Abstract
Purpose
To report fusional divergence in children with intermittent exotropia (XT).
Methods
We retrospectively studied 32 children with intermittent XT (aged 4 to 13 years) and 38 visually normal non-strabismic children. Fusional divergence break points were measured using a prism bar, starting from a naturally fused state. Distribution of divergence break points was evaluated. Subnormal fusional divergence was defined as below the 5th percentile in visually normal children. In children with intermittent XT, correlations were calculated between divergence break point and control score, angle of deviation, and convergence break point.
Results
The distribution of fusional divergence break points in intermittent XT was normal at near but bimodal at distance. 9% had subnormal divergence (<10 prism diopters [pd]) at near, and 48% (<6 pd) at distance. There was a moderate correlation between divergence and convergence break points at near (r=0.44; P=0.01) but only weak inverse correlations between divergence break point and control score (r=−0.29; P=0.11) and divergence break point and angle of deviation (r=0.08; P=0.68) at near.
Conclusions
Most children with intermittent XT have normal near fusional divergence but nearly half have reduced distance fusional divergence. We found the magnitude of near divergence break point correlates with the magnitude of near convergence break point. Measuring divergence may provide useful information about fragility of fusion in patients with intermittent XT. Future studies of fusional divergence are needed to establish whether common measurement methods represent true divergence amplitudes and whether such measures have prognostic significance.
Keywords: Control, Divergence, Fusional amplitudes, Intermittent Exotropia, Strabismus
INTRODUCTION
Severity of intermittent exotropia (XT) has typically been described in terms of angle of deviation, control of the exodeviation and stereoacuity. More recently, convergence fusion amplitudes have been found to correlate with control of the exodeviation, and poor convergence has been suggested as a marker of severity in intermittent XT. (Hatt et al., 2011) Nevertheless, there are few data describing fusional divergence in children with intermittent XT. Fusional divergence has been reported to be subnormal in intermittent XT, but the clinical significance of subnormal versus normal fusional divergence has not been explored. It is possible that larger fusional divergence amplitudes allow better control of the exodeviation when changing from near to distance fixation, or that more severe intermittent XT is characterized by both diminished divergence and convergence amplitudes. We evaluated fusional divergence in children with intermittent XT and examined correlations with exodeviation control score, angle of deviation, and fusional convergence. We also compared fusional divergence in children with intermittent XT and visually normal children.
MATERIALS AND METHODS
Institutional review board approval was obtained for this study. All procedures and data collection were conducted in a manner compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Visually normal non-strabismic cohort
To study fusional divergence in children with intermittent XT, we first recruited a reference normal population of 38 subjects (5 to 13 years, median 10 years) without strabismus and with no history of strabismus, from community pediatric clinics. Visual acuity was at least 20/25 in each eye and any horizontal phoria was 6 prism diopters (pd) or less. For all subjects stereoacuity using the Preschool Randot test was at least 60 seconds of arc. Testing was performed in habitual refractive correction if prescribed (5 of 38). Exclusion criteria included presence of any eye disease, amblyopia and any vertical phoria. Verbal consent and written HIPAA authorization were obtained for each patient. To minimize testing burden, normal subjects were randomly selected for fusion testing at either distance (n=23) or near (n=15).
Intermittent exotropia cohort
Thirty-two subjects (4 to 13 years, median 7 years) diagnosed with intermittent XT and undergoing measures of fusional divergence at near fixation, were identified using departmental databases and data were analyzed retrospectively. All consecutive patients with a measurement of near fusional divergence were included, and we only used data from their first examination at which near fusional divergence was measured. Convergence data from 13 of these subjects have been reported previously (Hatt et al., 2011) but not from the same clinic visit. Patients with convergence insufficiency type intermittent XT (near deviation more than 10 pd greater than distance) were excluded because convergence insufficiency may be a different condition from distance intermittent XT. All patients also had an assessment of exodeviation control using a previously described control scale (Mohney & Holmes, 2006) and measures of angle of deviation using prism and alternate cover test (PACT). We excluded patients with a visual acuity worse than 20/40 in either eye. Twenty-seven of 32 intermittent XT patients had measures of fusional divergence at distance as well as at near and 31 of 32 had measures of fusional convergence at near. Median angle of deviation by PACT was 25 prism diopters (pd) (range 12 pd to 55 pd) at distance and 16 pd (range 6 pd esodeviation to 30 pd exodeviation) at near. Testing was performed in habitual refractive correction if prescribed (8 of 32). None were in overminus. Fusional amplitudes were assessed after control of the exodeviation was assessed and alignment was measured. Fusional divergence was measured before fusional convergence.
