osteogenic differentiation of hMSCs was abrogated in the presence of PSB603 but not in the presence of DPCPX, akin to mineralized matrices, thus corroborating the role of extracellular adenosine as a signaling molecule.
Discussion
A number of studies have shown that CaP biomaterials like bioactive glasses, ceramics, and mineralized matrices promote bone healing (13–15). In addition to the physical cues provided, studies have shown that the dissolution kinetics and the ability of the CaP minerals to undergo dissolution/formation modulating the extracellular mineral environment play a key role in osteogenic functions of CaP materials (11, 18, 21). In this study, by using a mineralized matrix, we investigated the metabolic mechanisms by which the CaP-rich microenvironment contributes to osteogenic commitment of hMSCs. We chose nondegradable matrix because it allows us to eliminate the interference of matrix degradation on osteogenic differentiation, which has previously been shown to play a role in bone tissue formation (3, 40).
The finding that osteogenic differentiation of hMSCs via mineralized matrix and high  medium can be negated through SLC20a1 knockdown suggests that
medium can be negated through SLC20a1 knockdown suggests that  content in the extracellular milieu, and its transport through SLC20a1, is an important mediator of mineralized matrix-induced osteogenic differentiation of hMSCs. However, a fundamental question remains as to how
content in the extracellular milieu, and its transport through SLC20a1, is an important mediator of mineralized matrix-induced osteogenic differentiation of hMSCs. However, a fundamental question remains as to how 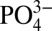 from the extracellular milieu promotes the osteogenic phenotype of hMSCs. Phosphate serves as the primary substrate for the F1F0-ATPase production of ATP in the mitochondria, regulates the production of mitochondrial ATP through activation of mitochondrial NADH, and improves the distribution of energy between cyto-b and cyto-c (41, 42). The increases in intracellular and intramitochondrial
from the extracellular milieu promotes the osteogenic phenotype of hMSCs. Phosphate serves as the primary substrate for the F1F0-ATPase production of ATP in the mitochondria, regulates the production of mitochondrial ATP through activation of mitochondrial NADH, and improves the distribution of energy between cyto-b and cyto-c (41, 42). The increases in intracellular and intramitochondrial  provide an explanation for the observed higher ATP synthesis in hMSCs cultured on mineralized matrices or in high
provide an explanation for the observed higher ATP synthesis in hMSCs cultured on mineralized matrices or in high  medium. This is in accordance with studies showing that inhibition of SLC20a1 and ATP synthesis disturbed endochondral ossification and suppressed mineralization in conjunction with reduced
medium. This is in accordance with studies showing that inhibition of SLC20a1 and ATP synthesis disturbed endochondral ossification and suppressed mineralization in conjunction with reduced  uptake in chondrocytes (43) and that ATP production affects the osteogenic commitment of hMSCs (44). The role of extracellular ATP during osteogenic differentiation, however, remains uncertain because ATP, acting through purinergic receptors, has been implicated both in promoting osteogenic differentiation (38, 39) and in inhibiting formation of mineralized nodules (45, 46). The lack of detectable extracellular ATP, together with the fact that pharmacological inhibition of purinergic receptors did not have any significant effect on mineralized matrix-mediated osteogenesis of hMSCs, suggests that the increase in intracellular ATP promotes osteogenic commitment through routes other than extracellular ATP acting on its own.
uptake in chondrocytes (43) and that ATP production affects the osteogenic commitment of hMSCs (44). The role of extracellular ATP during osteogenic differentiation, however, remains uncertain because ATP, acting through purinergic receptors, has been implicated both in promoting osteogenic differentiation (38, 39) and in inhibiting formation of mineralized nodules (45, 46). The lack of detectable extracellular ATP, together with the fact that pharmacological inhibition of purinergic receptors did not have any significant effect on mineralized matrix-mediated osteogenesis of hMSCs, suggests that the increase in intracellular ATP promotes osteogenic commitment through routes other than extracellular ATP acting on its own.
