Abstract
Altered cerebral metabolism and mitochondrial function have been identified in experimental and clinical studies of pediatric traumatic brain injury (TBI). Metabolic changes detected using 1H (proton) magnetic resonance spectroscopy correlate with long-term outcomes in children after severe TBI. We previously identified early (4-h) and sustained (24-h and 7-day) abnormalities in brain metabolites after controlled cortical impact (CCI) in immature rats. The current study aimed to identify specific alterations of cerebral glucose metabolism at 24 h after TBI in immature rats. Rats (postnatal days 16–18) underwent CCI to the left parietal cortex. Sham rats underwent craniotomy only. Twenty-four hours after CCI, rats were injected (intraperitoneally) with [1,6-13C]glucose. Brains were removed, separated into hemispheres, and frozen. Metabolites were extracted with perchloric acid and analyzed using 1H and 13C-nuclear magnetic resonance spectroscopy. TBI resulted in decreases in N-acetylaspartate in both hemispheres, compared to sham contralateral. At 24 h after TBI, there was significant decrease in the incorporation of 13C label into [3-13C]glutamate and [2-13C]glutamate in the injured brain. There were no differences in percent enrichment of [3-13C]glutamate, [4-13C]glutamate, [3-13C]glutamine, or [4-13C]glutamine. There was significantly lower percent enrichment of [2-13C]glutamate in both TBI sides and the sham craniotomy side, compared to sham contralateral. No differences were detected in enrichment of 13C glucose label in [2-13C]glutamine, [2-13C]GABA (gamma-aminobutyric acid), [3-13C]GABA, or [4-13C]GABA, [3-13C]lactate, or [3-13C]alanine between groups. Results suggest that overall oxidative glucose metabolism in the immature brain recovers at 24 h after TBI. Specific reductions in [2-13C]glutamate could be the result of impairments in either neuronal or astrocytic metabolism. Future studies should aim to identify pathways leading to decreased metabolism and develop cell-selective “metabolic rescue.”
Key words: : developmental brain injury, metabolism, mitochondria, NMR spectroscopy, trauma
Introduction
Traumatic brain injury (TBI) is the leading cause of long-term disability in children ages 1–18 years. Despite recent advances in neurointensive care and reduction in overall mortality rate, long-term morbidity of severe TBI in children remains high.1,2 In addition to regaining lost skills, young children are expected to rapidly be developing new skills, making the burden of TBI even greater. Additional studies elucidating the age-specific mechanisms of secondary injury and recovery are essential to target these mechanisms for effective neuroprotective treatments in pediatric TBI.
Alterations in brain metabolism and mitochondrial function have been identified in both experimental studies in immature animal models3–7 and in infants and children after TBI.3–10 These alterations can occur early after injury and persist for days or weeks.6,11 In clinical studies, metabolic changes detected using 1H (proton) magnetic resonance spectroscopy correlate with long-term functional outcomes in children after severe TBI.12 Our previous work identified increases in lactate in the ipsilateral hemisphere after controlled cortical impact (CCI) in immature rats that began at 4 h after injury and persisted to 24 h.11 We also found decreases in N-acetylaspartate (NAA) starting at 24 h and persisting to 7 days after TBI. Based on this initial work, we designed the current study to obtain a more in-depth assessment of cerebral oxidative metabolism at 24 h after TBI, the time point when maximal metabolic disturbances were observed by 1H spectroscopy. We hypothesized that oxidative glucose metabolism would be diminished at 24 h after CCI in immature rats, resulting in a shift to anaerobic metabolism, with the most pronounced abnormalities noted in the ipsilateral hemisphere of injured rats.
Methods
Animals
This study was approved by the University of Maryland (Baltimore, MD) Animal Care and Use Committee. All care of rats was in compliance with the National Institutes of Health guidelines. Postnatal days (PNDs) 16–18, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in all studies. Rats were housed with littermates both before surgery and after recovery from anesthesia.
Traumatic brain injury
The TBI model used in this study was previously described.5 Briefly, anesthesia was induced in a Plexiglass chamber with isoflurane (4% for induction). After induction, anesthesia was maintained using a nose cone with 2% isoflurane and 30% oxygen for the duration of the surgical procedure. The rat's head was secured in a stereotactic device, and a rectal probe was placed. Rectal temperature was maintained at 37.0±0.5°C using a heating blanket connected to the rectal probe. After a mid-line scalp incision, a left parietal craniotomy was performed with a high-speed dental drill. Brain temperature was maintained at 37.0±0.5°C with a heating lamp, using a temperature probe placed in the contralateral temporalis muscle. After a 20-min period of temperature stabilization, CCI was induced using a 6-mm flat metal impactor tip at 5.5 m/sec, duration of 50 msec, and depth of 1.5 mm. The craniotomy was then resealed with acrylic mixture, and the scalp incision was closed with interrupted sutures. Anesthesia was discontinued, and the rat was awakened and returned to the dam with littermates. Sham animals underwent all surgical procedures without sustaining the cortical impact.
