Abstract
Abstract Calcineurin inhibitors, such as cyclosporin A and tacrolimus (FK506), have played a pivotal role in the preservation of allograft function. However, these drugs can cause unexplained severe pain in patients, often referred to as calcineurin inhibitor-induced pain syndrome (CIPS). Although calcineurin can regulate NMDA receptor (NMDAR) activity, the causal relationship between spinal synaptic plasticity and CIPS remains unknown. In this study, we showed that systemic administration of FK506 (1.5 mg kg−1 day−1) for 7 days in rats led to long-lasting nociceptive and mechanical hypersensitivity. Whole-cell patch-clamp recordings in spinal cord slices revealed that FK506 treatment caused a large increase in the amplitude of NMDAR-mediated excitatory postsynaptic currents (EPSCs) of dorsal horn neurons evoked by dorsal root stimulation. The amplitude of NMDAR currents elicited by puff NMDA application to dorsal horn neurons was also significantly greater in FK506-treated than in vehicle-treated rats. The frequency of spontaneous and miniature EPSCs in most dorsal horn neurons was profoundly increased in FK506-treated rats and was reduced by blocking NMDARs. Furthermore, blocking GluN2A or GluN2B subunits similarly reduced the amplitude of evoked EPSCs and the frequency of miniature EPSCs in dorsal horn neurons of FK506-treated rats. In addition, intrathecal injection of an NMDAR antagonist or systemic administration of memantine effectively reversed nociceptive and mechanical hypersensitivity in FK506-treated rats. Our findings indicate that calcineurin inhibition increases glutamate-mediated nociceptive input by potentiating presynaptic and postsynaptic NMDAR activity in spinal cords. NMDAR antagonists may represent a new therapeutic option for the treatment of CIPS.
Key points.
We developed an animal model to study the mechanisms underlying the pain syndrome commonly seen in organ transplant patients receiving calcineurin inhibitors.
Systemic treatment with the calcineurin inhibitor induces long-lasting pain hypersensitivity and increases glutamate receptor activity in the spinal cord.
Blocking glutamate receptor activity at the spinal cord level effectively reduces pain hypersensitivity induced by the calcineurin inhibitor.
This information advances our understanding of the molecular basis of pain caused by calcineurin inhibitors and identifies new strategies for treating such pain syndrome in transplant patients.
Introduction
Calcineurin inhibitors, such as cyclosporin A and tacrolimus (FK506), are the most commonly used immunosuppressive drugs to prevent rejection of transplanted organs and tissues. However, these drugs can cause unexplained severe pain and pain hypersensitivity, often referred to as calcineurin inhibitor-induced pain syndrome (CIPS; Grotz et al. 2001; Collini et al. 2006; Kakihana et al. 2012). CIPS frequently occurs in bilateral ankles, knees and feet, although cutaneous tactile allodynia and hyperalgesia have also been reported (Fujii et al. 2006; Noda et al. 2008). The pain associated with CIPS is typically symmetrical, sharp, stinging and intermittent and increases when standing or walking (Grotz et al. 2001; Kida et al. 2004; Collini et al. 2006; Fujii et al. 2006; Noda et al. 2008). Although CIPS seems to affect about 5–10% of patients who undergo kidney, heart, lung, liver, pancreas, or bone marrow transplantation, it is probably much more common in transplant patients because CIPS is frequently diagnosed as reflex sympathetic dystrophy syndrome (Muñoz-Gomez et al. 1991; Grandtnerova et al. 1998; Puig et al. 2000; Ybarra et al. 2003). Also, the concurrent use of L-type Ca2+ channel blockers (to treat hypertension caused by calcineurin inhibitors) may mask CIPS in many patients, because L-type Ca2+ channels contribute to regulating glutamatergic input from primary afferents to the spinal dorsal horn (Bao et al. 1998).
