Abstract
Abstract A loud acoustic stimulus (LAS) presented during movement preparation can induce an early release of the prepared action. Because loud sound has been found to have an inhibitory effect on motor cortex excitability, it is possible that the motor cortex plays little role in the early release of prepared responses. We sought to shed new light on this suggestion by probing changes in corticospinal excitability after LAS presentation during preparation for an anticipatory action. Unexpectedly, we show that the changes in corticospinal excitability after LAS presentation are not fixed. Based on the magnitude of motor-evoked potentials elicited by transcranial magnetic and electric stimulation of the motor cortex, we demonstrate that the effects of auditory stimuli on corticospinal excitability depend on the level of readiness for action: inhibition in early preparation and facilitation close to movement onset. We also show that auditory stimuli can regulate intracortical excitability by increasing intracortical facilitation and reducing short-interval intracortical inhibition. Together, these findings indicate that, at least in part, the early release of motor responses by auditory stimuli involves the motor cortex.
Key points.
Unexpected loud auditory stimuli can trigger the involuntary release of motor actions during preparation to move.
Because acoustic stimulation can suppress motor cortex excitability during action, this early release could be independent of the motor cortex, and interpreted as pre-planned action stored and triggered from subcortical areas.
In contrast, we show that corticospinal excitability in response to the loud auditory stimuli was increased when people were highly prepared to move, and reduced otherwise.
Our results also indicate that auditory stimuli can affect intracortical excitability by increasing intracortical facilitation and reducing short-interval intracortical inhibition.
Together, our findings demonstrate that the early release of motor responses by auditory stimuli involves the motor cortex.
Introduction
It is a matter of common experience that a loud, abrupt noise like a car back-firing can elicit a startle response (the acoustic startle reflex; Leitner et al. 1980; Brown et al. 1991). Less widely known is the finding that when someone is ready to perform a particular behaviour, a loud acoustic stimulus (LAS) can elicit the prepared behaviour with a latency substantially shorter than normal voluntary reaction times (Valls-Sole et al. 1999). This has been termed the ‘StartReact effect’ (Valls-Sole et al. 1999; Carlsen et al. 2004) and may explain some false starts in sprint races where the starting pistol report serves as the startling stimulus.
It has been suggested that the StartReact effect only occurs if the person is truly startled, which can be assessed by whether or not the LAS reflexively evokes activity in muscles associated with the true startle reflex such as sternocleidomastoid (Carlsen et al. 2007). However, although startle-evoked activity in such muscles may be sufficient for early initiation of prepared responses by a LAS, it is not necessary (Valls-Sole et al. 2005; Kumru et al. 2006; Delval et al. 2012; Maslovat et al. 2012; Marinovic et al. 2013). In addition, in contrast to the StartReact, the startle reflex can be extinguished by a prepulse stimulus of lower intensity, indicating that the early release of prepared responses by acoustic stimuli and the startle reflex involve different physiological mechanisms (Valls-Sole et al. 2005; Kumru et al. 2006; Maslovat et al. 2012). Thus, the precise role of the startle reflex in the StartReact effect is unclear and to avoid confusion we will refer to the early release of motor actions – startled or not – simply as a ‘facilitated-reaction effect’. Our focus will be on the effects of loud auditory stimuli (LAS) on corticospinal excitability.
One suggested explanation for the facilitated-reaction effect is that when people ready themselves to make a response, they prepare a motor programme that is stored in subcortical motor circuits. The LAS evokes neural activity that rapidly reaches these circuits via subcortical pathways, and triggers response production (Valls-Sole et al. 1999; Carlsen et al. 2004). There are several findings that support this explanation. For example, a number of studies have reported that a LAS can transiently suppress activity in motor cortical areas (Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011). This finding is incompatible with facilitation of cortical response generation, but compatible with subcortical facilitation. Furthermore, the suppression of cortical activity has been found to be associated with increased excitability of subcortical motor pathways (Furubayashi et al. 2000; Ilic et al. 2011), consistent with facilitation of subcortical response generation.
The picture is not completely clear, however, since a subcortically stored programme idea is inconsistent with recent results showing that commands for well-learned ballistic acts are most likely generated in the cortex (Bullock et al. 1998; Ashby et al. 2010; Desmurget & Turner, 2010), and there are studies which are consistent with cortical involvement in the facilitated-reaction effect. For instance, a recent study found that transcranial magnetic stimulation (TMS) of the primary motor cortex (M1) delivered just prior to a loud stimulus reduced the facilitated-reaction effect, but did not influence the subcortical startle reflex pathways (Alibiglou & MacKinnon, 2012). A reduction of the facilitated-reaction effect, however, may also be caused by disruption of the subcortical stored programme by TMS. More specifically, it has been shown recently in anaesthetized macaque monkeys that TMS may also affect the ponto-medullary reticular formation in the brainstem (Fisher et al. 2012), casting some doubt about the involvement of M1 based on Alibiglou and MacKinnon’s ()2012 findings. Here we sought to shed new light on the debate by testing changes in corticospinal excitability – reflected by changes in motor-evoked potentials (MEPs) – after LAS presentation during different states of preparation for an action.
The inconsistency of findings relating to a cortical role in the facilitated-reaction effect may be due, in part, to differences in the preparatory processes leading to response production. In experiments that demonstrated motor cortical suppression by acoustic stimuli (Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011), the auditory stimuli were delivered after movement initiation, at a time when participants were not only in a low state of readiness but also maintained a low level of muscular contraction. In the study where the results were consistent with cortical involvement (Alibiglou & MacKinnon, 2012), participants were preparing for action and likely to be in a high state of readiness, meaning that the levels of response-related activation in cortical areas was probably close to movement threshold (see also Carpenter & Williams, 1995; Hanes & Schall, 1996). A reduced MEP amplitude shortly after the presentation of a LAS during the period of response preparation would be strong evidence against Alibiglou and MacKinnon’s ()2012 proposal that there is cortical involvement in the facilitated-reaction effect.
We show, using TMS delivered during preparation for an anticipatory timing task (Marinovic et al. 2011), that a LAS can have opposite effects on the net corticospinal excitability depending on the level of readiness for action: increased excitability when readiness is high, and decreased excitability when readiness is low. Our results also suggest that corticospinal excitability is modulated in response to loud acoustic stimuli by a combination of two mechanisms responsible for cortical regulation: intracortical disinhibition and facilitation (Kujirai et al. 1993; Nakamura et al. 1997). Finally, we show using transcranial electrical stimulation (TES) that the modulation of the net corticospinal excitability cannot be simply attributed to subcortical changes induced by acoustic stimuli. Taken together, these findings indicate cortical involvement in the early release of motor response by an auditory stimulus.
Methods
Participants
Seven volunteers (2 women) participated in Experiment 1 (mean age = 30.5, SEM = 2.8 years). Nine volunteers (2 women) participated in Experiment 2 (mean age = 30.1, SEM = 2.4 years), seven of whom had already participated in Experiment 1. Eight (2 women) of the nine volunteers in Experiment 2 also participated in Experiment 3 (mean age = 30.8, SEM = 2.6 years). Six participants (all male) of the nine volunteers in Experiment 2 also took part in Experiment 4 (mean age = 28.3, SEM = 3.2 years). Seven participants (all male) – five of whom took part in Experiment 4 – participated in Experiment 5 (mean age = 27.1, SEM = 3.2 years). Participants gave written informed consent prior to commencement of the study, which was in accordance with the Declaration of Helsinki and approved by the local Ethics Committee of the University of Queensland. All participants reported normal or corrected to normal vision and stated they were right-handed.
Procedures
Participants were seated in a chair in front of a monitor screen with support for the arms and hands as shown in Fig. 1A. Support for the hands was built so as to allow the participants to remain with their hands in a pronated position throughout the experiments. The participants were required to abduct their right index finger against a force transducer, placed beside their right hand on the hand rest, in anticipation of the moment they believed the moving target would make contact with a stationary target on the right side of the monitor screen. They were required to exert a comfortable, brief and constant contraction throughout the experiment. The displacement of the moving target was occluded from view 500 ms prior to its arrival at the contact position with the stationary target but participants were instructed to react as if the target was still in motion (constant speed was used). At the moment of contact between stationary and moving targets, the stationary target colour changed from green to red. Feedback about temporal error was provided after all practice trials and only after Control trials during the experiments (trials without LAS or TMS). The moving target was stationary for 1 s on its initial position and after motion onset it took 2 s to move from the left to the right side of a 19 inch monitor screen (60 Hz refresh rate, 1280 × 1024 (pixel) resolution) located 0.9 m away from the participants as shown in Fig. 1A. Visual stimuli were generated with Cogent 2000 Graphics running in MATLAB 7.5.
Figure 1. Experimental protocol.