The presence of a significant amount of adenosine, an ATP metabolite, in the extracellular milieu suggests that membrane-bound ectonucleotidases (CD39), such as ectonucleoside triphosphate diphosphohydrolase, ectonucleotide pyrophosphatase/phosphodiesterase, and ecto-5′nucleotidases (CD73), rapidly metabolized ATP to adenosine (47). These findings, in conjunction with the pharmacological inhibition studies, clearly identify the role of adenosine signaling through A2b receptor on mineralized environment-assisted osteogenic differentiation of hMSCs. The role of exogenous adenosine is further corroborated by the findings that hMSCs on nonmineralized matrices undergo osteogenesis in the presence of medium containing adenosine and that this phenomenon is abrogated upon pharmacological inhibition of A2b adenosine receptor. These results are consistent with emerging studies that show the pivotal role of adenosine signaling via A2b adenosine receptor in both in vivo and in vitro bone development and osteogenic differentiation of stem cells (48–50). A recent study by He et al. (51) showed the role of adenosine on bone metabolism in normal humans and patients with multiple myeloma. Furthermore, these authors have shown that osteoblast cells from A2b receptor and CD39-KO mice exhibit diminished osteogenic differentiation.
It is important to note that although the focus of the current study is phosphate metabolism associated with a mineralized environment, it does not refute the beneficial effect of Ca moieties of the CaP minerals. As stated earlier, the CaP moieties of the mineralized matrix undergo dissolution/precipitation responding to the concentration of Ca2+ or  ions in the surrounding environment, thus creating a dynamic environment. This is very similar to exogenous supplementation of Ca2+ or
ions in the surrounding environment, thus creating a dynamic environment. This is very similar to exogenous supplementation of Ca2+ or 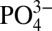 , which leads to CaP precipitation as the ion concentration in the medium increases to establish equilibrium. The importance of such a dynamic mineral environment and the interdependency between the extracellular Ca2+,
, which leads to CaP precipitation as the ion concentration in the medium increases to establish equilibrium. The importance of such a dynamic mineral environment and the interdependency between the extracellular Ca2+, 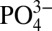 , and CaP was demonstrated in a study by Khoshniat et al. (52), where these authors showed that inhibiting the formation of CaP minerals abrogates the extracellular
, and CaP was demonstrated in a study by Khoshniat et al. (52), where these authors showed that inhibiting the formation of CaP minerals abrogates the extracellular 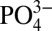 -promoted osteogenesis of osteoblasts even though CaP minerals were not endocytosed. As mentioned earlier, CaP minerals also function as a reservoir for growth factors (22, 23). It is likely that the osteoinductive factors adsorbed onto the mineralized matrices can also contribute to phosphate metabolism. Previous studies have shown that BMP-2–mediated differentiation of MC3T3-E1, preosteoblasts, and their ECM mineralization involves intracellular phosphate uptake, wherein BMP-2 promotes PO34− transport through up-regulation of SLC20a1 (53). Similar findings were also observed in calcification of human vascular smooth muscle cells (54). In addition to BMPs, other osteoinductive molecules (e.g., NEL-like molecule-1) have been shown to promote preosteoblast mineralization through SLC20a1 (55). Conversely, supplementation of PO34− in growth medium has been shown to up-regulate BMP-2 expression of various cells similar to other metal ions, such as Ca2+ and strontium (18, 24, 25, 56).
-promoted osteogenesis of osteoblasts even though CaP minerals were not endocytosed. As mentioned earlier, CaP minerals also function as a reservoir for growth factors (22, 23). It is likely that the osteoinductive factors adsorbed onto the mineralized matrices can also contribute to phosphate metabolism. Previous studies have shown that BMP-2–mediated differentiation of MC3T3-E1, preosteoblasts, and their ECM mineralization involves intracellular phosphate uptake, wherein BMP-2 promotes PO34− transport through up-regulation of SLC20a1 (53). Similar findings were also observed in calcification of human vascular smooth muscle cells (54). In addition to BMPs, other osteoinductive molecules (e.g., NEL-like molecule-1) have been shown to promote preosteoblast mineralization through SLC20a1 (55). Conversely, supplementation of PO34− in growth medium has been shown to up-regulate BMP-2 expression of various cells similar to other metal ions, such as Ca2+ and strontium (18, 24, 25, 56).
