Abstract
Abstract Age-related changes in circadian rhythms may contribute to the sleep disruption observed in older adults. A reduction in responsiveness to photic stimuli in the circadian timing system has been hypothesized as a possible reason for the advanced circadian phase in older adults. This project compared phase-shifting responses to 2 h of broad-spectrum white light at moderate and high intensities in younger and older adults. Subjects included 29 healthy young (25.1 ± 4.1 years; male to female ratio: 8: 21) and 16 healthy older (66.5 ± 6.0 years; male to female ratio: 5: 11) subjects, who participated in two 4-night and 3-day laboratory stays, separated by at least 3 weeks. Subjects were randomly assigned to one of three different time-points, 8 h before (−8), 3 h before (−3) or 3 h after (+3) the core body temperature minimum (CBTmin) measured on the baseline night. For each condition, subjects were exposed in a randomized order to 2 h light pulses of two intensities (2000 lux and 8000 lux) during the two different laboratory stays. Phase shifts were analysed according to the time of melatonin midpoint on the nights before and after light exposure. Older subjects in this study showed an earlier baseline phase and lower amplitude of melatonin rhythm compared to younger subjects, but there was no evidence of age-related changes in the magnitude or direction of phase shifts of melatonin midpoint in response to 2 h of light at either 2000 lux or 8000 lux. These results indicate that the acute phase-shifting response to moderate- or high-intensity broad spectrum light is not significantly affected by age.
Key points.
Ageing is characterized by changes in circadian rhythms.
Reduced light exposure or reduced responsiveness to light in older adults may contribute to age-related circadian changes.
We hypothesized that the aged circadian clock would exhibit a decreased response to light at a lower intensity (2000 lux) but not to light at a higher intensity (8000 lux). Here, we assessed phase-shifting responses to 2 h of broad-spectrum white light at two different intensities in 29 healthy younger and 16 healthy older subjects.
Older subjects had a significantly earlier phase and lower amplitude of melatonin rhythm compared with younger subjects.
There was no evidence of age-related changes in the magnitude or direction of phase shifts of melatonin midpoint in response to 2 h of broad-spectrum white light at either 2000 lux or 8000 lux; this indicates that the acute phase-shifting response to light is not significantly affected by age.
Introduction
Ageing is characterized by changes in sleep quality and sleep pattern. The characteristics of sleep in older adults include decreased sleep time, reduced deep sleep amount, increased wakefulness during sleep, early morning awakening, and earlier sleep timing (Campbell & Dawson, 1992; Duffy & Czeisler, 2002; Lack et al. 2008). These changes in sleep in older adults may be attributable to changes in the circadian timing system, such as an advanced circadian phase and an altered phase relationship between sleep and temperature rhythm (Duffy et al. 1998; Dijk et al. 2000). A number of authors have proposed that the observed changes in circadian rhythm in ageing, such as phase advances or reduced amplitude, may contribute to sleep disruption in older adults (Campbell & Dawson, 1992; Duffy & Czeisler, 2002; Lack et al. 2008). Previous studies in older adults have shown that timed morning light exposure with phase-advancing effects was associated with worsening sleep quality (Campbell & Dawson, 1992), whereas timed evening light exposure with phase-delaying effects improved sleep quality (Campbell et al. 1993). In general, an earlier waking time in older adults is accompanied by an advance of about 1 h in the rhythms of core body temperature and melatonin (Dijk et al. 2000).
Theoretically, an advanced circadian phase in older adults could be explained by a shortened period of the circadian clock (Morin, 1988; Duffy et al. 2002) because the phase angle difference between environmental synchronizers and circadian rhythms depends on the intrinsic period of the endogenous circadian clock (Klerman et al. 2001). Although a shortened period of free-running time with ageing has been observed in some animal studies (Rosenberg et al. 1991; Brown et al. 2011), experimental human studies under a forced desynchrony protocol have not shown a difference between younger and older adults in the intrinsic circadian period (Duffy et al. 1998; Brown et al. 2011).
Alternatively, reduced light exposure or reduced responsiveness to light in older adults may contribute to age-related circadian changes (Campbell et al. 1988; Ancoli-Israel et al. 1997; Duffy et al. 2007). There is also evidence that age-related structural changes in the circadian and/or visual systems, such as in ageing eyes, may contribute to a reduction in light sensitivity, including reduced light transmission through the eye (Charman, 2003; Herljevic et al. 2005), and a reduction in the number of circadian photoreceptors (Semo et al. 2003). There is a steady increase in the lenticular absorption of light and thus a decrease in light transmission with age (Pokorny et al. 1987; Weale, 1988; Xu et al. 1997; Van Best et al. 1998). The decrease in transmission of shorter wavelengths of light is particularly dramatic over the human lifespan as a result of the ‘yellowing’ of the crystalline lens (Brainard et al. 1997; Charman, 2003). Ex vivo studies have shown that lenses from humans aged 60–69 years transmit significantly less blue (440 nm) and to a lesser extent less green (540 nm) light than do lenses from adults aged 20–29 years, although the average transmission of longer visible wavelengths does not differ substantially between these age groups (Brainard et al. 1997). Although there is individual variability in lenticular transmittance, these data suggest that the level of short wavelength light reaching the retina, and therefore the illuminance-detecting photoreceptive system, will be reduced in older adults. Daily exposure to light has also been reported to be significantly reduced in older adults, particularly in institutionalized patients with dementia (Campbell et al. 1988; Ancoli-Israel et al. 1997). As a result, decreased light exposure has been suggested to contribute to both the disturbance of circadian rhythms and sleep difficulties in individuals with dementia (Van Someren et al. 1997; Liu et al. 2000). A recent study in healthy older adults reported that differences in natural light exposure may contribute to the age-related phase advance of the circadian pacemaker and its later timing relative to the sleep–wake cycle (Scheuermaier et al. 2010).
It has been proposed that, taken together, these changes with advancing age may be associated with a reduction in photic responsiveness in the circadian timing system (Benloucif et al. 2006; Duffy et al. 2007; Sletten et al. 2009). In line with this reasoning, studies in rodents found phase delays to be reduced in older mice following photic stimulation at 30–1000 lux (Benloucif et al. 1997), and phase advances at low light levels to be reduced in older hamsters (Zhang et al. 1996). A recent study in humans showed a significant reduction in responsiveness to the acute effects of short wavelength blue light in terms of subjective mood and alertness, but no significant difference in phase-shifting responses in older people (Sletten et al. 2009). The photic resetting of the circadian system is determined by several factors, including the timing, intensity, duration and wavelength of light exposure, and prior light exposure history. Previous studies that assessed the effect of illumination on the circadian system have not clearly shown an age-related reduction in the magnitude of phase shifts (Klerman et al. 2001; Benloucif et al. 2006; Duffy et al. 2007; Kripke et al. 2007). As a reasonable explanation, we could consider the possibility that exposure to a saturating duration (>4 h) or intensity of light exposure (Klerman et al. 2001; Chang et al. 2012) obscures age-related differences. A study by Duffy et al. ()2007 suggested that the phase-delay response was less sensitive to low-to-moderate levels of light (∼50–1000 lux) in older subject, and a study by Kripke et al. ()2007 found an age-related change in crossover time (transition from delays to advances).