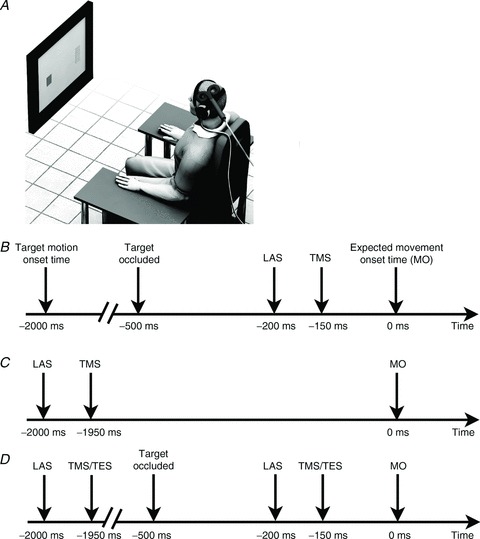
A, depiction of the experimental setup. B, sequence of events during probe trials with magnetic stimulation of M1 in Experiments 1 and 2. C, sequence of events during probe trials with magnetic stimulation of M1 in Experiment 3. D, sequence of events during probe trials with magnetic and electric stimulation of M1 in Experiment 5.
Procedures and design
Prior to the experimental block of trials, the participants were trained to abduct their index fingers at the correct time. Before the experiment started participants were presented with the LAS six times. Participants performed 60 trials during practice before Experiments 1 and 2. In Experiment 1, participants performed 120 trials where they tried to synchronize their contractions with the arrival of the moving target at the arrival position. In 24 of those, a LAS was presented and participants were asked to ignore it and perform the task normally. The onset time of the LAS was always 200 ms prior to the expected time of movement onset (Fig. 1B). In half of the trials in which a LAS was present, a single-pulse TMS was delivered over the left motor cortex 50 ms after LAS onset (Fig. 1B). In another 12 trials, a single-pulse TMS was delivered alone (without the LAS). The timing of these pulses matched the time of those where a LAS was presented. The remaining 84 trials (70% of total) were Control trials in which participants received feedback about synchronization errors. The order of presentation of the trials was pseudo-randomized so that trials with a loud auditory stimulus could not be presented twice in a row. Probe trials (LAS + TMS and TMS alone) were interleaved in one single block. The inter-trial interval was set to 4 s (end of one trial and beginning of the next trial). After the experimental block, participants were subject to the same probe trials – LAS + TMS, TMS alone and LAS alone – but were asked to remain at rest while looking at a fixation point on the monitor screen. Probe trials were again interleaved in one single block. The inter-trial interval for these trials at rest was set to 10 s.
In Experiment 2, participants performed 220 trials. In 40 of those, a LAS was presented. In 30 of these LAS trials, single (10 trials) or paired TMS (10 intracortical facilitation (ICF) and 10 short-interval intracortical inhibition (SICI)) pulses were applied over the left motor cortex. In another 30 trials, single, paired ICF or paired SICI pulses were applied at times matching those with LAS. The timing of the test stimulus was always 50 ms after LAS onset. The remaining 150 trials (68% of total) served as Control trials. The order of presentation of the trials was again controlled so that trials with a LAS could not be presented sequentially. Probe trials were interleaved in one single block. The inter-trial interval was set to 4 s. Experiment 2 took place 2 weeks after the completion of Experiment 1.
Experiment 3 was conducted 10 min after the end of Experiment 2. Only experimental probes (LAS + TMS, TMS alone and LAS alone) were presented and the timing of LAS presentation was 2000 ms prior to the expected time of movement onset (when the moving target initiated its motion as shown in Fig. 1C). Participants performed 56 trials – 8 for each probe condition – and feedback about temporal error was provided as there was no reason to believe LAS or TMS could interfere with performance when delivered so early into the trial.
In Experiment 4, the procedures were identical to those for Experiment 1 with the following exception. TES was used instead of TMS. Experiment 4 took place 4 weeks after the completion of Experiment 3.
In Experiment 5, the procedures were identical to those for Experiment 4 with the following exceptions. We employed both TES and TMS in the same block of trials (interleaved trials). Furthermore, because we did not test changes in corticospinal excitability with TES during early preparation, we included this condition in this experiment as shown in Fig. 1D. To reduce the number of trials in which a LAS was presented, we had no control trials where a LAS was delivered alone. Participants performed 240 trials in Experiment 5. In 40 of those, a LAS was presented during preparation (20 early and 20 late; 16.66% of total). Half of these trials were followed by a TMS or a TES pulse 50 ms after LAS onset. In another 40 trials, single pulse TMS or TES stimuli were presented during early and late preparation. The remaining 160 trials (66.66% of total) served as Control trials.
Auditory stimuli
The auditory stimuli were bursts of 50 ms broadband white noise with a rise/fall time shorter than 1 ms. Stimuli were generated on a digital computer and presented binaurally via high-fidelity stereophonic headphones (Sennheiser model HD25-1 II; frequency response 16 Hz to 22 kHz; Sennheiser Electronics GmbH & Co. KG, Wedemark, Germany). The input signal to the headphones had a bandwidth of approximately 10 Hz to 30 kHz. Auditory stimuli had a peak loudness of 114 dB. Sound intensity was measured with a Brüel and Kjaer sound level meter (type 2205, A-weighted; Brüel & Kjaer Sound & Vibration Measurement, Naerum, Denmark) placed 2 cm from the headphone speaker.
TMS stimulation
Before practice trials, the TMS intensity was established for each participant by finding the resting motor threshold (RMT) for the first dorsal interosseous muscle (FDI, primary agonist in the task). The intensity of the test stimulus was set to 120% of RMT, while the intensity of the conditioning stimulus in Experiment 2 and 3 was set to 70% of RMT. In Experiments 2 and 3, the intensity of the test stimulus was the same for trials with LAS and TMS alone as adjusting the intensity of the test stimulus to compare directly MEPs evoked with paired-pulses is known to alter the relationship between conditioning and test stimulus (see Garry & Thomson, 2009). The interstimulus interval between conditioning and test stimulus was adjusted to elicit maximal inhibitory (SICI) and excitatory (ICF) effects at rest, before the beginning of the experiment. These values ranged between 2 and 3 ms for SICI and 12–13 ms for ICF. The position of the coil over the specific site of stimulation was held in place by one experimenter. MEPs were recorded from the FDI and the abductor digiti minimi (ADM) muscles of the right hand using disposable Ag–AgCl electrodes. The electromyogram (EMG) signal was amplified, band-pass filtered between 30 and 1 kHz (Grass P511 isolated amplifier), sampled at 2000 Hz, and stored on computer. The torque transducer data were time locked to the collection of the EMG signal and also sampled at 2000 Hz.
TES stimulation
Transcranial electrical stimulation was performed using a Digitimer D180A stimulator (Digitimer, L. Welwyn Garden City, UK). The cathode was placed over the vertex, and the anode at the optimal position to elicit motor-evoked potentials using TMS (about 6 cm lateral to the vertex). The stimulus intensity was adjusted to the minimum that could elicit a noticeable MEP in 4 out of 4 consecutive stimuli without any acoustic stimulus. This procedure resulted in MEPs in Control trials (no LAS) that were similar in magnitude to those observed in Control trials using TMS (see Results). Details of MEP data recording were identical to those employed for TMS stimulation (see above).
Data analysis
The variables of interest were: constant temporal error, peak EMG, EMG duration, time to peak EMG, root mean squared (RMS) EMG and amplitude of the peak-to-peak motor-evoked potentials. Temporal error was defined as the difference between movement onset and the time the moving target arrived at the designated position (negative = early). Movement onsets were estimated from the tangential speed time series (derived by numerical differentiation from the filtered torque data) using the algorithm recommended by Teasdale et al. ()1993. Peak EMG was defined as the maximum value of the rectified EMG signal of the FDI muscle. EMG duration was defined as the time between EMG onset and EMG offset on the FDI muscle. Movement offset was estimated using the same algorithm used to determine movement onset. RMS EMG was defined as the root mean square signal over an interval of 250 ms preceding the test TMS pulse. The amplitude of the peak-to-peak MEP is presented as the raw value obtained when we compare responses obtained with single-pulse TMS. Ratios between MEPs obtained with paired pulse and single pulse are used to assess intracortical changes induced by the LAS (SICI and ICF). MEPs were inspected visually so that we could discard any trial in which a voluntary contraction preceding the test pulse was visible. Means of temporal error, peak EMG, EMG duration, time-to-peak EMG and RMS EMG obtained in Control trials and in LAS trials without TMS were compared using paired tests. Means of MEPs obtained in trials with and without LAS were also analysed using paired tests. For ratios, means were log-transformed before statistical analysis to reduce the detrimental effect of right-skewed outliers. All variables were first checked for normality using the Shapiro–Wilk test. When the assumption of normality was met we tested for differences between means using paired t tests. When the normality assumption was violated we employed the Wilcoxon test and reported the exact P values. α was set to 0.05 for all comparisons. We report r effect sizes for all pairwise comparisons.