The pathogenesis of CIPS is poorly understood at the present time. Because increased bone uptake and mild bone marrow oedema in the femur of some patients with CIPS have been reported, some studies have suggested that CIPS results from calcineurin-induced vascular changes that disturb bone perfusion and permeability (Grotz et al. 2001; Collini et al. 2006; Kakihana et al. 2012). In other studies, however, radiological examinations and magnetic resonance imaging of the bone structures in patients with CIPS have revealed no abnormalities (Fujii et al. 2006; Noda et al. 2008). Calcineurin is a Ca2+- and calmodulin-dependent serine/threonine protein phosphatase (leading to dephosphorylation), which is present in the T cells of the immune system, the dorsal root ganglion (Lukyanetz, 1997; Wu et al. 2005), and the spinal dorsal horn (Strack et al. 1996). The first sensory synapse between the central terminals of primary sensory neurons and spinal dorsal horn neurons is critically involved in the transmission and transformation of sensory information. Glutamate is an essential excitatory neurotransmitter for synaptic transmission, and NMDARs play a critical role in spinal synaptic plasticity associated with chronic pain conditions (Chaplan et al. 1997; Zhou et al. 2012). It has been shown that calcineurin negatively affects NMDAR activity in brain slice experiments (Lieberman & Mody, 1994; Tong et al. 1995). However, little is known about whether calcineurin inhibitors affect the activity of spinal NMDARs in vivo or whether increased NMDAR activity contributes to pain hypersensitivity associated with CIPS.
In this study, we developed a rat model of CIPS and tested the hypothesis that chronic treatment of the calcineurin inhibitor causes pain hypersensitivity by increasing synaptic NMDAR activity and glutamatergic input to spinal dorsal horn neurons. Our study provides new information about the important contribution of spinal NMDARs to CIPS and suggests NMDARs as a potential new target for treating CIPS.
Methods
Animal model
Male Sprague–Dawley rats (6–7 weeks old) were purchased from Harlan Laboratories (Indianapolis, IN, USA). A total of 97 rats were used for the entire study. FK506, a highly specific calcineurin inhibitor (Liu et al. 1991), was dissolved in dimethyl sulfoxide (DMSO) and injected intraperitoneally (i.p.) at 1.5 mg kg−1 once a day for 7 days. This dose of FK506 was selected because it can suppress the immune function in rat models of organ transplantations at 1–2 mg kg−1 day−1 (Murase et al. 1993; Tze et al. 1997; Takama et al. 2011). The vehicle control group was treated with DMSO for 7 days. All the final electrophysiological experiments and behavioural tests were performed 3–5 days after the last FK506 or vehicle injection. At the end of the study, rats were killed by being given an intraperitoneal injection of phenobarbital (200 mg kg−1).
Intrathecal catheters were implanted in some FK506-treated rats anaesthetized with 2–3% isoflurane. Briefly, a small incision was made at the back of the animal’s neck. A small opening was made in the atlanto-occipital membrane of the cisterna magna, and a PE-10 catheter (∼8.0 cm) was inserted such that the caudal tip reached the lumbar spinal cord (Chen et al. 2000). The rats were allowed to recover for 4–5 days after surgery. Animals displaying signs of motor or neurological dysfunction were excluded from the study. All surgical preparation and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines for the ethical use of animals.
Nociceptive behavioural tests
To quantify tactile allodynia, rats were placed in individual plastic boxes on a mesh floor and allowed to acclimate for 30–45 min. A series of calibrated von Frey filaments was applied perpendicularly to the plantar surface of the hindpaw with sufficient force to bend the filaments for 6 s. Brisk paw withdrawal or flinching was considered a positive response. The tactile stimulus producing a 50% likelihood of withdrawal was determined by using the ‘up-down’ method (Chaplan et al. 1994; Chen et al. 2000).
To measure the mechanical nociceptive threshold of the hindpaw, the paw pressure (Randall–Selitto) test was used (Chen & Pan, 2006). The test was performed by applying a pressure stimulus to the hindpaw using a Ugo Basil Analgesimeter (Varese, Italy). When the animal displayed pain by either withdrawing the paw or vocalization, the pedal was immediately released, and the rat’s nociceptive threshold was read on the scale. A maximum of 400 g of pressure was used as a cutoff to avoid potential tissue injury to the rats.
FK506 and d-2-amino-5-phosphonopentanoate (AP5) were purchased from Tocris Bioscience (Ellisville, MO, USA). Memantine was obtained from Forest Research Institute (Jersey City, NJ, USA).
Spinal cord slice preparation and electrophysiological recordings
The lumbar spinal cord at the L5–L6 level was removed through laminectomy after rats were anaesthetized with 2–3% isoflurane, and the spinal tissues were immediately placed in an ice-cold sucrose artificial cerebrospinal fluid (aCSF) presaturated with 95% O2 and 5% CO2. The aCSF contained (in mm) 234 sucrose, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 12.0 glucose, and 25.0 NaHCO3. The spinal cord tissue was then glued onto the stage of a vibratome, and transverse slices (400 μm) of spinal cords were cut in ice-cold sucrose aCSF and preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 34°C for at least 1 h before being transferred to the recording chamber. The Krebs solution contained (in mm) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3.