Based on the questions raised by the existing literature as noted above, the present study was designed to investigate age-related differences in the phase-shifting response to light at two different intensities when the duration of light is non-saturating. We hypothesized that the aged circadian clock would exhibit a decreased response to light at a lower intensity (2000 lux), but not to light at a higher intensity (8000 lux). To determine whether the phase response curve (PRC) to light was altered by age, we examined the phase-shifting response to light administered at three different times relative to the core body temperature minimum (CBTmin).
Methods
Ethical approval
The study protocol was approved by the institutional review board at Northwestern University. Written informed consent was obtained from each enrolled subject. All experimental procedures were carried out in accordance with the principles of the Declaration of Helsinki.
Subjects
A total of 30 healthy younger subjects aged 20–35 years and 18 healthy older subjects aged 60–80 years participated in this study. Younger subjects were recruited through advertisements and flyers posted at local universities. Older subjects were recruited using a database of demographic and health information for 1500 elderly people living in our community and a pool of over 160 cognitively healthy control participants enrolled in the Clinical Core of the Cognitive Neurology and Alzheimer’s Disease Center (CNADC) at Northwestern University Feinberg School of Medicine. In addition, community-dwelling volunteers were recruited through word of mouth, advertising and community presentations at retirement and senior citizen facilities.
A telephone screening was conducted to obtain details of each subject’s medical history, including any conditions, current medications and sleep habits. Subjects whose habitual bedtime occurred between 21.00 h and 24.00 h were included in this study. Younger subjects with concurrent illnesses and those using psychoactive medications were excluded from participation. Older subjects were required to have been stable on their medications for at least 3 months. Older subjects were also required to be living independently in the community, to be independent in their conduct of activities of daily living and to be within normal limits of cognitive functioning. Each subject was screened using a mini-mental state examination (MMSE) and four functional or behavioural assessment scales [the Functional Assessment Staging Tool (FAST), the Activities of Daily Living Scale (ADLS), the Neuropsychiatric Inventory Questionnaire (NPI-Q) and the Geriatric Depression Scale (GDS)]. To establish that cognitive functioning was within normal ranges for this age group, older subjects underwent a battery of neuropsychological tests conducted at the CNADC. Subjects were included if they achieved an MMSE score of ≥26 and were within normal ranges on tests in the neuropsychological test battery of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (semantic fluency; Boston Naming Test; word list memory; word list recall; word list recognition; constructional praxis; Trail-Making Test Parts A and B) and additional neuropsychological tests (logical memory; visual reproduction; visual verbal test; target cancellation test; judgment of line orientation). Older subjects underwent an ophthalmologic examination conducted by an ophthalmologist. Ophthalmologic conditions were evaluated as the following: colour deficiency; visual field defect; glaucoma; cataracts; astigmatism; hyperopia; myopia; presbyopia, and amblyopia. Subjects with significant cataracts that clinically affected their vision were excluded. Older subjects also underwent screening polysomnography to rule out primary sleep disorders.
The Morningness-Eveningness Questionnaire (MEQ) was administered to each subject. Subjects were categorized according to the standard scores on the MEQ proposed by Horne & Ostberg ()1976. All except three older subjects, who were scored as definite morning types, did not represent extreme types.
Exclusion criteria included: (i) a prior history of cognitive, neurological or major psychiatric disorders including substance abuse; (ii) current medical illness in younger subjects, and unstable or serious medical illness in older subjects; (iii) current use of psychoactive medications including antidepressants, anxiolytics, neuroleptics, anticonvulsants, hypnotics and stimulants; current use of β-blockers or Ca channel antagonists in older subjects; daily caffeine intake of >200 mg; use of tobacco amounting to more than three sticks per week; (iv) cognitive impairment (MMSE scores of <26 or ≥2 s.d. below the mean of norm for the subject’s age and education on tests in the neuropsychological test battery); (v) depression (Center for Epidemiologic Studies Depression Scale score of >22); (vi) primary sleep pathology (apnoea index of ≥10 or movement arousal index of ≥15); (vii) an ophthalmological condition affecting vision; (viii) haematocrit of ≤32, and (ix) shift work or travel across more than two time zones within 90 days of the study.
Of the 62 subjects (younger, n= 37; older, n= 25) who were randomized, 48 subjects (younger, n= 30; older, n= 18) participated in our experimental study; 43 of the 48 subjects completed both assessments. Data with artefacts in the melatonin profiles were excluded. Data for 29 younger subjects (mean ±s.d. age: 25.1 ± 4.1 years; male to female ratio: 8: 21) and 16 older subjects (mean ±s.d. age: 66.5 ± 6.0 years; male to female ratio: 5: 11) were included in the final analysis (Fig. 1).
Figure 1. Consort diagram showing the flow of participants.
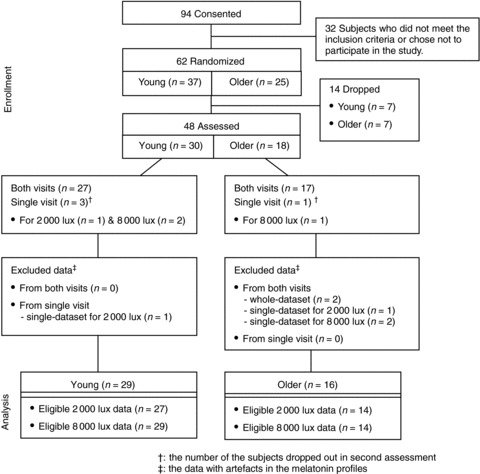
Experimental protocols
Subjects were asked to maintain sleep logs and to observe a regular sleeping and waking schedule for the 3 weeks prior to each admission by keeping their actual bedtime within ± 30 min of their habitual bedtime. On the week prior to admission, subjects wore an activity monitor on the non-dominant wrist (Actiwatch; Mini-Mitter, Inc., Bend, OR, USA). Sleeping and waking times were averaged from actigraphic recordings over the week prior to admission using Actiware-Sleep software (Mini-Mitter, Inc.).