Results
Movement timing and EMG
We examined the timing of movement onset in Experiments 1, 2 and 4 – where the auditory stimulus was delivered late in preparation (200 ms to movement onset) – to determine whether the auditory stimulus used could trigger the early release of the prepared movement. Since the auditory stimulus was presented during early preparation in Experiment 3 – 2 s to movement onset – we did not analyse the effect of the LAS on timing accuracy in this experiment as no differences were expected. As shown in Fig. 2, responses in Experiments 1 and 2 were initiated significantly earlier in the presence of a LAS than those produced without it (Experiment 1: paired t test, t6= 8.88, P= 0.0001, r= 0.95; Experiment 2: paired t test, t8= 7.30, P= 0.0001, r= 0.93). In Experiment 4, which served as a control using transcranial electrical stimulation (TES) with a subsample of the participants of Experiment 3, responses produced with a LAS were also initiated earlier than those without the acoustic stimulus (mean temporal error: LAS =−24.4 ms; Control = 17.41 ms; paired t test, t5= 3.17, P= 0.024, r= 0.82). EMG duration of early responses was not different from Control trials and neither was the time to peak EMG (see Table 1). Peak EMG, on the other hand, was greater in responses triggered by the auditory stimulus (see Table 1). Overall, these results indicate that the auditory stimulus triggered the prepared pattern of movement earlier compared to normal Control trials. It is also worth noting that responses to LAS at rest in Experiment 1, while participants simply observed a fixation point on the monitor screen, showed that the auditory stimulus failed to elicit any measurable response on the FDI muscle that could be easily differentiated from background noise (peak EMG: mean (SEM) = 0.0055 (0.0009) mV). Thus, the LAS by itself did not seem to trigger a reflex response in the absence of preparation for the FDI muscle, which could be mistaken for the release of the prepared response during preparation.
Figure 2. Response onset time relative to arrival of the moving target at the contact location with the stationary target in Experiments 1 and 2 (TMS trials not included).
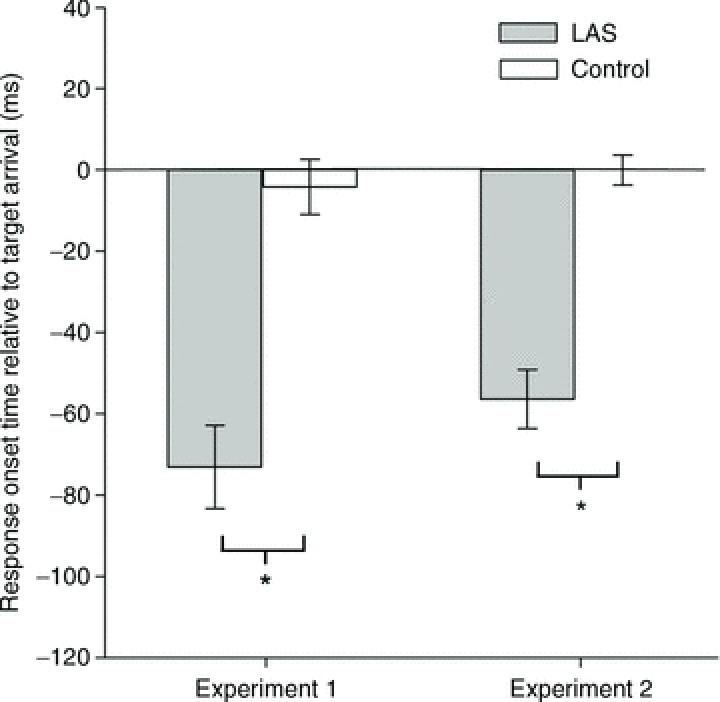
Error bars represent the SEM. *P < 0.05.
Table 1.
Means of EMG and temporal variables analysed in Experiments 1, 2, 3 and 4
| Variable | Control mean (SEM) | LAS mean (SEM) | t or z | P | r |
|---|---|---|---|---|---|
| Experiment 1 (n= 7) | |||||
| Peak EMG (mV) | 0.52 (0.06) | 0.57 (0.06) | 3.41 | 0.014* | 0.79 |
| EMG duration (ms) | 192.8 (24.5) | 194.3 (31.3) | 0.17 | 0.865 | 0.33 |
| Time to peak EMG (ms) | 87.5 (12.7) | 95.9 (44.7) | 1.21 | 0.272 | 0.44 |
| FDI RMS (mV) | 0.0035 (0.0001) | 0.0036 (0.0001) | 0.18 | 0.856 | 0.07 |
| ADM RMS (mV) | 0.0055 (0.0002) | 0.0057 (0.0003) | 1.95 | 0.098 | 0.62 |
| Experiment 2 (n= 9) | |||||
| Peak EMG (mV) | 0.60 (0.06) | 0.73 (0.08) | 6.08 | 0.001* | 0.90 |
| EMG duration (ms) | 202.8 (15.7) | 203.6 (15.5) | 0.12 | 0.907 | 0.04 |
| Time to peak EMG (ms) | 92.7 (8.13) | 87.1 (10.9) | 1.04 | 0.326 | 0.34 |
| FDI RMS (mV) | 0.0092 (0.001) | 0.0101 (0.001) | 0.99 | 0.349 | 0.33 |
| ADM RMS (mV) | 0.01669 (0.010) | 0.0171 (0.009) | 0.81 | 0.441 | 0.27 |
| Experiment 3 (n= 8) | |||||
| Peak EMG (mV) | 0.556 (0.07) | 0.591 (0.08) | 1.61 | 0.149 | 0.51 |
| Temporal error (ms) | −6.35 (7.97) | −6.50 (8.31) | 0.01 | 0.988 | 0.00 |
| Experiment 4 (n= 6) | |||||
| Peak EMG (mV) | 0.23 (0.07) | 0.28 (0.09) | 2.23 | 0.075 | 0.70 |
| EMG duration (ms) | 177.6 (20.0) | 182.9 (23.8) | 1.01 | 0.356 | 0.41 |
| Time to peak EMG (ms) | 64.8 (10.5) | 64.9 (15.4) | 0.02 | 0.983 | 0.02 |
| FDI RMS (mV) | 0.0111 (0.002) | 0.0047 (0.002) | 0.10† | 0.916 | 0.04 |
Statistically significant difference between LAS and Controls trials, P < 0.05. †Wilcoxon test was used.
Motor-evoked potentials elicited by single-pulse magnetic stimulation
Next, we examined the effect of the auditory stimulus on corticospinal excitability. In Experiment 1, during late response preparation, we found that motor-evoked potentials elicited shortly after a LAS by single-pulse TMS were significantly larger than those observed in Control trials (trials without a LAS) for the primary muscle (first dorsal interosseous, FDI) involved in the task (paired t test, t6= 4.19, P= 0.006, r= 0.86, see Fig. 3A). However, larger MEPs were not observed for the ADM muscle, which was not involved in the motor task (paired t test, t6= 0.83, P= 0.438, r= 0.32). At rest, there was no effect observed for either the FDI (paired t test, t6= 0.75, P= 0.477, r= 0.29, see Fig. 3A) and ADM (paired t test, t6= 0.40, P= 0.70, r= 0.27) muscles. As shown in Fig. 3B, these results were replicated in Experiment 2. More specifically, when accompanied by the LAS, motor-evoked potentials elicited in the FDI muscle were again significantly larger than those obtained in Control trials (Wilcoxon test, z= 2.66, P= 0.004, r= 0.88). Similar results late in preparation were observed for the FDI muscle in Experiment 5 (paired t test, t6= 4.446, P= 0.004, r= 0.87), where TMS and TES trials were interleaved. As in Experiment 1, this effect was not observed in the ADM muscle (paired t test, t8= 1.26, P= 0.242, r= 0.40) in Experiment 2. Interestingly, in a third experiment (see Fig. 3C) where we tested for changes in corticospinal excitability earlier on during preparation – 2 s prior to movement onset – we found some evidence for corticospinal suppression rather than facilitation (Wilcoxon test, z= 1.96, P= 0.055, r= 0.69) in response to the loud auditory stimulus. This effect during early preparation was also replicated in Experiment 5 (paired t test, t6= 2.74, P= 0.034, r= 0.74). The suppressive effect, however, was not observed in the ADM muscle (paired t test, t7= 0.67, P= 0.524, r= 0.24). The pattern of results obtained with single-pulse stimulation late in preparation is the reverse of what one would predict based on previous reports in the literature (Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011). More precisely, our results suggest corticospinal facilitation late in preparation (Experiments 1, 2 and 5) and corticospinal inhibition in early preparation (Experiments 3 and 5).
Figure 3. Motor-evoked potentials (MEPs) on the FDI muscle elicited by single-pulse stimulation over the motor cortex.
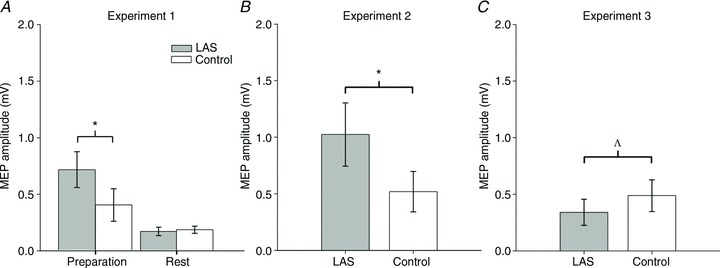
A, MEPs obtained in Experiment 1 during late preparation (150 ms to movement onset) and at rest. B, MEPs obtained in Experiment 2 during late preparation. C, MEPs obtained in Experiment 3 during early preparation. Error bars represent the SEM. *P < 0.05. ΛP= 0.055.