The spinal cord slice was placed in a glass-bottomed chamber and continuously perfused with Krebs solution at 5.0 ml min−1 at 34°C maintained by an inline solution heater and a temperature controller. The spinal lamina II, a translucent region in the superficial dorsal horn, was identified on an upright fixed-stage microscope with differential interference contrast/infrared illumination (Li et al. 2002; Pan & Pan, 2004). Lamina II outer zone neurons were visualized and selected for whole-cell patch-clamp recordings because they predominantly receive nociceptive input from unmyelinated sensory nerves (Pan et al. 2003; Pan & Pan, 2004). It has been shown that most neurons in lamina II are glutamate-releasing excitatory interneurons (Santos et al. 2007). These neurons form a network that plays a critical role in modulating nociceptive information from the primary afferents. We used a glass pipette (5–10 MΩ) filled with internal solution containing (in mm) 135.0 potassium gluconate, 5.0 TEA, 2.0 MgCl2, 0.5 CaCl2, 5.0 Hepes, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, and 10 lidocaine (lignocaine) N-ethyl bromide (adjusted to pH 7.2–7.4 with 1 m KOH; 290–300 mosmol l−1). The input resistance was monitored, and the recording was abandoned if it changed more than 15%. All signals were recorded using an amplifier (MultiClamp700B; Axon Instruments Inc., Union City, CA, USA), filtered at 1–2 kHz, digitized at 10 kHz, and stored for off-line analysis.
To evoke monosynaptic excitatory postsynaptic currents (EPSCs), the attached dorsal root was electrically stimulated by using a stimulating electrode (0.4–0.6 ms, 0.6 mA and 0.1 Hz; Li et al. 2002). The AMPA receptor (AMPAR)-EPSCs were recorded at a holding potential of −60 mV in the presence of 10 μm bicuculline and 1 μm strychnine, whereas NMDAR-EPSCs were recorded at +40 mV in the presence of 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione, 10 μm bicuculline, and 1 μm strychnine. Spontaneous EPSCs (sEPSCs) were recorded in the presence of 1 μm strychnine and 10 μm bicuculline at a holding potential of −60 mV, whereas miniature EPSCs (mEPSCs) were recorded in the presence of 1 μm tetrodotoxin. In some experiments, NMDAR currents were elicited by puff application of 100 μm NMDA using a positive pressure system (4 p.s.i., 15 ms; Toohey Company, Fairfield, NJ, USA). The tip of the puff pipette was place 150 μm away from the recorded neuron, and the recordings were performed in the extracellular solution containing 0.1 mm Mg2+, 10 μm glycine, and 1 μm tetrodotoxin at a holding potential of −60 mV and using the pipette internal solution containing (in mm) 110.0 Cs2SO4, 2.0 MgCl2, 0.1 CaCl2, 1.1 EGTA, 10.0 Hepes, 2.0 MgATP, and 0.3 Na2GTP (pH was adjusted to 7.25 with 1.0 m CsOH; 280–300 mosmol l−1). In electrophysiological experiments, 4–6 rats were used for each protocol.
Statistical analysis
Data are presented as means ± standard error of the mean. The amplitude of EPSCs and NMDAR currents was analysed with Clampfit 9.2 (Axon Instruments). The sEPSCs and mEPSCs were analysed off-line with a peak detection program (MiniAnalysis, Synaptosoft Inc., Decatur, GA, USA). We used an unpaired Student’s t test to compare the statistical differences in puff NMDAR currents and the ratio of NMDAR-EPSCs to AMPAR-EPSCs between the vehicle- and FK506-treated groups. One-way analysis of variance (with Dunnett’s or Tukey’s post hoc test) was used to compare the effects of AP5 on sEPSCs and mEPSCs and the behavioural data. The effects of NMDAR antagonists on paw withdrawal thresholds were determined by ANOVA followed by Dunnett’s post hoc test. The level of significance was set at P < 0.05.
Results
Chronic systemic administration of FK506 causes mechanical hypersensitivity
Systemic injection of FK506 (1.5 mg kg−1, i.p.) once a day for 7 days caused a gradual decrease in the baseline tactile and pressure withdrawal thresholds in 11 rats (Fig. 1. Notably, the FK506-induced reduction in the paw withdrawal thresholds persisted for at least another 10 days after cessation of FK506 treatment. Both the tactile and pressure withdrawal thresholds returned to pretreatment levels 2 weeks after the discontinuing FK506 administration. In another 9 rats, systemic treatment with the vehicle (DMSO, i.p.) for 7 days did not alter the paw withdrawal thresholds, measured with von Frey filaments and the pressure stimulus (Fig. 1).