Measurement of fusional amplitudes
Fusional divergence was defined as the ability to fuse a base-in prism, starting from a state of spontaneous fusion. For intermittent XT patients we did not neutralize the angle of deviation with prism before starting fusion amplitude measurements. Convergence was defined analogously. Divergence and convergence break points were measured at near (1/3 meter) and at distance (3 meters) using a prism bar and an accommodative target. Testing commenced with the 1 diopter prism and the prism strength was then slowly increased in 2 pd increments from 2 pd to 20 pd and 5 pd increments from 20 pd to 45 pd. Patients were encouraged to concentrate on the target and asked to report when it appeared double. Brief cover tests were used when necessary, particularly for younger children, to confirm whether binocular alignment was maintained or whether fusion had broken. The fusion break point was recorded as the prism strength at which a tropia developed with no subsequent recovery to motor fusion i.e. when the patient reported persistent diplopia and/or a tropia was confirmed by cover test. The prism strength was then decreased until the patient was again able to fuse. The prism strength at which fusion was regained was defined as the recovery point. When a patient was exotropic at the commencement of fusion testing, the break point was recorded as 0.
Measurement of control of the exodeviation in intermittent XT patients
Control of the exodeviation was measured using a previously described 0- to 5-point control scale (Mohney & Holmes, 2006) at distance (3 meters) and at near (1/3 meter) using an accommodative target. The control score rated the proportion of time any spontaneous exotropia was present and, if no spontaneous tropia occurred, the recovery rate after dissociation. Spontaneous tropia was rated during a 30-second period of observation (levels 5 to 3 on the scale; 5=constant exotropia) and the speed of recovery was rated as the worst of three 10-second dissociations, (levels 2 to 0 on the scale; 0= <1 second recovery). If control of the exodeviation was measured more than once during the exam, the first measure of control score was used.
Analysis
Distribution of divergence break points at near was compared by visual inspection of the frequency histograms for normals and subjects with intermittent XT. In addition, divergence break point values in visually normal children were used to define subnormal thresholds in children with intermittent XT. For the purposes of this study, we defined subnormal divergence as below the 5th percentile for visually normal children. We then evaluated non-parametric Spearman rank correlations between near divergence break point and near and distance exodeviation control score, near divergence break point and angle of deviation (by PACT), and near divergence and convergence break points. divergence break points (near and distance) and the following parameters: exodeviation control score (near and distance), convergence break points (near and distance) and angle of deviation by PACT (near and distance).
RESULTS
Distribution of divergence break points
In visually normal children, the magnitude of the near fusional divergence break point ranged from 10 pd to 20 pd (Figure 1A), with a 5th percentile of 10 pd. Distance fusional divergence break point in normals ranged from 6 pd to 12 pd (Figure 1C) with a 5th percentile of 6 pd.
Figure 1.
Distribution of fusional divergence break point in visually normal children and in children with intermittent XT. Near divergence break point in normals (top left) and in children with intermittent XT (top rightbottom left). Distance divergence break point in normals (bottom lefttop right) and in children with intermittent XT (bottom right) showing a bimodal distribution in children with intermittent XT (peaks at 1 pd to 5 pd and 16 pd to 20 pd).
For children with intermittent XT, near fusional divergence break points ranged from 1 pd to >40 pd (Figure 1B) and appeared normally distributed. Three (9%) of 32 had subnormal near divergence (<10 pd). Distance fusional divergence break points ranged from 0 pd to 40 pd (Figure 1D) and appeared to follow a bimodal distribution with one peak frequency at 1 to 5 pd and a second peak frequency at 16 to 20 pd (Figure 1D). Thirteen (48%) of 27 had subnormal distance divergence (<6 pd).