Motor-evoked potentials elicited by paired-pulse magnetic stimulation – intracortical facilitation
The increase in MEP amplitude in Experiment 1 and the reverse pattern in Experiment 3 could be brought about by changes in intracortical facilitation (ICF). We examined the involvement of this mechanism by probing the excitability of the corticospinal pathway using paired pulses (interstimulus interval, 12–13 ms) with and without the auditory stimulus delivered during late (Experiment 2) and early (Experiment 3) preparation. A larger MEP ratio between paired-pulse and single-pulse MEPs for LAS (LAS /LAS
/LAS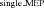 ) trials would indicate increased intracortical facilitation, whereas the opposite result would indicate reduced intracortical facilitation. In Experiment 2, we found no evidence for differences in motor-evoked potential ratios between LAS trials and Control trials for either FDI (paired t test, t8= 0.67, P= 0.521, r= 0.23, see Fig. 4A) or ADM (paired t test, t8= 0.96, P= 0.362, r= 0.32) muscles when the probe occurred late during the trial. This suggests that the increase in MEP amplitude obtained with single-pulse TMS may not be accounted for by this mechanism. Nevertheless, one should be careful to rule out this mechanism in late preparation because MEPs were so massively increased in response to single-pulse stimulation that there is little room for further increases in MEP amplitude using the ICF technique. In Experiment 3, we observed that MEP ratios obtained when a LAS was presented were significantly larger than those obtained in Control trials for the FDI muscle (Wilcoxon test, z= 2.52, P= 0.011, r= 0.89, see Fig. 4B). This effect was extended to the ADM muscle (paired t test, t7= 2.91, P= 0.022, r= 0.74), which was not the primary muscle involved in the task. Contrary to what would be expected if inhibition was achieved through decreased intracortical facilitation during preparation, the results from Experiment 3 indicate that an auditory stimulus can increase intracortical facilitation.
) trials would indicate increased intracortical facilitation, whereas the opposite result would indicate reduced intracortical facilitation. In Experiment 2, we found no evidence for differences in motor-evoked potential ratios between LAS trials and Control trials for either FDI (paired t test, t8= 0.67, P= 0.521, r= 0.23, see Fig. 4A) or ADM (paired t test, t8= 0.96, P= 0.362, r= 0.32) muscles when the probe occurred late during the trial. This suggests that the increase in MEP amplitude obtained with single-pulse TMS may not be accounted for by this mechanism. Nevertheless, one should be careful to rule out this mechanism in late preparation because MEPs were so massively increased in response to single-pulse stimulation that there is little room for further increases in MEP amplitude using the ICF technique. In Experiment 3, we observed that MEP ratios obtained when a LAS was presented were significantly larger than those obtained in Control trials for the FDI muscle (Wilcoxon test, z= 2.52, P= 0.011, r= 0.89, see Fig. 4B). This effect was extended to the ADM muscle (paired t test, t7= 2.91, P= 0.022, r= 0.74), which was not the primary muscle involved in the task. Contrary to what would be expected if inhibition was achieved through decreased intracortical facilitation during preparation, the results from Experiment 3 indicate that an auditory stimulus can increase intracortical facilitation.
Figure 4. MEPs obtained on FDI with paired-pulses (SICI and ICF) expressed as a ratio relative to MEPs obtained with single-pulse stimulation in the same experimental session.
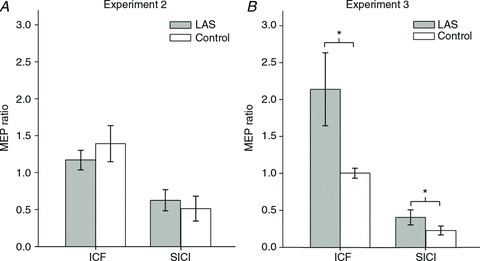
A, MEP ratios for ICF and SICI in Experiment 2. B, MEP ratios for ICF and SICI in Experiment 3. Error bars represent the SEM. *P < 0.05. Note that ratios were log-transformed for analysis and the figure above depicts the untransformed values.
Motor-evoked potentials elicited by paired-pulse magnetic stimulation – intracortical inhibition
Given that the increased net excitability of the corticospinal pathway could be associated with decreased inhibition, we tested the effect of LAS on short-interval intracortical inhibition during late (Experiment 2) and early (Experiment 3) preparation. The rationale for the interpretation of these results is that a decrease in MEP ratio for LAS trials (LAS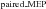 /LAS
/LAS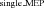 ) in relation to Control trials (Control
) in relation to Control trials (Control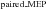 /Control
/Control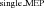 ) would indicate increased short-interval intracortical inhibition, whereas an increase would suggest a reduction in short-interval intracortical inhibition. In Experiment 2, the mean MEP ratio elicited with a LAS was not statistically different from that obtained in Control trials for the FDI (paired t test, t8= 0.95, P= 0.368, r= 0.32, see Fig. 4A) or ADM (paired t test, t8= 0.61, P= 0.559, r= 0.21) muscles. These results indicate that a reduction in short-interval intracortical inhibition is unlikely to explain the large increase in MEP amplitude in response to single-pulse stimulation (Experiments 1 and 2). Early in preparation (Experiment 3), however, we found that the mean MEP ratio obtained with a LAS was significantly greater than that observed in Control trials for the FDI muscle (paired t test, t7= 3.28, P= 0.013, r= 0.76, see Fig. 4B). This effect was not observed for the ADM muscle (Wilcoxon test, z= 0.98, P= 0.326, r= 0.34). Again, the results obtained in Experiment 3 are the opposite of what one would predict in the case of cortical suppression after LAS presentation as they indicate a reduction in short-interval intracortical inhibition.
) would indicate increased short-interval intracortical inhibition, whereas an increase would suggest a reduction in short-interval intracortical inhibition. In Experiment 2, the mean MEP ratio elicited with a LAS was not statistically different from that obtained in Control trials for the FDI (paired t test, t8= 0.95, P= 0.368, r= 0.32, see Fig. 4A) or ADM (paired t test, t8= 0.61, P= 0.559, r= 0.21) muscles. These results indicate that a reduction in short-interval intracortical inhibition is unlikely to explain the large increase in MEP amplitude in response to single-pulse stimulation (Experiments 1 and 2). Early in preparation (Experiment 3), however, we found that the mean MEP ratio obtained with a LAS was significantly greater than that observed in Control trials for the FDI muscle (paired t test, t7= 3.28, P= 0.013, r= 0.76, see Fig. 4B). This effect was not observed for the ADM muscle (Wilcoxon test, z= 0.98, P= 0.326, r= 0.34). Again, the results obtained in Experiment 3 are the opposite of what one would predict in the case of cortical suppression after LAS presentation as they indicate a reduction in short-interval intracortical inhibition.
Contrasting motor-evoked potentials elicited by single-pulse magnetic and electrical stimulation
It is conceivable that the large increase in MEP magnitude soon after LAS presentation could be due to massive spinal facilitation outweighing M1 inhibition. A comparison between MEPs obtained with TMS (Fig. 5A) and TES (Fig. 5B) allows a direct assessment of this possibility. It has been suggested that transcranial electrical stimulation is likely to activate the axons of corticospinal tract fibres in the subcortical white matter (Rothwell et al. 1994; Di Lazzaro et al. 2004). Thus, MEPs evoked by TES should be less affected by cortical suppression than MEPs evoked by TMS in the presence of an acoustic stimulus (see Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011). Therefore, we hypothesize that, in the case of subcortical facilitation, the ratio between LAS and Control MEPs obtained with TES would be larger than the ratio (LAS/Control) evoked by TMS. In contrast, a larger ratio between LAS and Control trials with TMS would suggest cortical facilitation. As shown in Fig. 5C, the mean ratio of MEP increase was 1.85 (SEM 0.45) with TES, whereas it was 3.09 (SEM 1.19) with TMS across the six participants that took part in both Experiments 2 and 4. Consistent with facilitation and/or disinhibition of cortical neurons in M1, the mean magnitude of MEP increase (expressed as a ratio between LAS MEPs/Control MEPs) was significantly larger using TMS than TES (paired t test, t5= 3.55, P= 0.016, r= 0.85, see Fig. 4C). A comparison between the mean amplitude of control MEPs – obtained without a LAS – obtained with TMS and TES indicated no significant difference (paired t test, t5= 0.97, P= 0.37, r= 0.39, see Fig. 2B and D). This suggests that the greater ratio of increase obtained with TMS cannot be attributed to differences in the size of the neural populations activated by the two different techniques.
Figure 5. MEPs on the FDI muscle elicited by single-pulse stimulation over the motor cortex for participants that took part in both Experiments 2 and 4 (A and B).