Twenty-four hours after CCI, rats were injected (intraperitoneally; i.p.) with [1,6-13C]glucose (200 mg/kg; 99% 13C enriched; Cambridge Isotope Laboratories, Woburn, MA). This method of injection (i.p.) shows comparable pharmacokinetics data to intravenous injections of glucose analogs in the rat13 and has been used in previous studies by our group6 and others.14 One hour after injection, rats were euthanized by decapitation, then brains were rapidly removed and placed on a cooled brain matrice. The most rostral 3 mm of brain tissue (uninjured) was discarded. The remaining peritrauma segment was separated into ipsi- (injured) and contralateral (control) hemispheres and snap-frozen in liquid nitrogen, with a total ischemic time of <30 sec from brain excision to liquid nitrogen for all samples. Samples were stored at −80°C until extraction.
Brain tissue extraction and protein quantification
Frozen brain tissue samples were weighed and homogenized in 2 mL of ice-cold perchloric acid (PCA; 7%) and extracted for nuclear magnetic resonance (NMR) spectroscopy, as described by Richards and colleagues.15 Neutralized, lyophilized samples were stored at −20°C until spectra were obtained.
The pellet from the PCA extraction was saved for protein assay. For protein measurements, the PCA pellet was digested overnight with 1 N of NaOH and the concentration was determined by the methods of Lowry and colleagues.16
Carbon-13 nuclear magnetic resonance spectroscopy and proton nuclear magnetic resonance spectroscopy
Lyophilized samples were dissolved in 0.8 mL of D2O (deuterated water) containing 0.4% dioxane and 0.02% sodium 3-(trimethylsilyl)propionate-2,2,3,3-d4 (TMSP) as internal standards.
Protein-decoupled 125.5-MHz carbon-13 (13C) NMR spectra were obtained on a Varian Inova 500-MHz spectrometer (Varian Medical Systems, Inc., Palo Alto, CA) with a broad-band detection probe. Spectra were acquired with a 35-degree pulse angle, with an acquisition time of 1.3 sec and relaxation time of 4.3 sec and total number of scans typically 11,000–13,000. A line broadening of 5 Hz was used. Optimum shims were obtained before each spectra was run, and factors for the nuclear Overhauser effects were applied to all spectra. 1H NMR spectra, obtained using the same spectrometer, were acquired with a pulse angle of 90 degrees, acquisition time of 1.36 sec, and relaxation delay of 10 sec. Total number of scans was 256.
Chemical shifts and amounts of 13C and 1H in the different metabolites were reported from the integrals of the relevant peaks obtained relative to dioxane peak at 67.4 ppm (13C experiments) and TMSP peak at 0 ppm.
Carbon-13 labeling in brain from metabolism of [1,6-13C]glucose
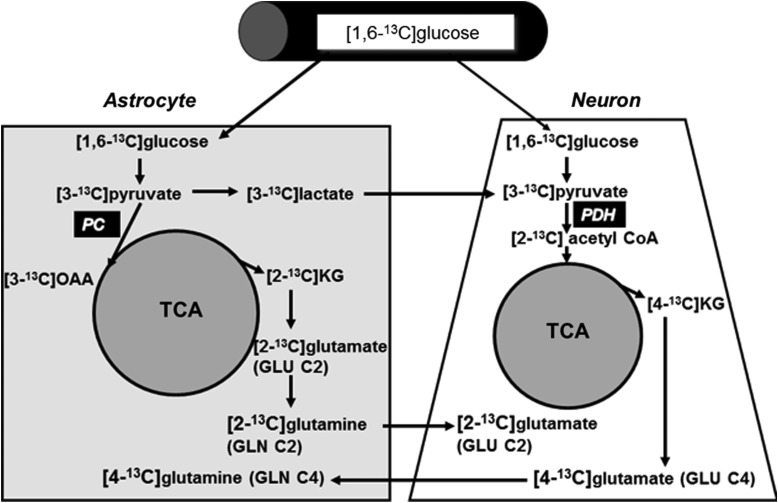
The labeling pattern of brain metabolites after [1,6-13C]glucose injection is well documented (Fig. 1).17 [1,6-13C]Glucose forms [3-13C]pyruvate through glycolysis, which can be converted to [3-13C]lactate or [3-13C]alanine or enter the tricarboxylic acid (TCA) cycle by pyruvate dehydrogenase and lead to the formation of [4-13C]glutamate (GLU C4) in neurons or [2-13C]GABA (gamma-aminobutyric acid). The GLU C4 can be released by neurons taken up by astrocytes and converted to the corresponding [4-13C]glutamine (GLN C4). If the 13C label stays in the TCA cycle for a second turn, it may go on to form [3-13C]glutamate (GLU C3), [3-13C]glutamine (GLN C3), and [3-13C]GABA (not shown in Fig. 1).18,19 In astrocytes, [3-13C]pyruvate formed from [1,6-13C]glucose can enter the TCA cycle by the anaplerotic pyruvate carboxylase (PC) pathway and result in the formation of [2-13C]glutamate (GLU C2), [2-13C]glutamine (GLN C2) from [2-13C]α-ketoglutarate formed in the first turn. After release of GLN C2 by astrocytes and uptake by neurons, GLU C2 and [4-13C]GABA (GABA C4) can be formed.20 After the second TCA cycle turn, glutamate and glutamine will be labeled in the [3-13C] positions (not shown in Fig. 1; please see previously publish reports18,19 for details of subsequent TCA cycle turns). We calculated cycling ratios, which reflect relative labeling from the second and first turns of the TCA cycle. These were calculated for GLU, GLN, and GABA using the following ratios: GLU C3/GLU C4; GLN C3/GLN C4; and GABA C3/GABA C2.21 Finally, we calculated total TCA cycle metabolism using the following formulas: TCA1=GLU C4+GLN C4+GABA C2+ASP C3 and TCA2=GLU C3+GLN C3+GABA C3+ASP C3.
