Abstract
The strength of the Ag receptor signal influences development and negative selection of B cells, and it might also affect B-cell survival and selection in the GC. Here, we have used mice with B-cell-specific deletion of the 5′-inositol phosphatase SHIP as a model to study affinity selection in cells that are hyperresponsive to Ag and cytokine receptor stimulation. In the absence of SHIP, B cells have lower thresholds for Ag- and interferon (IFN)-induced activation, resulting in augmented negative selection in the BM and enhanced B-cell maturation in the periphery. Despite a tendency to spontaneously downregulate surface IgM expression, SHIP deficiency does not alter anergy induction in response to soluble hen-egg lysozyme Ag in the MDA4 transgenic model. SHIP-deficient B cells spontaneously produce isotype-switched antibodies; however, they are poor responders in immunization and infection models. While SHIP-deficient B cells form GCs and undergo mutation, they are not properly selected for high-affinity antibodies. These results illustrate the importance of negative regulation of B-cell responses, as lower thresholds for B-cell activation promote survival of low affinity and deleterious receptors to the detriment of optimal Ab affinity maturation.
Keywords: Affinity maturation, B cell, Negative selection, SHIP
Introduction
BCR signaling is essential for the initiation of humoral responses and regulation of B-cell development and maturation [1-3]. It is generally believed that in the absence of foreign Ags, BCR continuously receives activation signals [4], presumably through the binding of self-ligands. The strength of BCR signaling is determined both by the extent of Ag engagement and by the involvement of coreceptors and intracellular effector molecules that modulate signaling events. Modifications that alter the strength of BCR signaling are expected to have an effect on final B-cell activation outcomes; however, these alterations also affect development, activation, and interactions with other cell types, making it difficult to ascertain whether each particular stage in the B-cell activation process requires modulation of BCR signal [5-7]. Enhancement of BCR signaling intensity in several KO mice leads to alterations in humoral responses that have been interpreted to be a consequence of developmental skewing or homing defects. For example, deficiency in the tyrosine phosphatase SHP-1 results in reduced isotype switched B-cell responses, which has been attributed to severe skewing of the B-cell repertoire toward a B1 cell differentiation path and reduced development of more effective responders, B2 cells [8]. CD22 deficiency also detrimentally affects humoral responses, though in this case alterations are attributed to the lower production of marginal zone (MZ) B cells and lack of recirculating B-cell populations [9]. Targeted deletion of Grb2, an adaptor protein that plays a significant role in negative regulatory processes, to the B-cell lineage results in reduced Ab responses that has been ascribed to the lack of lymphoid organization and GC defects [10, 11]. These examples illustrate the range of functional dysregulation that accompanies removal of various negative regulators of B-cell activation, making it difficult to discern which of these effects is responsible for the poor overall response.
The 5′-inositol phosphatase SHIP is another well-characterized modulatory factor in B cells that can associate with Grb2 and CD22 [12,13]. SHIP regulates cell responses in lymphocytes and myeloid cells by its ability to hydrolyze the second messenger PI(3,4,5) trisphosphate and prevent downstream signaling pathways that lead to activation [12, 14-16]. In B cells, SHIP is recruited to the phosphorylated immunoreceptor tyrosine-based inhibitory motif of FcγRIIB upon coaggregation with the BCR [17, 18] where its enzymatic activity depletes PI(3,4,5) trisphosphate and prevents membrane localization of PH-domain-containing factors such as Tec kinases, Akt, and PLCγ [19-22]. SHIP can also regulate BCR signaling in the absence of FcγRIIB engagement [23-26]. Overall, SHIP’s inhibitory activity leads to the modulation of BCR induced calcium influx and prevention of cellular activation [23]. B cells purified from SHIP-deficient mice are hyperresponsive to activating signals and more resistant to apoptosis in vitro [26-30]. SHIP-null mice develop alterations in B-cell development, such as reduced immature B-cell numbers in BM and increased numbers of mature B cells in the spleen [26]. However, there are caveats to studies performed on B cells purified from mice with germline deletion of SHIP (SHIPnull/null). Those mice exhibit early mortality from a myeloproliferative-like syndrome characterized by profound splenomegaly and massive myeloid infiltration of the lung [31], making it difficult to study the role of SHIP in lymphocytes without the influence of the inflammatory environment. For our current studies, we have analyzed mice with B-cell-specific deletion of SHIP as a model system to assess the effect of enhanced BCR signaling strength and enhanced responses to cytokines, in the Ab selection process. Our analysis confirms a role for SHIP as a negative regulator of B-cell selection and activation, while it also uncovers a requirement for SHIP in ensuring efficient GC responses and appropriate Ab affinity maturation.
Results
Increased negative selection and B-cell maturation in mice with B-cell-specific deletion of SHIP
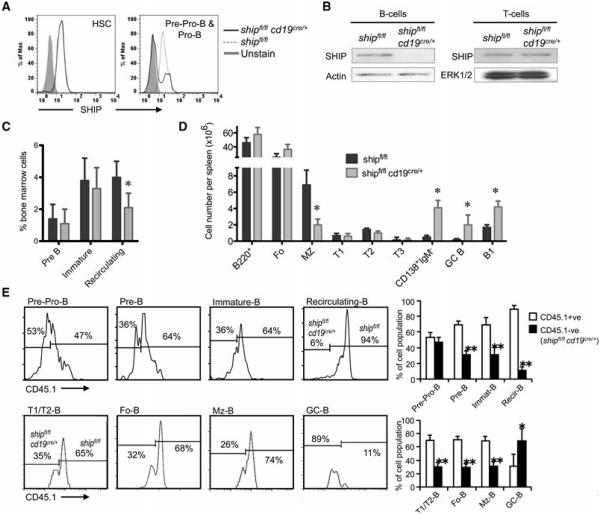
Mice with B-cell-specific deletion of SHIP were generated by crossing floxed-ship mice [32] with mice in which the Cre recombinase is driven by the CD19 promoter [33]. The resulting mice were designated shipfl/flcd19cre/+, while shipfl/fl littermates were used as controls. We observed that SHIP protein expression was greatly reduced at the pro-B-cell stage, although a few cells maintained SHIP expression (Fig. 1A). This may be a result of a small contaminating population of CD19− pre-pro B cells in our CD43+B220+HSA+ gate or it may indicate less than 100% efficiency of ship gene deletion at this stage. However, SHIP expression in mature CD19+ splenic B cells is completely ablated in shipfl/flcd19cre/+ mice (Fig. 1B). We tested whether B-cell-specific deletion of SHIP would alter B-cell development. Immature B-cell populations were found at the same frequency, while the number of recirculating B cells was reduced in BM of shipfl/flcd19cre/+ mice compared with control shipfl/fl (Fig. 1C and Supporting Information Fig. 1). B-cell-specific deletion of SHIP did not alter the number of transitional or follicular B cells in the spleen, but it reduced the number of MZ B cells and increased the number of B1, GC B cells, and isotype switched plasmablasts (CD138+IgM−) (Fig.1D and Supporting Information Fig. 2).