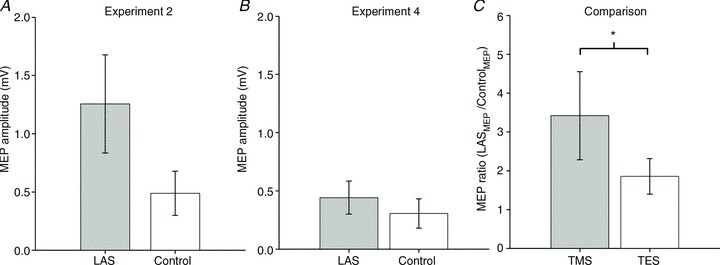
A, MEPs obtained in Experiment 2 during late preparation using TMS. B, MEPs obtained in Experiment 4 during late preparation using TMS. C, MEP amplitude ratio (LASMEP/ControlMEP) in Experiment 2 (TMS) and 4 (TES). Error bars represent the SEM. *P < 0.05. Note that ratios were log-transformed for analysis and C depicts the untransformed values.
Control experiment
Transcranial electric stimulation (TES) can be rather unpleasant and the expectation of such a stimulus could mean a different type of mechanism (e.g. sensitization or habituation) may have affected preparation and, as result, our comparison between Experiments 2 and 4. Thus, we ran a control experiment to observe the pattern of results we would obtain if MEPs induced by TES and TMS were obtained randomly in the same block. Furthermore, because we did not test the effect of TES early during preparation, the locus of suppression during early preparation was less certain. Therefore, we also obtained MEPs using both TES and TMS during early preparation in Experiment 5. We used the one-sample Wilcoxon signed rank test and compared the ratios against a reference value of 1 (no change) as the number of observations was uneven between conditions. As before, we report exact P values.
As shown in Fig. 6A and C, during early preparation MEPs decreased similarly with TMS (n= 7, one-sample Wilcoxon test, z= 2.36, P= 0.018, r= 0.89) and TES (n= 6, one-sample Wilcoxon test, z= 1.78, P= 0.094, r= 0.73). These results confirm our previous finding that the magnetic-evoked MEPs decrease early in preparation and suggest that the same occurs for electric-evoked MEPs. In Fig. 6B and D, we show a pattern of results very similar to those obtained in separate experiments (2 and 4). That is, we replicated the previous findings that MEPs increase substantially during late preparation for TMS (n= 7, one-sample Wilcoxon test, z= 2.36, P= 0.018, r= 0.89) but less so for electric-evoked MEPs (n= 5, one-sample Wilcoxon test, z= 1.48, P= 0.188, r= 0.66).
Figure 6.
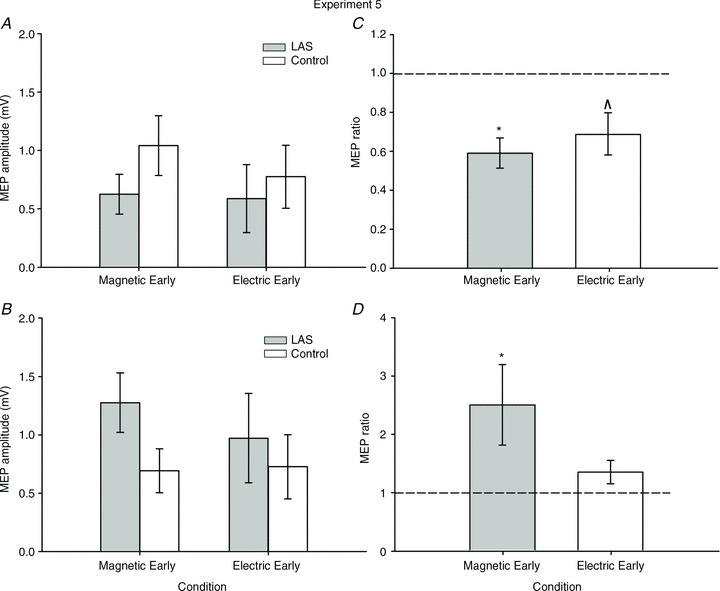
A, MEPs on the FDI muscle elicited by single-pulse magnetic and electric stimulation over the motor cortex during early preparation in Experiment 5. B, MEPs on the FDI muscle elicited by single-pulse magnetic and electric stimulation over the motor cortex during late preparation. C, MEP amplitude ratio (LASMEP/ControlMEP) during early preparation in Experiment 5 for both TMS and TES MEPs. D, MEP amplitude ratio (LASMEP/ControlMEP) during late preparation in Experiment 5 for both TMS and TES MEPs. Error bars represent the SEM. *P < 0.05, ΛP= 0.09.
Discussion
Our study sought to test the effect of a loud acoustic stimulus (LAS) on corticospinal excitability during the final preparation phase of a self-initiated anticipatory timing action (Marinovic et al. 2008, 2009a,2011). A pervasive idea in the field of sensorimotor control is that ballistic and brief actions, such as those involved in anticipatory actions (e.g. hitting a baseball), are programmed cortically and stored subcortically (Valls-Sole et al. 1995,1997,1999; Carlsen et al. 2003; Queralt et al. 2008), from where they can be triggered earlier than normal by a LAS without further involvement of cortical areas (Valls-Sole et al. 1999,2008). Alternatively, earlier responses could be prepared and triggered from the motor cortex via a fast conducting transcortical pathway (Alibiglou & MacKinnon, 2012). One possible way to tease apart these two possibilities is to investigate the effects of auditory stimuli on corticospinal excitability. Auditory stimuli have well-documented differential effects on cortical and spinal excitability during muscular contractions: cortical suppression and spinal facilitation (Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011).
The observation of decreased amplitude of motor-evoked potentials together with an early release of the response following the presentation of a LAS during preparation would be strong evidence in favour of the subcortical storage of motor programmes. However, we found that during late preparation the net excitability of the corticospinal pathway is enhanced shortly after an acoustic stimulus is presented (see Fig. 3A and B). We also found evidence that the increase in corticospinal pathway excitability can be brought about by a combination of reduced intracortical inhibition and increased intracortical facilitation as shown in early preparation (see Fig. 4B). Finally, we show that a combination of cortical suppression and subcortical facilitation cannot account for the strong increase in net corticospinal excitability induced by loud auditory stimuli.
Early triggering and EMG pattern of activation
The short latencies with which responses can be triggered by a LAS together with an unaltered morphology are the hallmark of what has been termed the StartReact effect (Valls-Sole et al. 1999,2008). We observed similar results in our studies despite the relatively lower intensity of the auditory stimulus used. We found that following a LAS, responses were triggered at about 50 ms earlier than normal (see Fig. 2). While an effect of 50 ms may seem small in the context of the StartReact phenomenon in simple reaction time tasks, this is quite a large effect for our task which required anticipation of the response. These types of actions are normally triggered by a stimulus event that occurs about 150 ms prior to movement onset (Marinovic et al. 2009a2009a,b2009b), which leaves little room for an even earlier response onset with an auditory stimulus which is presented 200 ms prior to the expected time of movement onset. In other words, an effect of 50 ms is possibly the largest effect that could be seen in this particular task. Moreover, the duration and time to peak EMG burst were unaffected by the LAS, indicating that these responses could not be simply the superimposition of a reflexive activation of the FDI muscle added to the voluntary contraction being prepared. In the absence of preparation, the auditory stimulus we employed failed to elicit an EMG response in the FDI muscle. Differences in MEP amplitudes cannot be attributed to differences in baseline EMG activity preceding the magnetic test stimulus as these values were not only very similar but also within reported values for RMS EMG at rest (Moller et al. 2009; see Table 1).
The early release of the prepared response and the build-up of response-related activation
It is well known that temporal predictability affects the time course of preparation for an action (Requin et al. 1991): the greater the temporal certainty regarding the time to act, the better a person can prepare in advance. The idea that temporal preparation affects readiness levels for upcoming movements fits well with the notion of activation models that we and others have recently proposed to explain the effects of LAS in simple reaction time and anticipatory timing tasks (Tresilian & Plooy, 2006; Maslovat et al. 2011; Alibiglou & MacKinnon, 2012; Marinovic et al. 2013). Generally, these models propose that a motor action is triggered when the level of activation of the action reaches a certain threshold (see also Carpenter & Williams, 1995; Hanes & Schall, 1996). We have recently shown that the activation increases gradually over time and peaks just prior to the moment the motor action must be triggered (Marinovic et al. 2013). According to the model, motor actions can be released early by a LAS as it increases the rate of rise to movement threshold and pushes the level of activation above the threshold for response initiation sooner than normal (Tresilian & Plooy, 2006; Marinovic et al. 2013). The activity evoked by a LAS adds to that which has already built up during preparation; when the combined activity exceeds threshold for activation the response is triggered early and its magnitude depends on the amount to which the threshold is exceeded (see also Jaskowski et al. 1995; Ulrich et al. 1998). This model explains quite well the early release of motor responses by LAS and also accounts for another frequently overlooked characteristic of these responses that we also observed in our own data, namely more vigorous responses (see Table 1). Thus, there may be a causal relationship between LAS presentation and the increase in corticospinal excitability we observed in the experiments reported here.
