Abstract
Background
Prospective studies of serum HCC biomarkers in patients with advanced hepatitis C are lacking.
Aims
To determine frequencies and performance of elevated alpha-fetoprotein (AFP), AFP-L3, and des-gamma-carboxy prothrombin (DCP) levels as HCC biomarkers in advanced hepatitis C.
Methods
Patients in the HALT-C Trial were tested every 3 months for 42 months. Screening ultrasound was performed every 12 months. Levels of biomarkers were compared in patients in whom HCC did or did not develop.
Results
855 patients were evaluated; HCC developed in 46. Among patients without HCC, 73.2% had AFP consistently <20, 24.5% had at least one AFP between 20-199, while 2.3% had at least one AFP value ≥200 ng/mL; 73.7% had DCP consistently <90, 11.6% had at least one DCP between 90-149, and 14.7% had at least one DCP value ≥150 mAU/mL. AFP-L3 ≥10% was present at least once in 9.0% and in 17.1% of those with AFP >20 ng/mL. Among all patients with elevated biomarkers, a diagnosis of HCC was made in 0-31.6% (depending on the biomarker and cutoff) during the subsequent 24 months. AFP ≥200 ng/mL had the highest specificity (99%), but sensitivity was ≤20%. DCP ≥40 mAU/mL had the highest sensitivity (76%), but specificity was ≤58%. Independent predictors of elevated AFP were gender (female), race (Black), more advanced disease, and HCC. Elevated DCP was associated with more advanced disease and HCC.
Conclusions
Mild-moderate elevations in total AFP and DCP but not AFP-L3 occur frequently in patients with chronic hepatitis C and advanced fibrosis, are related to factors other than HCC, and are poor predictors of HCC.
Keywords: interferon, cirrhosis, alpha fetoprotein, des-gamma-carboxy prothrombin
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide, and its incidence in the United States has more than doubled in the past 25 years [1], principally a consequence of hepatitis C virus (HCV) infection [2]. The five-year survival rate of patients with HCC is below 10% [1], because of late detection and lack of treatment options for patients with advanced-stage disease. Detection of early HCC (single nodule <2 cm) at a stage of disease amenable to treatment (liver transplantation, surgical resection, chemoembolization, or percutaneous ablation) [3, 4] is a goal of clinical management. Screening strategies for detection of early HCC have relied primarily on radiologic imaging [5-9] and serum biomarkers. Alpha-fetoprotein (AFP) is the most commonly used biomarker, but its sensitivity and specificity in detecting HCC are poor. AFP levels are increased more often in persons with cirrhosis without HCC than in patients with HCC [10-12]. Accordingly, the American Association for the Study of Liver Diseases (AASLD) Practice Guidelines Committee recommended the use of ultrasound alone, without AFP, for HCC surveillance in patients with cirrhosis [13].
Other tumor biomarkers have been proposed to complement or substitute for AFP in HCC detection [14, 15]. The two biomarkers currently used clinically are Lens culinaris agglutinin (LCA)-reactive fraction of alpha-fetoprotein (AFP-L3) [16-19] and des-gamma-carboxy prothrombin (DCP), an abnormal prothrombin molecule that is generated as a result of an acquired defect in the posttranslational carboxylation of the prothrombin precursor in malignant cells [20-27]. These tumor markers have been adopted routinely in Japan since the late 1990s, but few prospective studies evaluating their usefulness in North American populations have been performed [28]. Because AFP and DCP represent independent tumor proteins, several studies have shown that they may be complementary in the detection of HCC [29-32].
Few studies have involved cohorts of at-risk persons followed prospectively to determine and compare the frequency of elevated biomarkers among those in whom HCC did and did not develop. In the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) Trial, patients with chronic hepatitis C and bridging fibrosis or cirrhosis were followed prospectively, allowing us to examine the relationships of serum biomarkers to incidence of HCC. In a previous nested case control study that included 39 HCC cases and 77 matched controls, we focused on the performance of biomarkers in the diagnosis of HCC [32], In the current analysis, we measured AFP, AFP-L3, and DCP serially and determined the frequency and factors associated with elevated HCC biomarkers (AFP, AFP-L3 and DCP) and their test characteristics (sensitivity, specificity, predictive value, and area under the receiver operating characteristic curve [AUROC]) in all HALT-C subjects with and without HCC.
Patients and Methods
HALT-C Trial design, HCC definition, and surveillance
The HALT-C Trial was a multicenter, prospective study conducted at 10 clinical sites of the safety and efficacy of half-dose, long-term maintenance peginterferon treatment in patients with histologically advanced hepatitis C [33]. Inclusion criteria were age ≥18 years, chronic hepatitis C with advanced fibrosis (Ishak fibrosis score ≥3), nonresponse to prior interferon ± ribavirin treatment, and absence of laboratory markers or clinical events associated with hepatic decompensation. All patients were required to have an ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) with no evidence of hepatic mass lesions suspicious for HCC and a serum AFP <200 ng/mL prior to enrollment (protocol exceptions were allowed for three patients who had AFP values of 206, 212, and 315 ng/mL, respectively, and negative imaging).
Patients in whom HCV RNA remained detectable after 20 weeks of lead-in treatment with peginterferon alfa-2a, 180 μg weekly, and ribavirin, 1000/1200 mg/day, were randomized at week 24 to either no treatment (control group) or to continue peginterferon alfa-2a monotherapy at a dose of 90 μg weekly (treatment group). Patients were seen every 3 months through month 48 of the randomized trial to assess for clinical endpoints and adverse events. Blood samples were collected at each visit and tested for AFP immediately after the visit at the clinical laboratory of each participating hospital. For this study, in contrast to our prior report [34], frozen serum samples collected at each visit through Month 48 (42 months after randomization) were tested for all biomarkers, (AFP, AFPL3 and DCP) at a central laboratory (Wako Laboratories, Richmond, VA) in all patients. Results of these assays were not available to investigators during the trial. Per-protocol ultrasound examinations of the liver were repeated 6 and 12 months after enrollment and again every 12 months. Patients with an elevated or rising AFP by local laboratory and those with new lesions on ultrasound were evaluated further by CT or MRI, at the discretion of the site investigator. After Month 48, patients were tested for AFP at the local laboratory and ultrasounds were performed at 6-month intervals but blood samples were not sent to the central laboratory for testing of AFP, AFPL3 and DCP; therefore, only patients diagnosed to have HCC by month 60 were included in this analysis.