Figure 1.
B-cell-specific deletion of SHIP uncovers its role in negative selection. (A) Intracellular flow cytometric analysis of the expression of SHIP in BM cells (HSC and Pro-B cells) isolated from shipfl/flcd19cre/+ (solid line) or shipfl/f (dashed line). (B) Western blot analysis of SHIP expression in B cells (CD43 negative selection) and T cells (pan T cells isolation) isolated from spleen of mice of the indicated genotype. (C and D) B-cell development in (C) BM and (D) spleen was compared between shipfl/flcd19cre/+ and shipfl/f mice. Data are shown as mean SD of n = 4. *p < 0.05 (Student’s t-test). (E) BM cells isolated from shipfl/flcd19cre/+ (expressing CD45.2) and B6.Ptprca/b mice (expressing CD45.1 and CD45.2) were = mixed in a ratio of 1:1 and transferred to lethally irradiated (960 rads) C57BL/6 mice. Reconstituted BM cells (top histograms and bar graph) and total spleen cells (bottom histograms and bar graph) were collected after 2 months and analyzed by flow cytometry. Data are shown as mean + SD of n = 4 and are representative of four independent BM transfers.*p < 0.05, **p < 0.0005 (Student’s t-test). See Supporting Information Fig. 1 and 2 for the flow cytometric gating of different developmental stages and types of B cells from BM and spleen.
SHIP-deficient B cells could have repopulated back to normal levels even if their development was somewhat impaired. To test this possibility, we performed a BM reconstitution experiment in which shipfl/flcd19cre/+ cells compete with WT cells throughout development. An equal mix of shipfl/flcd19cre/+ and WT BM cells was injected into lethally irradiated WT recipients. Donor WT cells harbored the CD45.1 allotype to differentiate them from SHIP-deficient cells. Two months after the transfer, mice contained an equal ratio of WT:SHIP-deficient cells at the pre-pro-B stage, but a 2:1 ratio in all the subsequent populations (Fig. 1E). This skewing in favor of WT cells was even more pronounced (16:1) in the recirculating population in the BM. In contrast, the majority of the B cells present in spontaneous GCs (~ 80%) were SHIP-deficient. Thus, we determined that SHIP-deficient B cells are more likely to be negatively selected at the immature stage and in the MZ, but they are also more likely to spontaneously mature into GC B cells, possibly precluding their homing back to the BM upon activation.
Spontaneous IgG2a/b switch and enhanced interferon sensibility in SHIP-deficient B cells
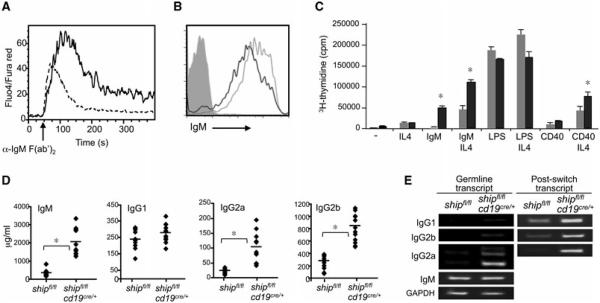
As previously reported for Ship−/− DT40 B cells [23], we observed enhanced Ca2+ flux in SHIP-deficient B cells upon stimulation with anti-IgM F(ab’)2 (Fig. 2A) even though they expressed lower surface levels of IgM (Fig. 2B). SHIP-deficient B cells from shipfl/flcd19cre/+ were hyperproliferative upon IgM and CD40 stimulation (Fig. 2C). Consistent with their lower threshold for activation, SHIP-deficient B cells spontaneously produced antibodies in vivo: steady state levels of serum IgM, IgG2a, and IgG2b were three- to fivefold higher in shipfl/flcd19cre/+ than in shipfl/fl control mice. In contrast, IgG1 levels in serum were not significantly different (Fig. 2D). Serum Ab levels correlated with transcript expression of the various isotypes in these mice, both for germline and postswitch transcripts (Fig. 2E).
Figure 2.
B-cell conditional deletion of SHIP results in B cells hyperresponsive to BCR and IFN-γ stimulation. (A) Purified B cells from shipfl/flcd19cre/+ (solid line) and shipfl/fl (dashed line) mice (n = 3) were loaded with 5 μg/mL Fluo-4 and FuraRed. Activation of calcium flux was induced by 10 μg/mL of anti-IgM F(ab′)2 and the resulting changes were monitored by flow cytometry. Result shown are representative from one experiment out of three performed. (B) IgM surface expression detected by flow cytometry. Solid line: shipfl/flcd19cre/+; dashed line: shipfl/f; gray histogram: isotype control. Data shown are representative of three independent analyses performed (n = 3). (C) Proliferation assay of purified B cells (n = 3) (CD43-negative fraction of spleen) 3 days after activation with the indicated stimulus. Tritiated thymidine was added for the last 6 h. Gray bar: shipfl/fl; black bar: shipfl/flcd19cre/+. (D) Total serum immunoglobulin concentration of indicated isotypes was detected by ELISA in shipfl/fl and shipfl/flcd19cre/+ mice (n = 10). (E) Germline and postswitch transcripts that appeared during isotype switching were detected by RT-PCR. Results shown are representative of one out of three independent experiments. *p < 0.001 (Student’s t-test).