Cortical or subcortical effects of LAS in late preparation?
The increase in motor-evoked potentials elicited by single-pulse TMS (Experiments 1 and 2) 50 ms after LAS onset is consistent with augmented excitability in the motor cortex. Alternatively, it is possible that there is suppression of the motor cortex and that the effect may be due to a substantial facilitation at the level of the spinal cord that is greater than the suppression. Three lines of evidence strongly refute this possibility. First, previous research investigating the effects of auditory stimuli using TES found increased facilitation only 80–120 ms after LAS onset (Delwaide & Schepens, 1995; Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011), indicating that spinal facilitation induced by sound can be attributed to activation of slow conducting descending pathways (Delwaide & Schepens, 1995). Since the asynchrony between the LAS and TMS pulses we employed (50 ms) was shorter than the minimum required for detection of subcortical facilitation (Fisher et al. 2004), it seems unlikely that such a large spinal facilitation could be seen at this time. Second, the fact that we found a trend towards cortical suppression early in preparation (Experiment 3, Fig. 3C) suggests that the detection of cortical suppression is possible with the LAS–TMS asynchrony we used. Last, and more importantly, in Experiments 4 and 5 we showed that motor-evoked potentials elicited with TMS increased significantly more in response to auditory stimuli than those elicited with TES. TES is assumed to activate the axons of corticospinal neurons bypassing the soma (Rothwell et al. 1994; Di Lazzaro et al. 2004) and therefore reflect changes in excitability below the level of the motor cortex. The difference in response to TMS compared to that of TES indicates that the additional facilitation seen with TMS must be brought about by an increase in excitation at a cortical level. While MEPs induced by single-pulse TMS do not indicate the specific intracortical mechanisms involved in corticospinal facilitation, changes in response to paired-pulse TMS, however, can reveal intracortical modulation that would occur soon after the presentation of an auditory stimulus.
Intracortical changes induced by LAS
There is a range of intracortical mechanisms involved in regulating the resultant level of excitability of M1 and, consequently, the overall output we can measure with TMS (Hallett, 2007). Here we tested whether short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) could be involved in the observed changes in M1 excitability associated with exposure to loud noises. Short-interval intracortical inhibition (SICI) can be assessed when a subthreshold conditioning stimulus precedes a suprathreshold test stimulus by 2–5 ms (Kujirai et al. 1993; Nakamura et al. 1997). While the subthreshold conditioning stimulus is not sufficient for the corticospinal neurons to discharge, it activates the inhibitory interneurons in M1, producing inhibitory effects on the corticospinal neurons. Intracortical facilitation can be assessed in a similar manner but at relatively longer intervals between conditioning and test stimuli (12–15 ms; Kujirai et al. 1993). Other mechanisms of intracortical regulation may interact with SICI and ICF measurements (Kujirai et al. 1993; Peurala et al. 2008), we therefore interpret our results with caution, recognizing the possibility that these other mechanisms may have affected our results.
We found early in preparation (i.e. Experiment 3) that intracortical facilitation was greater in LAS trials than in Control trials. As it would be expected in the case of a global effect of the LAS on the motor cortex, this effect was observed in a muscle not involved in the task (ADM). We also observed that early in preparation, short-interval intracortical inhibition for the FDI muscle was reduced (i.e. MEPs elicited by paired pulses delivered close in time (2–3 ms) were also relatively larger following LAS than those observed in Control trials). Thus, at least in relation to the time course of the effect, an increase in net excitability of the motor cortex when an auditory stimulus is present could be explained by these two mechanisms of intracortical regulation (ICF and SICI). Nonetheless, the fact that the effects of loud auditory stimuli are reversed in response to single-pulse TMS as preparation develops suggests that other mechanisms may regulate cortical excitability during motor preparation. Next, we consider a neural mechanism which may be involved in cortical facilitation during late preparation.
Possible neural pathways by which a LAS influences cortical preparation
There are two known routes by which auditory-evoked activity can reach motor areas of the cerebral cortex: (1) via the auditory pathways that transmit activity evoked in the cochlea to the primary auditory cortex via the medial geniculate nucleus of the thalamus (Fig. 7A) and then from the auditory cortex to premotor areas (as demonstrated in non-human primates, e.g. Arikuni et al. 1988; Petrides & Pandya, 1988; Deacon, 1992; Romanski et al. 1999; Petrides & Pandya, 2006); (2) via a pathway that transmits activity directly to motor areas via the pontomedullary reticular formation (PMRF) and the thalamus (Fig. 7B). Alibiglou & MacKinnon ()2012 suggested a cortical locus for motor command generating processes (motor programmes) and that pathway 2 (Fig. 7B) may be responsible for the facilitated-reaction effect as it provides a fast route to motor cortex: the PMRF receives short latency auditory input (Davis et al. 1982; Koch et al. 1992; Lingenhöhl & Friauf, 1992,1994; see also Yeomans & Frankland, 1995) and it has thalamocortical projections that terminate in motor areas, bypassing the auditory cortex. However, transmission of auditory activity from the cochlea nuclei to the auditory cortex can also be fast as evidenced by the finding that the middle latency response of auditory-evoked magnetic fields attributable to cortical activity has components with latencies between 11 and 33 ms (Kuriki et al. 1995). Such short latency cortical-evoked activity is probably the result of transmission via trisynaptic components of the classical auditory pathway (see Hackett & Kaas, 2003; Moller, 2003). Given the direct connections between auditory cortex and premotor areas, auditory-evoked activity could reach the premotor areas very quickly via pathway 1 (Fig. 7A). Thus, either one or both pathways shown in Fig. 7 could mediate the facilitated-reaction effect.
Figure 7.
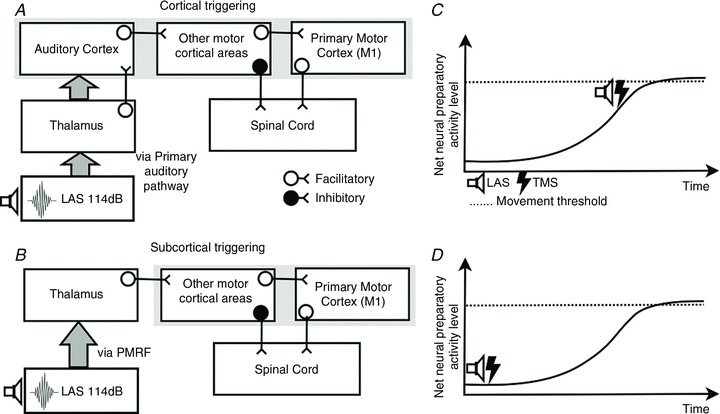
A, hypothetical subcortical triggering circuit of responses initiated by loud auditory stimuli. B, hypothetical cortical triggering circuit of responses initiated by loud auditory stimuli. C, during late preparation a LAS causes the early release of the anticipatory action as the net neural preparatory activity is close to movement threshold. D, during early preparation a LAS causes no early release of the anticipatory action as the net neural preparatory activity is well below movement threshold.
In order to explain the suppression of corticospinal excitability evoked by the LAS early in preparation (Experiments 3 and 5), LAS-evoked activity must facilitate descending inhibition. Recent work by Duque and colleagues (Duque & Ivry, 2009; Duque et al. 2012) has demonstrated that descending inhibition can originate from the premotor cortex. They showed that repetitive TMS over the dorsal premotor cortex (PMd) selectively reduced response inhibition before the onset of an imperative stimulus, which they suggested arises via inhibitory projections from the PMd to subcortical areas and/or spinal cord (see also Cohen et al. 2010). Auditory activation can potentially facilitate PMd since connections to this area from auditory cortex have been shown to exist in primates (see Zatorre et al. 2007). The function of the descending inhibition may be to prevent false starts while the motor system prepares for an impending action (Duque & Ivry, 2009; Duque et al. 2012). Thus, this connectivity should only be active early in preparation when the response is not yet required: in order for the response to occur, the inhibition would need to be withdrawn. This is consistent with animal (Tanji et al. 1988; Nakayama et al. 2008) and human (Liuzzi et al. 2010) work showing that activity of the PMd precedes M1 activity during motor preparation.
In summary, the circuits shown in Fig. 7 can account for our results if it is supposed that LAS-evoked activity facilitates those processes taking place in motor cortical areas at the time the activity arrives. Evidence from studies of response preparation indicate that preparatory processes in response-generating circuits develop from the moment the person can predict the time at which a response will be required, e.g. from presentation of a warning stimulus or the initiation of target motion (see Posner & Petersen, 1990; Los & Schut, 2008). The graphs in panels C and D of Fig. 7 illustrate this progressive increase in response-related activation over the course of a trial. LAS-evoked activity can result in early triggering of command generation if the level of preparatory activity is sufficiently high (as it is late in a trial, panel C). The finding that triggering by the LAS is not possible when it is presented early in a trial implies that preparatory cortical activity is insufficiently developed at this time (panel D). Since the preparatory process is active throughout the trial, a LAS may facilitiate it at any time, which accounts for why intracortical changes in M1 (release of intracortical inhibition and an increase in intracortical facilitation) were observed at early LAS presentation times without causing early response onsets. In contrast, the generation of inhibitory descending output from PMd is only active early in the preparatory period, so only at this time can it be facilitated by the LAS, which accounts for our finding of suppression of corticospinal excitability early in a trial, but not later (Experiment 3).