HCC was defined as previously described in the HALT-C Trial [34]. Definite HCC was defined by histologic confirmation or by the appearance of a new mass lesion on imaging with AFP levels increasing to ≥1,000 ng/mL. Presumed HCC was defined by the appearance of a new mass lesion on ultrasound in the absence of histology and with AFP <1,000 ng/mL in conjunction with one of the following: a) two liver imaging studies showing a mass lesion with characteristics of HCC (arterial enhancement ± wash out), b) progressively enlarging lesion(s) on ultrasound leading to death, or c) one additional imaging study showing a mass lesion with characteristics of HCC that either increased in size over time or was accompanied by an AFP level rising to >200 ng/mL and more than a tripling of the baseline value. All cases of HCC were adjudicated by an Outcomes Review Panel, blinded to study site and randomization allocation, to ascertain that cases met predefined diagnostic criteria and to determine the date when these criteria were first met. The radiology reports of all HCC cases were reviewed retrospectively by the HCC working group to determine the date when the last imaging showed no hepatic lesion, the date when imaging first revealed a suspicious nodule, and the tumor stage at diagnosis. This analysis focused on patients without HCC (definite or presumed) at any time during the HALT-C Trial, we included HCC cases diagnosed through Month 60 (12 months after last biomarker evaluation) for comparison.
Definition of biomarker cut-off values and time periods
For AFP, we defined three “absolute” cutoff values and one “relative” cutoff value. The absolute cutoff values were an AFP ≥20, ≥50, or ≥200 ng/mL. The relative AFP cutoff value had to meet the following two criteria: 1) AFP ≥20 ng/mL and 2) AFP value more than twice the average AFP values during the prior 12-month period. For DCP, we also defined three absolute cutoff values (≥40, ≥90, and ≥150 mAU/mL) and one relative cutoff value. We chose 90 mAU/mL as the middle cutoff value, because prior analysis of the HALT-C Trial data had suggested that a DCP cutoff of 87 mAU/mL had the best test performance in differentiating HCC cases from controls [34]. The relative DCP cutoff value had to meet the following two criteria: 1) DCP ≥40 and 2) DCP value more than twice the average DCP values during the prior 12-month period (see below for time periods). An AFP-L3 ≥10% was considered abnormal as per the manufacturer.
We divided the first 60 months of the HALT-C study into consecutive 12 month time periods (months 1-12, 13-24, 25-36, 37-48, and 49-60) to account for the variable time periods that patients were followed. Results in the first 12-month period were not included in this analysis, because randomization occurred at month 6, and patients who met the criteria for HCC in the first 6 months would not have been randomized. In addition, full-dose peginterferon and ribavirin treatment had been shown to decrease AFP levels [12]. According to the study protocol, patients should have had biomarker levels assessed four times during each 12-month period. For the purposes of these analyses, we used the highest value for each biomarker during the 12-month period, and for HCC cases, we used only the biomarker values up to the HALT-C Trial visit immediately prior to the diagnosis of HCC. Also, to be included in this analysis, patients had to be followed for at least 6 months after randomization, had to have at least one visit in the first 18 months after randomization, and, if they had an AFP or DCP elevation, had to be followed for at least one year after the first elevation. In assessing the occurrence of HCC, we determined the number of patients in whom presumed or definite HCC was identified during the same 12-month time period as the biomarker was drawn and the number of patients in whom HCC was identified during the subsequent 12-month time period. Thus, for our determination of the sensitivity and specificity of the biomarkers, the interval between the biomarker testing and the diagnosis of HCC ranged from 0 to 24 months.
Statistical analyses
Statistical analyses were performed at the Data Coordinating Center with SAS release 9.1 (SAS® Institute, Cary, NC). All reported P values are 2-sided. We used Cox proportional hazards regression to assess the relationship between baseline characteristics and elevated AFP or DCP during months 13 to 48 and logistic regression to assess the association between patient characteristics and elevations in AFP or DCP during a 12-month period. For this analysis, we used clinical and laboratory data at month 12, 24, or 36 to predict the likelihood of AFP or DCP elevation during months 13-24, 25-36, and 37-48, respectively. To determine the accuracy of biomarker elevations in predicting the diagnosis of HCC during the same time period or the next 12 months, we classified HCC cases into four groups according to the date of the last study visit before the diagnosis of HCC, months 13-24, 25-36, 37-48, and 49-60. Sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, area under the receiver operating characteristic curve (AUROC), and P value from a logistic regression were calculated to evaluate the association between an elevated biomarker during a given 12-month period and the development of HCC in that time period or the next 12-month period. For this analysis, patients in whom HCC developed more than a year after the end of the current study period (i.e., after month 60) were excluded. Because we previously reported that increased AFP can be seen in the absence of HCC [12] and that HCC can occur in the absence of cirrhosis [34], we included all patients with advanced fibrosis rather than just those with cirrhosis.
Results
Baseline characteristics of the patients
A total of 1,050 patients were randomized in the HALT-C Trial. Of these, 54 met criteria for definite or presumed HCC by month 60. Eight HCC cases were excluded from these analyses for one of the following reasons: (a) prevalent HCC, defined as HCC diagnosed within 6 months after randomization (n = 4), (b) missing laboratory data prior to diagnosis (n = 2), and (c) patients with a diagnosis of presumed HCC who did not receive treatment for HCC and were followed for at least 24 months with no evidence of radiologic or clinical progression of their liver nodules (n = 2). An additional 187 patients without HCC by month 60 were excluded because they were followed for fewer than 6 months after randomization (n = 41), missed all visits in the first 18 months after randomization (n = 40), were taking Coumadin, which can markedly elevate DCP in the absence of HCC by inhibiting vitamin k coagulation (n = 11), did not have an AFP or DCP value 1 year after their first AFP or DCP elevation (n = 59), or had HCC between month 60 and end of the study (n = 36). In total, 46 patients with HCC diagnosed by month 60 and 809 patients without HCC at any time during the course of the HALT-C Trial were included in this analysis. The baseline characteristics of the patients with and without at least one elevated AFP and/or DCP during months 13 to 48 are shown in Table 1. Those with total AFP ≥20 ng/dL on ≥1 occasion during months 13 to 48 were less likely male, less likely non-Hispanic white, with higher values of BMI, AST, ALT, and alkaline phosphatase, and more advanced disease (lower platelet count, higher AST-platelet ratio index [APRI], model for end-stage liver disease [MELD], and cirrhosis) compared to those with total AFP persistently <20 ng/dL. In those with elevated AFP, HCC was also more likely to develop (10% versus 3%; P <0.0001). No differences were apparent in mean baseline DCP values between those with AFP < or ≥20 ng/mL. Conversely, those with DCP ≥90 mAU/mL were more likely male and had higher total AFP compared to those with DCP <90 mAU/mL. Similarly, in subjects with elevated DCP, liver disease was more advanced and HCC was more likely to develop (11% versus 3%; P <0.0001).
Table 1.
Baseline data for patients with and without elevated HCC biomarkers at least once during months 13-48 [data presented as mean (SD) or percent].
| AFP mg/dL | DCP mAU/mL | |||||
|---|---|---|---|---|---|---|
| Baseline Variables | <20 (N = 613) |
≥20 (N = 242) |
P value* | <90 (N = 616) |
≥90 (N = 239) |
P value** |
| Age | 50.2 (7.3) | 49.5 (6.7) | 0.22 | 50.1 (7.1) | 49.9 (7.3) | 0.20 |
| Female, % | 26% | 36% | 0.003 | 31% | 23% | 0.03 |
| White-non-hispanic, % | 78% | 57% | <0.0001 | 72% | 72% | 0.54 |
| Hispanic, % | 6% | 12% | 7% | 10% | ||
| Black, % | 14% | 28% | 19% | 16% | ||
| Other race/ethnicity, % | 2% | 3% | 2% | 2% | ||
| Body Mass Index, kg/m2 | 29.6 (5.3) | 30.6 (5.7) | 0.03 | 29.8 (5.2) | 30.1 (6.1) | 0.28 |
| Diabetes, % | 23% | 28% | 0.16 | 25% | 24% | 0.84 |
| Log HCV RNA (IU/mL) | 6.46 (0.51) | 6.38 (0.54) | 0.05 | 6.46 (0.50) | 6.36 (0.56) | 0.002 |
| Platelets ×1000/mm3 | 175 (66) | 145 (57) | <0.0001 | 172 (63) | 153 (67) | <0.0001 |
| Albumin, g/dL | 3.9 (0.4) | 3.8 (0.4) | <0.0001 | 3.9 (0.35) | 3.8 (0.45) | <0.0001 |
| AST, U/L | 79 (57) | 104 (59) | <0.0001 | 83 (56) | 96 (65) | 0.002 |
| ALT, U/L | 103 (78) | 118 (76) | 0.01 | 106 (72) | 109 (92) | 0.55 |
| Alkaline phosphatase, U/L | 94 (42) | 113 (52) | <0.0001 | 95 (44) | 109 (49) | <0.0001 |
| Total bilirubin, mg/dL | 0.76 (0.40) | 0.85 (0.42) | 0.005 | 0.76 (0.37) | 0.85 (0.48) | 0.0001 |
| Prothrombin time, INR | 1.03 (0.10) | 1.05 (0.10) | 0.009 | 1.02 (0.10) | 1.06 (0.11) | <0.0001 |
| AST/ALT ratio | 0.83 (0.27) | 0.95 (0.30) | <0.0001 | 0.83 (0.26) | 0.95 (0.31) | <0.0001 |
| APRI | 1.34 (1.35) | 2.14 (1.80) | <0.0001 | 1.44 (1.43) | 1.88 (1.75) | <0.0001 |
| MELD | 6.9 (1.4) | 7.2 (1.4) | 0.03 | 6.89 (1.26) | 7.29 (1.61) | <0.0001 |
| AFP, ng/mL | 6.0 (8.9) | 38.4 (50.8) | <0.0001 | 13.8 (28.5) | 18.9 (38.2) | 0.004 |
| DCP, mAU/mL | 28.3 (174) | 23.0 (38.2) | 0.16 | 15.3 (17.7) | 56.2 (277) | <0.0001 |
| Cirrhosis on biopsy, % | 35% | 55% | <0.0001 | 37% | 51% | <0.0001 |
| Esophageal varices, % | 24% | 26% | 0.45 | 21% | 35% | <0.0001 |
| Treatment group ***, % | 51% | 49% | 0.60 | 49% | 56% | 0.07 |
| HCC, % | 3% | 10% | <0.0001 | 3% | 11% | <0.0001 |
APRI = AST to platelet ratio index; MELD = Model for End Stage Liver Disease; HCC = hepatocellular carcinoma
Cox proportional hazards regression comparing those with AFP <20 and ≥ 20 ng/mL,
Cox proportional hazards regression comparing those with DCP <90 and ≥ 90 mAU/mL
Patients randomized to long-term treatment with peginterferon alfa-2a monotherapy at a dose of 90 μg weekly
Frequency of AFP, AFP-L3 and DCP elevations
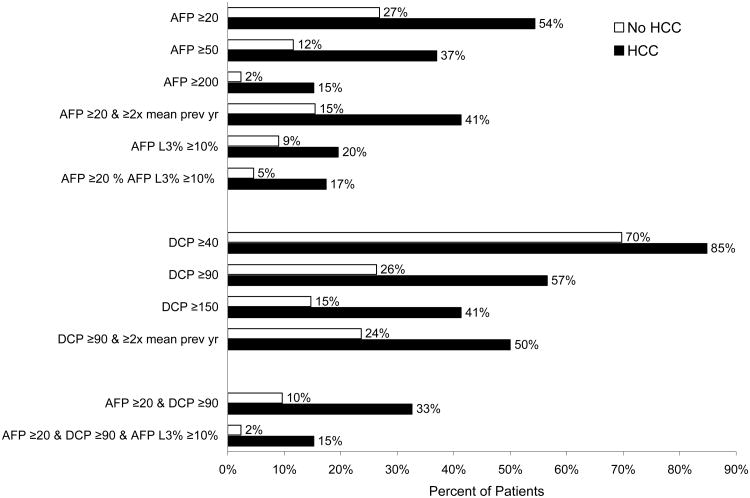
Table 2 shows the frequency of elevated AFP, DCP and AFP-L3 levels during the entire 36 months of the study (months 13 to 48) for all patients. The average number of measurements per patient was 10.5 (out of a possible 12), with 80% having more than 9 measurements. Overall, a higher percent of patients had elevated DCP than elevated AFP values.
Table 2. Percent of patients with biomarker elevations during months 13- 48*.
| Biomarker Elevation, % | (N=855) |
|---|---|
| AFP ≥20 ng/ml | 28.3% |
| AFP ≥50 ng/ml | 13.0% |
| AFP ≥200 ng/ml | 3.0% |
| AFP ≥20 & 2× mean prev yr, % | 16.8% |
| AFP L3% ≥10%, % | 9.6% |
| AFP ≥20 & AFP L3% ≥10%, % | 5.3% |
| DCP ≥40 mAU/mL, % elevated | 70.5% |
| DCP ≥90 mAU/mL, % elevated | 28.0% |
| DCP ≥150 mAU/mL, % elevated | 16.1% |
| DCP ≥90 & 2× mean prev yr, % | 25.0% |
| AFP ≥20 & DCP ≥90, % | 10.9% |
| AFP ≥20 & DCP ≥90 & AFP L3% ≥10%, % | 3.0% |
Percent of patients, cases and controls combined, with a biomarker above the specified limits at any one of the 12 study visits from month 13 to month 48 (0.5 to 3.5 years after randomization).
Of the 855 patients tested during months 13-48, 28.3%, 13.0%, and 3.0% had peak AFP values ≥20, ≥50, and ≥200 ng/mL, respectively; while 70.5%, 28.0%, and 16.1% had peak DCP values ≥40, ≥90, and ≥150 mAU/mL, respectively. When biomarker elevation was defined based not only on absolute values but also on an increase to ≥2-fold the mean value in the previous year, 16.8% of patients had peak AFP ≥20 ng/mL and ≥2 fold the mean value in the previous year, while 25.0% of patients had peak DCP ≥90 mAU/mL and ≥2 fold the mean value in the previous year. Contrary to the high frequency of elevated AFP or DCP values, only 9.6% of all patients regardless of total AFP levels had AFP-L3 ≥10%. Analysis of the combination of these biomarkers showed that 10.9% had peak AFP ≥20 ng/mL and peak DCP ≥90 U/mL, 4.4% had elevated total AFP and AFP-L3% values, and only 3.0% had peak AFP ≥20 ng/mL, peak DCP ≥90 U/mL, and peak AFP-L3 ≥10%.
Factors associated with AFP or DCP elevations
To determine baseline demographic and laboratory factors associated with AFP or DCP elevations, we compared patients with AFP ≥20 ng/mL on at least one occasion during each time period to the patients who had AFP persistently <20 ng/mL, and we compared patients with DCP ≥90 mAU/mL on at least one occasion during each time period to the patients who had DCP persistently <90 mAU/mL during the same time period (Table 3). Multivariate logistic regression analysis showed that AFP elevation during months 37-48 was associated independently with female gender, Black ethnicity, a low platelet count at month 36, high AST at month 36, high alkaline phosphatase at month 36, and HCC during that time period or the following time period (i.e., months 37 to 60). Logistic regression analysis revealed that DCP elevation during months 37-48 was associated independently with non-Black ethnicity, randomization to peginterferon, low albumin at month 36, high prothrombin time/international normalized ratio (INR) at month 36, and HCC during that time period or the subsequent time period. Similar associations were noted when regression analyses were performed to determine predictors of elevated AFP or DCP during months 13-24 and month 25-36, except that a diagnosis of HCC at months 13-36 was not associated with biomarker elevations at months 13-24 (data available in supplementary Table A).
Table 3. Predictors at month 36 of elevated biomarkers, DCP ≥90 and AFP ≥20 during months 37-48.
| Month 36 | DCP ≥90 | AFP ≥20 | ||||||
|---|---|---|---|---|---|---|---|---|
| Predicting | 14.9% (108/724), AUC = 0.76 | 20.4% (148/724), AUC = 0.81 | ||||||
| Months 37-48 | OR | 95% CI | p | OR | 95% CI | p | ||
| Case | 4.26 | 1.75 | 10.39 | 0.002 | 3.51 | 1.35 | 9.12 | 0.01 |
| Treatment | 1.66 | 1.06 | 2.61 | 0.03 | 0.73 | 0.47 | 1.12 | 0.15 |
| Female | 0.72 | 0.43 | 1.20 | 0.20 | 2.41 | 1.56 | 3.72 | <0.0001 |
| Black | 0.50 | 0.26 | 0.96 | 0.04 | 2.41 | 1.47 | 3.96 | 0.0005 |
| Platelets* (100) | 0.46 | 0.32 | 0.68 | <0.0001 | ||||
| Albumin** (1) | 0.40 | 0.24 | 0.68 | 0.0006 | ||||
| AST* (100) | 2.54 | 1.76 | 3.67 | <0.0001 | ||||
| Alk Phos* (100) | 1.83 | 1.22 | 2.74 | 0.004 | ||||
| INR^ (0.5) | 5.21 | 2.01 | 13.50 | 0.0007 | ||||
Stepwise logistic regressions were performed including all of the variables listed. The first four were kept in all models; the other variables were retained if the P value was less than 0.05. Values in bold are statistically significant.
per 100 platelets, AST U/L and alkaline phosphatase U/L.
per 1 g/dL albumin,
per 0.5 INR units
Biomarker elevation in patients with and without HCC
The frequency of elevated biomarkers at any time between months 13 and 48 during a mean study period of 34 months for patients without HCC and 27 months for HCC cases and a mean number of 11 tests for patients without HCC and seven for HCC cases, is shown in Figure 1. Although the frequency of biomarker elevation was more common in those with HCC, importantly, biomarker elevation was also common in those without HCC. Fifty-three percent of patients without HCC and 22% of HCC cases had no biomarker elevations throughout the study. Only 17.1% of those without HCC and with total AFP values >20 ng/dL had increased levels of AFP-L3%. Therefore, the negative predictive value for AFP-L3 in those with elevated total AFP ≥20 ng/mL was 92%.
Figure 1. Frequency of DCP, AFP, and AFP-L3 elevations in 809 controls and 46 HCC cases during months 13 - 48.
To control for the effect of varying durations of follow-up, we analyzed the percentage of patients who met HALT-C Trial criteria for HCC during the same time period and during the subsequent 12 months. When biomarker elevations at months 37-48 were evaluated as predictors of a diagnosis of HCC during months 37-60, the percent of patients with a diagnosis of HCC ranged from 4% to 63% (Table 4). The frequency of HCC diagnosis was highest among patients with DCP ≥90, AFP ≥20 and AFP-L3 ≥10% (5/8, 63%), second highest among those with AFP ≥20 and AFP-L3 ≥10% (6/19, 31.6%), and third highest among those with AFP ≥200 ng/mL (5/16, 31.3%). When biomarker elevations earlier in the course of the HALT-C Trial were analyzed, the frequency of HCC diagnosis was lower (Supplementary Table B).
Table 4.
Performance of biomarker elevations during months 37 to 48 in predicting HCC during months 37-60 in the entire cohort (table 4a) and in those with cirrhosis (table 4b).
| Table 4a All Patients |
No HCC | HCC | Total | Sens* | Spec* | PPV | NPV | % Elev | AUC | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 718 | 25 | 743 | |||||||
| Maximum M37-M48 | ||||||||||
| AFP >=20, % | 138 | 15 | 153 | 60% | 81% | 10% | 98% | 21% | 0.70 | <0.0001 |
| AFP >=50, % | 57 | 10 | 67 | 40% | 92% | 15% | 98% | 9% | 0.66 | <0.0001 |
| AFP >=200, % | 11 | 5 | 16 | 20% | 98% | 31% | 97% | 2% | 0.59 | <0.0001 |
| AFP >=20 & 2× mean prev yr, % | 31 | 8 | 39 | 32% | 96% | 21% | 98% | 5% | 0.64 | <0.0001 |
| AFP L3% >=10%, % | 31 | 6 | 37 | 24% | 96% | 16% | 97% | 5% | 0.60 | <0.0001 |
| AFP >=20 & AFP L3% >10%, % | 13 | 6 | 19 | 24% | 98% | 32% | 97% | 3% | 0.61 | <0.0001 |
| DCP >=40, % | 358 | 16 | 374 | 64% | 50% | 4% | 98% | 50% | 0.57 | 0.17 |
| DCP >=90, % | 100 | 12 | 112 | 48% | 86% | 11% | 98% | 15% | 0.67 | <0.0001 |
| DCP >=150, % | 58 | 11 | 69 | 44% | 92% | 16% | 98% | 9% | 0.68 | <0.0001 |
| DCP >=90 & 2× mean prev yr, % | 71 | 10 | 81 | 40% | 90% | 12% | 98% | 11% | 0.65 | <0.0001 |
| AFP >=20 & DCP >=90, % | 22 | 7 | 29 | 28% | 97% | 24% | 97% | 4% | 0.63 | <0.0001 |
| AFP >=20 & DCP >=90 & AFP L3% > 10%, % | 3 | 5 | 8 | 20% | 100% | 63% | 97% | 1% | 0.60 | <0.0001 |
| Table 4b Patients with cirrhosis at S00 or M24 | No HCC | HCC | Total | Sens* | Spec* | PPV | NPV | % Elev | AUC | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 358 | 20 | 378 | |||||||
| Maximum M37-M48 | ||||||||||
| AFP >=20, % | 93 | 11 | 104 | 55% | 74% | 11% | 97% | 28% | 0.65 | 0.007 |
| AFP >=50, % | 43 | 8 | 51 | 40% | 88% | 16% | 96% | 13% | 0.64 | 0.001 |
| AFP >=200, % | 9 | 4 | 13 | 20% | 97% | 31% | 96% | 3% | 0.59 | 0.0005 |
| AFP >=20 & 2× mean prev yr, % | 21 | 5 | 26 | 25% | 94% | 19% | 96% | 7% | 0.60 | 0.003 |
| AFP L3% >=10%, % | 18 | 5 | 23 | 25% | 95% | 22% | 96% | 6% | 0.60 | 0.001 |
| AFP >=20 & AFP L3% >10%, % | 9 | 5 | 14 | 25% | 97% | 36% | 96% | 4% | 0.61 | <0.0001 |
| DCP >=40, % | 205 | 13 | 218 | 65% | 43% | 6% | 96% | 58% | 0.54 | 0.50 |
| DCP >=90, % | 65 | 11 | 76 | 55% | 82% | 14% | 97% | 20% | 0.68 | 0.0003 |
| DCP >=150, % | 40 | 10 | 50 | 50% | 89% | 20% | 97% | 13% | 0.69 | <0.0001 |
| DCP >=90 & 2× mean prev yr, % | 47 | 9 | 56 | 45% | 87% | 16% | 97% | 15% | 0.66 | 0.0004 |
| AFP >=20 & DCP >=90, % | 20 | 6 | 26 | 30% | 94% | 23% | 96% | 7% | 0.62 | 0.0002 |
| AFP >=20 & DCP >=90 & AFP L3% > 10%, % | 3 | 4 | 7 | 20% | 99% | 57% | 96% | 2% | 0.60 | <0.0001 |
This table includes 743 patients who had at least one biomarker measurement between months 37 and 48 and did not develop HCC before month 37. Twenty-five of these developed HCC between months 37 and 60. Patients are classified according to the maximum biomarker measurement during months 37-48. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), were calculated comparing patients with and without HCC. Area under the receiver operating characteristic curve (AUC) and P values are based on logistic regression analyses.
Test Characteristics of AFP, DCP and AFP-L3 in the detection of HCC
Because patients were followed for variable periods of time, we calculated estimates of sensitivity and specificity based on three 12-month time periods. Table 4 shows the sensitivity, specificity, positive and negative predictive values, and AUROC of biomarker elevations during months 37-48 in detecting HCC during the same time period and the ensuing 12 months, i.e., from month 37 to month 60 in all patients (table 4a) and in only those with cirrhosis (table 4b). We used cutoffs for DCP and AFP so that we could look at combinations of biomarkers. ROC curves for total AFP and DCP as continuous predictors are shown in Figures 2a (for the entire cohort) and in Figure 2b (just those with cirrhosis). In the entire cohort, DCP ≥40 mAU/mL had the highest sensitivity, 64%, but specificity was only 50%. AFP ≥200 ng/mL, AFP-L3 ≥10%, or a combination of all three biomarkers (AFP ≥20 ng/mL, DCP ≥40 mAU/mL, and AFP-L3 ≥10%) had the highest specificity, ≥98%, but sensitivity varied from 20% to 24%. AFP ≥20 ng/mL had the highest AUROC of 0.70, with a sensitivity of 60% and specificity of 81%. The negative predictive values (NPV) of all three biomarkers were high (≥97%), while the positive predictive values (PPV) for individual tests were poor. When individual biomarkers were evaluated, PPV for total AFP ≥200 ng/mL was 31%, for total AFP ≥20 ng/mL combined with AFP-L3 ≥10% was 32%, and for total AFP ≥20 ng/mL, AFP-L3 ≥10%, and DCP ≥90 mAU/mL was 63%, but the latter was based on only eight patients (Table 4a). Similar results were observed in those with cirrhosis (table 4b). The sensitivity of biomarker elevations during months 13-24 or months 25-36 in detecting HCC in the entire cohort was substantially lower (supplemental Table B), although the specificity was similar.
Figure 2.
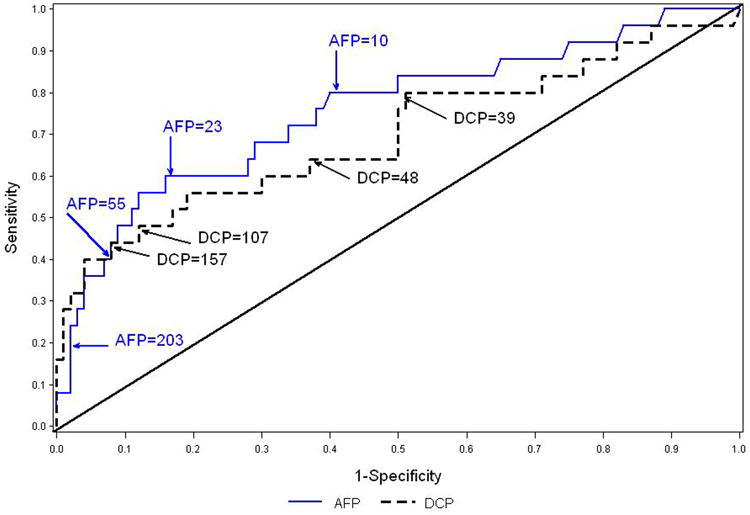
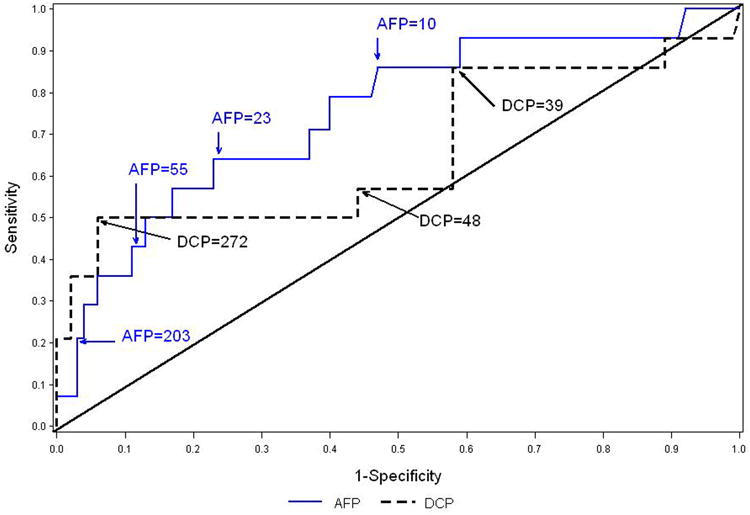
Figure 2a. ROC curves for maximum AFP and DCP between months 37 and 48 as predictors of HCC during months 37-60. The analysis includes 743 patients, 25 of whom developed HCC. The areas under the ROC curve are 0.76 for AFP and 0.70 for DCP. Figure 2b. ROC curves for maximum AFP and DCP between months 37 and 48 as predictors of HCC during months 37-60 for patients with cirrhosis at S00 or M24. The analysis includes 378 patients, 20 of whom developed HCC. . The areas under the ROC curve are 0.75 for AFP and 0.66 for DCP.
Discussion
The utility of biomarkers in HCC surveillance remains controversial. For decades, clinicians have relied on AFP for HCC surveillance, but the value of this marker is now doubted [10-13]. Two novel tumor markers—AFP-L3 and DCP—proposed to complement or substitute for AFP for the diagnosis of HCC [14, 15] have been adopted widely in Japan since the late 1990s. Few prospective studies, however, have been done to evaluate the usefulness of these new biomarkers in North American populations [33].
Earlier, we reported that AFP elevations identified at the time of routine study visits during the HALT-C Trial were substantially more likely to occur in the absence than in the presence of HCC. Elevation of AFP was not only nonspecific but also insensitive as a marker of HCC in our cohort of patients with advanced fibrosis and chronic hepatitis C [12], suggesting that the increased levels in such patients reflect the hepatocellular regeneration (hepatocyte turnover) that occurs in the setting of chronic liver injury and nodular architectural reorganization. Unlike our prior study of baseline AFP [12] or a nested case control study that focused on subjects with HCC [32], this analysis included all HALT-C subjects followed prospectively. We observed that AFP levels were higher in patients with cirrhosis than in those with bridging fibrosis and correlated with the level of hepatic inflammation, i.e., increased AST, high AST/ALT ratio, high log serum ferritin, high histologic activity index (grade of hepatic inflammation), as well as with degree of fibrosis, i.e., low platelet count and increased fibrosis score, and, unexpectedly, with Mallory bodies detected histologically [12]. A link between AFP elevation and hepatic inflammation was reinforced by the reduction in AFP during antiviral therapy [12].
Alpha fetoprotein has long been used as a screening test for HCC, but because most AFP elevations in chronic hepatitis C are not associated with HCC, AFP testing has the potential to generate anxiety for patients and physicians and prompt expensive, sometimes invasive additional tests. Therefore, improved biomarkers for the detection of HCC command a high priority. The HALT-C Trial, which involved patients with advanced chronic hepatitis C followed for a prolonged time period, provided an excellent population in which to assess the test characteristics of AFP and the two novel biomarkers purported to improve the accuracy of HCC detection. In this ancillary study of the prospective HALT-C Trial, we evaluated AFP, DCP and AFP-L3 as biomarkers for detection of HCC on serial frozen serum samples. The two newer biomarkers fared no better than AFP; AFP, DCP, and AFP-L3 elevations were common in patients without HCC and were influenced by race, gender, age, and severity of liver disease. and these other patient factors were more likely to account for biomarker elevation than HCCexplaining in part, the poor performance of these biomarkers in the detection of HCC.
Most prior studies supporting the value of HCC biomarkers in patients with chronic liver disease were cross-sectional in design [19, 26, 29, 30, 35]. In such cross-sectional studies, the frequency of elevated biomarkers and factors associated with their elevation in patients without HCC have remained poorly defined, and the value of these biomarkers in predicting the presence or absence of HCC in patients followed prospectively over time cannot be determined.
To assess the specificity of total AFP, AFP-L3% and DCP as biomarkers of HCC, we examined the levels of these biomarkers over time in 855 patients with chronic HCV infection and advanced fibrosis. In agreement with Nakamura et al [26] and Leerapun et al [19], we observed that 25.3% of HALT-C Trial subjects had a modest elevation of total AFP levels (20-199 ng/mL), and, like Nguyen et al [35], we found that an AFP value ≥200 ng/mL was rare (3.0%). Furthermore, in those that had total AFP ≥200 ng/mL, HCC was present in only 5/16 patients. Identified in our analysis but not reported previously, a ≥2-fold increase in total AFP over the average value during the preceding year was observed in 16.8%. Also, similar to data reported by Marrero [29], 11.6% of subjects in our study without HCC had modestly elevated DCP values (90-149 mAU/mL), while 14.7% had markedly increased values, ≥150 mAU/mL. With regard to AFP-L3, only 9.0% of subjects without HCC in this study had levels ≥10%, not dissimilar from the frequency reported by the Early Detection Research Network (EDRN) investigators [29], but lower than the 15% frequency among 169 cirrhotic patients without HCC reported by Volk et al [30]. Because other studies included patients with cirrhosis of a variety of causes, while ours was confined to patients with chronic hepatitis C, direct comparisons between prior data and ours is difficult.
In addition to being associated with the current or future presence of HCC, elevated levels of AFP and of the other biomarkers were more frequent in younger patients, women, blacks, and patients with more advanced fibrosis (Table 3). Nguyen et al [35] found that total AFP levels were lower in African Americans than in Caucasians with HCC, and, thus, our contradictory finding that being African American was associated independently with increased levels of AFP in those without HCC requires further investigation. Lower AFP levels in patients with HCC and higher AFP levels in those with advanced liver disease but no HCC would make AFP an even less desirable biomarker for HCC surveillance among African Americans with cirrhosis. As was the case for total AFP, one predictor of an increase in DCP was the presence of more advanced liver disease, as demonstrated by low albumin, high INR, and high AST/ALT ratio, but, unlike increased AFP levels, an increased DCP level was associated with male gender, suggesting gender differences between elevated AFP and DCP. Because elevations of each marker appear to be affected by different patient characteristics, biomarkers may be more useful when used in combination [28,32], supported by the observation that only 11% of subjects without HCC had elevations in levels of both AFP and DCP levels.
Because there were only 46 cases of HCC, this study was not powered to assess performance characteristics of these biomarkers nor for ultrasound for HCC surveillance. Of the patients with HCC, 46% had total AFP values <20 ng/mL at all visits prior to diagnosis, and only 41% had an AFP value >20 ng/mL associated with a ≥2-fold increase over the mean value observed during the year preceding diagnosis (data from Figure 1). Similarly, 43% of those with HCC had low (<90 mAU/mL) DCP values, and 50% had a value >90 mAU/mL associated with a ≥2-fold increase over the mean value observed during the year preceding diagnosis. Only nine (20%) patients with HCC had an elevated AFP-L3% values. One of these patients had a maximum AFP of 15; the other 8 had elevated AFP values ranging from 36 to 6540. These data suggest strongly that these tumor markers have limited sensitivity in the diagnosis of HCC when used alone, and they are not much better when used in combination, because only 15 (33%) patients with HCC had combined elevations of AFP (>20 ng/mL) and DCP (>90 mAU/mL). The high NPV of biomarkers in our study in part reflects the low incidence of HCC in our study population. Because the majority of patients with HCV and advanced fibrosis seen in every day practice do not have HCC, we believe our results are valid and are applicable to real life practice. We acknowledge that because we were blinded to the results of AFPL3 and DCP, and AFP value could be used as a criterion for HCC diagnosis, our results may have been biased in favor of total AFP; however, we would point out that only very high AFP values (>1000 ng/mL or >200 ng/mL and >3-times baseline value) in association with a new lesion on imaging qualify for the diagnosis of HCC.
In conclusion, mild-moderate elevations in total AFP and DCP occur frequently in patients with chronic HCV infection and advanced fibrosis in the absence of HCC, while AFP-L3 levels >10% are uncommon. In addition, several factors (gender, age, race, and presence of more advanced liver disease) are independent predictors of increased AFP and DCP. Finally, marked elevations in both AFP and DCP values were uncommon in subjects without HCC, suggesting that their specificity increases as cut-off values increase; however, we could not identify a high-cut-off value for these biomarkers with adequate test characteristics for a clinically useful screening assay. We conclude that AFP, AFP-L3, and DCP lack the sensitivity, specificity, and predictive values required for routine HCC surveillance.
Supplementary Material
What is current knowledge
Alpha-fetoprotein (AFP) is the most commonly used biomarker in surveillance for hepatocellular carcinoma (HCC), but its sensitivity and specificity in detecting HCC are poor.
Although other tumor biomarkers, des-carboxy prothrombin (DCP) and AFP-L3, have been proposed to complement or substitute for AFP in HCC detection, few prospective studies evaluating their usefulness in North American populations have been performed.
The utility of biomarkers in HCC surveillance remains controversial.
What is new
Mild-moderate elevations in total AFP and DCP occur frequently in patients with chronic HCV infection and advanced fibrosis in the absence of HCC, while AFP-L3 levels >10% are uncommon.
Several factors (gender, age, race, and presence of more advanced liver disease) are independent predictors of increased AFP and DCP.
Marked elevations in both AFP and DCP values were uncommon in subjects without HCC, suggesting that their specificity increases as cut-off values increase.
AFP, AFP-L3, and DCP lack the sensitivity, specificity, and predictive values required for routine HCC surveillance.
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc. (now Genentech), and Wako Chemicals USA, Inc. through Cooperative Research and Development Agreements (CRADAs) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Herbert L. Bonkovsky, MD, Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01), Gregory T. Everson, MD, Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: Marc G. Ghany, MD, T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, MPH, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) Chihiro Morishima, MD, David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Hae-Young Kim, DrPH
Inova Fairfax Hospital, Falls Church, VA: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- AFP
alpha fetoprotein
- DCP
des-gamma-carboxy prothrombin
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis
- CT
computed tomography
- MRI
magnetic resonance imaging
- AUROC
area under receiver operating characteristic curve
Footnotes
This is publication #67 from the HALT-C Trial Group.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
- R.K. Sterling is a consultant and receives research support;
- T.R. Morgan receives research support;
- J.C. Hoefs is on the Speaker's Bureau;
- A.M. Di Bisceglie is a consultant and receives research support; and
- A.S. Lok is a consultant and receives research support.
- R.K. Sterling is a consultant; and
- T.R. Morgan receives research support.
- E.C. Wright,
- L.B. Seeff, and
- J.L. Dienstag.
Contributor Information
Richard K. Sterling, Email: RKSterli@vcu.edu.
Elizabeth C. Wright, Email: wrightel@niddk.nih.gov.
Timothy R. Morgan, Email: timothy.morgan@va.gov.
Leonard B. Seeff, Email: seeffl@extra.niddk.nih.gov.
John C. Hoefs, Email: jchoefs@uci.edu.
Adrian M. Di Bisceglie, Email: dibiscam@slu.edu.
Jules L. Dienstag, Email: jdienstag@partners.org.
Anna S. Lok, Email: ASLok@umich.edu.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–26. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–7. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Saitoh S, Koida I, et al. Imaging diagnosis of small hepatocellular carcinoma. Hepatology. 1994;20:82–7. doi: 10.1016/0270-9139(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 6.Oka H, Tamori A, Kuroki T, et al. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–6. [PubMed] [Google Scholar]
- 7.Takayasu K, Furukawa H, Wakao F, M, et al. S CT diagnosis of early hepatocellular carcinoma: sensitivity, findings, and CT-pathologic correlation. AJR Am J Roentgenol. 1995;164:885–90. doi: 10.2214/ajr.164.4.7726041. [DOI] [PubMed] [Google Scholar]
- 8.Ebara M, Ohto M, Watanabe Y, et al. Diagnosis of small hepatocellular carcinoma: correlation of MR imaging and tumor histologic studies. Radiology. 1986;159:371–7. doi: 10.1148/radiology.159.2.3008213. [DOI] [PubMed] [Google Scholar]
- 9.Colli A, Fraquelli M, Conte D. Alpha-fetoprotein and hepatocellular carcinoma. Am J Gastroenterol. 2006;101:1939. doi: 10.1111/j.1572-0241.2006.00684_3.x. author reply 1940-1. [DOI] [PubMed] [Google Scholar]
- 10.Di Bisceglie AM, Hoofnagle JH. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer. 1989;64:2117–20. doi: 10.1002/1097-0142(19891115)64:10<2117::aid-cncr2820641024>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis C. Am J Gastroenterol. 1998;93:2452–6. doi: 10.1111/j.1572-0241.1998.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–41. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrero JA, Lok AS. Newer markers for hepatocellular carcinoma. Gastroenterology. 2004;127:S113–9. doi: 10.1053/j.gastro.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis. 2006;26:385–90. doi: 10.1055/s-2006-951606. [DOI] [PubMed] [Google Scholar]
- 16.Taketa K, Sekiya C, Namiki M, et al. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology. 1990;99:508–18. doi: 10.1016/0016-5085(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 17.Taketa K, Endo Y, Sekiya C, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–23. [PubMed] [Google Scholar]
- 18.Oka H, Saito A, Ito K, et al. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–83. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 19.Leerapun A, Suravarapu SV, Bida JP, et al. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5:394–402. doi: 10.1016/j.cgh.2006.12.005. quiz 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makuuchi M, Kokudo N, Arii S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 21.Aoyagi Y, Oguro M, Yanagi M, et al. Clinical significance of simultaneous determinations of alpha-fetoprotein and des-gamma-carboxy prothrombin in monitoring recurrence in patients with hepatocellular carcinoma. Cancer. 1996;77:1781–6. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1781::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Nomura F, Ishijima M, Kuwa K, et al. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol. 1999;94:650–4. doi: 10.1111/j.1572-0241.1999.00930.x. [DOI] [PubMed] [Google Scholar]
- 23.Mita Y, Aoyagi Y, Yanagi M, et al. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643–8. doi: 10.1002/(sici)1097-0142(19980501)82:9<1643::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Ishii M, Gama H, Chida N, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–40. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamamura K, Shiratori Y, Shiina S, et al. Unique clinical characteristics of patients with hepatocellular carcinoma who present with high plasma des-gamma-carboxy prothrombin and low serum alpha-fetoprotein. Cancer. 2000;88:1557–64. doi: 10.1002/(sici)1097-0142(20000401)88:7<1557::aid-cncr9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura S, Nouso K, Sakaguchi K, et al. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–43. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 27.Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–21. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 28.Sterling RK, Jeffers L, Gordon F, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104–13. doi: 10.1016/j.cgh.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 29.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–8. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volk ML, Hernandez JC, Su GL, et al. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79–87. doi: 10.3233/cbm-2007-3202. [DOI] [PubMed] [Google Scholar]
- 31.Carr BI, Kanke F, Wise M, Satomura S. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci. 2007;52:776–82. doi: 10.1007/s10620-006-9541-2. [DOI] [PubMed] [Google Scholar]
- 32.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen MH, Garcia RT, Simpson PW, et al. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–7. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.