Significance
Sympatric speciation, suggested by Darwin (1859) as a mode of the origin of species, is still controversial. We demonstrated that Acomys cahirinus, spiny mice, at Evolution Canyon (EC), Israel, support Darwin’s suggestion. At EC, the south-facing “African” slope receives high solar radiation, and hence is a dry, hot African-like savanna. The abutting north-facing “European” slope, at a distance of 200 m on average, is humid, cool, and forested. A. cahirinus is significantly divergent interslope phenotypically and genotypically in mtDNA and nuclear amplified fragment length polymorphism. In complete mtDNA, 25% of haplotypes were slope-biased. Habitat selection and preliminary demonstrated mate-choice overrule ongoing low interslope gene flow, suggesting incipient sympatric speciation in A. cahirinus, as in other phylogenetically diverse taxa at EC, dubbed the “Israeli Galapagos.”
Keywords: ecological-speciation, adaptation, microscale, rodents
Abstract
Does the paucity of empirical evidence of sympatric speciation in nature reflect reality, despite theoretical support? Or is it due to inappropriate searches in nature with overly restrictive assumptions and an incorrect null hypothesis? Spiny mice, Acomys, described here at Evolution Canyon (EC) incipiently and sympatrically speciate owing to microclimatic interslope divergence. The opposite slopes at EC vary dramatically, physically and biotically, representing the dry and hot south-facing slope savannoid-African continent [“African” slope (AS)], abutting with the north-facing slope forested south-European continent [“European” slope (ES)]. African-originated spiny mice, of the Acomys cahirinus complex, colonized Israel 30,000 y ago based on fossils. Genotypically, we showed significantly higher genetic diversity of mtDNA and amplified fragment length polymorphism of Acomys on the AS compared with the ES. This is also true regionally across Israel. In complete mtDNA, 25% of the haplotypes at EC were slope-biased. Phenotypically, the opposite slope’s populations also showed adaptive morphology, physiology, and behavior divergence paralleling regional populations across Israel. Preliminary tests indicate slope-specific mate choices. Colonization of Acomys at the EC first occurred on the AS and then moved to the ES. Strong slope-specific natural selection (both positive and negative) overrules low interslope gene flow. Both habitat slope selection and mate choices suggest ongoing incipient sympatric speciation. We conclude that Acomys at the EC is ecologically and genetically adaptively, incipiently, sympatrically speciating on the ES owing to adaptive microclimatic natural selection.
Can mammals speciate sympatrically (i.e., the origin of a new species within a freely interbreeding population) without geographic isolation (1)? We have recently argued that blind subterranean mole rats, Spalax, possibly display incipient sympatric ecological speciation within the range of Spalax galili (2n = 52) in the eastern Upper Galilee in Israel (2). Sympatric speciation (SS) has been controversial since it was first proposed as a mode of speciation by Darwin (3). Skepticism about extensive SS in nature, except polyploidy in plants, has been recently expressed in a treatise on speciation (1). Clearly, natural selection in the Spalax case (2) overruled gene flow, as was the general case at Evolution Canyon (4). We concluded in the spalacid study that the constrained local gene flow, overruled by natural adaptive edaphic selection, permits incipient SS to occur. Moreover, we hypothesized that SS may indeed be abundant in nature, as visualized by Darwin (3), because abutting divergent ecologies are abundantly derived from geological, edaphic, and climatic divergences in nature (2). Here, we describe a similar case of incipient SS in the spiny mice Acomys at Evolution Canyon (EC), based on sharp local microclimatic divergence.
The EC model reveals divergent interslope evolution in action involving biodiversity across life from bacteria to mammals, followed by divergent genomic, proteomic, and phenomic adaptive complexes (4–9). In the EC (details are given in Supporting Information) major adaptive complexes on the “African” slope (AS) are due to solar radiation, heat, and drought, whereas those on the “European” slope (ES) relate to shade or light stress and photosynthesis. Remarkably, interslope species divergence led to incipient SS in bacteria, fungi, plants (wild barley), insects (Drosophila and beetles), and mammals such as the spiny mice Acomys (7) to be described here in detail. Owing to this global pattern of incipient SS across life, the EC was dubbed the “Israeli Galapagos” (7). This local adaptive and speciational interslope microclimatic divergence also occurs in the other three “Evolution Canyons” studied in Israel in the Galilee, Golan, and Negev Mountains (8) as well as in other evolution canyons worldwide (9).
Spiny mice of the genus Acomys are tropical murid rodents involving about 19 species ranging in Africa and southwest Asia in rocky habitats (10). Acomys cahirinus, belonging to the A. cahirinus– Acomys dimidiatus complex (11, 12), is referred to as A. cahirinus in this paper. Acomys is widely distributed across Israel and Sinai, ranging in both xeric and mesic environments comprising the Mediterranean, steppe, and desert climatic regimes and thus climatically is a wide-ranging species complex. However, it lives only in rocky habitats and is relatively stenotopic (i.e., restricted in niche preference). The three karyotypes of Acomys in Israel (11) seem to represent two speciation events, an old one, generating Acomys russatus, 2n = 66, ranging in the Sinai, Negev, and Judean deserts, and a new one involving the Acomys cahirinus complex, with 2n = 36 in xeric Sinai, and the derivative 2n = 38 in the mesic north, differing by a single Robertsonian change. The 2n = 38 occurs in Sinai and Israel, respectively (13), including the 2n = 38 karyotype at EC on both slopes based on 34 karyotypes analyzed (Fig. S1A). Allozyme analysis estimated the origin of speciation of A. russattus as 1,500,000 ± 50,000 y ago, and the divergence of the two new karyotypes’ speciation in A. cahirinus to 115,000 ± 40,000 y (14).