Note that existing results do not allow unambiguous conclusions regarding the extent to which one and/or the other circuit in Fig. 7 underlies the facilitated-reaction effect and associated phenomena. Irrespective of the contributions of the two pathways, our results strongly indicate that motor cortical areas, and M1 in particular, are part of the circuit involved in the facilitated-reaction effect.
StartReact, intersensory facilitation or both?
Previous research has demonstrated transient suppression of motor cortex excitability following a loud acoustic stimulus (Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011). It has been assumed that this suppressive effect of the LAS occurs regardless of preparatory state, which is inconsistent with a cortical basis for the StartReact effect as proposed by Alibiglou & MacKinnon ()2012 and suggests that it is due to triggering a subcortically stored programme (Valls-Sole, 2012). We have shown here that the suppressive effect of the LAS is not independent of preparatory state: a LAS can increase cortical excitability when the moment of response initiation is imminent. This result would be expected if the StartReact effect has a cortical origin according to an account of the effect based on enhancement of temporal preparation that we have presented elsewhere (Tresilian & Plooy, 2006; Marinovic et al. 2013). This is consistent with the recent finding that the StartReact effect can be reduced (delay of early triggering) by TMS over the motor cortex (Alibiglou & MacKinnon, 2012) and is closely related to the preparation enhancement explanation of the intersensory facilitation effect on reaction time (Nickerson, 1973; Schmidt et al. 1984; Arieh & Marks, 2008). An example of this effect is that when people are asked to respond as quickly as possible to a visual stimulus, their reaction time is typically shorter when the visual stimulus is accompanied by a task-irrelevant acoustic stimulus (e.g. Simon & Craft, 1970; Los & Van der Burg, 2013). In the preparation enhancement account of this effect, activity evoked by the acoustic stimulus adds to (enhances) preparatory activation in response production circuits moving them closer to threshold as shown in Fig. 8. According to the preparation enhancement idea (see Fig. 8), the StartReact effect is the result of an enhancement which is large enough to push the response production circuits over threshold and hence initiate motor command generation (Marinovic et al. 2013). Of course, the results that have been presented do not refute the subcortical programme-triggering account of the StartReact effect, but they do establish the plausibility of a cortical origin for the effect. If subsequent study ultimately establishes that the effect is due to subcortical triggering, then the results presented here may reflect the neurophysiological mechanism of preparation enhancement by task-irrelevant acoustic stimuli.
Figure 8.
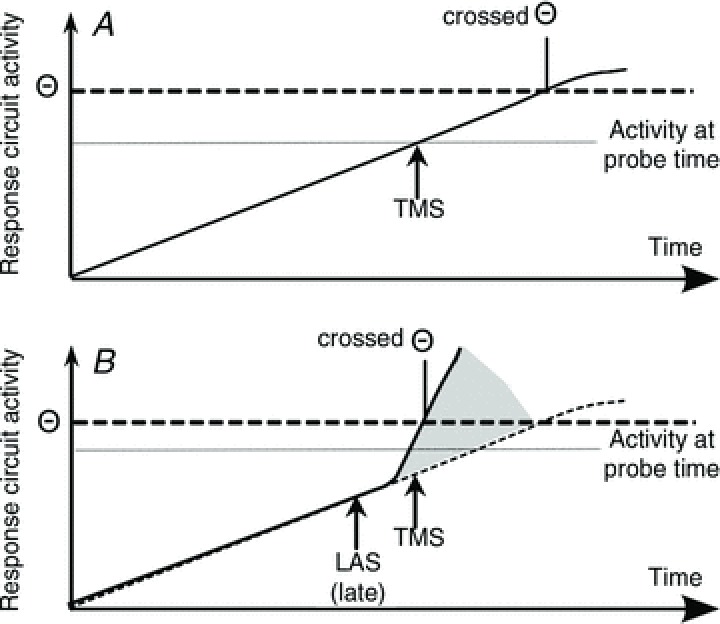
A, preparatory activation increases monotonically until it crosses the threshold for command generation (Θ). B, preparatory activation increases monotonically as in A but the activation introduced by the LAS adds to that already built up. This makes the level of activity in the response circuit reach threshold much sooner than in the absence of the LAS. As a result, the level of response-related activity in the circuits responsible for movement triggering differs significantly at the time of TMS probing.
Conclusions
It is important to emphasize that our results were obtained with a task that allows greater anticipation than reaction time tasks with variable and/or long foreperiods commonly used to investigate preparatory motor processes. Recent reports have indicated that temporally predictable tasks, such as the one used in our experiments, can heighten preparatory activity in the cortical networks that mediate movement initiation (Cui & MacKinnon, 2009; Ackerley et al. 2011). This suggests that our task choice may have been critical to determine the results we report here.
The results we report indicate that the effects of auditory stimuli on motor cortex excitability during late preparation are different compared to those at rest (Fig. 2A and B), early preparation (Fig. 3C) and sustained contraction (Furubayashi et al. 2000; Fisher et al. 2004; Kuhn et al. 2004; Ilic et al. 2011). More precisely, a LAS delivered during the final phase of preparation of a self-initiated timing action induces increased activation of the circuits involved with response programming. This increase in activation of cortical circuits would explain the systematic increase in response vigour usually associated with responses triggered by auditory stimuli (Kumru & Valls-Sole, 2006; Tresilian & Plooy, 2006; MacKinnon et al. 2007; Rogers et al. 2011; Marinovic et al. 2013).
Our results do not conclusively rule out the possibility that triggering of prepared motor programmes by loud auditory stimulation occurs entirely subcortically, but they do demonstrate cortical effects of such stimulation, consistent with a cortical locus for triggering. We have briefly described two pathways by which loud auditory stimulation could trigger cortical motor command generation at short latencies. Based on recent anatomical and electrophysiological evidence for the connectivity of the auditory and motor systems, both pathways provide plausible neural substrates for the facilitated-reaction effect and are compatible with evidence that motor command generation in voluntary action has a cortical locus.
Glossary
- ADM
abductor digiti minimi
- FDI
first dorsal interosseous
- ICF
intracortical facilitation
- LAS
loud acoustic stimulus
- M1
primary motor cortex;
- MEP
motor-evoked potential
- PMd
dorsal premotor cortex
- RMS
root mean square
- SICI
short-interval intracortical inhibition
- TES
transcranial electrical stimulation
- TMS
transcranial magnetic stimulation
Additional information
Competing interests
None declared.
Author contributions
W.M. was involved in the conception and design of the experiments, collection, analysis and interpretation of data, and drafting and revising of the article. J.R.T. was involved in the conception and design of the experiments, interpretation of data as well as drafting and revising the article. A.deR. was involved in the conception and design of the experiments, and in critical revision of the article. S.S. was involved in data collection and revising of the article. S.R. was involved in data collection, design of the experiments and drafting and revising the article. All experiments were performed in the Sensorimotor Neuroscience Centre, Brisbane (AU). All authors approved this manuscript.
Funding
W.M. thanks the Australian Research Council for their support in the form of a Discovery Early Career Research Award.