Figure 1. Chronic systemic administration of FK506 induces pain hypersensitivity in rats.
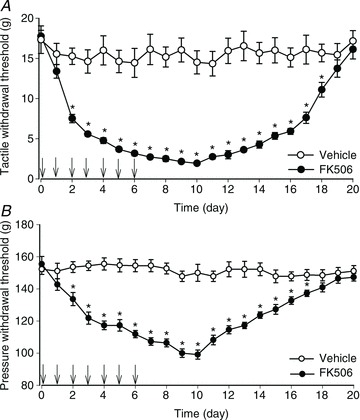
A and B, time course of changes in the hindpaw withdrawal thresholds of rats treated with FK506 (1.5 mg kg−1 day−1, i.p.; n= 11 rats) or vehicle (DMSO, n= 9 rats) for 7 days. Daily injections were indicated by arrows. The baseline hindpaw withdrawal thresholds were tested with von Frey filaments (A) or a noxious pressure stimulus (B). *P < 0.05 compared with the baseline value.
FK506 treatment potentiates postsynaptic NMDAR activity of spinal dorsal horn neurons
To determine changes in NMDAR activity of spinal dorsal horn neurons in CIPS, the spinal cord slices were obtained from vehicle- and FK506-treated rats at 3–5 days after the last treatment. We recorded monosynaptic NMDAR-EPSCs and AMPAR-EPSCs in lamina II neurons evoked by electrical stimulation of the dorsal root. Monosynaptic EPSCs were identified on the basis of the constant latency of evoked EPSCs and the absence of conduction failure of evoked EPSCs in response to a brief 20 Hz electrical stimulation (Li et al. 2002). The ratio of NMDAR-EPSCs to AMPAR-EPSCs of lamina II neurons in FK506-treated rats was significantly higher than that in vehicle-treated rats (0.24 ± 0.04 vs. 0.14 ± 0.02; Fig. 2A and B). The decay time of evoked NMDAR-EPSCs did not differ significantly between FK506-treated and vehicle-treated rats (107.0 ± 8.8 vs. 106.1 ± 12.1 ms).
Figure 2. Calcineurin inhibition increases NMDAR activity in the spinal dorsal horn.
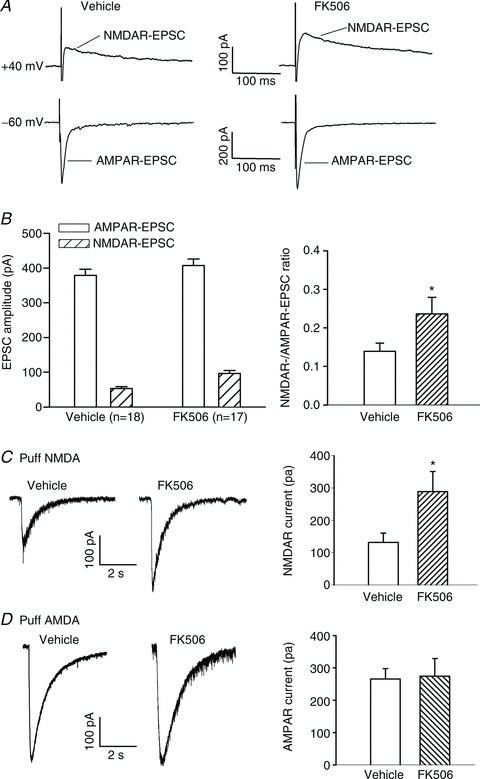
A and B, representative current traces (averaged responses from 6 EPSCs, A) and group data (B) show that FK506 treatment increased the ratio of NMDAR-EPSCs to AMPAR-EPSCs of lamina II neurons. The spinal cord slices were obtained from rats treated with FK506 or vehicle for 7 days. The holding potential was indicated on the left in A. C, original traces and summary data show NMDAR currents elicited by puff application of NMDA to lamina II neurons in vehicle-treated (n= 10 neurons) and FK506-treated (n= 11 neurons) rats. D, representative traces and group data show AMPAR currents elicited by puff application of AMPA to lamina II neurons in vehicle-treated (n= 18 neurons) and FK506-treated (n= 16 neurons) rats. *P < 0.05 compared with the value in the vehicle-treated group.