Participants were randomly assigned to one of three groups, each of which was exposed to 2 h of light centred at one of three different time-points based on the time at which the individual’s core body temperature reached a minimum (CBTmin) measured on the baseline night: 8 h before (−8); 3 h before (−3), or 3 h after (+3). Each subject was admitted to the General Clinical Research Center (GCRC) on two different occasions for an intervention in which the individual was exposed to light at 2000 lux or 8000 lux; each visit was separated by at least 3 weeks. The order in which the light interventions (2000 lux or 8000 lux) were administered was randomized. Each admission lasted 4 nights and 3 days and consisted of a habituation night, a 24 h baseline phase assessment, a treatment night, and a 24 h post-treatment phase assessment (Fig. 2).
Figure 2. Schematic of the experimental protocol.
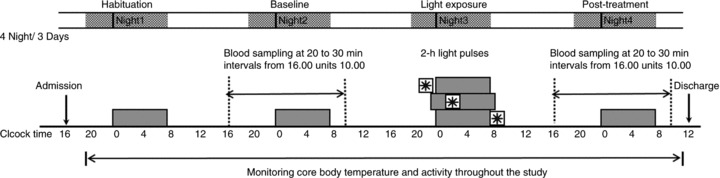
Subjects were admitted for 4 nights and 3 days, were maintained under dim light during daytime hours and were allowed 8 h of sleep in darkness at their habitual time (dark bars). Blood samples were taken throughout the baseline and post-treatment nights to assess light-induced changes in the circadian melatonin rhythm. On night 3, subjects were exposed to light at one of three time-points (−8 h, −3 h, 3 h) relative to the core body temperature minimum (CBTmin) measured on the baseline night. They were exposed to a 2 h light pulse (including a 15 min ramp up and ramp down) of 2000 lux on one laboratory stay and 8000 lux on a different laboratory stay, in a randomized order, separated by at least 3 weeks.
Habituation (night 1) Subjects were admitted to the GCRC in the evening in order to allow them to acclimatize to the dim light of the hospital setting and underwent a history interview and physical examination performed by Northwestern Hospital staff. Women of childbearing age took a urine pregnancy test. Lights were turned out for sleep according to each subject’s normal sleep/wake schedule. Subjects were awoken 8 h later and were allowed to remain quietly awake and to eat regular snacks throughout the following day.
Baseline (night 2) Blood samples were collected at 20–30 min intervals from 16.00 h until 10.00 h the next day to give a total of 35 blood draws for the night. The catheter was inserted at least 2 h before the beginning of sample collection in order to reduce the effect of vein-puncture stress on hormone levels. During sleep periods, samples were taken through tubing extended to an adjacent room in order not to disturb the subject’s sleep. Lights were turned out during the sleep period according to each subject’s normal sleep/wake schedule. In order to provide an estimate of baseline circadian phase, core body temperature was continuously measured at 1 min intervals throughout the stay using a flexible rectal thermistor connected to a lightweight data recording unit (Minilogger; Mini-Mitter, Inc.). The 24 h profile of temperature was edited to remove obvious artefacts and quantitatively described using both the Cleveland regression procedure and simple cosine analysis with software provided by C. Eastman (Cleveland, 1979; Martin & Eastman, 1998). In addition to estimates of CBTmin based on the edited raw data, demasking procedures were used to adjust for the decrease in body temperature associated with sleep, according to the method of Martin & Eastman ()1998.
Light exposure (night 3) Participants were exposed to light for a period of 2 h, the first and last 15 min of which were used, respectively, to ramp up from darkness to light and to gradually decrease the light to complete darkness. Subjects were awoken during the bright light exposure, but were allowed to sleep for 8 h around the period of light exposure (Fig. 3).
Figure 3. Timing of light exposure, sleeping and waking when the midpoint of 2 h light exposure (including 15 min ramp-up and ramp-down periods) was targeted at 8 h or 3 h before or 3 h after each subject’s baseline core body temperature minimum (CBTmin).
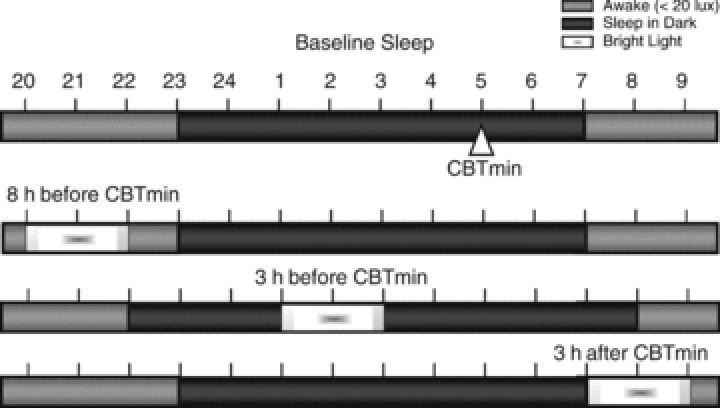
In this example the CBTmin is at 05.00 h, habitual bedtime is at 23.00 h and habitual waking time is at 07.00 h. With light scheduled 8 h before CBTmin, lights out was scheduled at each subject’s usual bedtime; with light exposure scheduled during the usual sleep period (e.g. 3 h before CBTmin), lights out was scheduled 1 h earlier than the subject’s habitual bedtime, and lights on was scheduled l h later than each subject’s habitual waking time; with light scheduled 3 h after CBTmin, lights out and lights on were scheduled at the subject’s habitual times.
Post-treatment (night 4) Treatment-induced changes in the timing of the melatonin profile were assessed from blood samples collected on the fourth night from 16.00 h until 10.00 h the following morning using the same method as in night 2.
During the GCRC stay, light levels, posture, meals and activity were controlled to minimize any masking of circadian phase measures. Participants remained in dim light (∼10 lux) during waking hours and were subjected to an 8 h enforced rest period in darkness at their reported habitual bedtime. Subjects were woken by staff and lighting increased to 10 lux at the scheduled wake time. Subjects remained seated or semi-reclined in bed during the 16 h wake period other than when they used the toilet facilities and took a daily shower. Isocaloric snacks (150–250 kcal, depending on normal food intake, comprising 50% carbohydrate, 20% protein and 30% fat) were provided every 2 h in place of regular meals. Caffeine intake during the GCRC stay was prohibited. The subjects were allowed to participate in a variety of activities that allowed them to remain quietly awake in the dim light, including reading, watching TV or videos, talking on the telephone, and using a laptop computer.