FIG. 1.
Labeling pattern from the metabolism of [1,6-13C]glucose in the brain. Specific details are discussed in the Methods section. PDH, pyruvate dehydrogenase complex; PC, pyruvate carboxylase; OAA, oxaloacetate; KG, α-ketoglutarate; TCA, tricarboxylic acid cycle.
Statistical analysis
Data are expressed as mean±standard error of the mean for metabolites and ratios. A power analysis was performed based on effect size from previous studies,11 indicating that a sample size of 5–11 per group would give a power of 0.8 using an alpha of 0.05.22 Based on these estimates, we selected a sample size of 8 per group. Data were analyzed using one-way analysis of variance, controlling for the paired nature of ipsi- and contralateral samples, with p<0.05 using Stata statistical software (StataCorp LP, College Station, TX).
Results
TBI resulted in significant decreases in NAA in both ipsi- and contralateral hemispheres, compared to sham contralateral. The concentration of other amino acids was not different between groups. The concentration of total GABA tended to be decreased in TBI rats in both sides and sham ipsilateral (craniotomy only), but did not reach statistical significance (Table 1).
Table 1.
Metabolite Concentrationa Measured by1H NMR Spectroscopy
| Sham | TBI | |||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| Lactate | 152.8±8.23 | 150.1±16.4 | 141.2±10.9 | 139.6±9.9 |
| Glutamate | 176.1±5.8 | 144.5±19.8 | 156.4±11.3 | 168.5±12.0 |
| GABA | 57.4±2.3 | 71.2±7.7 | 54.9±4.2 | 57.8±3.6 |
| Glutamine | 95.6±4.5 | 102.8±10.4 | 96.9±8.0 | 91.2±5.9 |
| Aspartate | 39.4±8.1 | 52.3±6.3 | 36.0±3.9 | 39.3±2.9 |
| NAA | 121.8±5.5 | 144.5±14.2* | 103.2±6.9 | 121.9±9.1 |
| Succinate | 11.6±0.4 | 12.8±0.9 | 11.1±0.6 | 11.3±0.7 |
nmol/mg protein.
p<0.05, sham contralateral versus TBI ipsilateral and TBI contralateral.
NMR, nuclear magnetic resonance; GABA, gamma-aminobutyric acid; NAA, N-acetylaspartate; TBI, traumatic brain injury.
Incorporation of carbon-13 into glutamate, glutamine, and gamma-aminobutyric acid at 24 h after traumatic brain injury
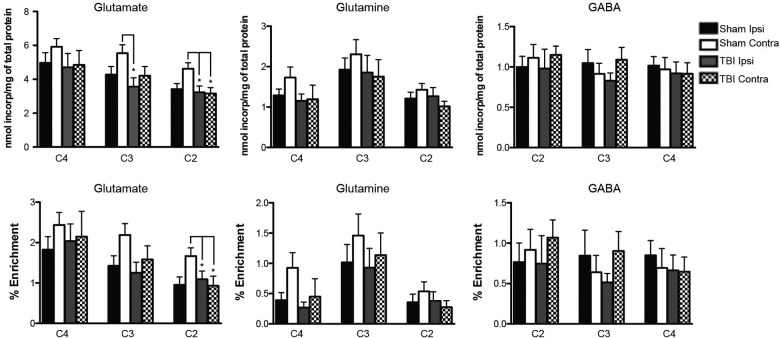
At 24 h after TBI, 60 min after injection of [1,6-13C]glucose, there was no difference in labeling of GLU C4 between TBI and sham rats, on both ipsi- and contralateral sides. However, labeling of GLU C3 was decreased in the TBI ipsilateral side, and label incorporation into GLU C2 was significantly decreased in both ipsi- and contralateral sides on TBI rats (Fig. 2). No difference in 13C label incorporation was observed in GLN C4, GLN C3, and GLN C2 and GABA C4, GABA C3, and GABA C4.