To directly determine whether the postsynaptic NMDAR activity is increased by FK506 treatment, we recorded NMDAR currents elicited by puff application of 100 μm NMDA directly to the recorded lamina II neuron. The amplitude of puff NMDAR currents of lamina II neurons was much larger in FK506-treated rats than in vehicle-treated rats (131.7 ± 28.9 vs. 288.6 ± 62.2 pA, P < 0.05; Fig. 2C). In contrast, the AMPAR current elicited by puff application of 200 μm AMPA to lamina II neurons did not differ significantly between FK506-treated and vehicle-treated rats (265.8 ± 32.2 vs. 274.5 ± 54.5 pA; Fig. 2D). These data indicate that chronic inhibition of calcineurin potentiates postsynaptic NMDAR activity in spinal dorsal horn neurons.
FK506 treatment increases presynaptic NMDAR activity in the spinal cord
We first determined how FK506 alters the overall glutamatergic input and whether NMDARs play a role in controlling glutamatergic input to dorsal horn neurons by recording glutamatergic sEPSCs, which reflect both action potential-dependent and independent glutamate release from primary afferents and interneurons. In lamina II neurons of vehicle-treated rats, the baseline frequency of sEPSCs was 3.05 ± 0.54 Hz in 14 neurons recorded. Bath application of AP5 (50 μm), a specific NMDAR antagonist, had no significant effects on the frequency and amplitude of sEPSCs in these neurons (Fig. 3). In 16 neurons recorded from FK506-treated rats, the baseline frequency of sEPSCs (5.90 ± 0.88 Hz) was significantly higher than that in vehicle-treated rats (Fig. 3A–D). Also, bath application of 50 μm AP5 significantly reduced the frequency, but not the amplitude, of sEPSCs in these 16 neurons (Fig. 3). Interestingly, the inhibitory effect of AP5 on sEPSCs was generally limited to those neurons with a baseline frequency of sEPSCs >3 Hz in FK506-treated rats (Fig. 3D).
Figure 3. Inhibition of calcineurin augments synaptic glutamate release to spinal dorsal horn neurons.
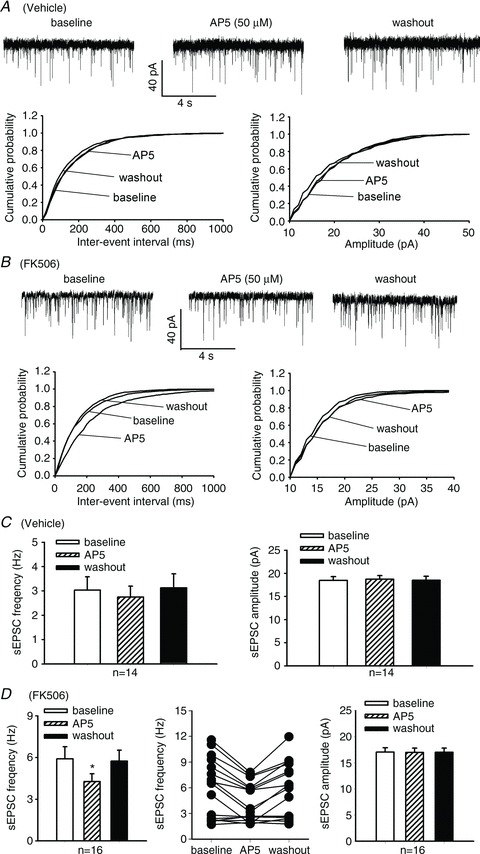
A and B, original recordings and cumulative plots show changes in the frequency of glutamatergic sEPSCs in lamina II neurons and the effect of bath application of 50 μm AP5 on sEPSCs in one vehicle-treated (A) and one FK506-treated (B) rat. C and D, summary data show changes in the frequency of sEPSCs and the effect of 50 μm AP5 on sEPSCs in lamina II neurons recorded from vehicle-treated (C) and FK506-treated (D) rats. A scattered line plot (middle pane in D) was provided to show the AP5 effect on sEPSCs in individual neurons recorded from FK506-treated rats. *P < 0.05 compared with the respective baseline value.
Furthermore, in a total of 18 lamina II neurons from FK506-treated rats, bath application of 50 μm AP5 significantly decreased the frequency of mEPSCs (Fig. 4A and B). Notably, the inhibitory effect of AP5 was only observed in 11 neurons with a baseline frequency of mEPSCs >3 Hz (Fig. 4B).