The fossil record and zoogeographical evidence suggest that Acomys colonized the Near East from Africa in the Pliocene. However, A. cahirinus, based on fossil evidence (15), colonized mesic Israel only in the Upper Pleistocene some 30,000 y ago, conforming roughly to the allozyme data (14). Acomys carmeliensis, akin to A. cahirinus, was described by Georg Haas in the Natufian-Neolithic site of the Abu Usba cave at the upper ES slope of EC, lower Nahal Oren, Mount Carmel (15). The oldest fossil record of Acomys out of Africa, similar in morphology to those from the Abu Usba cave and to the recent A. cahirinus at EC, was found in the Levalloiso-Mousterian of the Kebara cave about 20 km south of EC (15). Here we provide evidence strongly suggesting that Acomys speciated incipiently and sympatrically at EC.
Results
Interslope Differences in Animal Density.
Altogether, we analyzed 54 individuals of A. cahirinus at EC. Forty-nine animals were collected during 2007–2009 and five animals in 2004 (Table S1). Out of the total, 38 individuals originated at the AS and 16 individuals at the ES (Table S1). Our results showed that in an interyear comparison between the same slopes the genetic variability was smaller than in the comparison of the opposite slopes. Because the intensity of sampling was identical on both slopes (measured by an equal number of used Sherman’s traps and by equal sampling time), the significant interslope difference in the number of sampled animals (binomial test, P = 0.003) indicates the interslope difference in abundance (Table S1).
Amplified Fragment Length Polymorphism.
From the total sample of A. cahirinus, 4 animals in the ES and 17 in the AS collected in 2009 and 2008 were chosen for amplified fragment length polymorphism (AFLP) analysis. Their AFLP analysis provided 364 dominant markers (bands, considered alleles). In 2009, the AS sample was more polymorphic than the ES sample (Fig. S1 B and C) as indicated by the following comparisons: number of polymorphic loci, 5% and 1% criteria [PAS/PES (5%) = 0.911/0.473] and PbAS/PbES = 1.573/1.473. However, the number of private (unique) bands was higher at the ES than at the AS (UAS/UES = 0/17) in 2009. An AFLP-based neighbor-joining dendogram of A. cahirinus samples shows clear interslope divergence at EC (Fig. S1C). In 2008 the AS sample composed of four animals showed [PAS (5%) = 0.948] and PbAS = 1.758. The interyear comparison showed higher genetic variability at the AS than at ES.
Interslope Differences and Natural Selection.
Nei’s genetic distance 0.034 and both ΘI (ΘI corresponds directly to Wright’s FST) = 0.32 (95% credible interval: 0.25–0.38), and ΘII (measures divergence among contemporaneous populations similar to Nei’s GST) = 0.11 (95% credible interval: 0.07–0.16) showed significant interslope divergence, as indicated by the lower boundary of the 95% interval in both indices (higher than zero). Genetic diversity was slightly higher at the AS (Hs = 0.286; 95% credible interval: 0.27–0.30) but overlapping with the ES (Hs = 0.284; 95% credible interval: 0.27–0.30).
The tests for candidate AFLP markers for interslope differential selection identified 61 significant (P < 0.05) cases (16). We can reject, with 95% confidence, the possibility that all significant results are false positives because the number of 61 significant cases represents 21.4% from the total number of the polymorphic markers. At least 53 recorded cases of the positive selection are significantly different between the slopes.
Mitochondrial DNA.
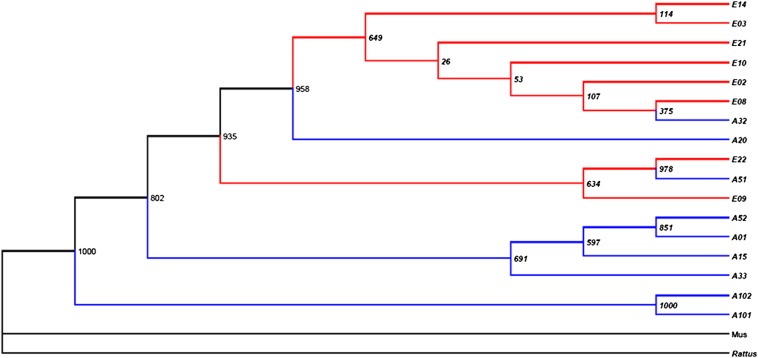
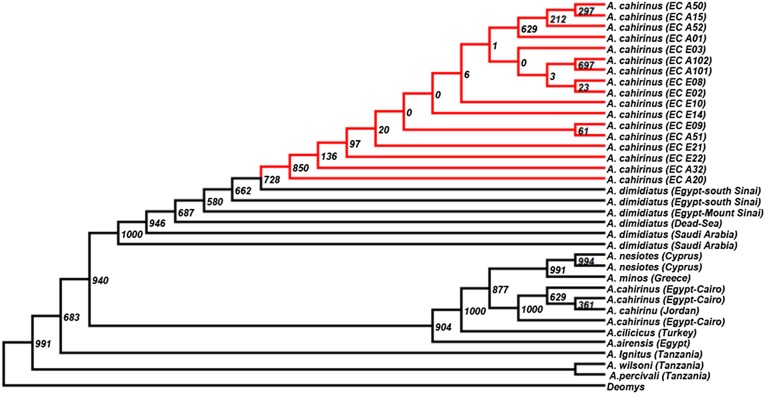
Complete mtDNA sequencing was conducted in nine animals at the AS and eight animals at the ES. The list of sequenced genes and control region (16,258 bp) including their specifications is given in Table S2. Base frequencies in all complete mtDNA sequence samples are A = 0.3339, C = 0.2782, G = 0.1279, and T = 0.2600. Ten haplotypes differed by 42 variable sites (SNPs, indels, and insertions) (Tables S2–S5). The most parsimonious substitution nucleotide model for the dataset was HKY85 (17) (010010). The maximum parsimony cladogram of mtDNA shows clear interslope divergence at EC (Fig. 1) with only three potential migrants from the AS to ES. The constructed Cytochrome b (Cytb) chronogram, including many species (Fig. 2 and Table S6), showed that Acomys samples from the EC homogeneously cluster together. Fig. 2 also indicates that A. cahirinus in Egypt might be different from A. cahirinus at EC (12).
Fig. 1.
A maximum likelihood-phylogenetic tree of Acomys cahirinus from complete mtDNA sequences at EC. The bootstrap values are based on 1,000 cycles. A, AS samples collected from AS; E, ES samples collected from ES.
Fig. 2.
A maximum likelihood chronogram based on Cytb from different Acomys species. Deomys was used as an outgroup. The bootstrap values are based on 1,000 cycles. The bootstrap values equal zero or very small ones are showing some individuals from EC are genetically identical or very similar.
Genetic interslope differences.
Genetic parameters for the ES and AS and pooled samples at EC are given in Table S5. Haplotype diversity was significantly higher at the AS than at the ES (binomial probability test, P = 0.0005). Nucleotide and phylogenetic diversities were also higher at the AS than at the ES, but the difference did not reach a significant level. The genetic distances ΘI = 0.042 (95% credible interval: 0. 0.007–0.09) and ΘII = 0.02 (95% credible interval: 0.0008–0.06) indicate that slopes are significantly divergent, as already indicated for AFLP data. The maximum possible Cohen’s unweighted kappa (κ = 0.73) indicates that at least 27% of the detected haplotype variability could be attributed to slope effects.
Rat and mouse were chosen as out-groups because they are regarded phylogenetically older than Acomys (Fig. 1). Both the tree (Fig. 1) and spanning network (Fig. 3) indicate that the phylogenetically oldest haplotypes were collected at the AS.
Fig. 3.
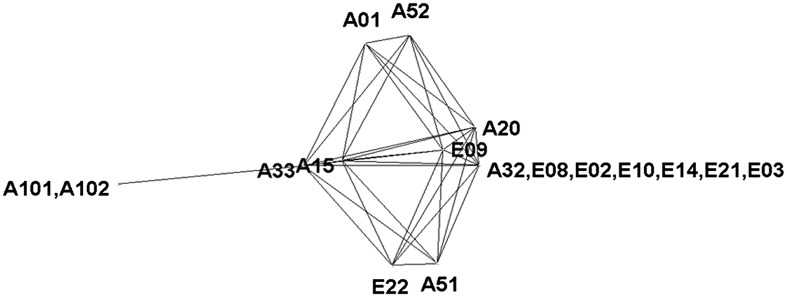
A minimum spanning network constructed by means of SplitsTree (54) from 17 Acomys cahirinus samples from EC.
Interslope selection pressure.
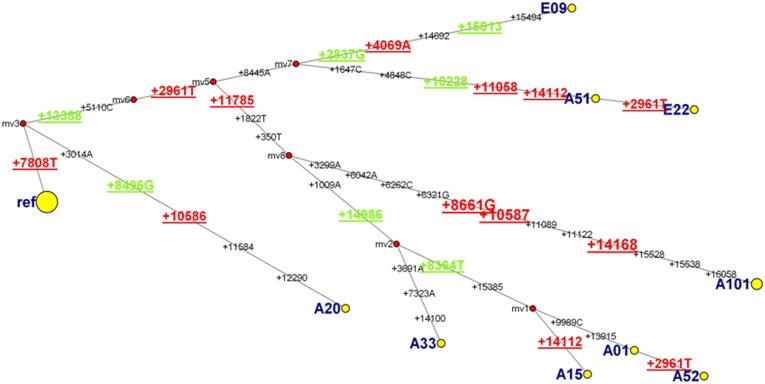
There is evidence for positive global selection pressure on the neighbor-joining phylogenetic tree. The overall PARRIS test indicated a significantly higher likelihood for the model with dN > dS (M2) than the null model (M1) (18). The sites under positive or negative selection, as detected by the integrative test, are summarized in Fig. 4. Altogether, there are 13 sites under positive selection and 7 sites under negative selection in 42 total identified mutations (Fig. 4).
Fig. 4.
A median joining network representing all complete mtDNA sequences in A. cahirinus at EC. Numerals describe position of polymorphic sites. Nucleotide position in red and green refers to the positive and negative selections, respectively. Ref refers to the ES abundant haplotype Y1 (Table S3 gives details).
Discussion
Origin of Species.
The origin of species, the “mystery of mysteries” (3) and the most important event in evolution (19), is still deeply controversial despite much theoretical (20) and empirical progress (1). A major ongoing debate relates to the mode of speciation, and especially that of SS. Darwin, who first proposed SS (3), considered it an important mode of biodiversity origin, where new species originate in sympatry to fill empty niches in nature. Mayr, the main antagonist of SS (19, 21), claimed that SS “is unproven and unlikely that reproductive isolation can develop between contiguous populations.” However, even Mayr changed his views in his last book, admitting that “so many well analyzed cases of sympatric speciation were found, particularly among fishes and insects, that there is now no longer any doubt about the frequency of sympatric speciation” (22). Nevertheless, skepticism about the high frequency of SS, particularly in animals, still prevails (1). SS still remains an extremely controversial issue, primarily in animals.
Evolution Canyon Is a Microscale Cradle of SS.
Theory (20) does not consider that SS occurs easily, but, under biologically realistic conditions, it is certainly possible. Clearly, it can occur when natural selection overrules gene flow (4) and habitat and mate choice evolve (Table S7), as we have shown in EC (7) and in our recent demonstration of a possible edaphic incipient SS in the subterranean rodent Spalax (2). The easiest sites to locate such natural potential situations are those in which drastic ecological divergence occurs in abutting divergent geologies, soils, or climates (2, 4–9, 23). Adaptations across the genome (24) provide fertile background for genomic divergence during speciation (24–29). Surprisingly, and in contrast to theoretical expectations, the examples of unequivocal SS in animals are scanty (1), although quite abundant in polyploid plants. Recently, a flow of novel SS cases, proven or hypothesized, have been proposed (2, 7, 29–34). The EC case clearly demonstrates how incipient sympatric climatic ecological speciation (35) occurs across life from bacteria through fungi, plants (wild barley), insects (Drosophila and the beetle Oryzaephilus surinamensis), to mammals [spiny mice Acomys described here and in (7)].
EC is an optimal model for the sympatric origin of new species at a microsite. There is no geographical barrier between the abutting slopes, separated on average by 200 m, but representing the divergence between two continents (i.e., dry Africa and humid southern Europe) owing to interslope microclimatic differential stresses (4–9, 23). At EC microclimatic variation (36) rather than geological variation (2) leads to SS. The most important factor driving incipient sympatric (7) ecological speciation (35) at EC relates to strong microclimatic niche-specific interslope divergence. This occurs through strong interslope genomic natural selection for slope-specific resources and habitat selection coupled with constrained gene flow (4), sometimes complemented by disruptive sexual selection and positive assortative mating. Adaptive complexes against high UV radiation, high temperature, drought, and for high DNA repair on the AS are contrasted with adaptive complexes against shade and light deprivation on the ES in all model organisms studied (8). These adaptive contrasts are clearly displayed by whole genome transcription divergent patterns of the crucifer Ricotia lunaria, revealed by tiling array hybridizations* (37). They unfold contrasting adaptive complexes caused by differential climatic stresses on the opposite slopes (8).
Significant ecological niche-divergent abutting slopes lead to incipient SS at a surprisingly short distance (7). This is true in all five major taxa examined at EC [bacteria, soil fungi including yeast (38), flowering plants (7), Drosophila (30), and Acomys rodents described here]. In all of these distant taxa, strong interslope natural microclimatic selection overrides the homogenizing effects of the ongoing restricted gene flow (4, 7). Remarkably, the flying Drosophila migrates between the slopes (6) but displays multiple slope-specific adaptive complexes to the abutting slope’s stresses (30). It speciates sympatrically based on mate and habitat choices, positive assortative mating (39) reinforced by slope-specific courtship songs (40). The preliminary analysis of mate choice indicates positive slope assortative mating in Acomys (discussed in Supporting Information). This experiment suggests that positive female mate choice operates for a female of the “European” slope. The European estral female each time visited the European males—more than the African one in six, two-choice mate experiments, when the female had to choose between two different alternative males in a Y maze. That choice result is significant (P < 0.05) (Table S7).
A remarkable case is that of the drosophilid Zaprionus tuberculatus, which invaded Israel, including the EC, from India in the early 1980s. It rapidly evolved significant interslope allozymic divergence with higher allozymic diversity on the more stressful AS for Z. tuberculatus than on the milder ES (41). This indicates that SS is local and not derived from earlier allopatric sites and highlights the rapidity of the local process. Drosophilid fauna at EC (two genera and nine species) is almost entirely allochtonous, composed of colonizing species (41). If a colonizing species such as Z. tuberculatus succeeded to evolve significant interslope allozymic diversity in about 30 or more years, it could also evolve mate choice, which is directly dependent on habitat and resource choice, as was the case with D. melanogaster. In the latter, peculiarities in courtship song patterns and nonrandom mating (i.e., mate choice) were observed at EC, despite a very small interslope distance of 200 m (39, 40). Single- and multiple-mate choice tests with Drosophila melanogaster from the opposite slopes displayed highly significant assortative mating, with a preference for slope-specific sexual partners, based on variation in courtship songs (40). Significant assortative mating occurs also in Drosophila simulans at EC. Thus, interslope divergent ecological speciation was rapid and caused incipient SS. The final fate of these newly evolving species with slope-specific adaptations (42) is unknown, as is the fate of established species owing to environmental stresses, such as global warming (9) or that of climatic speciation on the blind mole rat (43).
Interslope Gene Flow.
Rodent species diversity and microhabitat distribution across opposing slopes were assessed by the capture-mark-recapture method at EC, Mount Carmel, during 1 y (44) and 3 y (Table S8). Trapped animals on the AS were almost exclusively the African-originated A. cahirinus. By contrast, on the ES, the vast majority of rodents were the European-originated Apodemus mystacinus and Apodemus flavicolis, with almost half the sample size of A. cahirinus, and even fewer Mus macedonicus and Rattus rattus on the ES. Activity of A. cahirinus during the winter on the AS decreased with decreasing elevation. On the ES, Acomys densities decreased from the rocky top to the bottom. The evidence of a low interslope gene flow suggests indirect high levels of habitat selection, an important hallmark of speciation.
Our data indicate that the colonization of Acomys started on the African niche AS, and only later Acomys migrated from the AS to the European niche ES (Fig. 1). In addition, the sampled Acomys (Fig. 3) have common origin. This allows us to exclude the possibility that the Acomys population in each slope originated from a different outside region. This is indicative of substantial evidence supporting incipient SS.
Our evidence (Figs. 1 and 3 and Fig. S1C) strongly supports the primary SS hypothesis and highly likely rejects the secondary contact hypothesis. As is evident in Fig. 2, all genotypes from EC tested for Cyt b are clustered together, whereas the Dead Sea and south Sinai populations of A. cahirinus (A. dimidiatus complex) are distant, as are also all other Acomys species.
In allozymes, in locus Me1 there is a significant difference between the slopes. Allele c appears only on the north-facing slope (ES), and allele b is more frequent there. That difference is significant by Fisher’s exact test (45).
Recapture data indicate that individuals tended to remain in the same or in adjacent altitudinal transects within the same slope (44). Not a single marked animal was recovered on the opposite slope during February, May, and September 1992 when tests were conducted in that study (44). However, an advanced study of 460 Acomys marked by chips, conducted for 3 y (1996–1999) (Table S8), found five long-term migrations of Acomys from extreme stations between the slopes. This result indicates that a very low and limited interslope gene flow occurs by migrants, both males and females, from AS to ES, and from ES to AS. This limited gene flow permits free interbreeding (i.e., confirms sympatry) (Table S8). The interslope rodent community’s dissimilarities are attributed to the different microclimates and ecologies on the opposing slopes.
Regional (Across Israel) and Interslope (EC) Phenomic Differences in Acomys.
Predation by Acomys on European Pomatias land snails exceeds the proportion of Pomatias shells among the large land snail fauna at EC (46). The ratios of consumed to unconsumed Pomatias were ∼4:1 and 7:1 in the ES and AS, respectively (46), indicating the need of AS Acomys for snail food rich in water. Remarkably, oxygen consumption of the AS Acomys was adaptively 20% lower than that of ES Acomys, 200 m apart, as expected in a desert rodent, thereby economizing energy† (47, 48). This extraordinary interslope differential in resting metabolic rate is similar in value to the difference between xeric southern Negev desert Acomys in Eilat and mesic north Galilee Acomys in Metula, separated by 600 km (×3,000 in transect length). This observation highlights the dramatic interslope differential ecologies of the dry tropical AS against the humid temperate ES, across 200 m only. Likewise, the urine volume collected after 24 h from trapping was 70% higher in ES animals than that of AS animals in which urine concentration was higher, and this was similar to desert rodents (47). Differential osmoregulation of AS and ES Acomys populations is retained in their offspring, suggesting long-term xeric adaptations in AS populations (48). Likewise, nonshivering thermogenesis indicated that ES and AS populations are “heat producers” and “heat conservers,” respectively (48). Thus, the ES and the AS Acomys populations display dramatically contrasting mesic and xeric adaptations across 200 m, respectively.
Genomic SS in Other Organisms Sweeping Across the Genome.
Insights obtained from the long-term laboratory selection experiments as to how adaptation sweeps through the genome (28, 29) could potentially reveal insight into the mutational dynamics that most likely occur in natural populations under similar situations, such as, for example, global warming (9). These changes could involve de novo nonrandom or adaptive mutations, or those already present in the population as existing genetic diversity. Widespread genomic divergence during SS on numerous independent genomic regions, characterized by the fly Rhagoletis (32), may be abundant in nature. Isolated genomic islands of speciation (32) may be the rule rather than the exception, as is also the rapid evolution of host fruit odor discrimination in Rhagoletis flies (32, 34). Hybrid sterility over tens of meters between ecotypes adapted to serpentine and nonserpentine soil highlight genomic differentiation at a microscale (33).
Conclusions and Prospects
Conclusions.
Our results demonstrate both genotypic and phenotypic divergence of A. cahirinus at the (EC) microsite at Mount Carmel. EC is a natural laboratory of evolution in action, sharply contrasting dramatically microclimatic divergent ecologies, imitating abutting dry tropical African and temperate European continents, separated on average by only 200 m. The opposite slopes can be regarded as hosting differential levels of freely interbreeding populations from viruses to mammals. Thus, EC is a cradle for SS across life [see Fitzpatrick et al. (49) for a critical review of SS]. The interslope divergent adaptive complexes of organisms across phylogeny evolve incipiently into the new incipient sister species on the ES. They display differential levels of adaptive genomic, proteomic, and phenomic complexes plus variable habitat selection and mate choice in sympatry. These divergent interslope niches and ecological resources represent differential phases of incipient sympatric ecological speciation in action.
A. cahirinus from dry, hot Africa colonized Israel and the dry tropical African slope at EC some 30,000 y ago based on fossils. Later, possibly around 20,000–30,000 y ago, tropical AS Acomys colonized the abutting temperate ES from the AS, demonstrating ecological speciation in action (35). During this incipient speciation, divergent mtDNA and nuclear genomic adaptations evolved in the 200-m distant, humid, and cooler ES. Therefore, the ES represents a new and stressful temperate niche for Acomys. This might be the fossil A. carmeliensis (15) found in the Abu Usba cave of the ES. The microallopatric null hypothesis is negated by the ongoing little gene flow and by the strong overruling natural selection. This prevents gene flow from the AS to homogenize the ES colonized niche, despite ongoing free interbreeding. Speciation is advanced and supported by both slopes’ specific genomic, proteomic, and phenomic adaptive complexes coupled with habitat and mate choice behavior, promoting premating reproductive isolation. Adaptation and speciation are coupled in sympatry, generating new incipient species whose fates remain unknown. This ecological theater has been dubbed the Israeli Galapagos. This is the second case of incipient SS we have described in mammals (see ref. 2). Our hypothesis is that SS may be prevalent in nature, as suggested by Darwin. However, its unfolding may be expedited by exploring sharply abutting divergent ecologies caused by geological, edaphic, or climatic, geographically very close, barrierless contacts. The case for SS is dramatically reinforced if it occurs in parallel across phylogeny from bacteria to mammals (4–9, 36) (see ref. 50 for the probability of genetic parallelism and convergence in natural populations). For this adaptive incipient sympatric ecological process across life to proceed, strong selection must overrule gene flow, as is the case at EC (4). Strong selection is the only known evolutionary driving force that can overrule gene flow and explains the very close interslope divergence in mitochondrial (17, 51) and nuclear genomes, and the evolving premating reproductive isolating mechanisms revealed here in preliminary tests in Acomys. This is also presumably true at EC for other incipiently speciating organisms across life (bacteria, plants, insects, and mammals). All live under divergent climates at the microscale but share rocks and soils on both slopes (7). The EC case of possible incipient SS is different from that of the Spalax case owing to divergence by rocks and soils but sharing climate (2). The Spalax case of possible incipient SS described earlier (2) and the Acomys case described here at EC with supportive evidence from other taxa across life support Darwin’s (3) initial claim that SS may be much more frequent in nature than generally believed. In fact, it may occur frequently as in other model organisms at the EC microsite.
Prospects.
What next? Determining how frequent SS actually occurs in nature will require future studies in sharply subdivided local microsites displaying barrierless ecological heterogeneity in geology (2) or climate as in EC described here. The genomic revolution now permits in-depth studies of whole genome sequence comparisons of both nuclear and mtDNA population genomics in freely interbreeding populations subject to sharp divergent ecologies, which is also described by Moyle et al. (33) based on serpentines. Populations should be explored across the geological or climatic interfaces coupled with phenomic studies in morphology, physiology, and behavior followed by field experimentation. Comparisons should involve the exploration of genes and regulatory noncoding protein elements, possibly focusing on the regulatory elements recently discovered by the ENCODE program in humans (52). ENCODE revealed that about 80% of the noncoding human elements, which do not code for proteins, code for small RNA and abound with repeated elements, such as transposons and retrotransposons. The latter could be substantial in channeling both adaptation and speciation processes (53). Experimentation is vital to assess the dynamics and differential rates of the evolutionary processes in different taxa. Transplant experiments between the slopes, especially in prokaryotes such as cyanobacteria, soil bacteria, but also in eukaryotes such as soil fungi, including yeast (38), among others, could provide natural experiments in adaptation and speciation and unfold their evolutionary rate. The recent (30-y) colonization and allozymic differentiation of the drosophilid fly Z. tuberculatus at EC suggest that evolutionary rates could also be very fast.
EC is a fitting natural laboratory to track the origin of new incipient species and follow their dynamics and fate of genomic elements (DNA and RNA), proteomics, and regulatory elements of the noncoding genome (ENCODE), such as nonrandomly jumping transposons and retrotransposons. Ecological speciation (35) could now be coupled to functional ecological genomics to highlight the still unclear picture surrounding the modes and rates of the origin of new species in nature. Darwin’s evolutionary deep insights on the origin of species and adaptations could now be explored empirically, experimentally, and theoretically and advance from the qualitative to the quantitative phase of exploring nature. The still persisting dilemma of how much SS occurs in nature is now readily resolvable—partly from the evidence suggested in ref. 2 and here as well as in other studies mentioned earlier. Evolution canyons in Israel and the world are exciting sites for highlighting the origin of species in nature (3) by exploring genomic divergence (24, 27) during adaptive ecological speciation (35), along with its causes and consequences (27).
Materials and Methods
The study of incipient SS in spiny mice Acomys was conducted at a microscale, the Evolution Canyon natural laboratory in Mount Carmel, which is the focus of studies on evolution in action across life from viruses and bacteria through fungi, plants, and animals (Supporting Information). The sample of genetic analysis contained 54 adult spiny mice belonging to the A. cahirinus complex. All animals were treated according to guidelines of the Ethics Committee, University of Haifa. We analyzed complete mtDNA sequences (16,258 bp) (Table S9) in a sample of 17 animals (9 from AS and 8 from ES). We also examined 32 animals (10 from ES and 22 from AS) for AFLP genome diversity. Details of DNA extraction, sequencing, bioinformatics software, and statistical analysis are given in SI Materials and Methods. Earlier we also conducted physiological (resting metabolic rate) and preliminary behavioral (mate choice) tests on Acomys populations from EC. Likewise, the flora and fauna have been thoroughly studied in other published studies (4–9).
Supplementary Material
Acknowledgments
We thank Daphna Halevi, Alma Joel, Tami Krugman, and Robin Permut for technical assistance in our work. We thank the Ministry of Science and Technology and Council for Higher Education in Israel for providing scholarships (Y.H.). We thank the Ancell–Teicher Research Foundation for Genetics and Molecular Evolution for financial support for all studies (E.N.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JN571139–JN571155).
*Brodsky L, Kossover O, Jacob E, Korol AB (2008) Genome transcription divergent patterns of Ricotia lunaria on opposite slope of “Evolution Canyon” revealed by tiling array hybridizations. Proceedings of the Plant and Animal Genome XVI Conference, January 12–16, San Diego.
†Haim A, et al. (1997) [Comparative physiology in populations of common spiny mice, Acomys cahirinus, from the same locality but different habitats of “Evolution Canyon”, Local Nahal Oren, Mount Carmel.] Annual Meeting of the Israel Science Society of Ecology and Environmental Quality, June 16–17, 1997, University of Haifa, p 104 (abstr). Hebrew.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322301111/-/DCSupplemental.