FIG. 2.
Top panels show incorporation of label from the metabolism of [1,6-13C]glucose and bottom panels show percent 13C enrichment into glutamate (GLU C2, GLU C3, and GLU C4), glutamine (GLN C2, GLN C3, and GLN C4), and GABA (GABA C2, GABA C3, GABA C4) 24 h after CCI. Both incorporation of [1,6-13C]glucose and percent enrichment of 13C into [2-13C]glutamate (GLU C2) were higher in sham contralateral, compared to all other groups (*p<0.05 vs. sham contra, TBI ipsi, and TBI contra). In addition, incorporation of [1,6-13C]glucose into [3-13C]glutamate (GLU C2) was lower in TBI ipsilateral, compared to sham contralateral. There were no differences in [1,6-13C]glucose incorporation or 13C percent enrichment into GLU C4, GLN C3, or GLN C4 between groups, comparing trauma ipsilateral, trauma contralateral, sham ipsilateral, and sham contralateral hemispheres. Incorporation of [1,6-13C]glucose into [2-13C]GABA (GABA C2), [3-13C]GABA (GABA C3), and [4-13C]GABA (GABA C4), as well as percent enrichment of 13C into GABA C2, GABA C3, and GABA C4, were not different between groups. Values are mean±standard error of data for nmol 13C incorporated/mg protein and for percent 13C enrichment. GABA, gamma-aminobutyric acid; CCI, controlled cortical impact; TBI, traumatic brain injury.
After injection of [1,6-13C]glucose, there were no differences in percent enrichment of GLU C3 or GLU C4 between TBI and sham rats on both ipsi- and contralateral sides (Fig. 2). In addition, percent enrichment of GLN C3 and GLN C4, formed from the GLU C3 and GLU C4 in neurons, was the same in TBI and sham rats (Fig. 2). Although the mean percent enrichment of all four metabolites (GLU C3, GLU C4, GLN C3, and GLN C4) was consistently highest in sham contralateral samples, these were not statistically different from the other groups. Percent enrichment of GLU C2 was significantly decreased in both TBI sides and sham ipsilateral, compared to sham contralateral, samples (Fig. 2; p<0.05 sham contralateral vs. sham ipsilateral, TBI ipsilateral, and TBI contralateral). There were no significant differences in ipsi- or contralateral labeling of GABA C4, GABA C3, or GABA C2 between TBI and sham rats (data not shown).
Incorporation of carbon-13 into lactate and aspartate after traumatic brain injury
Values of LAC C3 percent enrichment in this study (1.98–3.42% enrichment [5.4±0.6 – 6.3±0.9 13C nmol/mg protein]) are comparable to those reported for both immature and adult rat brain.23 However, incorporation of [1,6-13C]glucose into LAC C3 was not different in ipsi- or contralateral samples between TBI and sham rats at 24 h after injury. There were no significant differences in ipsi- and contralateral labeling of aspartate (ASP) C3 between TBI and sham rats (data not shown). There were no significant differences in ratio of labeling in Lac/TCA cycle or ASP C3/GLU C4 ratio between groups (data not shown).
Metabolic cycling ratios and total tricarboxylic acid metabolism
Metabolic cycling ratios of metabolites labeled from the second turn/first turn of the TCA cycle, including GLU C3/GLU C4, GLN C3/GLN C4, and GABA C3/GABA C2, were all the same between groups (data not shown). Total TCA cycle metabolism was not different between TBI and sham rats (data not shown).
Discussion
The key finding of this study is that brain oxidative metabolism of glucose is maintained at 24 h after moderate-to-severe TBI, as opposed to a shift to anaerobic metabolism, and it is, for the most part, comparable to sham-operated animals. We hypothesized that oxidative glucose metabolism would be diminished at 24 h after TBI, because extensive histological data demonstrate that significant neuronal cell death occurs in the first 24 h after brain trauma.24,25 Our previous work showed early mitochondrial dysfunction (1–4 h) after CCI in immature rats5 and peak metabolic alterations observed at 24 h using 1H NMR spectroscopy.11 Specifically, we observed reductions in NAA in injured brain, suggesting neuronal mitochondrial dysfunction and/or neuronal death. Similar to these findings in current experiments, we found that NAA was decreased after TBI (Table 1). In addition, total GABA showed a trend to be decreased in both sides of the injured brain, but did not reach statistical significance. In contrast to our previous report, showing delayed metabolism and hypermetabolism in injured brain at 5–6 h after TBI,6 in this study, at 24 h after TBI, we did not observe differences in amount of glutamate, glutamine, aspartate, and lactate between injured and sham-operated animals. These results are consistent with data from Bartnik and colleagues,26 who demonstrated that adult rat brain after CCI had increased cerebral metabolism at 3.5 h after trauma and, at 24 h after TBI, observed no difference in total amount of glutamate and glutamine, as well as no difference in 13C label incorporation between injured and sham rats.
We determined that incorporation of 13C label in GLU C2 as well as percent enrichment of GLU C2 were reduced in TBI rats in both ipsi- and contralateral sides. GLU C2 is derived from the GLN C2 that is labeled from glucose metabolism by PC in astrocytes.27 GLU C2 can also be formed in neurons as a result of labeling from multiple turns of the TCA cycle. There were no differences in percent enrichment of GLN C2 between TBI and sham rats, suggesting that the decrease in GLU C2 may not be the result of early astrocytic dysfunction. However, label incorporation in GLU C3 was decreased in the injured side. These observed changes suggest that multiple turns of TCA cycle in neurons may be impaired, where astrocyte metabolism at 24 h is comparable to sham controls. Because we observed decreased NAA level in injured brains, results of these 13C experiments demonstrate that oxidative glucose metabolism in glutamatergic neurons at 24 h after TBI is impaired, when astrocytic and GABAergic metabolism is comparable to sham controls.
It is also possible that GLU C2 was reduced as a result of impairment of PC in astrocytes, especially considering that neuronal GLU C4 production was not decreased. Because we did not observe a correlative reduction in GLN C2, this might suggest that neuronally produced GLU C2 is compensating and this isotopomer is being supplied to astrocytes through the glutamate-glutamine cycle. However, this is not likely, because neurons are dependent on the anaplerotic reactions of astrocytes to replenish TCA cycle intermediates.28,29 Ultimately, if PC were to be impaired, with a reduction in GLU C2 production, overall neuronal metabolism would be impaired, because neurons are dependent on astrocytes for sustained oxidative metabolism and glutamate production.28
We determined that the 13C label incorporation into glutamate was decreased at 24 h after TBI, and the amount of 13C label incorporation into GABA was unchanged compared to sham. GABA synthesis occurs by glutamic acid decarboxylase (GAD), which is present in two isoforms, 65 or 67 (GAD65/67). GAD65 is enriched in nerve endings and is essential for biosynthesis of synaptic GABA from the precursor, glutamine, where GAD67, localized in the cytosol of GABAergic neurons, is important for GABA synthesis by TCA cycle and from the precursor, glutamine.30 No studies to date have assessed GAD65/67 expression in immature brain trauma; but, in adult rat brain TBI, O'Dell and colleagues reported no alteration in the amount of GAD65 messenger RNA at 12 and 24 h after fluid percussion injury (FPI).31 Further, Kobori and Dash demonstrated that TBI did not affect the level of GAD65 and significantly increased the level of GAD67 within hours and remained elevated for at least 28 days after injury.32
Glutamatergic and GABAergic neurons rely on a constant supply of the precursor, glutamine, supplied by astrocytes to sustain neurotransmitter homeostasis. This relationship, known as the glutamine-glutamate-GABA cycle, entails release of glutamate or GABA from neurons and prompt uptake into astrocytes, thus tightly controlling excitatory or inhibitory signal within the synaptic cleft.33,34 Astrocytes can use glutamate as a metabolic fuel or return the carbon skeleton to neurons as glutamine generated by glutamine synthetase.28,35 Thus, neurons are dependent on properly functioning astrocytes, and perturbations in astrocyte specific enzymes, including glutamine synthetase, have been described in many pathological conditions.36–38 Glutamine synthetase is localized in astrocytic cytoplasm, and one of its main roles is to detoxify glutamate and ammonia by converting them into glutamine. The rate of glutamine synthesis can be directly determined by label incorporation into glutamine. Our results did not show any difference in label incorporation and percent enrichment in glutamine at 24 h after immature brain trauma between injured and sham brains. Redell and Dash showed that the amount of glutamine synthetase was unchanged at 24 h after adult brain trauma in the same rat model (CCI).39 In immature rats, Kochanek and colleagues showed that glutamine synthetase was significantly increased at 14 days after TBI.40 It should be noted that the glutamate-glutamine cycle is not stoichiometric, and glutamate may have many fates in neurons, astrocytes, and other brain cells.33 One such fate is extensive use of glutamate for energy, thus resulting in loss of glutamate from the glutamate-glutamine cycle.33 Adenosine triphosphate production is significantly decreased in the TBI brain at 24 h in both the cortex and hippocampus,41 so it is possible that an observed decreased glutamate is a result of increased oxidation of glutamate.
A potentially important finding of this study is the relatively similar metabolic profile of the injured (ipsilateral) and uninjured (contralateral) hemispheres after TBI. Though there is no overt cell loss contralateral to CCI in our immature rat model,5 we hypothesized that there could be reversible metabolic alterations in the uninjured hemisphere. Other groups have demonstrated altered metabolism in the contralateral hemisphere after FPI in adult rats.42,43 These transient metabolic changes could reflect alterations in glucose transport and uptake, glutamate synthesis, or catecholamine release. An unexpected finding of the present results was that 13C glucose metabolism in the sham ipsilateral samples looked comparable to metabolism in the TBI samples. Although studies in adult rats have suggested mild histologic changes in the sham ipsilateral hemisphere44; to our knowledge, this study is the first to report minor metabolic changes. Our results and those of others show the importance of independently measuring metabolism in both hemispheres after focal injury models as well as comparing to both ipsi- and contralateral sham samples.6,42 Based on our findings, the sham contralateral hemisphere may be the best “control” in CCI studies.
One limitation of this study is that at the age studied (PNDs 16–18), rats are still suckling and therefore consuming a high-fat diet with significant brain uptake and utilization of ketones, which differs from human pediatric TBI in older children, where the brain is utilizing glucose. This transition from ketone to glucose utilization is summarized in detail in a recent review by Prins.45 Despite higher brain ketone utilization, many features of energy metabolism in the developing brain at this age (PNDs 16–18) are relevant to pediatric TBI. Mitochondrial function is still maturing at this age, with a peak in mitochondrial respiration rate and enzyme activity in the third to fourth week of life in the rat.46,47 Further, ketones account for only ∼30% of energy metabolism at PNDs 15–20,48 and glucose plays a large role in the synthesis of glutamate, glutamine, and GABA by this age.49 Another limitation of this study is that glucose metabolism was studied only at one time point after CCI. Previous work in our lab demonstrate that there are very early (1–4 h) metabolic alterations after CCI in immature rats.5,11 Results of this study would suggest that there is recovery of oxidative metabolism in surviving cells by 24 h. Future studies should therefore investigate additional times after injury (48 h to 14 days) to determine whether there is a subsequent decline in metabolic function, as was observed in adult rats after FPI.50 Confirmation of this phenomenon would suggest a “window of opportunity” for metabolic rescue in the first 24 h after TBI in the developing brain. It would also be important to correlate metabolic alterations with functional deficits. Prins and Hovda4 showed a correlation between glucose metabolism and spatial learning (Morris water maze) after TBI in PNDs 17 and 28 and adult rats. Earlier studies in adult rats showed a stronger correlation between longitudinal metabolic patterns and behavioral recovery.51
In conclusion, our results suggest that by 24 h after TBI, oxidative glucose metabolism recovers in 16- to 18-day-old rat brain. Specific reductions in [2-13C]glutamate could be the result of impairments in either neuronal or astrocytic metabolism. This suggests that the first 24 h are crucial in the metabolic support of the injured developing brain.
Acknowledgments
This work was supported by the National Institutes of Health (grants K08 NS42805, 5P01 HD016596, and K08 NS069815) and the the University of Maryland Department of Pediatrics (SRIS grant).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Keenan H.T., and Bratton S.L. (2006). Epidemiology and outcomes of pediatric traumatic brain injury. Dev. Neurosci. 28, 256–263 [DOI] [PubMed] [Google Scholar]
- 2.Tilford J.M., Aitken M.E., Anand K.J., Green J.W., Goodman A.C., Parker J.G., Killingsworth J.B., Fiser D.H., and Adelson P.D. (2005). Hospitalizations for critically ill children with traumatic brain injuries: a longitudinal analysis. Crit. Care Med. 33, 2074–2081 [DOI] [PubMed] [Google Scholar]
- 3.Thomas S., Prins M.L., Samii M., and Hovda D.A. (2000). Cerebral metabolic response to traumatic brain injury sustained early in development: a 2-deoxy-D-glucose autoradiographic study. J. Neurotrauma 17, 649–665 [DOI] [PubMed] [Google Scholar]
- 4.Prins M.L., and Hovda D.A. (2001). Mapping cerebral glucose metabolism during spatial learning: interactions of development and traumatic brain injury. J. Neurotrauma 18, 31–46 [DOI] [PubMed] [Google Scholar]
- 5.Robertson C.L., Saraswati M., and Fiskum G. (2007). Mitochondrial dysfunction early after traumatic brain injury in immature rats. J. Neurochem 101, 1248–1257 [DOI] [PubMed] [Google Scholar]
- 6.Scafidi S., O'Brien J., Hopkins I., Robertson C., Fiskum G., and McKenna M. (2009). Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J. Neurochem. 109, Suppl. 1, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson C.L., Scafidi S., McKenna M.C., and Fiskum G. (2009). Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp. Neurol. 218, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babikian T., Freier M.C., Tong K.A., Nickerson J.P., Wall C.J., Holshouser B.A., Burley T., Riggs M.L., and Ashwal S. (2005). Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr. Neurol. 33, 184–194 [DOI] [PubMed] [Google Scholar]
- 9.Babikian T., Freier M.C., Ashwal S., Riggs M.L., Burley T., and Holshouser B.A. (2006). MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J. Magn. Reson. Imaging 24, 801–811 [DOI] [PubMed] [Google Scholar]
- 10.Aaen G.S., Holshouser B.A., Sheridan C., Colbert C., McKenney M., Kido D., and Ashwal S. (2010). Magnetic resonance spectroscopy predicts outcomes for children with nonaccidental trauma. Pediatrics 125, 295–303 [DOI] [PubMed] [Google Scholar]
- 11.Casey P.A., McKenna M.C., Fiskum G., Saraswati M., and Robertson C.L. (2008). Early and sustained alterations in cerebral metabolism after traumatic brain injury in immature rats. J. Neurotrauma 25, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwal S., Babikian T., Gardner-Nichols J., Freier M.C., Tong K.A., and Holshouser B.A. (2006). Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch. Phys. Med. Rehabil. 87, S50–S58 [DOI] [PubMed] [Google Scholar]
- 13.Wong K.P., Sha W., Zhang X., and Huang S.C. (2011). Effects of administration route, dietary condition, and blood glucose level on kinetics and uptake of 18F-FDG in mice. J. Nucl. Med. 52, 800–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melo T.M., Nehlig A., and Sonnewald U. (2005). Metabolism is normal in astrocytes in chronically epileptic rats: a (13)C NMR study of neuronal-glial interactions in a model of temporal lobe epilepsy. J. Cereb. Blood Flow Metab. 25, 1254–1264 [DOI] [PubMed] [Google Scholar]
- 15.Richards E.M., Fiskum G., Rosenthal R.E., Hopkins I., and McKenna M.C. (2007). Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke 38, 1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry O. H., Rosebrough N. J., Farr A. L., and Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 17.Marin-Valencia I., Good L.B., Ma Q., Malloy C.R., Patel M.S., and Pascual J.M. (2012). Cortical metabolism in pyruvate dehydrogenase deficiency revealed by ex vivo multiplet (13)C NMR of the adult mouse brain. Neurochem. Int. 61, 1036–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melo T.M., Sonnewald U., Touret M., and Nehlig A. (2006). Cortical glutamate metabolism is enhanced in a genetic model of absence epilepsy. J. Cereb. Blood Flow Metab. 26, 1496–1506 [DOI] [PubMed] [Google Scholar]
- 19.Zwingmann C., and Leibfritz D. (2003). Regulation of glial metabolism studied by 13C-NMR. NMR Biomed. 16, 370–399 [DOI] [PubMed] [Google Scholar]
- 20.Sonnewald U., Westergaard N., Schousboe A., Svendsen J.S., Unsgard G., and Petersen S.B. (1993). Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem. Int. 22, 19–29 [DOI] [PubMed] [Google Scholar]
- 21.Qu H., Eloqayli H., Muller B., Aasly J., and Sonnewald U. (2003). Glial-neuronal interactions following kainate injection in rats. Neurochem. Int. 42, 101–106 [DOI] [PubMed] [Google Scholar]
- 22.Faul F., Erdfelder E., Lang A.G., and Buchner A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 [DOI] [PubMed] [Google Scholar]
- 23.Hassel B., Bachelard H., Jones P., Fonnum F., and Sonnewald U. (1997). Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J. Cereb. Blood Flow Metab. 17, 1230–1238 [DOI] [PubMed] [Google Scholar]
- 24.Hall E.D., Bryant Y.D., Cho W., and Sullivan P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 25.Whalen M.J., Dalkara T., You Z., Qiu J., Bermpohl D., Mehta N., Suter B., Bhide P.G., Lo E.H., Ericsson M., and Moskowitz M.A. (2008). Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 490–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartnik B.L., Sutton R.L., Fukushima M., Harris N.G., Hovda D.A., and Lee S.M. (2005). Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma 22, 1052–1065 [DOI] [PubMed] [Google Scholar]
- 27.Haberg A., Qu H., Haraldseth O., Unsgard G., and Sonnewald U. (1998). In vivo injection of [1-13C]glucose and [1,2-13C]acetate combined with ex vivo 13C nuclear magnetic resonance spectroscopy: a novel approach to the study of middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 18, 1223–1232 [DOI] [PubMed] [Google Scholar]
- 28.Hertz L., and Zielke H.R. (2004). Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 27, 735–743 [DOI] [PubMed] [Google Scholar]
- 29.Hertz L., Dringen R., Schousboe A., and Robinson S.R. (1999). Astrocytes: glutamate producers for neurons. J. Neurosci. Res. 57, 417–428 [PubMed] [Google Scholar]
- 30.Walls A.B., Eyjolfsson E.M., Smeland O.B., Nilsen L.H., Schousboe I., Schousboe A., Sonnewald U., and Waagepetersen H.S. (2011). Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J. Cereb. Blood Flow Metab. 31, 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Dell D.M., Raghupathi R., Crino P.B., Eberwine J.H., and McIntosh T.K. (2000). Traumatic brain injury alters the molecular fingerprint of TUNEL-positive cortical neurons In vivo: a single-cell analysis. J. Neurosci. 20, 4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobori N., and Dash P.K. (2006). Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J. Neurosci. 26, 4236–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna M.C. (2007). The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J. Neurosci. Res. 85, 3347–3358 [DOI] [PubMed] [Google Scholar]
- 34.Bak L.K., Schousboe A., and Waagepetersen H.S. (2006). The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 98, 641–653 [DOI] [PubMed] [Google Scholar]
- 35.McKenna M.C., Sonnewald U., Huang X., Stevenson J., and Zielke H.R. (1996). Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J. Neurochem. 66, 386–393 [DOI] [PubMed] [Google Scholar]
- 36.Smith C.D., Carney J.M., Starke-Reed P.E., Oliver C.N., Stadtman E.R., Floyd R.A., and Markesbery W.R. (1991). Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 88, 10540–10543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eid T., Behar K., Dhaher R., Bumanglag A.V., and Lee T.S. (2012). Roles of glutamine synthetase inhibition in epilepsy. Neurochem. Res. 37, 2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Hel W.S., Notenboom R.G., Bos I.W., van Rijen P.C., van Veelen C.W., and de Graan P.N. (2005). Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology 64, 326–333 [DOI] [PubMed] [Google Scholar]
- 39.Redell J.B., and Dash P.K. (2007). Traumatic brain injury stimulates hippocampal catechol-O-methyl transferase expression in microglia. Neurosci. Lett. 413, 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochanek A.R., Kline A.E., Gao W.M., Chadha M., Lai Y., Clark R.S., Dixon C.E., and Jenkins L.W. (2006). Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev. Neurosci. 28, 410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lifshitz J., Friberg H., Neumar R.W., Raghupathi R., Welsh F.A., Janmey P., Saatman K.E., Wieloch T., Grady M.S., and McIntosh T.K. (2003). Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 23, 219–231 [DOI] [PubMed] [Google Scholar]
- 42.Bartnik B.L., Lee S.M., Hovda D.A., and Sutton R.L. (2007). The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J. Neurotrauma 24, 1079–1092 [DOI] [PubMed] [Google Scholar]
- 43.Yoshino A., Hovda D.A., Kawamata T., Katayama Y., and Becker D.P. (1991). Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 561, 106–119 [DOI] [PubMed] [Google Scholar]
- 44.Cole J.T., Yarnell A., Kean W.S., Gold E., Lewis B., Ren M., McMullen D.C., Jacobowitz D.M., Pollard H.B., O'Neill J.T., Grunberg N.E., Dalgard C.L., Frank J.A., and Watson W.D. (2011). Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prins M.L. (2008). Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 28, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holtzman D., and Moore C.L. (1973). Oxidative phosphorylation in immature rat brain mitochondria. Biol. Neonate 22, 230–242 [DOI] [PubMed] [Google Scholar]
- 47.Holtzman D., and Moore C.L. (1975). Respiration in immature rat brain mitochondria. J. Neurochem. 24, 1011–1015 [DOI] [PubMed] [Google Scholar]
- 48.Cremer J.E., and Heath D.F. (1974). The estimation of rates of utilization of glucose and ketone bodies in the brain of the suckling rat using compartmental analysis of isotopic data. Biochem. J. 142, 527–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeVivo D.C., Leckie M.P., and Agrawal H.C. (1975). D-beta-Hydrozybutyrate: a major precursor of amino acids in developing rat brain. J. Neurochem. 25, 161–170 [DOI] [PubMed] [Google Scholar]
- 50.Bartnik-Olson B.L., Oyoyo U., Hovda D.A., and Sutton R.L. (2010). Astrocyte oxidative metabolism and metabolite trafficking after fluid percussion brain injury in adult rats. J. Neurotrauma 27, 2191–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore A.H., Osteen C.L., Chatziioannou A.F., Hovda D.A., and Cherry S.R. (2000). Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-microPET. J. Cereb. Blood Flow Metab. 20, 1492–1501 [DOI] [PubMed] [Google Scholar]