Together, the results propose a molecular mechanism, depicted in Fig. 5, in which the dynamic dissolution/precipitation of CaP minerals from the mineralized matrices dictates the concentrations of Ca2+ and 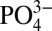 in the extracellular milieu. Extracellular
in the extracellular milieu. Extracellular  enters the cells through SLC20a1 and subsequently into the mitochondria, which serves as a substrate for ATP synthesis. ATP is then secreted and metabolized into adenosine, which subsequently promotes osteogenic differentiation of hMSCs through the A2b adenosine receptor via autocrine and/or paracrine signaling. The active function of
enters the cells through SLC20a1 and subsequently into the mitochondria, which serves as a substrate for ATP synthesis. ATP is then secreted and metabolized into adenosine, which subsequently promotes osteogenic differentiation of hMSCs through the A2b adenosine receptor via autocrine and/or paracrine signaling. The active function of 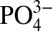 in this study reveals the underappreciated role of phosphate ions of the CaP minerals in the vicinity of osteoprogenitors during bone remodeling. The roles of
in this study reveals the underappreciated role of phosphate ions of the CaP minerals in the vicinity of osteoprogenitors during bone remodeling. The roles of  and ATP as precursors of osteogenic inducers in bone formation imply that their aberrant regulation could result in osteoporosis, a principal disease of imbalanced bone remodeling. Recent studies have found that mice lacking P2Y (13), a receptor of ADP, results in reduced bone turnover (57) and that polymorphisms in the P2X7 receptor gene are associated with reduced lumbar spine bone mineral density and accelerated bone loss in postmenopausal women (58). Validation of the
and ATP as precursors of osteogenic inducers in bone formation imply that their aberrant regulation could result in osteoporosis, a principal disease of imbalanced bone remodeling. Recent studies have found that mice lacking P2Y (13), a receptor of ADP, results in reduced bone turnover (57) and that polymorphisms in the P2X7 receptor gene are associated with reduced lumbar spine bone mineral density and accelerated bone loss in postmenopausal women (58). Validation of the  -ATP-adenosine signaling cascade in osteoporotic animal models could unravel new therapeutic targets.
-ATP-adenosine signaling cascade in osteoporotic animal models could unravel new therapeutic targets.
Fig. 5.
Schematic model of mineralized matrix-induced osteogenic differentiation.
In sum, by using an osteogenic, osteoinductive biomimetic matrix, we have unraveled a mechanism by which bone minerals contribute to bone tissue formation from bone marrow-derived stem cells. Furthermore, this study demonstrates the role of phosphate metabolism on osteogenic commitment of stem cells and the role of adenosine signaling in this process. These findings pave the way to new targets and approaches in treating critical bone defects and bone metabolic disorders.
Materials and Methods
Cell Culture.
The hMSCs (p7071L; Institute for Regenerative Medicine, Texas A&M University) were cultured on mineralized matrices, nonmineralized matrices, or tissue culture plates. More details about mineralized matrices, cell culture, and medium are provided in SI Text.
siRNA Knockdown.
For knockdown of SLC20a1, hMSCs were transfected with siRNA oligonucleotides (Invitrogen) according to the manufacturer’s instructions. Briefly, 30 nM siRNA targeting SLC20a1 (sense: GGGUGUCAAGUGGUCUGAACUGAUA, antisense: UAUCAGUUCAGACCACUUGACACCC) and scrambled control siRNA (medium GC content) were transfected with RNAimax transfection reagent (Invitrogen) under serum-free conditions for 5.5 h before cells were washed with PBS and changed to growth medium.
Characterization of Cell Phenotype.
The changes in cell phenotype responding to various culture conditions were analyzed by PCR, Western blot, and immunofluorescent staining as described in SI Text.
HPLC Experiments.
HPLC measurements were carried out to measure extracellular ATP and adenosine. Commercially available ATP and adenosine were used as controls. Fig. S5B shows the measurable threshold of ATP. Details are provided in SI Text.
Statistical Analysis.
Beyond the biological replicates, experiments were repeated independently at least twice. Statistical analyses were performed with one-way ANOVA, followed by a Bonferroni post hoc test or a two-tailed Student t test. Different letters and asterisks represent significance at P < 0.05.
Supplementary Material
Acknowledgments
We thank Colin Jamora and Samuel Suk for valuable discussions and Ruvi Chauhan for the schematics. This work is supported by the National Institutes of Health (NIH; Grant 1 R01 AR063184-01A1 to S.V.) and the University System of Taiwan–University of California, San Diego International Center of Excellence in Advanced Bioengineering sponsored by the Taiwan National Science Council International Research-Intensive Centers of Excellence (I-RiCE) Program under Grant NSC101-2911-I-009-101. The authors acknowledge the support of research grants from the Taiwan National Science Council (NSC 101-2314-B-038-022-MY3, NSC 98-2314-B-038-010-MY3, NSC 101-2120-M-010-002, NSC 100-2911-I-010-503, NSC 100-2314-B-010-030-MY3, NSC 101-2321-B-010-009, NSC 101-2911-I-010-503, and NSC 99-3114-B-002-005 to O.K.L.). The hMSCs used in this study were provided by the Institute for Regenerative Medicine, Texas A&M University, through Grant P40RR017447 from the National Center for Research Resources (NCRR) of the NIH.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321717111/-/DCSupplemental.
References
- 1.Hwang Y, Phadke A, Varghese S. Engineered microenvironments for self-renewal and musculoskeletal differentiation of stem cells. Regen Med. 2011;6(4):505–524. doi: 10.2217/rme.11.38. [DOI] [PubMed] [Google Scholar]
- 2.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15(2):205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutolf MP, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 4.Ayala R, et al. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials. 2011;32(15):3700–3711. doi: 10.1016/j.biomaterials.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalby MJ, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 7.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta N, et al. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103(8):2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phadke A, et al. Effect of scaffold microarchitecture on osteogenic differentiation of human mesenchymal stem cells. Eur Cell Mater. 2013;25:114–128, discussion 128–129. doi: 10.22203/ecm.v025a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phadke A, Shih YR, Varghese S. Mineralized synthetic matrices as an instructive microenvironment for osteogenic differentiation of human mesenchymal stem cells. Macromol Biosci. 2012;12(8):1022–1032. doi: 10.1002/mabi.201100289. [DOI] [PubMed] [Google Scholar]
- 12.Bhumiratana S, et al. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials. 2011;32(11):2812–2820. doi: 10.1016/j.biomaterials.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan H, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci USA. 2010;107(31):13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts SJ, et al. The combined bone forming capacity of human periosteal derived cells and calcium phosphates. Biomaterials. 2011;32(19):4393–4405. doi: 10.1016/j.biomaterials.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Johal HS, Buckley RE, Le IL, Leighton RK. A prospective randomized controlled trial of a bioresorbable calcium phosphate paste (alpha-BSM) in treatment of displaced intra-articular calcaneal fractures. J Trauma. 2009;67(4):875–882. doi: 10.1097/TA.0b013e3181ae2d50. [DOI] [PubMed] [Google Scholar]
- 16.Vaquette C, Ivanovski S, Hamlet SM, Hutmacher DW. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials. 2013;34(22):5538–5551. doi: 10.1016/j.biomaterials.2013.03.088. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Murphy WL. A screening approach reveals the influence of mineral coating morphology on human mesenchymal stem cell differentiation. Biotechnol J. 2013;8(4):496–501. doi: 10.1002/biot.201200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai YC, Roberts SJ, Schrooten J, Luyten FP. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng Part A. 2011;17(7-8):1083–1097. doi: 10.1089/ten.TEA.2010.0160. [DOI] [PubMed] [Google Scholar]
- 19.Chou YF, Huang W, Dunn JC, Miller TA, Wu BM. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26(3):285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Cowan CM, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22(5):560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Autefage H, et al. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J Biomed Mater Res B Appl Biomater. 2009;91(2):706–715. doi: 10.1002/jbm.b.31447. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Suarez-Gonzalez D, Murphy WL. Mineral coatings for temporally controlled delivery of multiple proteins. Adv Mater. 2011;23(37):4279–4284. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai YC, et al. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials. 2012;33(11):3127–3142. doi: 10.1016/j.biomaterials.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Barradas AM, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33(11):3205–3215. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Beck GR., Jr Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90(2):234–243. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- 27.Beck GR, Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97(15):8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen L, et al. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;424(3):439–445. doi: 10.1016/j.bbrc.2012.06.128. [DOI] [PubMed] [Google Scholar]
- 29.Yadav MC, et al. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: A unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2011;26(2):286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huitema LF, et al. Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc Natl Acad Sci USA. 2012;109(52):21372–21377. doi: 10.1073/pnas.1214231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jono S, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 32.Giachelli CM, et al. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38(4) Suppl 1:S34–S37. doi: 10.1053/ajkd.2001.27394. [DOI] [PubMed] [Google Scholar]
- 33.Yoshiko Y, Candeliere GA, Maeda N, Aubin JE. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol. 2007;27(12):4465–4474. doi: 10.1128/MCB.00104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Giachelli CM. Sodium-dependent phosphate cotransporters and vascular calcification. Curr Opin Nephrol Hypertens. 2007;16(4):325–328. doi: 10.1097/MNH.0b013e3281c55ef1. [DOI] [PubMed] [Google Scholar]
- 35.Phadke A, Zhang C, Hwang Y, Vecchio K, Varghese S. Templated mineralization of synthetic hydrogels for bone-like composite materials: Role of matrix hydrophobicity. Biomacromolecules. 2010;11(8):2060–2068. doi: 10.1021/bm100425p. [DOI] [PubMed] [Google Scholar]
- 36.Raynaud S, Champion E, Bernache-Assollant D, Thomas P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials. 2002;23(4):1065–1072. doi: 10.1016/s0142-9612(01)00218-6. [DOI] [PubMed] [Google Scholar]
- 37.Novak I. ATP as a signaling molecule: The exocrine focus. News Physiol Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- 38.Nakano Y, Addison WN, Kaartinen MT. ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone. 2007;41(4):549–561. doi: 10.1016/j.bone.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Zippel N, et al. Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev. 2012;21(6):884–900. doi: 10.1089/scd.2010.0576. [DOI] [PubMed] [Google Scholar]
- 40.Alsberg E, et al. Regulating bone formation via controlled scaffold degradation. J Dent Res. 2003;82(11):903–908. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 41.Boyer PD. The ATP synthase—A splendid molecular machine. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 42.Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: Multiple roles of inorganic phosphate. J Biol Chem. 2003;278(40):39155–39165. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- 43.Sugita A, et al. Cellular ATP synthesis mediated by type III sodium-dependent phosphate transporter Pit-1 is critical to chondrogenesis. J Biol Chem. 2011;286(4):3094–3103. doi: 10.1074/jbc.M110.148403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 45.Hoebertz A, Mahendran S, Burnstock G, Arnett TR. ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: A novel role for the P2Y2 receptor in bone remodeling. J Cell Biochem. 2002;86(3):413–419. doi: 10.1002/jcb.10236. [DOI] [PubMed] [Google Scholar]
- 46.Orriss IR, et al. The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of p2x receptors. Bone. 2012;51(3):389–400. doi: 10.1016/j.bone.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4-5):299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 48.Costa MA, et al. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J Cell Physiol. 2011;226(5):1353–1366. doi: 10.1002/jcp.22458. [DOI] [PubMed] [Google Scholar]
- 49.Carroll SH, et al. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem. 2012;287(19):15718–15727. doi: 10.1074/jbc.M112.344994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gharibi B, Abraham AA, Ham J, Evans BA. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26(9):2112–2124. doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- 51.He W, Mazumder A, Wilder T, Cronstein BN. Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 2013;27(9):3446–3454. doi: 10.1096/fj.13-231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoshniat S, et al. Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone. 2011;48(4):894–902. doi: 10.1016/j.bone.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki A, et al. Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res. 2006;21(5):674–683. doi: 10.1359/jbmr.020603. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98(7):905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 55.Cowan CM, et al. NELL-1 increases pre-osteoblast mineralization using both phosphate transporter Pit1 and Pit2. Biochem Biophys Res Commun. 2012;422(3):351–357. doi: 10.1016/j.bbrc.2012.04.077. [DOI] [PubMed] [Google Scholar]
- 56.Nakade O, Takahashi K, Takuma T, Aoki T, Kaku T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J Bone Miner Metab. 2001;19(1):13–19. doi: 10.1007/s007740170055. [DOI] [PubMed] [Google Scholar]
- 57.Wang N, et al. Reduced bone turnover in mice lacking the P2Y(13) receptor of ADP. Mol Endocrinol. 2012;26(1):142–152. doi: 10.1210/me.2011-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gartland A, et al. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur J Hum Genet. 2012;20(5):559–564. doi: 10.1038/ejhg.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.