Figure 4. Calcineurin inhibition increases presynaptic NMDAR activity in the spinal cord.
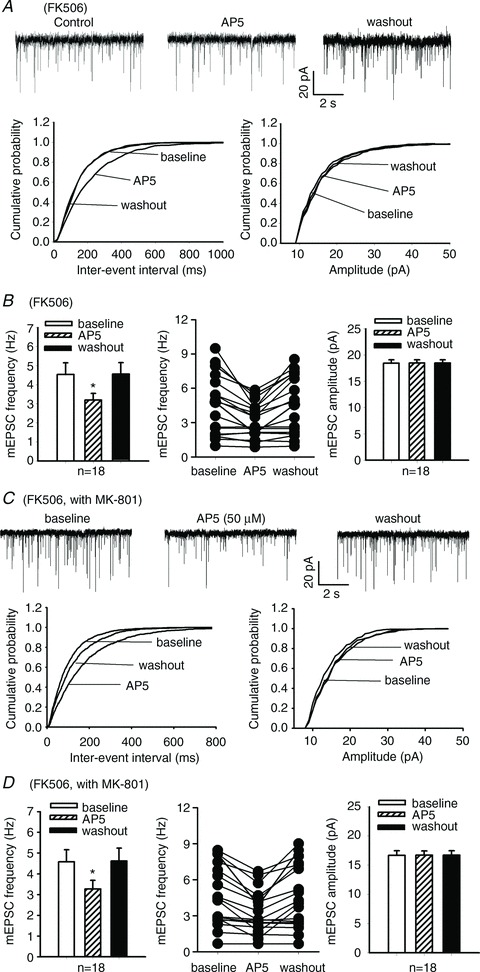
A, original recordings and cumulative plots show that bath application of 50 μm AP5 reduced the frequency, but not the amplitude, of glutamatergic mEPSCs in one lamina II neuron of an FK506-treated rat. B, group data show the effect of 50 μm AP5 on the frequency and amplitude of mEPSCs in lamina II neurons of FK506-treated rats. C, representative traces and cumulative plots show that 50 μm AP5 reduced the frequency of mEPSCs in one lamina II neuron of an FK506-treated rat. D, summary data show the effect of 50 μm AP5 on the frequency and amplitude of mEPSCs in lamina II neurons of FK506-treated rats. In C and D, mEPSCs were recorded in lamina II neurons using pipette electrode solution containing 1 mm MK-801 to block postsynaptic NMDARs. In B and D, a scattered line plot (middle pane) was inserted to show the AP5 effect on mEPSCs in individual neurons recorded from FK506-treated rats. *P < 0.05 compared with the respective baseline value.
In additional lamina II neurons recorded from FK506-treated rats, we blocked postsynaptic NMDARs through intracellular dialysis of 1 mm MK-801, a specific NMDAR channel blocker. In a total of 18 neurons tested, application of 50 μm AP5 significantly reduced the frequency, but not the amplitude, of mEPSCs (Fig. 4C and D). Also, the inhibitory effect of AP5 was evident only in those neurons with a frequency of mEPSCs >3 Hz (Fig. 4D). Together, these results suggest that chronic treatment with the calcineurin inhibitor also increases the presynaptic activity of NMDARs in a subpopulation of primary afferent terminals, which potentiates synaptic glutamate release and glutamatergic input to spinal dorsal horn neurons.
Role of GluN2A and GluN2B in increased NMDAR activity of dorsal horn neurons by FK506 treatment
To determine the relative contribution of GluN2A and GluN2B subunits to the increased NMDAR activity of lamina II neurons induced by FK506 treatment, we used 0.6 μm Ro25-6981, a highly specific GluN2B-containing NMDAR antagonist (Fischer et al. 1997; Zhao et al. 2012), as well as a low concentration of AP5 (5 μm), which preferentially blocks GluN2A-containing NMDARs at this concentration (Kimura & Matsuki, 2008; Zhao et al. 2012). In our experiments, we first tested the effect of Ro25-6981 through bath application and then applied 5 μm AP5 in the presence of Ro25-6981. In 11 lamina II neurons from vehicle-treated rats, bath application of 0.6 μm Ro25-6981 and 5 μm AP5 plus Ro25-6981 caused a similar reduction in the amplitude of monosynaptic NMDAR-EPSCs electrically evoked from the dorsal root (Fig. 5). In eight lamina II neurons tested from FK506-treated rats, application of 0.6 μm Ro25-6981 or 5 μm AP5 plus Ro25-6981 also significantly reduced the augmented amplitude of evoked NMDAR-EPSCs (Fig. 5).