Correlations with control scores
Overall there was a weak, inverse correlation between near divergence break point and near control score (r=−0.29; P=0.11, Figure 2A). Specifically, worse near divergence was associated with numerically high (worse) control score. Low near divergence break point was associated with near poor control of the exodeviation while normal divergence could be associated with either good or poor control of the exodeviation. There was a moderate inverse correlation between distance divergence break point and distance exodeviation control score (r=−0.41; P=0.03, Figure 2B).
Figure 2.
Relationship between fusional divergence break point and control score in children with intermittent XT at near (left) and distance (right), showing a weak inverse correlation at near and a moderate inverse correlation at distance (r=−0.29, p=0.11 and r=−0.41, p=0.03 respectively).
Correlations with convergence break point
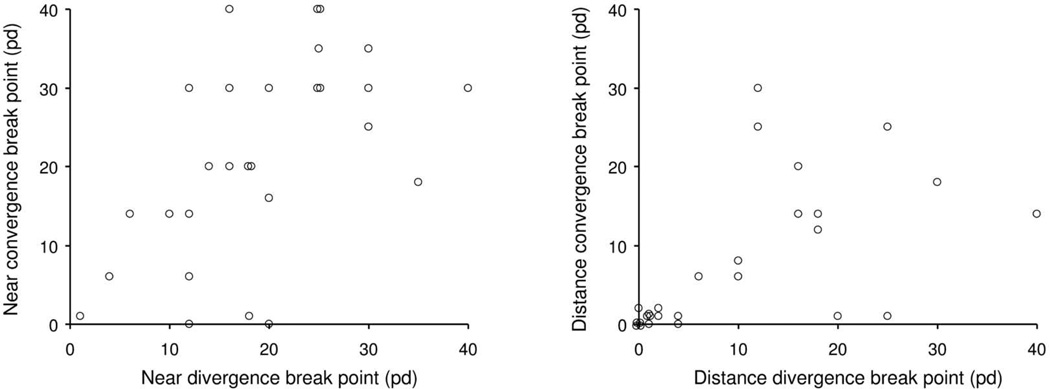
There was a moderate correlation between near divergence break point and near convergence break point (r=0.44, P=0.01, Figure 3A). Low near divergence break point was associated with a low near convergence break point, while normal near divergence could be associated with either near good convergence or near poor convergence. There was a strong correlation between distance divergence break point and distance convergence break point (r=0.72, P<0.0001, Figure 3B).
Figure 3.
Relationship between fusional divergence break point and fusional convergence break point in children with intermittent XT, at near fixation (left) and at distance fixation (right), showing a moderate positive correlation at near and a strong positive correlation at distance (r=0.44, p=0.01 and r=0.72, p<0.0001 respectively).
Correlations with angle
There was a weak correlation between near divergence break point and near angle of deviation (r=0.08, P=0.68). Any amount of strabismic near deviation was associated with any amount of near fusional divergence. There was also a weak correlation between distance divergence break point and distance angle of deviation (r=−0.14; P=0.48)
DISCUSSION
In children with intermittent XT fusional near divergence break point showed a similar distribution to normals, but distance divergence break points showed a bimodal distribution, with nearly half of subjects showing subnormal divergence break point at distance. Near divergence break point was only weakly inversely correlated with near control score, but there was a moderate inverse correlation between distance divergence break point and distance control score. Near divergence break point and near convergence break point showed a moderate correlation while distance divergence break point and distance convergence break point showed a strong correlation.
In the present study, we measured fusional divergence using the standard clinical method of progressively increasing base-in prisms from a state of spontaneous binocular fusion. Nevertheless, in patients with intermittent XT, it is unclear whether or not such testing actually measures true fusional divergence amplitudes. Jampolsky (Jampolsky, 1970) has postulated that in intermittent XT, binocular alignment is achieved by convergence mechanisms and that therefore, when assessed from a point of spontaneous fusion, base-in prisms initially result in a relaxation of convergence or “de-convergence.” (Jampolsky, 1970) Nevertheless, there is emerging evidence that convergence-related neurons and divergence-related neurons are anatomically separate (Rambold et al., 2004; Sander et al., 2009; Gamlin & Clarke, 1995; Mays et al., 1986; Nitta et al., 2008; Zhang & Gamlin, 1996) and it is possible that true fusional divergence is utilized in intermittent XT, but the distinction between fusional de-convergence and fusional divergence cannot yet be established on clinical grounds.