References
- 1.Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Assoc; 2004. [Google Scholar]
- 2.Hadid Y, et al. Possible incipient sympatric ecological speciation in blind mole rats (Spalax) Proc Natl Acad Sci USA. 2013;110(7):2587–2592. doi: 10.1073/pnas.1222588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darwin C. The Origin of Species by Means of Natural Selection, or the Preservation of Favored Races in the Struggle for Life. New York: Modern Library; 1859. [Google Scholar]
- 4.Nevo E. Selection overrules gene flow at “Evolution Canyons,” Israel. Adv Genet Res. 2011;5:67–89. [Google Scholar]
- 5.Nevo E. Asian, African and European biota meet at “Evolution Canyon”, Israel: Local tests of global biodiversity and genetic diversity patterns. Proc Biol Sci. 1995;262:149–155. [Google Scholar]
- 6.Pavlíček T, Frenkel Z, Korol AB, Beiles A, Nevo E. Drosophila at the “Evolution Canyon” microsite, Mt. Carmel, Israel: selection overrules migration. Isr J Ecol Evol. 2008;54:165–180. [Google Scholar]
- 7.Nevo E. “Evolution Canyon”: A microcosm of life’s evolution focusing on adaptation and speciation. Isr J Ecol Evol. 2006;52:485–506. [Google Scholar]
- 8.Nevo E. Evolution in action across life at “Evolution Canyons”, Israel. Trends Evol Biol. 2009;1:12–34. [Google Scholar]
- 9.Nevo E. “Evolution Canyon,” a potential microscale monitor of global warming across life. Proc Natl Acad Sci USA. 2012;109(8):2960–2965. doi: 10.1073/pnas.1120633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson DE, Reeder DM. Mammal Species of the World. Baltimore: Johns Hopkins Univ Press; 2005. [Google Scholar]
- 11.Wahrman J, Zahavi A. Intra-genetic difference in chromosome numbers of spiny mice (Rodentia: Murinae) Bull Res Counc Isr. 1953;3:265. [Google Scholar]
- 12.Volobouev V, Auffray JC, Debat V, Denys C, Gautun JC. Species delimitation in the Acomys cahirinus–dimidiatus complex (Rodentia, Muridae) inferred from chromosomal and morphological analyses. Biol J Linn Soc Lond. 2007;91:203–214. [Google Scholar]
- 13.Wahrman J, Goitein R, Nevo E. 1969. Comparative Mammalian Cytogenetics: An International Conference at Dartmouth Medical School, ed Benirschke, K (Springer, New York), pp 30–48. [DOI] [PubMed]
- 14.Nevo E. Genetic differentiation and speciation in spiny mouse, Acomys. Acta Zool Fenn. 1985;17:131–136. [Google Scholar]
- 15.Tchernov E. Succession of Rodent Faunas During the Upper Pleistocene of Israel. Hamburg, Germany: Paul Parey; 1968. [Google Scholar]
- 16.Joost S, Kalbermatten M, Bonin A. Spatial analysis method (sam): A software tool combining molecular and environmental data to identify candidate loci for selection. Mol Ecol Resour. 2008;8(5):957–960. doi: 10.1111/j.1755-0998.2008.02162.x. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Kishino HH, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22(2):160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 18.Delport W, Poon AFY, Frost SDW, Kosakovsky Pond SL. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26(19):2455–2457. doi: 10.1093/bioinformatics/btq429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayr E. Animal Species and Evolution. Cambridge, MA: Belknap; 1963. [Google Scholar]
- 20.Gavrilets S. Fitness Landscapes and the Origin of Species. Princeton: Princeton Univ Press; 2004. [Google Scholar]
- 21.Mayr E. Ecological factors in speciation. Evolution. 1947;1:263–288. [Google Scholar]
- 22.Mayr E. What Makes Biology Unique? Considerations on the Autonomy of a Scientific Discipline. Cambridge, UK: Cambridge Univ Press; 2004. [Google Scholar]
- 23.Nevo E. Evolution of genome-phenome diversity under environmental stress. Proc Natl Acad Sci USA. 2001;98(11):6233–6240. doi: 10.1073/pnas.101109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radwan J, Babik W. The genomics of adaptation. Proc Biol Sci. 2012;279(1749):5024–5028. doi: 10.1098/rspb.2012.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Via SC, Conte G, Mason-Foley C, Mills K. Localizing F(ST) outliers on a QTL map reveals evidence for large genomic regions of reduced gene exchange during speciation-with-gene-flow. Mol Ecol. 2012;21(22):5546–5560. doi: 10.1111/mec.12021. [DOI] [PubMed] [Google Scholar]
- 26.Kondrashov FA. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc Biol Sci. 2012;279(1749):5048–5057. doi: 10.1098/rspb.2012.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosil P, Feder JL. Genomic divergence during speciation: Causes and consequences. Philos Trans R Soc Lond B Biol Sci. 2012;367(1587):332–342. doi: 10.1098/rstb.2011.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke MK. How does adaptation sweep through the genome? Insights from long-term selection experiments. Proc Biol Sci. 2012;279(1749):5029–5038. doi: 10.1098/rspb.2012.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel AP, et al. Widespread genomic divergence during sympatric speciation. Proc Natl Acad Sci USA. 2010;107(21):9724–9729. doi: 10.1073/pnas.1000939107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korol AB, Rashkovetsky E, Iliadi K, Nevo E. Drosophila flies in “Evolution Canyon” as a model for incipient sympatric speciation. Proc Natl Acad Sci USA. 2006;103(48):18184–18189. doi: 10.1073/pnas.0608777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sikorski J, Nevo E. Adaptation and incipient sympatric speciation of Bacillus simplex under microclimatic contrast at “Evolution Canyons” I and II, Israel. Proc Natl Acad Sci USA. 2005;102(44):15924–15929. doi: 10.1073/pnas.0507944102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Powell T, Cha D, Jr CL, Feder J (2012) On the scent of standing variation for speciation: Behavioral evidence for native sympatric host races of Rhagoletis pomonella (Diptera:tephritidae) in the southern United States. Evolution 66(9): 2739–2756. [DOI] [PubMed]
- 33.Moyle LC, Levine M, Stanton ML, Wright JW. Hybrid sterility over tens of meters between ecotypes adapted to serpentine and non-serpentine soils. Evol Biol. 2012;39:207–218. [Google Scholar]
- 34.Sim SB, et al. A field test for host fruit odour discrimination and avoidance behaviour for Rhagoletis pomonella flies in the western United States. J Evol Biol. 2012;25(5):961–971. doi: 10.1111/j.1420-9101.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- 35.Rundle HD, Nosil P. Ecological speciation. Ecol Lett. 2005;8:336–352. [Google Scholar]
- 36.Pavlíček T, Sharon D, Kravchenko V, Saaroni H, Nevo E. Microclimatic interslope differences underlying biodiversity contrasts in “Evolution Canyon”, Mt. Carmel, Israel. Isr J Earth Sci. 2003;52:1–9. [Google Scholar]
- 37.Kossover O, Frenkel Z, Korol AB, Nevo E. Genetic diversity and stress of Ricotia lunaria in “Evolution Canyon,” Israel. J Hered. 2009;100(4):432–440. doi: 10.1093/jhered/esp014. [DOI] [PubMed] [Google Scholar]
- 38.Lidzbarsky G, Shkolnik T, Nevo E. Adaptive response to DNA-damaging agents in natural Saccharomyces cerevisiae population “Evolution Canyon”, Mt. Carmel, Israel. PloS One. 2009;4:e:5914. doi: 10.1371/journal.pone.0005914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SR, Rashkovetsky E, Iliadi K, Nevo E, Korol AB. Assortative mating in Drosophila adapted to a microsite ecological gradient. Behav Genet. 2005;35(6):753–764. doi: 10.1007/s10519-005-6119-2. [DOI] [PubMed] [Google Scholar]
- 40.Iliadi K, et al. 2009. [Peculiarities of the courtship song in the Drosophila melanogaster populations adapted to gradient of microecological conditions]. Zh Evol Biokhim Fiziol 45(5):478–485. Russian.
- 41.Harry M, et al. Fine-scale biodiversity of Drosophilidae in “Evolution Canyon” at the Lower Nahal Oren microsite, Israel. Biologia. 1999;54:685–705. [Google Scholar]
- 42.Zamorzaeva I, Rashkovetsky E, Nevo E, Korol AB. Sequence polymorphism of candidate behavioural genes in Drosophila melanogaster flies from ‘Evolution canyon’. Mol Ecol. 2005;14(10):3235–3245. doi: 10.1111/j.1365-294X.2005.02616.x. [DOI] [PubMed] [Google Scholar]
- 43.Nevo E. Mosaic Evolution of Subterranean Mammals: Regression, Progression and Global Convergence. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 44.Blaustein L, Kotler B, Nevo E. Rodent species diversity and microhabitat use along opposing slopes of Lower Nahal Oren, Mount Carmel Israel. Israel J Ecol Evol. 1996;42:327–333. [Google Scholar]
- 45.Nevo E, et al. Genotypic and phenotypic divergence of rodents (Acomy cahirinus and Apodemus mystacinus) at “Evolution Canyon”: Micro- and macroscale parallelism. Acta Theriol (Warsz) 1998;5:9–34. [Google Scholar]
- 46.Broza M, Nevo E. Selective land snail prediation by the spiny mouse, Acomys cahirinus, in Nahal Oren, Mt. Carmel Israel. Isr J Zool. 1994;40:173–176. [Google Scholar]
- 47.Shanas U, Afik D, Scantlebury M, Haim A. Differential osmoregulatory capabilities of common spiny mice (Acomys cahirinus) from adjacent microhabitats. J Zool. 2003;261:7–13. [Google Scholar]
- 48.Scantlebury M, Afik D, Shanas U, Haim A. Comparative non-shivering thermogenesis in adjacent populations of the common spiny mouse (Acomys cahirinus) from opposite slopes: the effects of increasing salinity. J Comp Physiol B. 2002;172(1):1–5. doi: 10.1007/s003600100220. [DOI] [PubMed] [Google Scholar]
- 49.Fitzpatrick BM, Fordyce JA, Gavrilets S. What, if anything, is sympatric speciation? J Evol Biol. 2008;21(6):1452–1459. doi: 10.1111/j.1420-9101.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 50.Conte GL, Arnegard ME, Peichel CL, Schluter D. The probability of genetic parallelism and convergence in natural populations. Proc Biol Sci. 2012;279(1749):5039–5047. doi: 10.1098/rspb.2012.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gershoni M, Templeton AR, Mishmar D. 2009. Mitochondrial bioenergetics as a major motive force of speciation. BioEssays 31:642–650. [DOI] [PubMed]
- 52.Bernstein BE, et al. ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shapiro JA. Evolution: A View from the 21st Century. Upper Saddle River, NJ: FT Press Science; 2011. [Google Scholar]
- 54.Huson DH. SplitsTree: Analyzing and visualizing evolutionary data. Bioinformatics. 1998;14(1):68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.