Figure 5. GluN2A and GluN2B subunits contribute to increased NMDAR activity of spinal dorsal horn neurons by FK506 treatment.
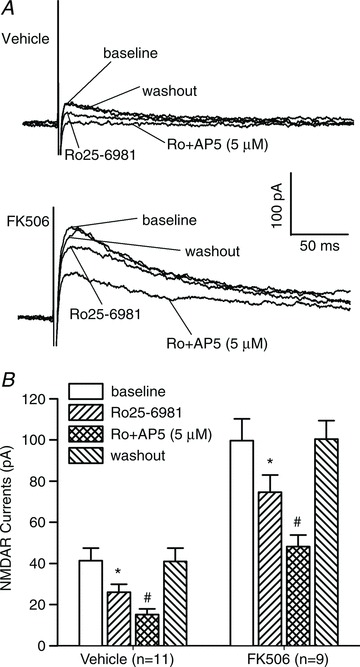
A, representative recording traces show changes in evoked NMDAR-EPSCs of lamina II neurons during baseline control, bath application of 0.6 μm Ro25-6981, and bath application of 5 μm AP5 plus 0.6 μm Ro25-6981 from one vehicle-treated rat and one FK506-treated rat. B, mean changes in the amplitude of NMDAR currents of lamina II neurons during baseline control, bath application of 0.6 μm Ro25-6981, bath application of 5 μm AP5 plus 0.6 μm Ro25-6981, and washout in vehicle-treated (n= 11 neurons) and FK506-treated (n= 9 neurons) rats. *P < 0.05 compared with the baseline control. #P < 0.05 compared with Ro25-6981 alone.
We then examined whether GluN2A and GluN2B subunits contribute to the increased presynaptic NMDAR activity in the spinal cord of FK506-treated rats. In these experiments, we recorded mEPSCs of lamina II neurons while blocking the postsynaptic NMDARs through intracellular dialysis of 1 mm MK-801. In all nine neurons tested, bath application of 0.6 μm Ro25-6981 or 5 μm AP5 plus Ro25-6981 similarly reduced the frequency, but not the amplitude, of mEPSCs (Fig. 6). Collectively, these data suggest that both GluN2A and GluN2B subunits contribute to increased NMDAR activity in the spinal cord by the calcineurin inhibitor treatment.
Figure 6. Contribution of GluN2A and GluN2B to increased presynaptic NMDAR activity in the spinal cord by FK506 treatment.
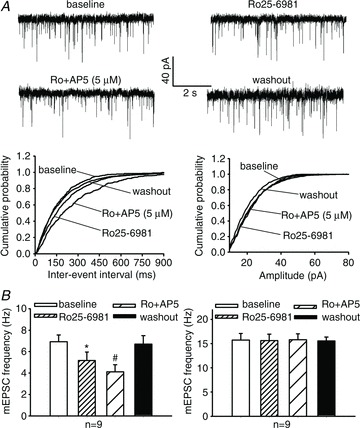
A, original traces and cumulative plots show that bath application of 0.6 μm Ro25-6981 alone and 5 μm AP5 plus 0.6 μm Ro25-6981 reduced the frequency, but not the amplitude, of mEPSCs in one lamina II neuron recorded from an FK506-treated rat. B, mean changes in the frequency and amplitude of mEPSCs in lamina II neurons during baseline control, bath application of 0.6 μm Ro25-6981, bath application of 5 μm AP5 plus 0.6 μm Ro25-6981, and washout in FK506-treated (n= 9 neurons) rats. mEPSCs were recorded in lamina II neurons using internal pipette solution containing 1 mm MK-801 to block postsynaptic NMDARs. *P < 0.05 compared with the baseline control. #P < 0.05 compared with Ro25-6981 alone.