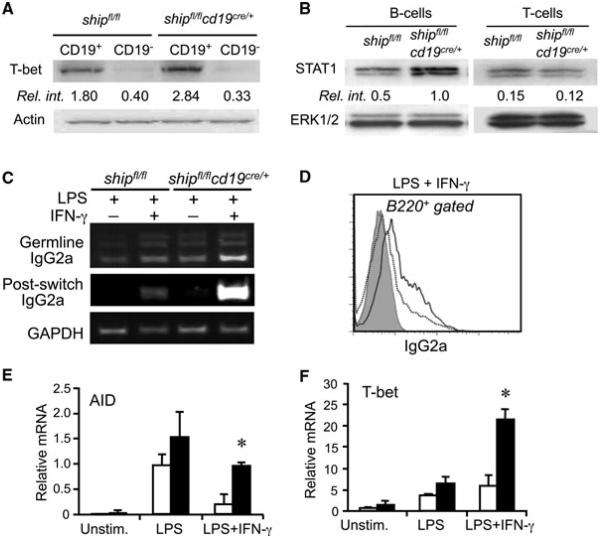
The observed IgG isotype preference in shipfl/flcd19cre/+ mice, mainly of IgG2a/b, indicated a Th1 skewing in the humoral response. Given that SHIP deletion is B-cell specific in these mice, we sought to find a reason for Th1 skewing that was B-cell intrinsic. We tested expression of T-bet and STAT1, both of which are transcription factors linked to Th1 bias. We observed that B cells, but not T cells, of shipfl/flcd19cre/+ had increased basal level expression of T-bet and STAT1 compared with shipfl/fl controls (Fig. 3A and B). These expression differences could be general to all B cells or due to changes in the proportion of certain B-cell populations that are more likely to express these transcription factors. We then tested the LPS/IFN-γ sensitivity of purified B cells for switching to Th1 IgG isotypes by incubating them for 24 h with LPS and IFN-γ. Indeed, SHIP-deficient cells were much more likely to induce IgG2a production (Fig. 3C and D), and to upregulate AID and T-bet expression upon LPS + IFN-γ activation compared with control B cells (Fig. 3E and F).
Figure 3.
SHIP negatively regulates IFN-γ-induced class switching. (A and B) Western blot analysis of T-bet and STAT-1 expression levels in lymphocytes from mice of the indicated genotype. Results shown are from one experiment representative of two independent experiments performed. (C) Germline and postswitch transcripts generated in B cells stimulated by IFN-γ and/or LPS during IgG2a class switching were detected by RT-PCR. (D) Surface IgG2a expression on B cells stimulated with IFN-γ and LPS. Solid line: shipfl/flcd19cre/+; dashed line: shipfl/fl, gray histogram: isotype control. (E and F) The expression of AID and T-bet in B cells upon LPS ± IFN-γ stimulation was analyzed by qRT-PCR. Data were normalized to GAPDH mRNA levels. (E and F) Data shown as mean + SD and (C–F) are representative of two independent experiments, n = 4 mice/group. *p < 0.001 (Student’s t-test).
SHIP deficiency does not alter B-cell tolerance to soluble Ag
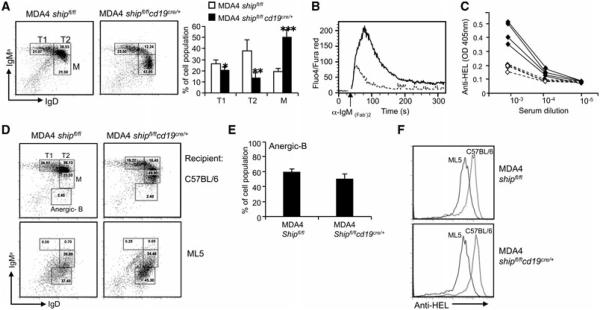
Mutations that alter the threshold for B-cell activation as SHIP deficiency does might override an anergic state driven by chronic stimulation with soluble Ag. To test the role of SHIP in B-cell tolerance to soluble Ag, we bred hen-egg lysozyme (HEL)-specific IgM/D transgenic mice (MDA4) to shipfl/flcd19cre/+ mice. As shown in Fig. 4A, when mice lacked soluble HEL Ag (sHEL), MDA4 shipfl/flcd19cre/+ were more likely to fully mature to IgMlo IgD+B cells than control MDA4 shipfl/fl cells. Their lower surface IgM expression level was not an indication of anergy, as SHIP-deficient MDA4 B cells were still hyperresponsive to IgM stimulation in calcium assays and spontaneously produced higher levels of HEL Ab than control B cells did (Fig. 4B and C). We then tested SHIP-deficient B cells for their ability to become anergized in the presence of soluble Ag. BM isolated from MDA4 shipfl/flcd19cre/+ or MDA4 shipfl/fl mice was transferred into lethaly irradiated sHEL Tg recipients (ML5). As shown in Fig. 4D, when WT recipient mice were reconstituted, B-cell populations resembled the ones present in the donor mice: more immature (IgMhiIgD+) when using MDA4 shipfl/fl donor controls and more mature (IgMloIgD+)when using MDA4 shipfl/flcd19cre/+ donor BM. When sHEL recipients received the BM transfers, reconstitution resulted in peripheral B cells that showed an anergic phenotype with very low IgMHEL-specific expression and decreased responses. This happened equally in mice reconstituted with MDA4 shipfl/flcd19cre/+ as with control cells (Fig. 4D–F). This result indicates that even though SHIP deficiency lowers the threshold for B-cell activation, it does not alter the establishment of B-cell tolerance against soluble HEL.
Figure 4.
Deletion of SHIP does not influence the establishment of B-cell tolerance to soluble HEL Ag. (A) FACS histogram and bar graph analysis of splenocyte populations: T1 (B220+, IgDlow, IgMahigh), T2 (B220+, I gDhigh, IgMahigh), and mature (B220+, IgDhigh, IgMalow) B cells. *p < 0.01, **p < 0.0005, ***p < 0.00001 (Student’s t-test). (B) Calcium release assay of MDA4 shipfl/flcd19cre/+ (solid line) and MDA4 shipfl/fl (dashed line), performed as in Figure 2A. (C) Anti-HEL Ab levels in serum from MDA4 shipfl/flcd19cre/+ (solid line) and MDA4 shipfl/fl (dashed line) mice were analyzed by ELISA. (D and E) BM cells were isolated from MDA4 shipfl/flcd19cre/+ or MDA4 shipfl/fl mice. A total of 1 × 107 cells were then transferred to lethally irradiated (960 rads) C57BL/6 (top) or ML5 (bottom) mice. Total spleen cells were collected after 2 months and the development of anergic B cells was analyzed by flow cytometry. (F) Surface anti-HEL expression on B cells isolated from the mice in (D) was analyzed by flow cytometry. (A and E) Data shown as mean + SD and (A–F) are representative of four independent experiments, n = 3 mice/group.
B-cell-specific SHIP deletion reduces isotype switched responses to T-cell-dependent Ags
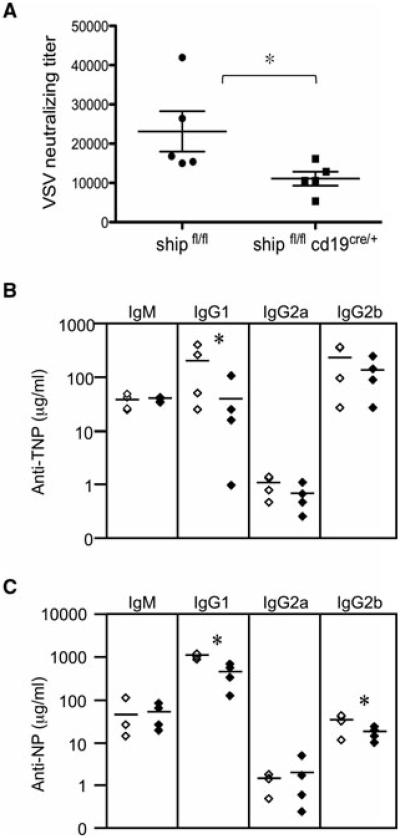
We tested the ability of SHIP-deficient B cells to mount a humoral response both in infectious and immunization settings. In response to a vesicular stomatitis virus (VSV) challenge, shipfl/flcd19cre/+ mice produced significantly lower neutralizing titers at day 14 compared with control mice (Fig. 5A). shipfl/flcd19cre/+ mice were also less efficient in mounting an isotype-switched response in both trinitrophenyl-keyhole limpet hemocyanin (TNP-KLH) and nitrophenyl-chicken gammaglobulin (NP-CGG) immunizations. While IgM and IgG2a responses were equal between SHIP-deficient mice and controls, Ag-specific titers of IgG1 and IgG2b were significantly reduced (Fig. 5B and C).
Figure 5.
B-cell conditional deletion of SHIP reduces humoral B-cell responses. (A) Serum analysis of VSV-specific Ab production in shipfl/flcd19cre/+ or shipfl/f mice (n = 5 per group) 14 days after VSV injection was performed by neutralization assay. (B–C) Six- to eight-week-old shipfl/flcd19cre/+ or shipfl/f mice (four mice per group) were immunized with 50 μg of TNP-KLH in RIBI (B) or NP-CGG in Alum (C). Ag-specific serum IgM was determined 2 weeks after the primary immunization and specific serum IgG was determined 1 week after a booster given on day 28. (A–C) Results shown are representative of three experiments. *p < 0.05 (Student’s t-test).
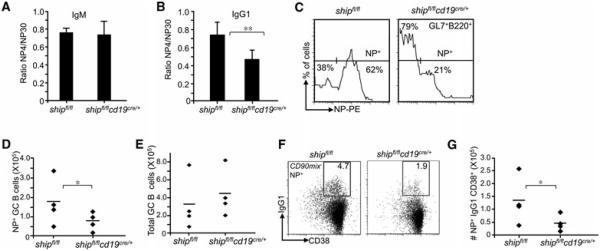
We tested Ab affinity in NP-CGG immunized mice by determining the ratio of Ab binding to low valency (NP4) and high valency (NP30) Ag in ELISA assays. As shown in Figure 6A and B, NP-specific IgM antibodies bound with equal efficiency to NP4 and NP30, while IgG1 antibodies raised in shipfl/flcd19cre/+ mice were less likely to bind the low valency Ag compared with shipfl/fl controls. Overall, we observed that Ag-specific antibodies raised in shipfl/flcd19cre/+ mice were produced in lower titers and with lower affinity for the immunizing Ag compared with those produced in shipfl/fl control mice.
Figure 6.