We defined “fusional divergence” as a patient’s ability to maintain binocular alignment through increasing base-in prisms, as this approach is commonly used in the clinical assessment of intermittent XT. This method of measuring divergence may not represent true divergence amplitudes, since the patient may not have a strong “fusion lock” (Jampolsky, 1970), especially at distance. (Jampolsky, 1970) Nevertheless, we suggest that the behavior of motor fusion under conditions of prism induced stress may convey information about the fragility of intermittent XT in an individual patient. Alternative methods of assessing fusional divergence such as first neutralizing the deviation with prisms or using the synoptophore may help to improve our understanding of the nature and role of fusional divergence in intermittent XT.
There are few previous data on divergence amplitudes in children with intermittent XT and none of the previous studies used the same methods or a comparable population. A study by Sharma and colleagues (Sharma et al., 2008) analyzed pre-operative and post-operative fusional divergence and fusional convergence amplitudes in 31 adults and children with intermittent XT and found mean divergence and convergence values were below normal pre-operatively. Nevertheless, Sharma and colleagues measured fusion amplitudes after first neutralizing the angle of deviation with prisms. Also, it is unclear how many children were included in their study (data for children are not reported separately). Such differences make it difficult to compare their outcomes with ours. In a review of intermittent XT Cooper and Medow (Cooper & Medow, 1993) stated that divergence amplitudes in intermittent XT are usually less than the angle of deviation. Analyzing our data with reference to angle of deviation, at distance, two thirds (67%) demonstrated fusional divergence less than their angle of deviation, while one third demonstrated divergence fusion equal to or greater than the angle of deviation. In contrast, at near, only 31% of our patients demonstrated fusional divergence less than their angle of deviation. Further study of the significance of fusional divergence in relation to the angle of deviation is warranted.
When changing from near fixation to distance fixation, the eyes de-convergence and / or diverge in order to maintain ocular parallelism. It might therefore be expected that patients with low fusional divergence would also show poorer control of the exodeviation when looking from near to distance and that those with larger divergence amplitudes would show better ability to maintain ocular alignment and control to exodeviation. Consistent with this hypothesis we found some correlation between divergence break point and control score, but the correlation was not strong. One possible reason for the absence of a strong correlation between divergence break point and control score, is that control of the exodeviation is mainly influenced by fusional convergence: we previously reported strong correlations between fusional convergence break point and control score. (Hatt et al., 2011) Alternatively, it may be that the method we employed for measuring fusional divergence, despite being a method used commonly in clinical practice, may not represent true divergence amplitudes, as discussed above.
The strongest correlation found in this present study, was between fusional divergence break point and fusional convergence break point. This correlation suggests that patients with reduced fusional divergence are also those with reduced fusional convergence. Given our previous report of a strong correlation between fusional convergence and exodeviation control score, it would seem possible that diminished horizontal fusional vergences are associated with poor control of the exodeviation and may be characteristic of patients at the more severe end of the intermittent XT spectrum. Whether intermittent XT signals a primary problem with slow vergence, or whether it overrides normal vergence mechanisms and corrupts clinical measurement of fusional divergence cannot be determined from our study. It is also interesting that some of our patients have divergence amplitudes greater than normals, which might be indicative of controlled de-convergence. It would be of interest to study whether or not treatment to increase fusional amplitudes may effect improvements in overall control of the exodeviation in these patients with “fragile” motor fusion.
Fusional divergence values in our visually normal cohort were comparable to previously reported values in visually normal children. Jiminez and colleagues (Jimenez et al., 2004) found a slightly lower mean divergence break points of 11 ± 3 pd at near, compared to our mean of 15 ± 3 pd, and 6 ± 2 pd, at distance, compared to our mean of 9 ± 2 pd. Nevertheless, the majority of our patients with intermittent XT who we classified with subnormal poor divergence would still be considered to have subnormal divergence using these alternative normal values.