Spinal NMDARs contribute to mechanical hypersensitivity induced by FK506 treatment
Having shown that the spinal NMDAR activity is increased in rats treated with FK506, we next determined whether increased NMDAR activity contributes to mechanical hypersensitivity caused by the calcineurin inhibitor. To examine the role of NMDARs at the spinal level in pain hypersensitivity induced by FK506, we tested the effect of intrathecal injection of AP5 on tactile allodynia and mechanical hyperalgesia at 3–5 days after chronic systemic treatment with FK506 (1.5 mg kg−1 day−1 for 7 days). We have shown that intrathecal injection of 5–10 μg of AP5 attenuates pain hypersensitivity induced by nerve injury in rats (Zhou et al. 2012). AP5 was dissolved in 30% DMSO in saline and administered in a volume of 5 μl, followed by a 10 μl flush with normal saline. AP5 (0.1, 0.5 and 2 μg) produced a dose-dependent reversal of tactile allodynia and mechanical hyperalgesia, measured with application of von Frey filaments and the noxious pressure stimulus to the hindpaw of FK506-treated rats (n= 7–8 rats in each group; Fig. 7). The AP5 effects reached maximal at 90 min and lasted for about 2.5 h after a single injection.
Figure 7. Blocking spinal NMDARs reduces mechanical hypersensitivity of rats treated with FK506.
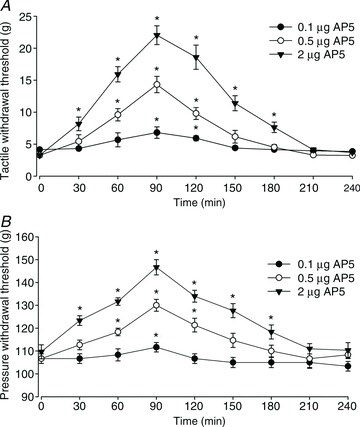
A and B, time course of the effect of intrathecal injection of 0.1–2 μg of AP5 on the paw withdrawal threshold measured with von Frey filaments (A) and a pressure stimulus (B) in rats treated with FK506 (1.5 mg kg−1 day−1 for 7 days). n= 7–8 rats per group. *P < 0.05 compared with the respective baseline value (time 0).
Intrathecal injection of 2 μg AP5 had no significant effect on the motor function of FK506-treated rats, quantified with a rotarod performance test (Cai et al. 2013). The fall latency, measured from the start of the acceleration period until the rat fell off the drum, was 204.2 ± 6.7 and 196.2 ± 8.2 s for vehicle- and AP5-treated rats (P > 0.05; n= 6 rats per group), respectively.
Systemic administration of memantine reverses mechanical hypersensitivity caused by FK506
Memantine, an orally active NMDAR antagonist, is used clinically to treat patients with Alzheimer’s disease and has relatively few adverse effects (Farlow & Cummings, 2007). Thus, we determined whether systemic administration of memantine could reduce the mechanical hypersensitivity caused by FK506. We showed previously that systemic administration of 20 mg kg−1 day−1 of memantine can achieve therapeutic plasma levels and attenuate neuropathic pain in rats (Chen et al. 2009). In FK506-treated rats (n= 7–8 rats in each group), intraperitoneal administration of memantine (1, 5 and 10 mg kg−1) significantly reduced tactile allodynia and mechanical hyperalgesia in a dose-dependent fashion (Fig. 8). At 10 mg kg−1, memantine produced a maximal effect at 90 min, which subsided 2.5 h after treatment.
Figure 8. Systemic administration of memantine alleviates mechanical hypersensitivity of rats chronically treated with FK506.
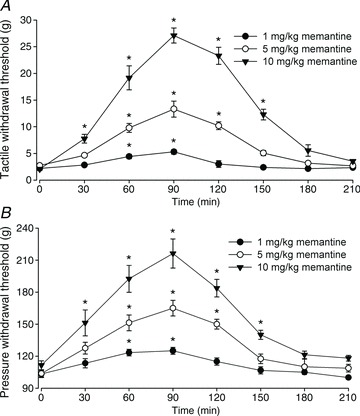
A and B, time course of the effect of a single injection of memantine (1–10 mg kg−1, i.p.) on the paw withdrawal threshold tested with von Frey filaments (A) and a noxious pressure stimulus (B) in rats treated with FK506 (1.5 mg kg−1 day−1 for 7 days). n= 7–8 rats per group. *P < 0.05 compared with the respective baseline value (time 0).
Systemic injection of 10 mg kg−1 memantine had no effect on the motor function of FK506-treated rats. The fall latency was 214.8 ± 6.4 and 221.6 ± 7.3 s in vehicle-treated and memantine-treated rats (P > 0.05; n= 8 rats per group), respectively, 60 min after drug or vehicle injection.
Discussion
Calcineurin inhibitors have been recognized clinically to cause spontaneous pain and pain hypersensitivity (Grotz et al. 2001; