Significance
The precise roles of E-cadherin in the liver and liver carcinogenesis are still unknown. Here we show that mice lacking E-cadherin in the liver develop spontaneous periportal inflammation via an impaired intrahepatic biliary network, as well as periductal fibrosis, which resembles primary sclerosing cholangitis. Inducible gene knockout studies identified E-cadherin loss in biliary epithelial cells as a causal factor of cholangitis induction, and dysregulated E-cadherin expression was also seen in patients with primary sclerosing cholangitis. E-cadherin loss also significantly accelerates genetically and chemically engineered liver cancer through epithelial–mesenchymal transition, up-regulation of stem cell markers, and ERK activation. Thus, E-cadherin plays critical roles in maintaining homeostasis and suppressing carcinogenesis in the liver.
Keywords: liver progenitor cell, cholangiocellular carcinoma, mixed type tumor
Abstract
E-cadherin is an important adhesion molecule whose loss is associated with progression and poor prognosis of liver cancer. However, it is unclear whether the loss of E-cadherin is a real culprit or a bystander in liver cancer progression. In addition, the precise role of E-cadherin in maintaining liver homeostasis is also still unknown, especially in vivo. Here we demonstrate that liver-specific E-cadherin knockout mice develop spontaneous periportal inflammation via an impaired intrahepatic biliary network, as well as periductal fibrosis, which resembles primary sclerosing cholangitis. Inducible gene knockout studies identified E-cadherin loss in biliary epithelial cells as a causal factor of cholangitis induction. Furthermore, a few of the E-cadherin knockout mice developed spontaneous liver cancer. When knockout of E-cadherin is combined with Ras activation or chemical carcinogen administration, E-cadherin knockout mice display markedly accelerated carcinogenesis and an invasive phenotype associated with epithelial–mesenchymal transition, up-regulation of stem cell markers, and elevated ERK activation. Also in human hepatocellular carcinoma, E-cadherin loss correlates with increased expression of mesenchymal and stem cell markers, and silencing of E-cadherin in hepatocellular carcinoma cell lines causes epithelial–mesenchymal transition and increased invasiveness, suggesting that E-cadherin loss can be a causal factor of these phenotypes. Thus, E-cadherin plays critical roles in maintaining homeostasis and suppressing carcinogenesis in the liver.
E-cadherin is an important adhesion molecule, and not only establishes the core of the epithelial adherens junction with neighboring cells but also participates in intracellular signaling (1, 2). E-cadherin knockout mice die early during embryogenesis due to failed blastocyst and trophectoderm formation (3). Conditional knockout of E-cadherin in skin impairs an epidermal water-barrier function that leads to perinatal lethality (4). In addition, E-cadherin deletion in the differentiating alveolar epithelial cells of mammary gland results in an impaired differentiation program during lactation (5). Thus, although E-cadherin is a key regulator of embryonic development and tissue homeostasis, its role varies depending on the organ. Adult liver parenchyma consists of two types of hepatic epithelial cells, hepatocytes and biliary epithelial cells (BECs), and both cell types express E-cadherin localizing at the junctional complex (6). However, the precise functional role of E-cadherin in the liver is still unknown, especially in vivo.
Dysregulation of E-cadherin also contributes to cancer progression. In fact, mutation or decreased expression of E-cadherin is associated with malignant progression in various cancers, such as gastric, breast, and skin cancer (7). Also in human liver cancers, such as hepatocellular carcinoma (HCC) and cholangiocellular carcinoma (CCC), E-cadherin expression is decreased by 20–60% and is associated with higher histological grade, invasiveness, and poor prognosis (8–10). These findings suggest that E-cadherin may be a tumor suppressor in liver tumorigenesis. However, down-regulation of E-cadherin in liver cancer is caused by several mechanisms, including loss of heterozygosity, methylation of the E-cadherin promoter region, transcriptional repressors, and gene-silencing microRNAs (miRs) (8, 11–13). Transcriptional repressors such as Snail, Slug, and Twist, as well as miR-9, play an important role in induction of the epithelial–mesenchymal transition (EMT), which is a major cancer progression-mediating process. E-cadherin is a major target of these factors; however, they also control other EMT-inducing molecules involved in junctional complexes, intermediate filament networks, and the actin cytoskeleton (14). Therefore, it is unclear whether the loss of E-cadherin is a real culprit or a bystander in EMT induction and liver cancer progression. In addition, expression of E-cadherin can be increased during the early stages of HCC (11), and it has been suggested that preservation of E-cadherin expression may be beneficial for tumor growth, invasion, and metastasis (11, 15). Thus, here we characterize the role of E-cadherin in liver homeostasis and carcinogenesis in vivo using liver-specific E-cadherin knockout mice.
Results
Spontaneous Portal Inflammation and Periductal Fibrosis in CDH1ΔL Mice.
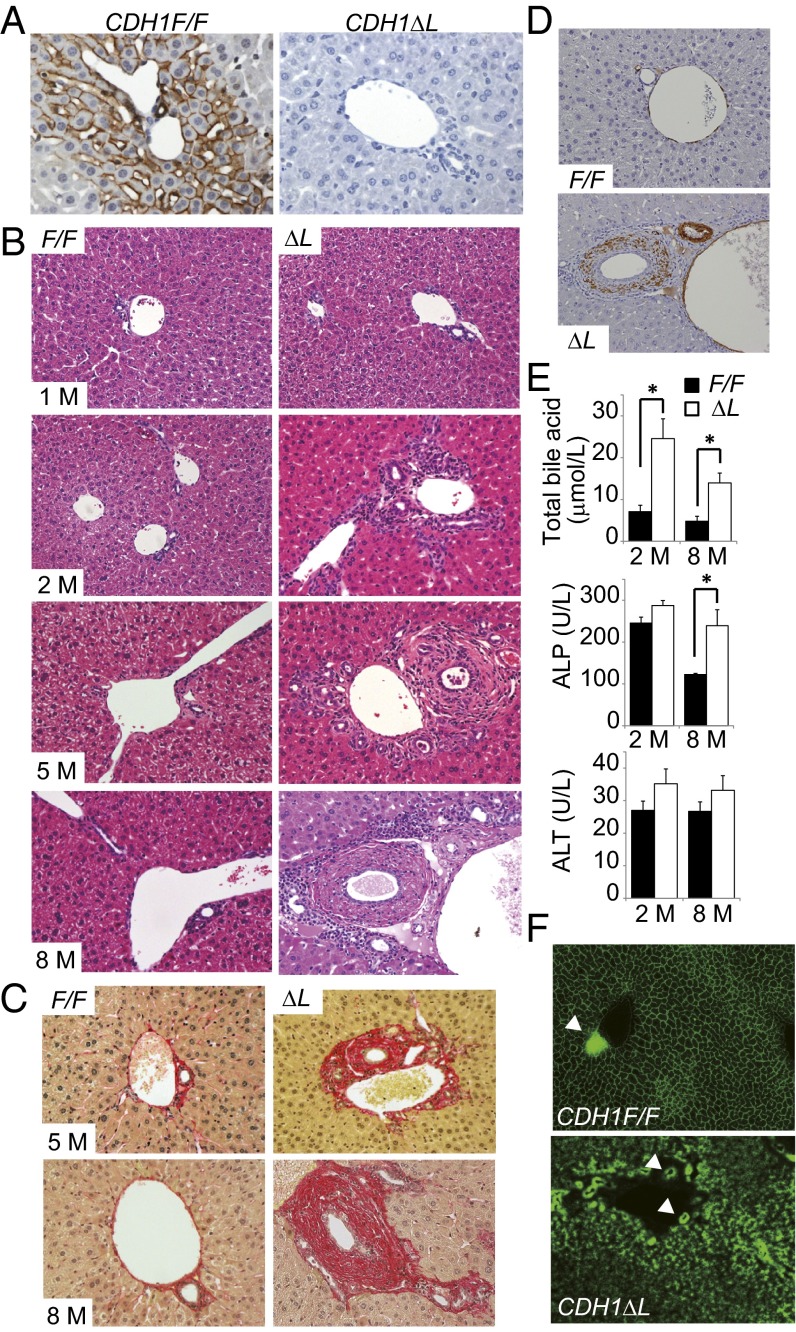
Liver-specific E-cadherin knockout mice (CDH1ΔL) were generated by crossing CDH1 flox/flox (CDH1F/F) mice with albumin-Cre transgenic (Alb-Cre) mice. Immunohistochemistry (IHC) and immunofluorescence (IF) revealed that E-cadherin was expressed in the membrane of hepatocytes, especially in zone 1, and in the interlobular BECs in 1-mo-old CDH1F/F mice, whereas it was completely absent from both hepatocytes and interlobular BECs in CDH1ΔL mice of the same age (Fig. 1A and Fig. S1A). On the other hand, E-cadherin expression was preserved in the large bile duct that is near the common bile duct (Fig. S1B). These results are consistent with recent reports of Cre expression in hepatocytes and interlobular BECs of Alb-Cre mice (16).
Fig. 1.
Spontaneous development of portal inflammation and periductal fibrosis in CDH1ΔL mice. (A) Analysis of E-cadherin expression by IHC of liver sections (×200) obtained from 1-mo-old CDH1F/F and CDH1ΔL mice. (B) H&E staining of 1-, 2-, 5-, and 8-mo-old mice (representative images, ×200). (C) Sirius red staining in 5- and 8-mo-old mice (×200). (D) IHC of α-smooth muscle actin in 8-mo-old mice (×200). (E) Serum levels of total bile acid, ALP, and ALT in 2- and 8-mo-old mice. Results are means ± SEM (n = 5–7 per group; *P < 0.05). (F) Functional analysis of the bile transport system in 2-mo-old mice injected with fluorescent-labeled bile acid in the tail vein for 15 min followed by analysis of staining in the bile canaliculi (×200). Arrowheads indicate interlobular bile duct lumen (n = 3 per group).
The histology of the liver appeared almost normal in 1-mo-old CDH1ΔL mice (Fig. 1B). However, at 2 mo of age, CDH1ΔL mice spontaneously developed periportal inflammation, followed by periductal fibrosis resembling primary sclerosing cholangitis (PSC) at 8 mo of age (Fig. 1 B and C). IHC for α-smooth muscle actin confirmed activation of fibroblasts in the periductal area (Fig. 1D). These histological changes were not seen in CDH1F/F mice. As is the case in cholestasis, serum levels of total bile acid and alkaline phosphatase (ALP) were significantly elevated in CDH1∆L mice compared with CDH1F/F mice (Fig. 1E). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining revealed that TUNEL-positive nonhepatocyte cells were noticeable in the periportal area in CDH1ΔL mice (Fig. S1C). Although these cells were mostly CD45+ leukocytes, they also contained K19-positive BECs, which were completely absent in CDH1F/F mice (Fig. S1 D–F). In comparison, there were no significant differences in serum alanine aminotransferase (ALT) and the number of TUNEL-positive hepatocytes between CDH1F/F mice and CDH1ΔL mice other than a small increase of TUNEL-positive hepatocytes in 2-mo-old CDH1ΔL mice (Fig. 1E and Fig. S1G). Hence, BEC injury could be one of the main factors contributing to development of periductal fibrosis.
Next, we postulated that cholestatic liver injury and periportal inflammation might be caused by disruption of the junction complex due to loss of E-cadherin. However, electron microscopy revealed no obvious morphological abnormalities in the adherens junctions, tight junctions, desmosomes, or bile canaliculi in 2-mo-old CDH1ΔL mice (Fig. S1H). To functionally analyze the bile transport system, we injected fluorescent-labeled bile acid into the tail vein of 2-mo-old mice. Fifteen minutes after the injection, we could see clear canalicular staining in CDH1F/F mouse liver and smooth transport of bile acid into the bile duct lumen. In contrast, the canalicular staining pattern in CDH1ΔL mice was very fuzzy, particularly in zone 1, and bile acid had not yet reached the bile duct lumen (Fig. 1E). These results suggest that the intrahepatic biliary network may be functionally impaired in CDH1ΔL mice, which could lead to cholestatic liver injury and subsequent inflammation.
Loss of E-Cadherin in BECs Rather than Hepatocytes Is a Causal Factor of Cholangitis Induction.
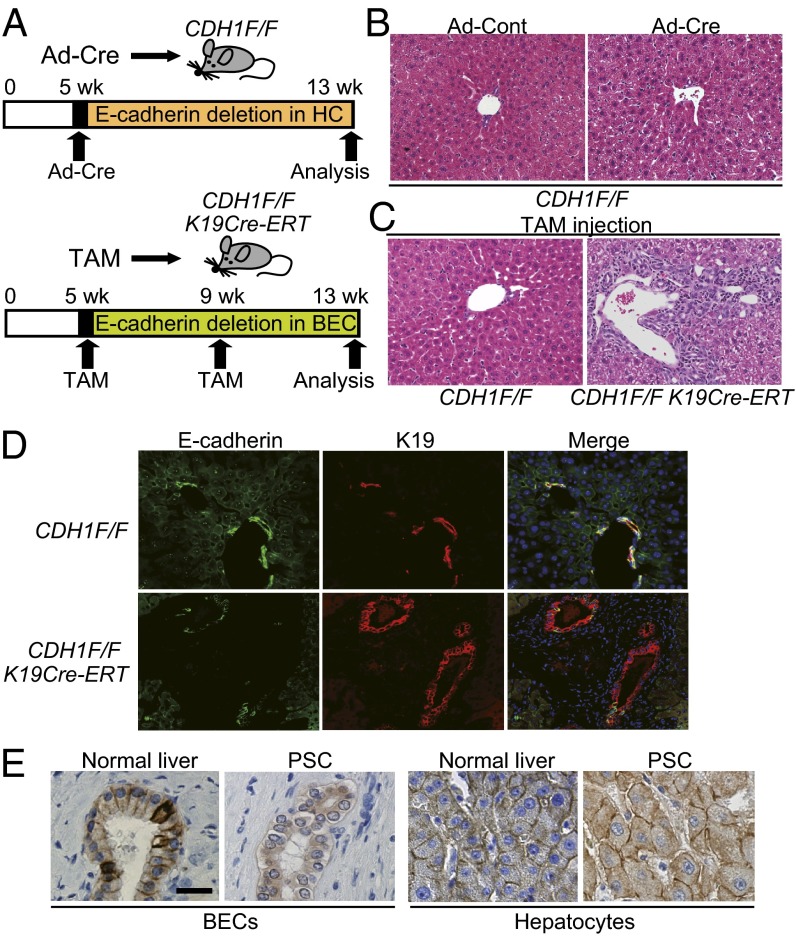
To distinguish the function of E-cadherin in hepatocytes and BECs maintaining liver homeostasis, we generated two different models of E-cadherin deletion in the liver (Fig. 2A). First, to delete E-cadherin only in hepatocytes, we i.v. injected 5-wk-old CDH1F/F mice with adenovirus expressing Cre-recombinase (Ad-Cre) or control adenovirus (Ad-Cont) (17). We confirmed that this method induced Cre-loxP recombination in 73.0 ± 4.2% (mean ± SD) of hepatocytes but no recombination in BECs at 1 wk after injection using Rosa26-lox-stop-lox-YFP mice (Fig. S2A). Although E-cadherin expression was still reduced significantly in Ad-Cre–injected CDH1F/F mice at 8 wk after injection (Fig. S2 B and C), there was no apparent periportal inflammation (Fig. 2B). Next, to delete E-cadherin only in BECs, we crossed CDH1F/F mice with K19CreERT mice in which tamoxifen (TAM)-inducible Cre ERT was knocked into the endogenous K19 locus (CDH1F/F/K19CreERT) (18). According to the previous study, which showed relatively low efficacy of recombination in BECs, we injected TAM twice, at 5 and 9 wk after birth. One week after the first TAM injection into CDH1F/F/K19CreERT mice, E-cadherin expression was deleted in 31.2 ± 7.2% of K19-positive BECs, whereas E-cadherin in the hepatocytes was well-preserved (Fig. S2D). Eight weeks after the first TAM injection, four of eight CDH1F/F/K19CreERT mice revealed significant periportal inflammation as seen in CDH1ΔL mice (Fig. 2C). Although the rate of E-cadherin loss in BECs varied widely in TAM-injected CDH1F/F/K19CreERT mice at this point (25.5 ± 17.1%), bile ducts with strong inflammation tended to show high rates of E-cadherin deletion (Fig. 2D and Fig. S2E). Thus, loss of E-cadherin in BECs rather than hepatocytes is a causal factor of periportal inflammation. Furthermore, in human liver samples, a clear membranous pattern of E-cadherin expression in the epithelial cells of medium-size bile ducts was seen in normal liver, whereas it mostly disappeared, with only fragmented cytoplasmic expression, in four of seven PSC samples (Fig. 2E). In contrast, E-cadherin expression in hepatocytes was well-preserved in all samples. Based on these data, abnormality of E-cadherin expression in BECs might contribute to the pathogenesis of PSC.
Fig. 2.
Loss of E-cadherin in BECs rather than hepatocytes is a causal factor in periportal inflammation. (A) Experimental protocol of E-cadherin deletion in hepatocytes (HC) (Upper) or BECs (Lower). (B) H&E staining of liver sections from Ad-Cont– or Ad-Cre–injected CDH1F/F mice at 8 wk after adenovirus injection (×200). (C and D) Histological analyses of CDH1F/F and CDH1F/F/K19CreERT mice at 8 wk after TAM injection. H&E staining (×200) (C) and double IF staining of E-cadherin (green) and K19 (red) (×400) (D). (E) IHC of E-cadherin in human normal liver and PSC samples (n = 7). (Scale bar, 20 μm.)
Ductular Reaction in CDH1ΔL Mice.
To further characterize CDH1ΔL mice, we conducted cDNA microarray analysis using whole-liver samples from young (2-mo-old) and aged (11-mo-old) CDH1F/F and CDH1ΔL mice. Interestingly, expression of progenitor cell markers such as Sox9, CD44, and Epcam, as well as inflammatory cytokines and chemokines, was up-regulated in both young and aged CDH1ΔL mice compared with age-matched CDH1F/F mice (Fig. S3A). The microarray results were confirmed by IHC of these markers, and we found numerous primitive duct cells expressing progenitor cell markers, the so-called ductular reaction, in the periportal area (Fig. S3B). IHC of Ki67 revealed significant ongoing proliferation of these primitive duct cells but not hepatocytes in CDH1ΔL mice (Fig. S3 B and C). Inflammatory cytokines have been implicated in hepatic progenitor cell induction (19), and CDH1ΔL mice revealed infiltration of numerous F4/80-positive macrophages in the periportal area (Fig. S3D). Macrophage depletion by liposomal clodronate remarkably decreased the expression of interleukin 6, which is one of the major progenitor cells inducing cytokines (19), and subsequently reduced the ductular reaction (Fig. S3 E–G). Thus, the ductular reaction was due, in part, to macrophage-mediated inflammatory response.
Loss of E-Cadherin Accelerates Liver Tumorigenesis.
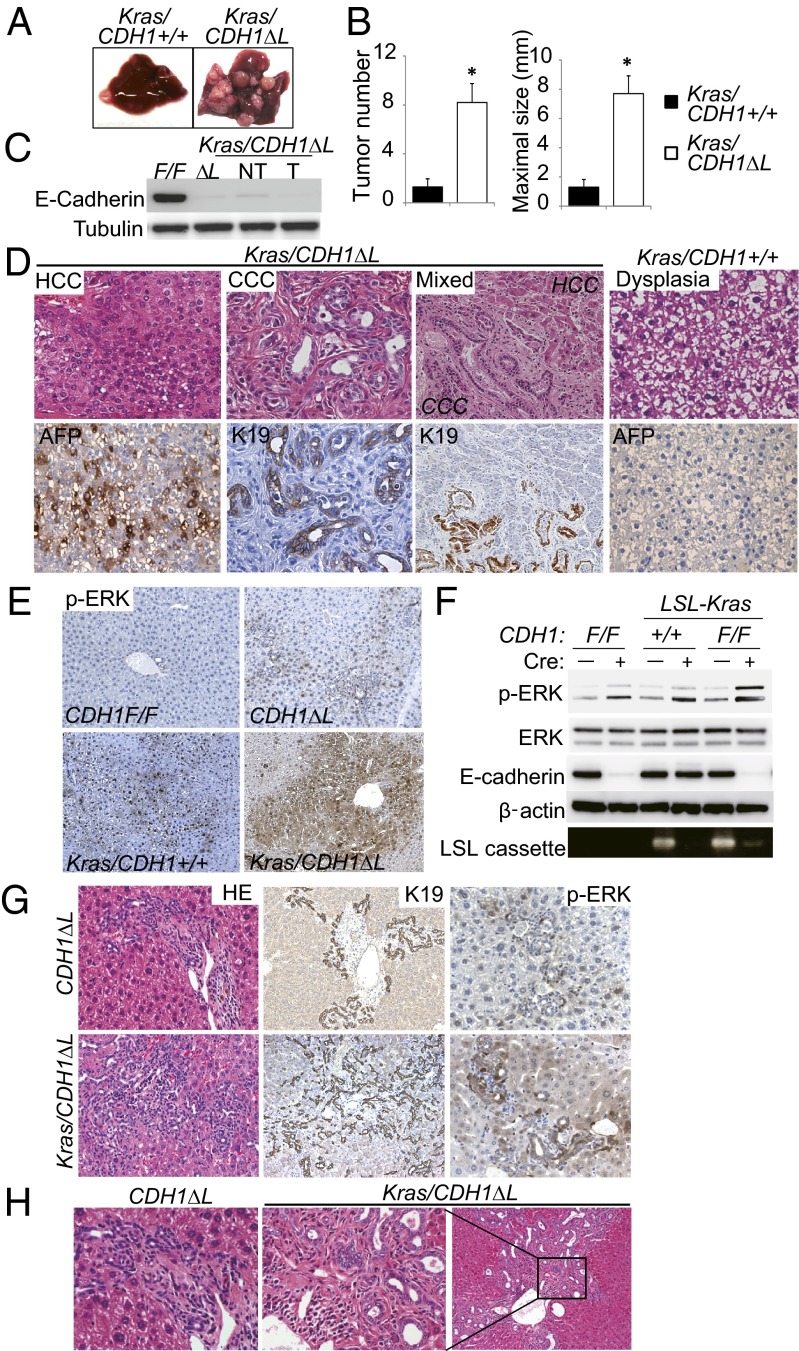
A few of the male CDH1ΔL mice (2/12, 16.7%) spontaneously developed liver tumors at 11 mo of age (Fig. S4 A and B). Most of the tumors were α-fetoprotein (AFP)–positive HCC, and negative for E-cadherin (Fig. S4 C–E). However, the tumor incidence rate in CDH1ΔL mice was too low to investigate the detailed mechanism of hepatocarcinogenesis. Because Ras signaling is frequently activated in human HCC, we generated liver-specific active Kras-expressing CDH1ΔL mice (Kras/CDH1ΔL) by crossing CDH1ΔL mice with KrasG12D conditional knockin (LSL-KrasG12D) mice. Strikingly, all male Kras/CDH1ΔL mice developed multiple liver tumors at 8 mo of age (n = 10), whereas only 4 of 10 male Alb-Cre/LSL-KrasG12D/CDH1 wild-type mice (Kras/CDH1+/+) developed a few macroscopically visible small tumors at the same age (Fig. 3A). Quantitative analyses also showed significant acceleration of liver tumorigenesis by E-cadherin loss (Fig. 3B). We confirmed the absence of E-cadherin in both nontumor and tumor tissue in Kras/CDH1ΔL mice (Fig. 3C). Most of the tumors arising in Kras/CDH1ΔL mice were AFP-positive HCC and ranged from the typical trabecular type to a poorly differentiated type (Fig. 3D). Importantly, CCC and mixed-type HCC/CCC tumors were also seen in 10% and 40% of Kras/CDH1ΔL mice, respectively (Fig. 3D). On the other hand, tumors in Kras/CDH1+/+ mice were mostly AFP-negative dysplastic nodules or well-differentiated HCC (Fig. 3D). These results suggest that loss of E-cadherin cooperates with Ras signaling in liver cancer development.
Fig. 3.
Loss of E-cadherin cooperates with Ras activation in liver cancer development. (A) Representative images of the liver from 8-mo-old Kras/CDH1+/+ and Kras/CDH1ΔL mice. (B) Bar graphs of tumor number and maximal tumor size in each mouse are shown. Data are means ± SEM (n = 10 per group; *P < 0.05). (C) WB analysis of E-cadherin expression in nontumor (NT) and tumor (T) tissues in Kras/CDH1ΔL mice. (D) H&E staining and IHC of AFP and K19 of tumors from Kras/CDH1ΔL and Kras/CDH1+/+ mice (mixed, ×200; others, ×400). (E) IHC analysis of ERK phosphorylation in nontumor areas of 8-mo-old mouse livers (×200). (F) Primary hepatocytes isolated from CDH1F/F, LSL-Kras, and Kras/CDH1F/F mice were infected with Ad-Cont or Ad-Cre. The indicated proteins were assessed by WB 48 h after infection. Cre-mediated recombination of the LSL-Kras allele (deletion of LSL cassette) was confirmed by PCR. (G) Ductular reaction assessment by H&E and IHC of K19 and ERK phosphorylation in primitive duct cells in 8-mo-old mice (H&E and K19, ×200; p-ERK, ×400). (H) H&E staining of the ductular reaction with dysplastic change in Kras/CDH1ΔL mice (Right, ×100; Left and Center, ×400).
Next, we assessed activation of extracellular signal-regulated kinase (ERK), which is a major downstream transducer of Ras, in nontumor tissue. ERK phosphorylation was almost undetectable by IHC in CDH1F/F mouse liver and faintly detected in periportal hepatocytes of CDH1ΔL mice. Kras/CDH1+/+ mice had diffuse ERK phosphorylation that was further increased in Kras/CDH1ΔL mice (Fig. 3E). These findings were confirmed by Western blotting (WB) (Fig. S5A). To eliminate the influence of environmental factors on ERK activation, we isolated primary hepatocytes from CDH1F/F, LSL-Kras/CDH1+/+, and LSL-KRas/CDH1F/F mice (all Cre-negative), and then induced gene recombination by Ad-Cre infection. Ad-Cre efficiently induced gene recombination, and Ras activation and E-cadherin loss cooperatively increased ERK phosphorylation (Fig. 3F). This finding was confirmed in an immortalized human normal hepatocyte (20) by transfection of active Ras and knockdown of E-cadherin (Fig. S5B). E-cadherin was reported to inhibit the phosphorylation of several receptor tyrosine kinases (RTKs) (21), and phospho-RTK array analysis revealed that Ad-Cre–mediated E-cadherin deletion significantly increased only epithelial growth factor receptor (EGFR) phosphorylation (Fig. S5C). Thus, the cooperative activation of ERK by Ras and E-cadherin loss-induced EGFR phosphorylation may explain the accelerated carcinogenesis in Kras/CDH1ΔL mice.
Furthermore, the ductular reaction was also significantly induced in Kras/CDH1ΔL mice, likely due to increased ERK activation in the primitive duct cells (Fig. 3G and Fig. S5D). In addition to quantitative change, morphology of proliferating duct cells in Kras/CDH1ΔL mice was sometimes dysplastic compared with that in CDH1ΔL mice (Fig. 3H). These findings, along with CCC and mixed-type tumors, suggested that some tumors in Kras/CDH1ΔL mice might originate from primitive duct cells, including bipotential progenitor cells induced by inflammation.
Spontaneous EMT, Vascular Invasion, and Intrahepatic Metastasis in Kras/CDH1ΔL Tumors.
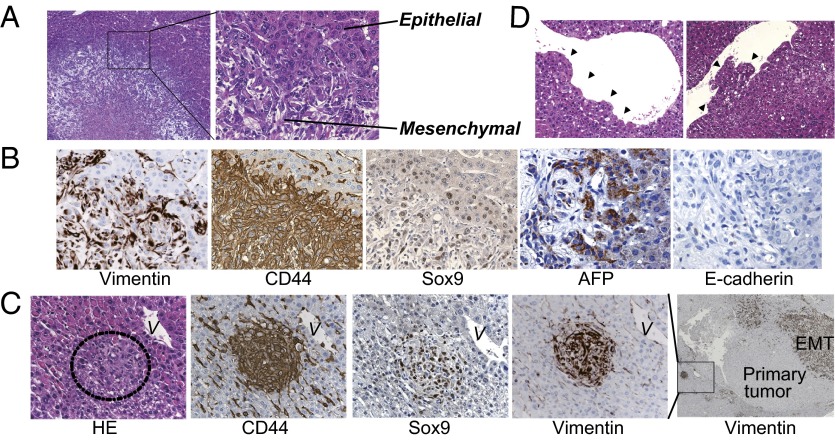
EMT is considered a key process driving tumor invasiveness, and loss of E-cadherin expression is a hallmark of EMT (14). However, whether loss of E-cadherin is a consequence or a cause of EMT remains controversial. Importantly, in some tumors arising in Kras/CDH1ΔL mice, HCC cells gradually transformed into fibroblast-like cells, and these cells are positive for both the HCC marker AFP and the mesenchymal marker vimentin, indicating that spontaneous EMT occurred in the tumors of these mice (Fig. 4 A and B). As expected, these cells were negative for E-cadherin (Fig. 4B). EMT was shown to be associated with a gain of stem cell properties (22). Indeed, evident expression of stem cell markers CD44 and Sox9 was seen in tumor cells undergoing EMT (Fig. 4B). We also found several tiny nodular lesions near the large tumors undergoing EMT. Despite their small size, these lesions resembled the adjacent larger tumor in that they were strongly positive for vimentin, CD44, and Sox9, suggesting that they might represent intrahepatic metastasis (Fig. 4C). However, although tumor cells frequently invaded the central vein lumen in Kras/CDH1ΔL mice (Fig. 4D), we could not find any apparent extrahepatic metastasis in any tissues, including the lymph nodes.
Fig. 4.
Spontaneous epithelial–mesenchymal transition in tumors of Kras/CDH1ΔL mice. (A) H&E staining of tumors from 8-mo-old Kras/CDH1ΔL mice in which HCC cells gradually undergo an EMT-like morphological change (Right, ×400; Left, ×100). (B) Expression of the indicated proteins was assessed by IHC using serial sections (×200). (C) Intrahepatic metastasis-like lesions were seen near the large tumors undergoing EMT. Expression of EMT and stem cell markers in tiny nodular lesions was assessed by IHC using serial sections. V, vein near the lesion (Right, ×50; others, ×400). (D) H&E staining of tumor cell invasion into the central vein (×400). Arrowheads indicate invading cells.
Loss of E-cadherin has been reported to induce β-catenin nuclear translocation that can promote carcinogenesis (23). Although the β-catenin expression was increased in the cancer cell membrane in Kras/CDH1ΔL mice, we could not detect any nuclear translocation in either nontumor or tumor areas (Fig. S6A). In addition, E-cadherin knockdown in HCC cell lines did not induce significant β-catenin nuclear translocation (Fig. S6B). Thus, this mechanism does not play a major role in enhanced carcinogenesis by loss of E-cadherin in the liver.
Loss of E-Cadherin Promotes Chemically Induced HCC.
To further examine the tumor-suppressive role of E-cadherin, CDH1F/F mice and CDH1ΔL mice were injected with diethylnitrosamine (DEN) on postnatal day 14 (20, 24). After 8 mo, CDH1ΔL mice had significantly increased number and size of liver tumors and developed histologically more advanced HCC compared with CDH1F/F mice (Fig. S7 A and B). As seen in Kras/CDH1ΔL mice, strong ERK phosphorylation was seen in tumors of DEN-treated CDH1ΔL mice (Fig. S7C). In addition, although an obvious fibroblast-like morphological change was not seen, some tumors in CDH1ΔL mice strongly expressed CD44 and vimentin, whereas very few tumors in CDH1F/F mice expressed these markers (Fig. S7D). This confirmed that loss of E-cadherin enhances activation of ERK and expression of stem cell and EMT markers in a chemically induced HCC model.
Correlating E-Cadherin Loss with Mesenchymal and Stem Cell Markers in Human HCC.
To investigate whether E-cadherin loss correlates with mesenchymal and stem cell markers in human HCC, we examined the expression of E-cadherin, CD44, and vimentin in human HCC cell lines. There were significant inverse correlations, particularly between E-cadherin and CD44 (Fig. 5A). Among these cell lines, we chose three that expressed E-cadherin, Hep3B, HuH7, and Alexander, and assessed the effect of E-cadherin knockdown with siRNA. After 6 d of E-cadherin knockdown, all three cell lines exhibited elevated expression of mesenchymal markers such as N-cadherin and vimentin and underwent morphological changes that resulted in an elongated mesenchymal-like appearance (Fig. 5 B and C). In addition, invasion capacity was significantly increased by E-cadherin knockdown (Fig. 5D), suggesting that loss of E-cadherin can be a causal factor of EMT and invasive phenotype of HCC.
Fig. 5.