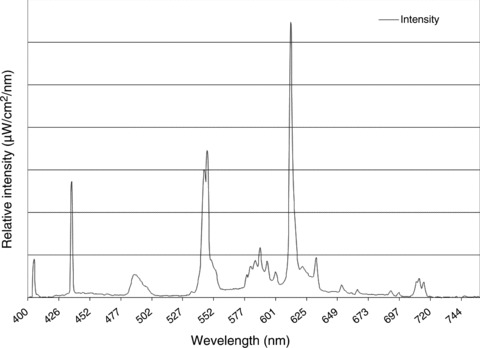
Light interventions were provided using broad-spectrum fluorescent light boxes (Sunbox, Co., Gaithersburg, MD, USA) (Fig. 4). In the 2000 lux condition, two light boxes were placed on the sides of the bed at a level between the subject’s knees and feet, and were angled toward the subject. In the 8000 lux condition, a second pair of light boxes were placed on the sides of the bed at a level between the subject’s elbows and knees, and were angled toward the subject. Each light box had a three-way switch that could turn on one, two or three light bulbs. For the 2000 lux condition, we used two light boxes with two of the three bulbs illuminated. For the 8000 lux condition, we used four light boxes at maximum intensity (three bulbs illuminated in each). The 15 min ramp-up and ramp-down periods in the 2000 lux condition were accomplished by increasing or decreasing the light by switching off one light bulb every 5 min. In the 8000 lux condition, a combination of light bulbs in the two pairs of lights were switched on or off in order to ramp up to or down from, respectively, maximum intensity for 15 min. Adjustments were made in the proximity of the lights to the subject so that the light reached the target intensity as measured by a photographic light meter placed at the subject’s forehead. A movie of the subject’s choosing was shown on a video monitor at the foot of the bed to facilitate a stable direction of gaze during light exposure.
Figure 4. Relative intensity at various wavelengths for the broad-spectrum fluorescent light.
Blood samples were collected at 20 min intervals from 16.00 h until 01.00 h and at 30 min intervals from 01.00 h until 10.00 h the next day on the baseline and post-treatment nights. The i.v. line was kept patent with a slow drip of heparinized saline (750 IU heparin in 9.0 g NaCl/L). Blood samples were centrifuged and plasma frozen at −80°C for subsequent measurement of melatonin. Plasma melatonin levels were measured with a double-antibody radioimmunoassay using commercially available reagents (Stockgrand Ltd, Guildford, UK) (Van Cauter et al. 1994). The lower limit of sensitivity of the assay was 2.5 pg ml−1. The intra-assay coefficient of variation averaged 17.5% for values of <10 pg ml−1, 8.6% for values in the range of 10–30 pg ml−1, and 5.2% for values of >30 pg ml−1. The inter-assay coefficient of variation averaged 20% for values of <10 pg ml−1 and 13.5% for values of ≥10 pg ml−1. All samples from the same subject were measured in the same assay.
Circadian phase assessments
Baseline phase markers of the dim light melatonin onset (DLMO) and dim light melatonin offset (DLMOff) were assessed across the nocturnal melatonin profile. The two absolute thresholds were DLMO at a level of 10 pg ml−1 (DLMO 10 pg) or DLMO at two standard deviations above baseline (2 s.d.), defined as the first sample to rise >2 s.d. above the average of melatonin levels sampled between 16.00 h and 18.00 h and to be followed by a continued rise in melatonin levels (Benloucif et al. 2005,2008). Values of 2.5 pg ml−1 and 200 pg ml−1 were used for samples that were, respectively, below and above the limit of sensitivity for this assay. The amplitude of the melatonin rhythm was defined as the peak of the nocturnal profile in pg/ml.
To assess the change in the time of the melatonin rhythm from baseline to post-treatment, melatonin levels (pg ml−1) were adjusted to a percentage of maximum (average of the three highest values). The raw data were then smoothed with the Lowess (Cleveland) curve-fitting procedure and interpolated at 1 min intervals using GraphPad Prism version 3.0 (Graphpad Software, Inc., San Diego, CA, USA). The times at which melatonin levels rose to 50% of maximum levels and declined to 50% of maximum levels were defined as DLMO-50% and DLMOff-50%, respectively. The time of the melatonin midpoint was computed as the time of the midpoint between DLMO-50% and DLMOff-50%. This measure incorporates the times of the rapid rise and rapid decline in melatonin synthesis with less baseline variability than other phase markers (Benloucif et al. 2005). In some of the data, the DLMO-50% or DLMOff-50% of the melatonin profile could not be determined. For example, in some instances melatonin had not declined sufficiently by 10.00 h to assess DLMOff-50%. In these cases, the melatonin midpoint based on DLMO-75% and DLMOff-75% was used (n= 5 at baseline; n= 7 at post-treatment).
In order to determine the variability in the markers of the baseline phase, we assessed the mean absolute difference in CBTmin and melatonin midpoint between the first and second visits for each subject, irrespective of the direction of the difference. The mean absolute difference provides a measure of individual variability in the timing of the phase markers, which is more informative than the average time of the phase marker for the group as a whole (Benloucif et al. 2005).
The phase shift in the melatonin rhythm was calculated by the difference in timing between the baseline melatonin midpoint and the post-treatment melatonin midpoint for each condition. The magnitude of phase shifts in response to each light intensity was averaged in 2 h bins for statistical analysis, based on the timing of light exposure relative to the melatonin midpoint.
Statistical analysis
Demographic variables, including age and gender ratios, were compared between younger and older subjects using an independent t test and a chi-squared test, respectively. Baseline values for sleep parameters and circadian phase markers were compared between younger and older subjects with independent t tests.
The PRC was created from the polynomial fourth-order, non-linear regression fitted through all of the data points (GraphPad Prism; Graphpad Software, Inc.).
To compare the effects of age on the direction and magnitude of phase shifts according to the intensity and timing of light pulses, we calculated the average magnitude of phase shifts in 2 h bins, and applied three-factor (age, intensity and time of pulse) repeated-measures ANOVA on only the subjects (n= 27) who had light pulses of both 2000 lux and 8000 lux within the same 2 h bin. Additionally, the effects of age and intensity on the direction and magnitude of phase shifts were compared in the entire dataset using two-factor (age and intensity) ANCOVA controlling for time of pulse. Two-sided P-values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using spss Version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline circadian phase markers
The average age, gender distribution and MEQ scores of the subjects, and values of the phase markers assessed at baseline are shown in Table 1. The baseline values of melatonin profiles at the two different interventions (2000 lux and 8000 lux) are presented separately. As the timing of light exposure on both visits was calculated according to the baseline CBTmin on the first visit, the baseline values of CBTmin are presented only for the first visit. Sleep onsets and offsets measured by actigraphy were averaged from recordings over the week prior to admission.
Table 1.
Baseline characteristics of sleep times and circadian phase markers in younger and older subjects
| Younger subjects | n | Older subjects | n | P-value | |
|---|---|---|---|---|---|
| Age, years | 25.14 ± 4.14 | 29 | 66.50 ± 6.00† | 16 | <0.001 |
| Male : female ratio | 8 : 21 | 29 | 5 : 11 | 16 | 0.728 |
| MEQ | 52.38 ± 6.65 | 26 | 62.00 ± 8.97† | 14 | <0.001 |
| CBTmin phase (first visit) | 04.57 ± 1.19 | 29 | 04.13 ± 1.00 | 16 | 0.063 |
| Assessment (2000 lux) | |||||
| Sleep onset time | 00.22 ± 1.07 | 24 | 23.34 ± 1.07* | 12 | 0.049 |
| Sleep offset time | 07.43 ± 1.03 | 24 | 06.43 ± 0.55† | 12 | 0.008 |
| Melatonin profiles | |||||
| DLMO-10 pg | 21.22 ± 1.44 | 27 | 20.19 ± 1.39 | 14 | 0.069 |
| DLMO-2 s.d. | 21.12 ± 1.48 | 27 | 20.10 ± 1.39 | 14 | 0.085 |
| DLMO-50% | 22.55 ± 1.33 | 27 | 21.53 ± 1.43 | 13 | 0.063 |
| DLMOff-50% | 07.27 ± 1.21 | 25 | 07.17 ± 1.37 | 14 | 0.585 |
| Melatonin midpoint | 03.04 ± 1.15 | 25 | 02.36 ± 1.36 | 13 | 0.275 |
| Melatonin amplitude, pg ml−1 | 63.14 ± 25.80 | 27 | 42.52 ± 27.50* | 14 | 0.023 |
| Assessment (8000 lux) | |||||
| Sleep onset | 00.16 ± 0.51 | 26 | 23.30 ± 1.07* | 14 | 0.019 |
| Sleep offset | 07.44 ± 0.49 | 26 | 06.21 ± 1.26† | 14 | <0.001 |
| Melatonin profiles | |||||
| DLMO-10 pg | 21.13 ± 1.44 | 29 | 19.49 ± 1.55* | 14 | 0.022 |
| DLMO-2 s.d. | 21.21 ± 1.28 | 29 | 19.49 ± 2.03† | 14 | 0.008 |
| DLMO-50% | 23.00 ± 1.31 | 29 | 21.27 ± 1.50† | 14 | 0.006 |
| DLMOff-50% | 07.31 ± 1.18 | 27 | 06.35 ± 2.07 | 14 | 0.090 |
| Melatonin midpoint | 03.09 ± 1.08 | 27 | 02.01 ± 1.52* | 14 | 0.022 |
| Melatonin amplitude, pg ml−1 | 67.53 ± 23.66 | 29 | 47.21 ± 30.37* | 14 | 0.021 |
Phases are denoted using 24 h clock time. Values are presented as the mean ±s.d. Abbreviations: MEQ, Morningness-Eveningness Questionnaire; CBTmin, core body temperature minimum; DLMO, dim light melatonin onset; DLMOff, dim light melatonin offset. *P < 0.05; †P < 0.01 (chi-squared test or independent t test).
The average MEQ score was higher in older subjects than in younger subjects (P < 0.001), indicating a greater tendency toward ‘morningness’.
Average sleep onset and offset were significantly earlier in older than in younger subjects on both assessments (2000 lux and 8000 lux conditions, P < 0.05). The average differences between younger and older subjects were about 50 min in sleep onset and >1 h in sleep offset.
The average time of melatonin onset including DLMO-10 pg, DLMO-2 s.d. and DLMO-50% was significantly advanced in older subjects on one of the assessments (8000 lux, P < 0.05) and showed an advance that approached statistical significance on the other assessment. The average time of these variables was around 1 h earlier in older than in younger subjects on both assessments. DLMOff-50% and melatonin midpoint were about 1 h earlier in older subjects (P= 0.07 and P= 0.015, respectively) on one of the assessments. In older subjects, of all the melatonin variables assessed, DLMOff-50% showed the greatest variability between the two assessments (≈40 min).
The mean ±s.d. absolute differences in the timing of CBTmin (cosine demasked) and melatonin midpoint on the two assessments were 50 ± 42 min and 21 ± 17 min, respectively, showing that there is less variability in the phase of the melatonin midpoint compared with estimates of CBTmin.
The phase angles between circadian phase markers (DLMO-10 pg, DLMO-2 s.d., DLMO-50%, melatonin midpoint, DLMOff-50%, CBTmin) and the timing of sleep (onset and offset) did not differ significantly between younger and older subjects (P > 0.05).
The amplitude of nocturnal melatonin production was significantly lower in older subjects for both the 2000 lux and 8000 lux conditions (P < 0.05). Older subjects showed a tendency toward earlier timing of CBTmin than younger subjects (P= 0.06). The average difference in CBTmin between younger and older subjects was about 40 min.
Phase response curves in response to 2 h light pulses
Phase response curves in response to 2 h light pulses of 2000 lux and 8000 lux for older and younger subjects, using baseline melatonin midpoints as the phase reference, are shown in Fig. 5A (2000 lux) and B (8000 lux). Combined data are shown in Fig. 5C. When plotted relative to the melatonin midpoint, light exposure that was timed to −8 h, −3 h and +3 h from CBTmin was distributed nearly continuously from 8 h prior to 8 h after the melatonin midpoint. The PRC followed the expected pattern with delays in the early subjective night and advances at the end of the subjective night.
Figure 5. Phase response curves for 2 h light pulses at 2000 lux and 8000 lux in younger and older subjects.
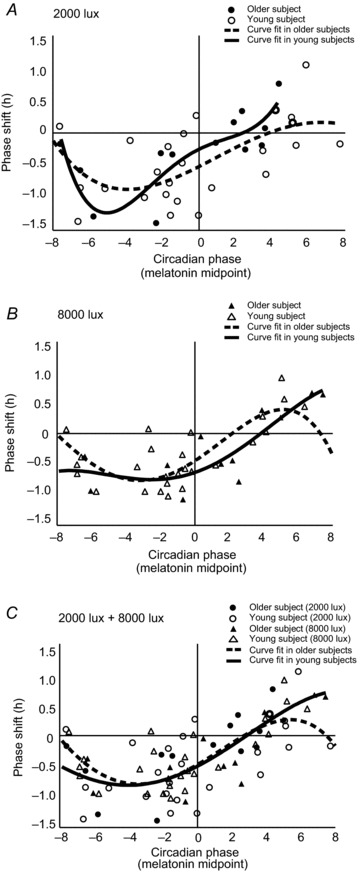
The magnitude and direction of phase shifts are plotted against the timing of the centre of the light exposure relative to the baseline melatonin midpoint following exposure to light of 2000 lux, A, and 8000 lux, B, at three different times relative to the core body temperature minimum (CBTmin). Positive values indicate a phase advance; negative values indicate a phase delay. The curved lines illustrate non-linear curve fit. C, the fitted curve from the combined data for exposure to light at 2000 lux and 8000 lux.
In the fitted curve for the combined data for the 2000 lux and 8000 lux conditions (Fig. 5C), peak times in delay shifts were similar in younger and older adults, with a maximum delay around 4 h prior to the melatonin midpoint (younger subjects: 3.4 h; older subjects: 4.0 h). The transition from delays to advances occurred about 3 h after the melatonin midpoint in both younger and older subjects. Peak advance shifts in older subjects could not be estimated, but peak advance shifts in younger subjects occurred 5.4 h after the melatonin midpoint.
Magnitude of phase shifts
The magnitude of phase shifts in response to each light intensity were averaged into 2 h bins (Fig. 4), based on the time of the centre of the light pulse relative to the melatonin midpoint. For subjects who received light pulses of both 2000 lux and 8000 lux within the same 2 h bin, analysis by three-factor repeated-measures ANOVA showed a significant effect for the time of pulse (F6,17= 3.08, P= 0.03), but not for intensity (F1,17= 0.90, P= 0.36), age (F1,17= 0.46, P= 0.51), or interactions between age and intensity (F1,17= 2.17, P= 0.16), or time of pulse and intensity (F6,17= 0.31, P= 0.93).
As the light pulses at 2000 lux and 8000 lux did not fall into the same bin for some subjects, three-factor ANCOVA controlling for time of pulse was also conducted on the entire dataset with intensity as a between-subjects factor. Similar to repeated-measures analysis, two-factor ANCOVA showed a significant effect on phase shifts of the melatonin midpoint for time of pulse (F1,76= 22.61, P < 0.0001) reflecting a significant PRC, but not for age (F1,76= 0.23, P= 0.64), intensity (F1,76 < 0.001, P= 0.99) or interactions of age with intensity (F1,76= 0.35, P= 0.56).
In the combined data averaged in 2 h bins (Fig. 6), the average magnitude in phase delays was largest at 6–4 h before the melatonin midpoint (younger : older: −0.96 : −1.37), and the largest average in phase advances occurred 4–8 h after the melatonin midpoint (younger : older: 0.42 : 0.70) in both age groups.
Figure 6. Magnitude of phase shifts within 2 h bins.
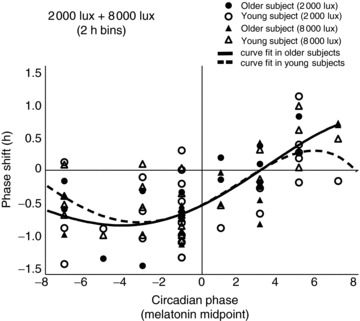
Phase shifts were binned into 2 h intervals based on time of exposure to light at 2000 lux and 8000 lux relative to the baseline melatonin midpoint in younger and older subjects.
Discussion
In the present study, we evaluated phase-shifting responses to bright light at lower and higher intensities in younger and older subjects to test the hypothesis that older adults would show a reduced response to 2 h of exposure to light at 2000 lux but not to light at 8000 lux. We found no group differences for the magnitude of phase shifts in the melatonin midpoint following exposure to 2 h of light at either 2000 lux or 8000 lux. In addition, as the fitted curve derived from the combined data for exposure to light at 2000 lux and 8000 lux shows, we did not find any significant differences between younger and older subjects in PRC, including the peak time of phase delays or the timing of the transition from delays to advances.
The results of the present study might be considered similar to those of Duffy et al. ()2007, who found no difference between younger and older adults in the magnitude of phase delays in melatonin rhythm following 6.5 h of exposure to light of >1000 lux. However, Duffy et al. ()2007 did report a difference between younger and older adults in the curve fit of data for exposure to light of 100–1000 lux, although this finding was based on results in only four older subjects and non-concurrent controls. Our results are partially consistent with those of Klerman et al. ()2001, who found no age-related differences in the magnitude of phase delays following exposure to 3 days of light at 10,000 lux for 5 h. Klerman et al. ()2001 did find a reduction in the magnitude of light-induced phase advances, which was not observed in either the present study or in two other studies (Kripke et al. 2007; Sletten et al. 2009) that assessed phase-advancing responses to light in younger and older adults.
Sletten et al. ()2009 compared the effectiveness of blue and green light in younger and older adults, and reported a significant effect of age in subjective alertness and sleepiness following exposure to blue light, but not after exposure to green light. Phase advances in response to blue light were slightly larger than advances in response to green light in both younger and older adults, but the difference did not reach statistical significance in the study’s small sample. In a previous study in our laboratory, we found there to be an age-related decrease in melatonin suppression following exposure to monochromatic blue light, but not to red or green light (Green et al. 2004). It remains possible that the greater power of long wavelength light relative to short wavelength light that occurs with the broad-spectrum light boxes used in the present study may have obscured any age-related differences in responses to short wavelength light, although the clinical significance of an age-related reduction in the response to short, but not long, wavelength light is unclear. In addition, our results do not exclude the possibility that there may be small differences in the magnitude of phase shifts between age groups and that we did not detect these differences under our experimental protocol designed to observe the acute effect of light. According to published data, a phase shift is often not completed immediately after a light pulse (Pittendrigh et al. 1958; Watanabe et al. 2001).
This study tested the hypothesis that the phase-shifting response to light in older adults would be lower than that in younger subjects following exposure to light of 2000 lux but not to light of 8000 lux. However, we did not observe significant differences between 2000 lux and 8000 lux in the magnitude of light-induced phase shifts in either younger or older subjects. A key question, therefore, is whether the 2000 lux light exposure used in this study was saturating (i.e. elicited a maximal response). In general, the phase-shifting response to light exposure is known to depend on the wavelength of the light source (Sletten et al. 2009), the light intensity (Zeitzer et al. 2000), the duration of the light pulse (Dewan et al. 2011; Chang et al. 2012), and the timing of the light pulse (Khalsa et al. 2003), as we observed in the present study. Light exposure targeted 8 h before CBTmin accounted for the observed phase delays (−0.72 ± 0.74 h for 2000 lux, −0.50 ± 0.41 h for 8000 lux), whereas light exposure targeted 3 h after CBTmin resulted in phase advances in the melatonin midpoint (0.05 ± 0.49 h for 2000 lux, 0.18 ± 0.57 h for 8000 lux). Light exposure targeted 3 h before CBTmin was distributed in both phase delay and advance regions (−3.2 h to +2.5 h) relative to the melatonin midpoint. In subjects exposed to light during the night, the sleep schedule (dark period) during the treatment night was adjusted to be both 1 h earlier and 1 h later than in the baseline and post-treatment nights. This alteration of the dark period could potentially result in a small phase shift, although the change in the time of the light/dark cycle was not great.
In addition, several studies have reported that prior light history affects the sensitivity of the circadian timing system to phase-shifting and melatonin-suppressing responses to subsequent light exposure (Hebert et al. 2002; Smith et al. 2004; Jasser et al. 2006; Chang et al. 2011).
In the present study, subjects had a single day to adapt to the dim light condition prior to the baseline phase assessment and 2 h light pulse. In comparison, Zeitzer et al. ()2000 reported that in subjects who spent 3 days at baseline in light of approximately 90 lux followed by a constant routine in about 5 lux, a 6.5 h exposure to light of 1000 lux was saturating. In addition, studies with a single long-duration, bright light pulse (>6 h, up to ∼10,000 lux) have reported phase delays of up to 3 h (Zeitzer et al. 2000; Khalsa et al. 2003; Duffy et al. 2007), compared with the 1 h phase shift obtained in the present study. In a 2012 paper from the same group, 10,000 lux was found not to be saturating when the duration of light exposure was <3 h (Chang et al. 2012), which is consistent with our recent finding that, in humans, the duration of light exposure is more important than the intensity (Dewan et al. 2011). Together, these results suggest that the 2 h light exposure of 2000 lux used in the present study did not elicit a maximal response.
The maximal delay of the melatonin midpoint was about 1 h in the present study. Similar to our study of the duration of light stimuli, previous studies using a stimulus consisting of exposure to 2 h or 3 h of bright light (4000 lux or 5000 lux) have reported an average phase delay of 1 h and a maximum delay of 2 h for CBTmin (Minors et al. 1991; Van Cauter et al. 1994) or salivary DLMO (Canton et al. 2009). We also found that in both younger and older subjects, the peak time for phase delays was observed within the range of 4–2 h before the melatonin midpoint, and a transition from delays to advances occurred at approximately 3 h after the melatonin midpoint. Previous studies (3 h light pulse at 5000 lux) conducted under conditions similar to those of the present study protocol reported the crossover to phase advances occurred about 1 h after CBTmin (Minors et al. 1991; Van Cauter et al. 1994). A study using a single long-duration, bright light pulse (>6 h, up to ∼10,000 lux) reported that a relatively rapid transition from phase delays to phase advances occurs near circadian phase 0 h of CBTmin (Khalsa et al. 2003). Considering that CBTmin is likely to occur approximately 2 h later than a melatonin midpoint according to our baseline data as well as data cited in previous studies (Khalsa et al. 2003; Dewan et al. 2011), our finding of the crossover time (transition between delays and advances) is consistent with previous results.
We found no evidence of a change in older adults in the PRC for the combined (2000 lux plus 8000 lux) data. This contrasts with a study by Kripke et al. ()2007, who reported that the transition from delays to advances was approximately 1.8 h earlier in older adults than in younger participants. Although the experimental protocols were substantially different, it is not clear why our results differ. To the best of our knowledge, the present study and that by Kripke et al. ()2007 are the only studies to have compared the difference in the transition from delays to advances between younger and older adults. Further studies are required to explore the discrepancies in the findings and to determine whether ageing may be associated with a change in transition time following exposure to bright light.
Consistent with other reports, in the present study the timing of sleep onset and offset, temperature and melatonin rhythm were earlier in older than in younger adults. In addition, melatonin amplitude was lower in older than in younger subjects. These findings support those of previous studies that have reported advanced phase or reduced amplitude of various physiological rhythms in older subjects (Duffy et al. 1998,2002; Dijk et al. 2000; Carrier et al. 2002; Yoon et al. 2003). It has also been reported that wake times and bedtimes occur earlier in older people than in younger people (Duffy et al. 1998). We found that the average difference between younger and older subjects was around 1 h for both sleep onset and offset and endogenous circadian markers, which is similar to findings reported in other studies of various endogenous markers in young adults (Duffy et al. 1998,2002). The reduction in the amplitude of melatonin synthesis is similar to those in previous studies that have reported age-related reductions in the circadian amplitude of many physiological variables including CBT, melatonin and cortisol rhythms (Jewett et al. 1991; Youngstedt et al. 2001), although some studies failed to demonstrate a difference in the amplitude of CBT or melatonin rhythms in very healthy older adults (Zeitzer et al. 1999; Klerman et al. 2001). We did not find evidence of an age-related change in the phase angle between sleep–wake time and circadian rhythms. Similarly, several other studies failed to find age-related differences in the phase angles between sleep timing and circadian phase markers (Carrier et al. 2002; Tozawa et al. 2003; Benloucif et al. 2006).
This present study has several limitations. Firstly, the peak time in phase advances could not be estimated for older subjects. This may reflect the fact that our sample size was relatively small in the context of the phase advanced stimulus. Secondly, the ‘dead zone’ of the PRC characterized by a region of relative insensitivity to light-induced resetting was not evaluated. Although there was no evidence of an age-related change in the magnitude of phase shifts on the PRC, the limited statistical power imposed by the modest sample size in the present study (n2000 lux + 8000 lux= 81) may have played a role in limiting the significance of some of the statistical comparisons (e.g. three-way ANOVA). A post hoc power analysis revealed that an n of approximately 103 is required to obtain statistical power at the recommended 0.80 level.
In conclusion, this study showed that older subjects, in whom melatonin phase and temperature rhythms were advanced in comparison with those in younger subjects, and in whom the amplitude of melatonin synthesis was lower than in younger subjects, showed no evidence of age-related changes in the magnitude or direction of phase shifts. These results suggest that the advances in circadian phase in older adults are unlikely to reflect an alteration in the phase-shifting response to broad-spectrum light.
Acknowledgments
We thank the study participants, the research coordinators of the Sleep and Circadian Rhythm Research Laboratory, and the staff of the General Clinical Research Center. Technical support in statistical analyses was provided by Joseph Kang, PhD, Assistant Professor in Preventive Medicine, Northwestern University Feinberg School Medicine, Chicago, IL, USA. This paper was presented at the 2013 Annual Meeting of the Associated Professional Sleep Societies, Baltimore, MD, USA.
Glossary
- ADLS
Activities of Daily Living Scale
- CBTmin
core body temperature minimum
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- CNADC
Cognitive Neurology and Alzheimer’s Disease Center
- DLMOff
dim light melatonin offset
- DLMO
dim light melatonin onset
- FAST
Functional Assessment Staging Tool
- GCRC
General Clinical Research Center
- GDS
Geriatric Depression Scale
- MEQ
Morningness-Eveningness Questionnaire
- MMSE
mini-mental state examination
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- PRC
phase response curve
Additional information
Competing interests
None declared.
Author contributions
All authors contributed extensively to the work presented and approved the final manuscript for publication. S.J.K. wrote the manuscript for important intellectual content, and conducted the analysis and interpretation of data. S.B. contributed to the conception and design of the study, oversaw the collection, analysis and interpretation of data, and revised the manuscript for language and intellectual content. K.J.R. was involved in the analysis and interpretation of data and the drafting of the manuscript. S.W. contributed to the critical revision of the article and to the clinical and neuropsychological characterization of the sample. N.K. contributed to data collection and to the clinical and neuropsychological characterization of the sample. L.F.W. conducted the history and physical examinations and was the attending physician for the inpatient studies. P.C.Z. conceived and designed the study and edited the paper; the experimental protocols were conducted in her laboratory.
Funding
This work was supported in part by a grant from the National Center on Clinical Research Resources (NCRR-0048), Public Health Service grants R01 HL67604 and P01 AG11412, and by grant AG13854 (Alzheimer’s Disease Core Center) from the National Institute on Aging.
References
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, Pat-Horenczyk R, Fell R. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Green K, L’Hermite-Baleriaux M, Weintraub S, Wolfe LF, Zee PC. Responsiveness of the aging circadian clock to light. Neurobiol Aging. 2006;27:1870–1879. doi: 10.1016/j.neurobiolaging.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Responsiveness to melatonin and its receptor expression in the aging circadian clock of mice. Am J Physiol-Reg I. 1997;273:R1855–R1860. doi: 10.1152/ajpregu.1997.273.6.R1855. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schmitt K, Eckert A. Aging and circadian disruption: causes and effects. Aging (Albany NY) 2011;3:813–817. doi: 10.18632/aging.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Dawson D. Aging young sleep: a test of the phase advance hypothesis of sleep disturbance in the elderly. J Sleep Res. 1992;1:205–210. doi: 10.1111/j.1365-2869.1992.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Kripke DF, Gillin JC, Hrubovcak J. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Canton JL, Smith MR, Choi H-S, Eastman CI. Phase delaying the human circadian clock with a single light pulse and moderate delay of the sleep/dark episode: no influence of iris colour. J Circadian Rhythms. 2009;7:8. doi: 10.1186/1740-3391-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J, Paquet J, Morettini J, Touchette É. Phase advance of sleep and temperature circadian rhythms in the middle years of life in humans. Neurosci Lett. 2002;320:1–4. doi: 10.1016/s0304-3940(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE, Czeisler CA. Human responses to bright light of different durations. J Physiol. 2012;590:3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman W. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol Opt. 2003;23:181–187. doi: 10.1046/j.1475-1313.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk D-J, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Green KA, Wolfe LF, Gaskill MB, Goldman N, Zee PC, Benloucif S. Comparison of melatonin suppression by blue wavelength light in young and older adults. Sleep. 2004;27:A76. [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herljevic M, Middleton B, Thapan K, Skene DJ. Light- induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21:394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350:59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008;12:307–317. doi: 10.1016/j.smrv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Liu R-Y, Zhou J-N, Hoogendijk WJ, van Heerikhuize J, Kamphorst W, Unmehopa UA, Hofman MA, Swaab DF. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–322. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–165. [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Morin LP. Age-related changes in hamster circadian period, entrainment, and rhythm splitting. J Biol Rhythms. 1988;3:237–248. [Google Scholar]
- Pittendrigh C, Bruce V, Kaus P. On the significance of transients in daily rhythms. Proc Natl Acad Sci U S A. 1958;44:965–973. doi: 10.1073/pnas.44.9.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- Rosenberg RS, Zee PC, Turek FW. Phase response curves to light in young and old hamsters. Am J Physiol Regul Integr Comp Physiol. 1991;261:R491–R495. doi: 10.1152/ajpregu.1991.261.2.R491. [DOI] [PubMed] [Google Scholar]
- Scheuermaier K, Laffan AM, Duffy JF. Light exposure patterns in healthy older and young adults. J Biol Rhythms. 2010;25:113–122. doi: 10.1177/0748730410361916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo MA, Lupi D, Peirson SN, Butler JN, Foster RG. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;18:3007–3017. doi: 10.1111/j.1460-9568.2003.03061.x. [DOI] [PubMed] [Google Scholar]
- Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metabol. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Tozawa T, Mishima K, Satoh K, Echizenya M, Shimizu T, Hishikawa Y. Stability of sleep timing against the melatonin secretion rhythm with advancing age: clinical implications. J Clin Endocrinol Metabol. 2003;88:4689–4695. doi: 10.1210/jc.2003-030147. [DOI] [PubMed] [Google Scholar]
- Van Best JA, Van Delft JL, Keunen JE. Long term follow-up of lenticular autofluorescence and transmittance in healthy volunteers. Exp Eye Res. 1998;66:117–123. doi: 10.1006/exer.1997.0417. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne M, Blackman J, Leproult R, Ofek G, L’Hermite-Baleriaux M, Refetoff S, Turek F, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Deboer T, Meijer JH. Light-induced resetting of the circadian pacemaker: quantitative analysis of transient versus steady-state phase shifts. J Biol Rhythms. 2001;16:564–573. doi: 10.1177/074873001129002259. [DOI] [PubMed] [Google Scholar]
- Weale R. Age and the transmittance of the human crystalline lens. J Physiol. 1988;395:577–587. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Pokorny J, Smith VC. Optical density of the human lens. J Opt Soc Am A Opt Image Sci Vis. 1997;14:953–960. doi: 10.1364/josaa.14.000953. [DOI] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001;31:264–272. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Daniels JE, Duffy JF, Klerman EB, Shanahan TL, Dijk D-J, Czeisler CA. Do plasma melatonin concentrations decline with age. Am J Med. 1999;107:432–436. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser J, Zee P, Mayo K, Takahashi J, Turek F. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]