References
- Ackerley SJ, Stinear CM, Byblow WD. Promoting use-dependent plasticity with externally-paced training. Clin Neurophysiol. 2011;122:2462–2468. doi: 10.1016/j.clinph.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol. 2012;590:919–936. doi: 10.1113/jphysiol.2011.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieh Y, Marks LE. Cross-modal interaction between vision and hearing: a speed-accuracy analysis. Percept Psychophys. 2008;70:412–421. doi: 10.3758/pp.70.3.412. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Watanabe K, Kubota K. Connections of area 8 with area 6 in the brain of the macaque monkey. J Comp Neurol. 1988;277:21–40. doi: 10.1002/cne.902770103. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 2010;14:208–215. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114:1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Bullock D, Cisek P, Grossberg S. Cortical networks for control of voluntary arm movements under variable force conditions. Cereb Cortex. 1998;8:48–62. doi: 10.1093/cercor/8.1.48. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res. 2007;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R. Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol. 2003;89:1857–1863. doi: 10.1152/jn.00852.2002. [DOI] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Cohen O, Sherman E, Zinger N, Perlmutter S, Prut Y. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Curr Opin Neurobiol. 2010;20:696–703. doi: 10.1016/j.conb.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui RQ, MacKinnon CD. The effect of temporal accuracy constraints on movement-related potentials. Exp Brain Res. 2009;194:477–488. doi: 10.1007/s00221-009-1725-5. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW. Cortical connections of the inferior arcuate sulcus cortex in the macaque brain. Brain Res. 1992;573:8–26. doi: 10.1016/0006-8993(92)90109-m. [DOI] [PubMed] [Google Scholar]
- Delval A, Dujardin K, Tard C, Devanne H, Willart S, Bourriez JL, Derambure P, Defebvre L. Anticipatory postural adjustments during step initiation: Elicitation by auditory stimulation of differing intensities. Neuroscience. 2012;219:166–174. doi: 10.1016/j.neuroscience.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Schepens B. Auditory startle (audio-spinal) reaction in normal man: EMG responses and H reflex changes in antagonistic lower limb muscles. Electroencephalogr Clin Neurophysiol. 1995;97:416–423. doi: 10.1016/0924-980x(95)00136-9. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Turner RS. Motor sequences and the basal ganglia: kinematics, not habits. J Neurosci. 2010;30:7685–7690. doi: 10.1523/JNEUROSCI.0163-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32:806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol. 2012;590:4045–4060. doi: 10.1113/jphysiol.2011.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Sharott A, Kuhn AA, Brown P. Effects of combined cortical and acoustic stimuli on muscle activity. Exp Brain Res. 2004;157:1–9. doi: 10.1007/s00221-003-1809-6. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Ugawa Y, Terao Y, Hanajima R, Sakai K, Machii K, Mochizuki H, Shiio Y, Uesugi H, Enomoto H, Kanazawa I. The human hand motor area is transiently suppressed by an unexpected auditory stimulus. Clin Neurophysiol. 2000;111:178–183. doi: 10.1016/s1388-2457(99)00200-x. [DOI] [PubMed] [Google Scholar]
- Garry MI, Thomson RH. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res. 2009;193:267–274. doi: 10.1007/s00221-008-1620-5. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Kaas JH. Auditory pathways in the primate brain. In: Gallagher M, Nelson RJ, Weiner IB, editors. Handbook of Psychology, Biological Psychology. Hoboken, NJ, USA: John Wiley & Sons; 2003. pp. 187–210. [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Potter-Nerger M, Holler I, Siebner HR, Ilic NV, Deuschl G, Volkmann J. Startle stimuli exert opposite effects on human cortical and spinal motor system excitability in leg muscles. Physiol Res. 2011;60(Suppl. 1):S101–106. doi: 10.33549/physiolres.932182. [DOI] [PubMed] [Google Scholar]
- Jaskowski P, Rybarczyk K, Jaroszyk F, Lemanski D. The effect of stimulus intensity on force output in simple reaction time task in humans. Acta Neurobiol Exp (Wars) 1995;55:57–64. doi: 10.55782/ane-1995-1061. [DOI] [PubMed] [Google Scholar]
- Koch M, Lingenhohl K, Pilz PK. Loss of the acoustic startle response following neurotoxic lesions of the caudal pontine reticular formation: possible role of giant neurons. Neuroscience. 1992;49:617–625. doi: 10.1016/0306-4522(92)90231-p. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Sharott A, Trottenberg T, Kupsch A, Brown P. Motor cortex inhibition induced by acoustic stimulation. Exp Brain Res. 2004;158:120–124. doi: 10.1007/s00221-004-1883-4. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Valls-Sole J. Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp Brain Res. 2006;169:427–432. doi: 10.1007/s00221-005-0156-1. [DOI] [PubMed] [Google Scholar]
- Kumru H, Valls-Sole J, Kofler M, Castellote J, Sanegre T. The effects of a prepulse on the StartReact phenomenon. Suppl Clin Neurophysiol. 2006;58:101–109. doi: 10.1016/s1567-424x(09)70062-5. [DOI] [PubMed] [Google Scholar]
- Kuriki S, Nogai T, Hirata Y. Cortical sources of middle latency responses of auditory evoked magnetic field. Hear Res. 1995;92:47–51. doi: 10.1016/0378-5955(95)00195-6. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Powers AS, Hoffman HS. The neural substrate of the startle response. Physiol Behav. 1980;25:291–297. doi: 10.1016/0031-9384(80)90219-x. [DOI] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the caudal pontine reticular formation receive short latency acoustic input: an intracellular recording and HRP-study in the rat. J Comp Neurol. 1992;325:473–492. doi: 10.1002/cne.903250403. [DOI] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit. J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi G, Horniss V, Hoppe J, Heise K, Zimerman M, Gerloff C, Hummel FC. Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cereb Cortex. 2010;20:1323–1331. doi: 10.1093/cercor/bhp196. [DOI] [PubMed] [Google Scholar]
- Los SA, Schut ML. The effective time course of preparation. Cogn Psychol. 2008;57:20–55. doi: 10.1016/j.cogpsych.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Los SA, Van der Burg E. Sound speeds vision through preparation, not integration. J Exp Psychol Hum Percept Perform. 2013 doi: 10.1037/a0032183. (in press) [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang YH, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Marinovic W, de Rugy A, Lipp OV, Tresilian JR. Responses to loud auditory stimuli indicate that movement-related activation builds-up in anticipation of action. J Neurophysiol. 2013;109:996–1008. doi: 10.1152/jn.01119.2011. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Plooy A, Tresilian JR. The time course of amplitude specification in brief interceptive actions. Exp Brain Res. 2008;188:275–288. doi: 10.1007/s00221-008-1360-6. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Plooy AM, Tresilian JR. Preparation and inhibition of interceptive actions. Exp Brain Res. 2009a;197:311–319. doi: 10.1007/s00221-009-1916-0. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Plooy AM, Tresilian JR. The utilisation of visual information in the control of rapid interceptive actions. Exp Psychol. 2009b;56:265–273. doi: 10.1027/1618-3169.56.4.265. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Reid CS, Plooy AM, Riek S, Tresilian JR. Corticospinal excitability during preparation for an anticipatory action is modulated by the availability of visual information. J Neurophysiol. 2011;105:1122–1129. doi: 10.1152/jn.00705.2010. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Hodges NJ, Chua R, Franks IM. Motor preparation and the effects of practice: evidence from startle. Behav Neurosci. 2011;125:226–240. doi: 10.1037/a0022567. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Kennedy PM, Forgaard CJ, Chua R, Franks IM. The effects of prepulse inhibition timing on the startle reflex and reaction time. Neurosci Lett. 2012;513:243–247. doi: 10.1016/j.neulet.2012.02.052. [DOI] [PubMed] [Google Scholar]
- Moller AR. Sensory Systems: Anatomy and Physiology. San Diego, CA, USA: Academic Press; 2003. [Google Scholar]
- Moller C, Arai N, Lucke J, Ziemann U. Hysteresis effects on the input-output curve of motor evoked potentials. Clin Neurophysiol. 2009;120:1003–1008. doi: 10.1016/j.clinph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, Tanji J, Hoshi E. Transformation of a virtual action plan into a motor plan in the premotor cortex. J Neurosci. 2008;28:10287–10297. doi: 10.1523/JNEUROSCI.2372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson RS. Intersensory facilitation of reaction time: energy summation or preparation enhancement. Psychol Rev. 1973;80:489–509. doi: 10.1037/h0035437. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fibre pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Müller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Queralt A, Weerdesteyn V, van Duijnhoven HJ, Castellote JM, Valls-Sole J, Duysens J. The effects of an auditory startle on obstacle avoidance during walking. J Physiol. 2008;586:4453–4463. doi: 10.1113/jphysiol.2008.156042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requin J, Brener J, Ring C. Preparation for action. In: Jennings JR, Coles MGH, editors. Handbook of Cognitive Psychophysiology: Central and Autonomic Nervous System Approaches. Chichester: Wiley; 1991. pp. 357–448. [Google Scholar]
- Rogers MW, Kennedy R, Palmer S, Pawar M, Reising M, Martinez KM, Simuni T, Zhang YH, MacKinnon CD. Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol. 2011;106:915–924. doi: 10.1152/jn.00005.2010. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical-stimulation of the motor cortex in man – further evidence for the site of activation. J Physiol. 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Gielen SC, van den Heuvel PJ. The locus of intersensory facilitation of reaction time. Acta Psychol (Amst) 1984;57:145–164. doi: 10.1016/0001-6918(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Simon JR, Craft JL. Effects of an irrelevant auditory stimulus on visual choice reaction time. J Exp Psychol. 1970;86:272–274. doi: 10.1037/h0029961. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Bard C, Fleury M, Young DE, Proteau L. Determining movement onsets from temporal series. J Mot Behav. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- Tresilian JR, Plooy AM. Effects of acoustic startle stimuli on interceptive action. Neuroscience. 2006;142:579–594. doi: 10.1016/j.neuroscience.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Rinkenauer G, Miller J. Effects of stimulus duration and intensity on simple reaction time and response force. J Exp Psychol Hum Percept Perform. 1998;24:915–928. doi: 10.1037//0096-1523.24.3.915. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J. Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin Neurophysiol. 2012;123:13–20. doi: 10.1016/j.clinph.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kofler M, Kumru H, Castellote JM, Sanegre MT. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res. 2005;165:541–548. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Sole A, Valldeoriola F, Munoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Valldeoriola F, Tolosa E, Marti MJ. Distinctive abnormalities of facial reflexes in patients with progressive supranuclear palsy. Brain. 1997;120:1877–1883. doi: 10.1093/brain/120.10.1877. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception and production. Nat Rev Neurosci. 2007;8:547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]