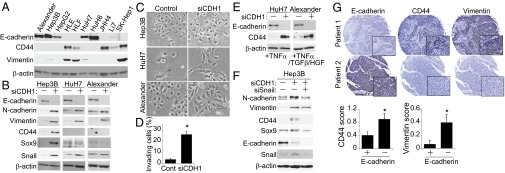
Correlating E-cadherin loss with mesenchymal and stem cell markers in human HCC. (A) WB analysis of the indicated proteins in human HCC cell lines. (B and C) Effect of E-cadherin knockdown on human HCC cell lines. Expression levels of the indicated proteins (B) and morphological changes (C) in HCC cell lines 6 d after transfection with E-cadherin siRNA or controls. (D) Invasion capacity was assessed by invasion assay using Alexander cells. Bars show the percentages of invaded cells (n = 3). Data are means ± SD; *P < 0.05. (E) The effect of E-cadherin knockdown on CD44 expression upon cytokine and/or growth factor stimulation in HuH7 and Alexander cells. Two days after transfection with E-cadherin or control siRNA, cells were stimulated with the indicated cytokines and/or growth factors (10 ng/mL TNF-α, 10 ng/mL HGF, or 1 ng/mL TGF-β). Four days later, the indicated proteins were assessed by WB. (F) Effect of E-cadherin and Snail double knockdown on the expression of mesenchymal and stem cell markers in Hep3B cells. Six days later, the indicated proteins were assessed by WB. (G) IHC of the expression pattern of E-cadherin, CD44, and vimentin using a human HCC tissue array that contains 60 HCC samples (E-cadherin–negative, n = 28; –positive, n = 32). When the number of E-cadherin–positive cells was <25% of the tumor cell population, the sample was defined as E-cadherin–negative. Representative images of two patients are shown (Magnifications: 40×; insets, 400×.). Data are means ± SD; *P < 0.05.
Furthermore, the expression of stem cell markers such as CD44 and Sox9 were also increased upon E-cadherin knockdown in Hep3B cells (Fig. 5B), as seen in mouse HCC models. However, HuH7 and Alexander cells did not show any significant changes in stem cell markers upon E-cadherin knockdown (Fig. 5B). As shown in microarray data (Fig. S3A), various inflammatory cytokines and chemokines were up-regulated in CDH1ΔL mouse livers in vivo. Interestingly, tumor necrosis factor α (TNF-α) stimulation significantly increased CD44 expression in E-cadherin–knocked down HuH7 cells but not in control cells (Fig. 5E). In E-cadherin–knocked down Alexander cells, not TNF-α alone but TNF-α in combination with transforming growth factor β (TGF-β) and hepatocyte growth factor (HGF) significantly increased CD44 expression (Fig. 5E). These findings suggest that loss of E-cadherin can up-regulate CD44 expression cooperatively with the inflammatory environment.
E-cadherin knockdown in Hep3B and HuH7 cells increased expression of Snail (Fig. 5B), which is an upstream transcriptional repressor of E-cadherin that plays important roles in EMT induction. Also in vivo, tumor cells undergoing EMT in Kras/CDH1ΔL mice strongly expressed Snail (Fig. S8A). Additional knockdown of Snail in Hep3B cells suppressed up-regulation of mesenchymal and stem cell markers caused by E-cadherin knockdown (Fig. 5F), suggesting that Snail may link E-cadherin loss to EMT.
Finally, we investigated the expression of E-cadherin, CD44, and vimentin using a human HCC tissue array. Expression of E-cadherin tended to be lost in advanced-stage HCC (percentage of E-cadherin loss: 33.3% in stage I/II and 55.6% in stage III; P = 0.088). CD44 and vimentin expression exhibited significant inverse correlations with E-cadherin expression (Fig. 5G).
Discussion
This study shows that E-cadherin plays critical roles in maintaining liver homeostasis. Although we assumed that periportal inflammation in CDH1ΔL mice might be caused by disruption of the junction complex, electron microscopy showed no morphological abnormalities. This is consistent with a previous report (25). However, our functional analysis of the bile transport system using fluorescent-labeled bile acid showed impaired bile acid flow around zone 1. E-cadherin deletion in BECs by crossing CDH1F/F mice with K19CreERT mice also triggered periportal inflammation similar to that seen in CDH1ΔL mice. Therefore, loss of E-cadherin, especially in the intrahepatic bile duct epithelium, functionally impairs biliary flow, and subsequently induces cholestatic liver injury and sclerosing cholangitis. Our analysis of human PSC samples showed dysregulated E-cadherin expression in BECs, and another group also reported similar findings (26). Thus, abnormality of E-cadherin expression in BECs might contribute to the pathogenesis of PSC. Of note, PSC is well-known as an extraintestinal manifestation of ulcerative colitis (UC), and a recent genome-wide association study showed that single-nucleotide polymorphism in the CDH1 gene was associated with susceptibility to UC (27). Combined with our finding, impaired function of E-cadherin may be a common factor of these coexisting diseases.
Our data also suggest that E-cadherin is a tumor suppressor in the liver. A few CDH1ΔL mice develop spontaneous HCC, and when knockout of E-cadherin is combined with Ras activation or chemical carcinogen administration, CDH1ΔL mice display markedly accelerated carcinogenesis and an invasive phenotype. Although it has not been clear whether the loss of E-cadherin is a consequence or a cause of EMT, we have definitively demonstrated its causal role in vivo and in vitro. Recent reports have established a direct link between EMT and a gain of stem/progenitor cell properties (22), which is supported by our mouse models because tumor cells undergoing EMT clearly expressed stem cell markers. The expression of stem cell markers such as CD44 and Sox9 has been reported to be associated with poor prognosis in patients with HCC (28, 29). Interestingly, E-cadherin knockdown was sufficient to up-regulate both EMT and stem cell markers in Hep3B cells, whereas additional supplementation of inflammatory cytokines and/or growth factors was necessary for up-regulation of CD44 in HuH7 and Alexander cells (Fig. 5E). Therefore, E-cadherin loss in the tumor cells and inflammatory environment might synergistically up-regulate stem cell markers in our mouse models.
E-cadherin interacts with various molecules and controls activation of related signaling pathways. In our study, increased ERK activation was implicated in accelerated tumorigenesis by loss of E-cadherin. It has been shown that E-cadherin can negatively regulate activation of divergent classes of RTKs such as EGFR, insulin-like growth factor 1 receptor, and c-Met by inhibiting receptor mobility (21); all of this can activate ERK signaling. Our analysis using the phospho-RTK array revealed that deletion of E-cadherin in primary hepatocytes increased EGFR phosphorylation without influencing other RTKs, suggesting that EGFR activation might be one explanation for E-cadherin loss-induced ERK phosphorylation in the liver.
The cellular source of liver cancers is a hot topic at present. Kras/CDH1ΔL mice revealed not only an increase of the ductular reaction but also dysplastic change of ductal cells. These findings, along with CCC and mixed-type tumors, suggested that some tumors might originate from primitive duct cells, including bipotential progenitor cells. However, we also found some well-differentiated hepatocellular lesions that were adjacent to the central vein and distant from proliferating duct cells (Fig. S8B), suggesting that they may be derived from mature hepatocytes. In Kras/CDH1+/+ mice, dysplastic foci were randomly found in zones 1, 2, and 3. Thus, we consider that the tumors in Kras/CDH1ΔL mice may originate from both proliferating duct cells, including progenitor cells induced by inflammation and mature hepatocytes transformed by Ras activation. Meanwhile, recent studies suggested that mature hepatocytes also could transform to CCC (30, 31). Therefore, further analyses such as cell-lineage tracing are needed to definitively determine this issue.
In summary, loss of E-cadherin in the liver, especially in BECs, causes impairment of the intrahepatic biliary network and subsequent inflammatory reactions that lead to progenitor cell proliferation and periductal fibrosis. In some cases, these progenitor cells may lead directly to tumor development through oncogenic mutations such as Kras. In mature hepatocytes, loss of E-cadherin leads to ERK activation, EMT induction, and up-regulation of stem cell markers, which eventually results in enhanced carcinogenesis and an invasive phenotype (Fig. S9). Thus, E-cadherin plays critical roles in maintaining homeostasis and suppressing carcinogenesis in the liver.
Materials and Methods
CDH1F/F, Alb-Cre, Lox-stop-lox KrasG12D, and Rosa26-lox-stop-lox-YFP mice were purchased from The Jackson Laboratory. K19CreERT mice were a generous gift from Guoqiang Gu (Vanderbilt University, Nashville, TN) (18). All mice were on the C57BL/6 genetic background. All animal experiments were approved by the Ethics Committee for Animal Experimentation at the Institute for Adult Diseases, Asahi Life Foundation, University of California, San Diego, and University of Tokyo, and conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals. For details of reagents, biochemical analyses, histology, microarray, cell culture, and statistical analyses, please see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Alan Hofmann for providing fluorescent-labeled bile acid and Dr. Shoen Kume (Kumamoto University) for handling the mice. This study was supported by a Grant-in-Aid for Scientific Research (25893042 to H.N.; 22300317 and 23114508 to S.M.; 23590933 to Y. Hirata), the Uehara Memorial Foundation (S.M.), and the Daiichi Sankyo Foundation of Life Science (H.N.). Work at the University of California, San Diego, was supported by the National Institutes of Health (CA118165 and CA155120).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322731111/-/DCSupplemental.
References
- 1.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6(8):622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 2.Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1(5):a002949. doi: 10.1101/cshperspect.a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tunggal JA, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24(6):1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115(1-2):53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 6.Ihara A, Koizumi H, Hashizume R, Uchikoshi T. Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology. 1996;23(6):1441–1447. doi: 10.1053/jhep.1996.v23.pm0008675162. [DOI] [PubMed] [Google Scholar]
- 7.Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1(6):a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura T, Makino R, Mitamura K. Frequent down-regulation of E-cadherin by genetic and epigenetic changes in the malignant progression of hepatocellular carcinomas. Clin Cancer Res. 2001;7(3):594–599. [PubMed] [Google Scholar]
- 9.Lim SO, et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: Methylation of the E-cadherin promoter. Gastroenterology. 2008;135(6):2128–2140. doi: 10.1053/j.gastro.2008.07.027. e8. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol. 2002;161(3):1015–1022. doi: 10.1016/S0002-9440(10)64262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, et al. Altered expression of E-cadherin in hepatocellular carcinoma: Correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology. 2002;36(3):692–701. doi: 10.1053/jhep.2002.35342. [DOI] [PubMed] [Google Scholar]
- 12.Yang MH, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50(5):1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osada T, et al. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24(6):1460–1467. doi: 10.1053/jhep.1996.v24.pm0008938181. [DOI] [PubMed] [Google Scholar]
- 16.Malato Y, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. 2011;121(12):4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akagi K, et al. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25(9):1766–1773. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46(6):318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137(2):466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa H, et al. Apoptosis signal-regulating kinase 1 inhibits hepatocarcinogenesis by controlling the tumor-suppressing function of stress-activated mitogen-activated protein kinase. Hepatology. 2011;54(1):185–195. doi: 10.1002/hep.24357. [DOI] [PubMed] [Google Scholar]
- 21.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23(8):1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112(Pt 8):1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 24.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121(7):977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Battle MA, et al. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci USA. 2006;103(22):8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rygiel KA, et al. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008;88(2):112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 27.Barrett JC, et al. UK IBD Genetics Consortium Wellcome Trust Case Control Consortium 2 Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41(12):1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang GH, et al. Osteopontin combined with CD44, a novel prognostic biomarker for patients with hepatocellular carcinoma undergoing curative resection. Oncologist. 2008;13(11):1155–1165. doi: 10.1634/theoncologist.2008-0081. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, et al. Expression features of SOX9 associate with tumor progression and poor prognosis of hepatocellular carcinoma. Diagn Pathol. 2012;7:44. doi: 10.1186/1746-1596-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan B, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122(8):2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122(11):3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.