Reduced production of NP-specific GC B cells and memory B cells in SHIP-deficient mice. (A, B) ELISA assay to detect NP-specific antibodies using either NP30-BSA or NP4-BSA as the coating Ags was performed 5 days (for IgM) and 14 days (for IgG1) after NP-CGG (Alum) immunization (n = 5 samples per group). The affinities of the NP-specific IgM or IgG1 were determined by the ratio NP4/NP30. (C–E) Total and NP-specific GC B cells were identified by gating GC B cells B220+GL7+ splenocytes 14 days after NP-CGG (Alum) immunization and staining with NP-PE (n = 4 mice per group). (F–G) NP-specific memory B cells were determined on day 55 after NP-CGG (Alum) immunization, defined as CD90mix−, NP+, IgG1+, and CD38+ cells. (A–G) Data are shown as mean ± SD and are representative of two independent experiments, (n = 4–5 per group). *p < 0.05, **p < 0.001 (Student’s t-test).
Although shipfl/flcd19cre/+ mice developed spontaneous GC B cells in the absence of Ag stimulation, it was still possible that these mice could not establish the specific microenvironment for the development of GC B cells in response to Ag challenge. To test this possibility, we measured the total number of NP-specific and total B cells in the GC of immunized mice at day 14 after injection. GC B cells were identified by flow cytometry as GL7+FAShi and B220+ cells. As shown in Fig. 6C–E, 60% of the B cells in shipfl/fl GC controls were NP+, but only 25% of the GC B cells in shipfl/flcd19cre/+ mice were NP-specific, even though the total number of GC B cells was comparable in both cases. Long-term responses were also impaired in shipfl/flcd19cre/+ mice, as measured by the number of NP-specific IgG1 memory B cells detected 55 days after the second challenge (Fig. 6F and G).
SHIP-deficient B cells have aberrant Ab affinity selection
To test if the lower affinity of antibodies generated in shipfl/flcd19cre/+ mice was due to an abnormal rate of somatic hypermutation, we sequenced antibodies generated in the course of an NP-CGG immunization. We purified GC B cells from shipfl/flcd19cre/+ and shipfl/fl controls 14 days after NP-CGG immunization and the V186.2 heavy chain was amplified by PCR and sequenced. V186.2 has been previously shown to dominate in response to T-dependent NP immunization; frequently joining the D segment DFL16.1 and the JH segment JH2 [34-37]. The extent of somatic hypermutation of individual clones was determined by comparing its sequence with the original nonmutated germline V186.2 gene sequence (Supporting Information Fig. 3) and the result was summarized in Table 1. Shipfl/flcd19cre/+ B cells had similar percentages of clones containing at least one mutation in germline V186.2 sequences compared with controls (78% versus 90%) and somewhat lower average number of mutations (2.0 ± 1.6 versus 5.3 ± 4.05). Remarkably, we observed that none of the shipfl/flcd19cre/+ GC B cells had a mutation at CDR 1 compared with 60% of GC B cells in control mice. In particular, the tryptophan to leucine substitution at position 33, which has been shown to increase the affinity of anti-NP antibodies by tenfold [38, 39], was present in five out of ten sequences in control mice but was detected in none of the shipfl/flcd19cre/+ sequences. We then compared CDR 3 sequences, which contain the rearranged D and JH segments (Supporting Information Fig. 4). Consistent with previous studies that showed DFL16.1 as the predominant D segment in combination with V186.2 in the anti-NP response [35,40], 60% of the GC B cells from our control mice contained this segment. In contrast, only 22% of the shipfl/flcd19cre/+ GC B cells included the DFL16.1 region. Uncharacteristically, 33% of the antibodies raised in shipfl/flcd19cre/+ mice contained an early stop codon mutation (Supporting Information Fig. 3), while no such mutation occurred in controls. Thus, SHIP-deficient B cells are more likely to encode nonfunctional antibodies, a result that points toward a defect in GC selection and affinity maturation mechanisms.
Table 1.
VH 186.2-D—JH2 gene sequence summary from GC B cells of NP immunized micea)
| Cell type | No. of VH
genes sequenced |
Sequencesb)
mutated Ratio (%) |
Mutationsc)
per VH gene Average ± SD |
Sequences mutated at CDR1 Ratio (%) |
Sequences mutated at CDR2 Ratio (%) |
|---|---|---|---|---|---|
| ship fl/fl | 10 | 9/10 (90) | 5.3 ± 4.05 | 6/10 (60) | 5/10 (50) |
| ship fl/fl cd19 cre/+ | 9 | 7/9 (78) | 2.0 ± 1.6 | 0/9 (0) | 1/9 (11) |
|
| |||||
| Cell type | Position 33 Trp → Leu Ratio (%) |
Stop codond)
mutation Ratio (%) |
R/S ratioe)
CDR1 and 2 |
R/S ratiof)
FWR |
DFL16.1 gene usage (%) |
|
| |||||
| ship fl/fl | 5/10 (50) | 0/10 (0) | 16/1 | 1.1/1 | 60 |
| ship fl/fl cd19 cre/+ | 0/9 (0) | 3/9 (33) | 2/0 | 6.5/1 | 22 |
The percentage of VH186.2 genes which had more than one mutation.
SD = standard deviation.
The percentage of genes containing mutations which resulted in the coding of early stop codon (TGA).
The ratio of replacement (R) to silent (S) mutations.
FWR: framework region.
Discussion
Modulation of B-cell activation by inhibitory pathways allows for fine-tuning and flexibility of responses to adapt them to specific needs. Mutational studies in inhibitory molecules of the BCR pathway such as SHIP, SHP-1, or Grb2 have established the importance of this regulation in B-cell development and activation [8, 10, 11, 26]. Most experiments aimed at elucidating the role of SHIP in B cells were performed in mice with complete deletion of the gene, usually giving rise to a number of pleiotropic phenotypes and a prominent inflammatory condition that complicates interpretation of B-cell-specific effects. [31]. In ship-null mice, lymphoid cell development is inhibited by increased production of IL-6 [41], thus preventing an accurate assessment of the role of SHIP in B cells. By analyzing shipfl/flcd19cre/+ mice, we have confirmed the B-cell intrinsic role for SHIP, both in regulating negative selection in the BM and activation in the periphery.
B-cell-specific SHIP deletion allowed us to study the consequences of hyperactive Ag- and cytokine-receptor pathways in the Ab selection process. We found that B-cell SHIP deficiency results in a lower BCR stimulation threshold and enhanced proliferative responses, consistent with the phenotype already observed in cells obtained from SHIP-null mice and another model of B-cell-specific SHIP deficiency [28, 29]. However, our data differ in some ways when compared with the recently described Cd79a-cre SHIP ablation model [42] as we did not find that anergy is SHIP dependent in B cells. This discrepancy may be explained by differing mechanisms of anergy induction in the mouse models that were used, as anergy in MD4 B cells has been suggested to be dependent on PTEN expression, while Ars/A1 transgenic B cells used in O’Neill et al. appear to be more SHIP dependent [42]. Our mice expressing SHIP-deficient B cells also developed autoantibodies at a much later timepoint than those in the Cd79a-cre system (data not shown). Overall, our data reinforce the view that the strength of BCR signal correlates with the rate of B-cell development. We also confirm the B-cell-intrinsic role of SHIP in balancing the development between Fo and Mz B cells in positive selection of the primary immune repertoire and in setting a threshold to prevent B cells from spontaneous activation. Additionally, we have extended the role of SHIP in B cells beyond the regulation of BCR signaling, as IFN-γ signaling was similarly affected by deletion of SHIP. This cytokine regulation might occur through SHIP binding to Shc by a mechanism similar to what has been reported for GM-CSF or IL-3 regulation by SHIP [43].
We detected reduced humoral responses in mice with B-cell-specific deletion of SHIP, a phenotype reminiscent of the ones observed in mice with B-cell-specific deletion of SHP-1 or Grb2 [8, 10, 11]. One main difference is that in the case of SHIP deficiency, the formation of GCs is unaltered or even enhanced in spontaneous conditions and thus this phenotype cannot be explained by a disruption of GC structure as suggested for Grb2 Ab responses. Also, SHIP deficiency in B cells does not lead to B1-type skewing to the extreme degree that B-cell-specific PTEN or SHP-1 deletion does and does not expand MZ B-cell populations as occurs in PTEN-deficient B cells [8, 44]. Thus, reduced numbers of follicular B cells cannot explain defective humoral responses. Although both SHIP-deficient B cells and PTEN-deficient B cells have defects in CSR and IgG production, they differ in that SHIP-deficient B cells have increased expression of AID upon stimulation, whereas PTEN-deficient B cells do not properly induce AID [44, 45]. Thus, our preferred hypothesis is that impaired affinity maturation leads to less effective humoral responses in SHIP-deficient B cells.
SHIP deficiency results in enhanced negative selection of B cells at immature stages, likely causing a shift in clonality of the B-cell repertoire toward cells bearing low-affinity BCRs. Enhancement of BCR signaling strength likely upregulates B-cell apoptosis in negative selection of auto-reactive B cells supporting the possible occurrence and rapid disappearance of high-affinity B cells in response to T-dependent immunization. We also cannot exclude the possibility that high-affinity B cells are able to escape to the periphery, but upon Ag encounter, they are subjected to apoptosis as a result of hyperactive BCR and/or cytokine signaling augmented by the absence of SHIP. However, absence of B cells with high-affinity BCRs from the starting pool of B-cell clones should not prevent the production of high-affinity antibodies in the GC. According to a study by Shih et al., B cells in GCs actually undergo a fixed mutation program which is independent of the initial affinity of the BCR [46], suggesting that the tendency for a B-cell to develop a high-affinity BCR is the same as that to acquire a low-affinity one in the GC. As our data show that SHIP-deficient B cells have increased AID expression upon IFN-γ + LPS stimulation but have a reduced propensity to acquire high-affinity BCRs upon Ag challenge when compared with control B cells, it is more likely that SHIP-deficient B cells are exempt from processes that normally dictate B-cell survival and selection of high-affinity clones.
Overall, it seems counterintuitive that SHIP deficiency enhances spontaneous switched Ab generation but reduces high-affinity GC responses. These results suggest that very low affinity responses drive spontaneous Ab production and would be favored by lack of negative regulation, while hyperactive low-affinity SHIP-deficient B cells may be deleted in the GC because they do not meet the minimum Ag affinity requirement for selection.
Materials and methods
Mice
The generation of shipfl/fl mice has been described previously [32]. C57BL/6 mice, cd19cre/+ mice, ML5 mice (mice expressing soluble HEL), MD4 mice (mice containing the anti-HEL IgMa transgene) and B6. Ptprca mice were obtained from The Jackson laboratory (Bar Harbor, ME, USA). All experiments involving mice were performed in accordance with National Institutes of Health (NIH) guidelines.
Cells and antibodies
Purified B cells were obtained by negative selection of splenocytes isolated from 6- to 8-week-old mice with magnetic beads specific for CD43 (Miltenyi Biotec, Auburn, CA, USA). For all assays, cells were maintained and stimulated in DMEM (Invitrogen, Carlsbad, CA, USA) with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. All of the antibodies for FACS analysis were from BD Biosciences (San Jose, CA, USA).
Intracellular calcium measurements
Purified B cells were loaded with 5 μg/mL Fluo-4 and 5 μg/mL FuraRed (Molecular Probes, Eugene, OR, USA). Ca2+ mobilization was triggered by 10 μg/mL F(ab′)2 fragment of anti-IgM and the resulting changes were monitored by FACS. The data were expressed as the ratio of Fluo-4/FuraRed.
IFN-γ and LPS B-cell stimulations
Purified B cells from shipfl/flcd19cre/+ or shipfl/fl mice were incubated with LPS (20 μg/mL) with or without IFN-γ (100 ng/mL) for 24 h. Stimulated B cells were analyzed for Th1 isotype switching by flow cytometry and RT-PCR. B-cell total RNA was extracted and cDNA was prepared for real-time PCR analysis of the expression of AID and T-bet.
Immunization and serum analysis
Mice were injected with 50 μg of TNP-KLH with RIBI (Sigma, St. Louis, MO, USA) or NP-CGG with Alum (Pierce, Rockford, IL, USA). Sera were collected 2 weeks after the immunization. The mice were given a second booster on day 28 and sera were collected 1 week after the booster. The levels of Ag-specific antibodies of different isotypes were determined by ELISA using TNP-BSA or NP-BSA (NP4-BSA or NP30-BSA) (5 μg/mL; Biosearch Technologies, Novato, CA, USA) coated 96-well plates and the Clonotyping System-HRP (SouthernBiotech, Birmingham, AL, USA).
VSV immunization and neutralization assay
VSV serotype Indiana (VSV-IND) was obtained from the laboratory of Drs. Jack R. Bennink and Jonathan W. Yewdell. For immunization, 6- to 8-week-old mice were i.p. injected with 2 × 106 PFU of VSV in 200 μL of PBS. Sera were collected on day 14 after immunization. Neutralizing titers of sera were determined as described [47, 48]. Briefly, the sera were heat inactivated for 30 min at 56°C. Serial diluted sera were mixed with 500 PFU/mL of VSV and incubated for 90 min at 37°C. The serum–virus mixture was subsequently transferred onto Vero cell monolayers in 96-well plates and incubated for 1 h at 37°C. An overlay of 100 μL MEM containing 1% methylcellulose (Sigma) was added. After incubation for 24 h at 37°C, the cells were fixed and stained with 0.5% crystal violet (Sigma) and the number of plaques was counted. Neutralization titer was determined as the dilution of serum that reduces the number of plaques by 50%.
Somatic hypermutation analysis
GC B cells from NP-immunized mice were sorted based on the expression of GL7, Fas, and B220 using FACS Aria (BD Biosciences). Genomic DNA was isolated with the DNeasy 96 kit from Qiagen. V186.2 genes were amplified by using iProof High-Fidelity DNA polymerase (Biorad) with primers V186.2 outer 5′-TCTTTACAGTTACTGAGCACACAGGAC-3′ and JH2 5′-GGGTCTAGAGG TGTCCCTAGTCCTTCATGACC-3′ for 20 cycles. Three microliters of product were used as template for a second round (nested PCR) of PCR with primers V186.2inner 5′-CAGTAGCAGGCTTGAGGTCTGGAC-3′ and JH2 in 30 cycles. PCR products were cloned using TOPO TA cloning kit (Invitrogen) and sequenced using the T3 universal primer.
BM reconstitution
Eight- to ten-week-old recipient mice (C57BL/6 or ML5 mice) were lethally irradiated (960 rads) and reconstituted 16–20 h later by intravenous injection with 1 × 107 BM cells isolated from shipfl/fl or shipfl/flcd19cre/+ mice. All mice were analyzed 2 months after reconstitution.
Supplementary Material
Acknowledgments
We thank Drs. Jack R. Bennink and Jonathan W. Yewdell (NIAID) for providing the VSV-IND, Bethany Scott for managing the mouse work. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- HEL:
hen-egg lysozyme
- MZ:
marginal zone
- NP:
nitrophenyl
- VSV:
vesicular stomatitis virus
Footnotes
 Additional supporting information may be found in the online version of this article at the publisher’s web-site
Additional supporting information may be found in the online version of this article at the publisher’s web-site
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Benschop RJ, Cambier JC. B cell development: signal transduction by antigen receptors and their surrogates. Curr. Opin. Immunol. 1999;11:143–151. doi: 10.1016/s0952-7915(99)80025-9. [DOI] [PubMed] [Google Scholar]
- 2.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 3.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, et al. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 4.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 5.King LB, Monroe JG. Immunobiology of the immature B cell: plasticity in the B-cell antigen receptor-induced response fine tunes negative selection. Immunol. Rev. 2000;176:86–104. doi: 10.1034/j.1600-065x.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaki T. Functional dissection of BCR signaling pathways. Curr. Opin. Immunol. 2000;12:276–281. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 7.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 8.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, et al. B cell-specific deletion of proteintyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Samardzic T, Marinkovic D, Danzer CP, Gerlach J, Nitschke L, Wirth T. Reduction ofmarginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Jang IK, Cronshaw DG, Xie LK, Fang G, Zhang J, Oh H, Fu YX, et al. Growth-factor receptor-bound protein-2 (Grb2) signaling in B cells controls lymphoid follicle organization and germinal center reaction. Proc. Natl. Acad. Sci. USA. 2011;108:7926–7931. doi: 10.1073/pnas.1016451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann JA, Radtke D, Maurberger A, Winkler TH, Nitschke L. Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling. EMBO J. 2011;30:1621–1633. doi: 10.1038/emboj.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM. Structure, function, and biology of SHIP proteins. Genes Dev. 2000;14:505–520. [PubMed] [Google Scholar]
- 13.Poe JC, Fujimoto M, Jansen PJ, Miller AS, Tedder TF. CD22 forms a quaternary complex with SHIP, Grb2, and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J. Biol. Chem. 2000;275:17420–17427. doi: 10.1074/jbc.M001892200. [DOI] [PubMed] [Google Scholar]
- 14.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 15.Kalesnikoff J, Sly LM, Hughes MR, Buchse T, Rauh MJ, Cao LP, Lam V, et al. The role of SHIP in cytokine-induced signaling. Rev. Phys. Biochem. Pharm. 2003;149:87–103. doi: 10.1007/s10254-003-0016-y. [DOI] [PubMed] [Google Scholar]
- 16.Rauh MJ, Kalesnikoff J, Hughes M, Sly L, Lam V, Krystal G. Role of Src homology 2-containing-inositol 5’-phosphatase (SHIP) in mast cells and macrophages. Biochem. Soc. Trans. 2003;31:286–291. doi: 10.1042/bst0310286. [DOI] [PubMed] [Google Scholar]
- 17.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Brauweiler A, Cambier JC. Effects of Src homology domain 2 (SH2)-containing inositol phosphatase (SHIP), SH2-containing phosphotyrosine phosphatase (SHP)-1, and SHP-2 SH2 decoy proteins on Fc gamma RIIB1-effector interactions and inhibitory functions. J. Immunol. 2000;164:631–638. doi: 10.4049/jimmunol.164.2.631. [DOI] [PubMed] [Google Scholar]
- 19.Bolland S, Pearse RN, Kurosaki T, Ravetch JV. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 20.Scharenberg AM, El-Hillal O, Fruman DA, Beitz LO, Li Z, Lin S, Gout I, et al. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carver DJ, Aman MJ, Ravichandran KS. SHIP inhibits Akt activation in B cells through regulation of Akt membrane localization. Blood. 2000;96:1449–1456. [PubMed] [Google Scholar]
- 22.Galandrini R, Tassi I, Mattia G, Lenti L, Piccoli M, Frati L, Santoni A. SH2-containing inositol phosphatase (SHIP-1) transiently translocates to raft domains and modulates CD16-mediated cytotoxicity in human NK cells. Blood. 2002;100:4581–4589. doi: 10.1182/blood-2002-04-1058. [DOI] [PubMed] [Google Scholar]
- 23.Okada H, Bolland S, Hashimoto A, Kurosaki M, Kabuyama Y, Iino M, Ravetch JV, et al. Role of the inositol phosphatase SHIP in B cell receptor-induced Ca2+ oscillatory response. J. Immunol. 1998;161:5129–5132. [PubMed] [Google Scholar]
- 24.Petrie RJ, Schnetkamp PP, Patel KD, Awasthi-Kalia M, Deans JP. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto A, Hirose K, Okada H, Kurosaki T, Iino M. Inhibitory modulation of B cell receptor-mediated Ca2+ mobilization by Src homology 2 domain-containing inositol 5’-phosphatase (SHIP) J. Biol. Chem. 1999;274:11203–11208. doi: 10.1074/jbc.274.16.11203. [DOI] [PubMed] [Google Scholar]
- 26.Brauweiler A, Tamir I, Dal Porto J, Benschop RJ, Helgason CD, Humphries RK, Freed JH, et al. Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5’ inositol phosphatase (SHIP) J. Exp. Med. 2000;191:1545–1554. doi: 10.1084/jem.191.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brauweiler AM, Tamir I, Cambier JC. Bilevel control of B-cell activation by the inositol 5-phosphatase SHIP. Immunol. Rev. 2000;176:69–74. doi: 10.1034/j.1600-065x.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 28.Helgason CD, Kalberer CP, Damen JE, Chappel SM, Pineault N, Krystal G, Humphries RK. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of b lymphocytes in ship -/- mice. J. Exp. Med. 2000;191:781–794. doi: 10.1084/jem.191.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Oliveira-Dos-Santos AJ, Mariathasan S, Bouchard D, Jones J, Sarao R, Kozieradzki I, et al. The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 1998;188:1333–1342. doi: 10.1084/jem.188.7.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Damen JE, Hughes MR, Babic I, Jirik FR, Krystal G. The Src homology 2 (SH2) domain of SH2-containing inositol phosphatase (SHIP) is essential for tyrosine phosphorylation of SHIP, its association with Shc, and its induction of apoptosis. J. Biol. Chem. 1997;272:8983–8988. doi: 10.1074/jbc.272.14.8983. [DOI] [PubMed] [Google Scholar]
- 31.Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady J, Radonovich M, Vodkin M, Natarajan V, Thoren M, Das G, Janik J, et al. Site-specific base substitution and deletion mutations that enhance or suppress transcription of the SV40 major late RNA. Cell. 1982;31:625–633. doi: 10.1016/0092-8674(82)90318-x. [DOI] [PubMed] [Google Scholar]
- 35.Cumano A, Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur. J. Immunol. 1985;15:512–520. doi: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- 36.Cumano A, Rajewsky K. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 1986;5:2459–2468. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss U, Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J. Exp. Med. 1990;172:1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohno S, Mori N, Matsunaga T. Antigen-binding specificities of antibodies are primarily determined by seven residues of VH. Proc. Natl. Acad. Sci. USA. 1985;82:2945–2949. doi: 10.1073/pnas.82.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen D, Simon T, Sablitzky F, Rajewsky K, Cumano A. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J. 1988;7:1995–2001. doi: 10.1002/j.1460-2075.1988.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bothwell AL, Paskind M, Reth M, Imanishi-Kari T, Rajewsky K, Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981;24:625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Kouro T, Kincade PW, Malykhin A, Maeda K, Coggeshall KM. Src homology 2-containing 5-inositol phosphatase (SHIP) suppresses an early stage of lymphoid cell development through elevated interleukin-6 production by myeloid cells in bone marrow. J. Exp. Med. 2004;199:243–254. doi: 10.1084/jem.20031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill SK, Getahun A, Gauld SB, Merrell KT, Tamir I, Smith MJ, Dal Porto JM, et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011;35:746–756. doi: 10.1016/j.immuni.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramshaw HS, Guthridge MA, Stomski FC, Barry EF, Ooms L, Mitchell CA, Begley CG, et al. The Shc-binding site of the betac subunit of the GM-CSF/IL-3/IL-5 receptors is a negative regulator of hematopoiesis. Blood. 2007;110:3582–3590. doi: 10.1182/blood-2007-01-070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat. Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 47.Wagner RR, Snyder RM, Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J. Virol. 1970;5:548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fehr T, Rickert RC, Odermatt B, Roes J, Rajewsky K, Hengartner H, Zinkernagel RM. Antiviral protection and germinal center formation, but impaired B cell memory in the absence of CD19. J. Exp. Med. 1998;188:145–155. doi: 10.1084/jem.188.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.