One potential limitation of our study is that we did not repeat the measures of fusional divergence, and there may be variability during the day. Nevertheless, despite possible variability, we still found correlations between fusional divergence and fusional convergence. We used a standard prism bar for fusion assessment, with 2 pd increment changes up to 18 pd and 5 pd increment changes from 20 pd to 40 pd. It is possible that this change in demand at the point of transition may have resulted in poorer overall amplitudes and that divergence fusion values would have been greater using a rotary prism. Also, we used a relatively small, local group of visually normal children for our reference normal population and our results may have differed somewhat had we used a different cohort for comparisons between intermittent XT patients and normals. One additional weakness is in the method we used to assess control of the exodeviation. We only had a single measure during the office visit and we have previously reported that single measures of control score vary throughout the day. (Hatt et al., 2008)
In conclusion, while most patients with intermittent exotropia have normal fusional divergence at near, approximately half show decreased fusional divergence at distance. Whether this signifies an intrinsic slow vergence defect or a confounding effect of the exodeviation is unclear. It is unknown whether patients with reduced divergence are more likely to deteriorate over time. Future studies are needed to determine whether poor fusional divergence has prognostic significance and whether exercises to improve fusional divergence may have therapeutic value.
ACKNOWLEDGMENTS
Financial support: Supported by National Institutes of Health Grant EY018810 (JMH), Research to Prevent Blindness, New York, NY (JMH as Olga Keith Weiss Scholar and an unrestricted grant to the Department of Ophthalmology, Mayo Clinic), and Mayo Foundation, Rochester, MN.
Footnotes
Disclosures: None of the sponsors or funding organizations had a role in the design or conduct of this research. No authors have any financial / conflicting interests to disclose.
REFERENCES
- 1.Cooper J, Medow N. Intermittent exotropia, basic and divergence excess type. Binocul Vis Strabismus Q. 1993;8:187–216. [Google Scholar]
- 2.Gamlin PD, Clarke RJ. Single-unit activity in the primate nucleus reticularis tegmenti pontis related to vergence and ocular accommodation. J Neurophysiol. 1995;73:2115–2119. doi: 10.1152/jn.1995.73.5.2115. [DOI] [PubMed] [Google Scholar]
- 3.Hatt SR, Mohney BG, Leske DA, Holmes JM. Variability of control in intermittent exotropia. Ophthalmology. 2008;115:371–376. doi: 10.1016/j.ophtha.2007.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatt SR, Leske DA, Mohney BG, Brodsky MC, Holmes JM. Fusional convergence in childhood intermittent exotropia. Am J Ophthalmol. 2011;152:314–319. doi: 10.1016/j.ajo.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jampolsky A. Ocular divergence mechanisms. Trans Am Ophthalmol Soc. 1970;68:730–822. [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez R, Perez MA, Garcia JA, Gonzalez MD. Statistical normal values of visual parameters that characterize binocular function in children. Ophthalmic Physiol Opt. 2004;24:528–542. doi: 10.1111/j.1475-1313.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 7.Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: Neurons encoding vergence velocity. J Neurophysiol. 1986;56:1007–1021. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- 8.Mohney BG, Holmes JM. An office-based scale for assessing control in intermittent exotropia. Strabismus. 2006;14:147–150. doi: 10.1080/09273970600894716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitta T, Akao T, Kurkin S, Fukushima K. Involvement of the cerebellar dorsal vermis in vergence eye movements in monkeys. Cereb Cortex. 2008;18:1042–1057. doi: 10.1093/cercor/bhm143. [DOI] [PubMed] [Google Scholar]
- 10.Rambold H, Neumann G, Helmchen C. Vergence deficits in pontine lesions. Neurology. 2004;62:1850–1853. doi: 10.1212/01.wnl.0000125331.95849.62. [DOI] [PubMed] [Google Scholar]
- 11.Sander T, Sprenger A, Neumann G, et al. Vergence deficits in patients with cerebellar lesions. Brain. 2009;132:103–115. doi: 10.1093/brain/awn306. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Saxena R, Narvekar M, Gadia R, Menon V. Evaluation of distance and near stereoacuity and fusional vergence in intermittent exotropia. Indian J Ophthalmol. 2008;56:121–125. doi: 10.4103/0301-4738.39116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HY, Gamlin PD. Single-unit activity within the posterior fastigial nucleus during vergence and accommodation in the alert primate. Soc Neurosci Abstr. 1996;22:2034. [Google Scholar]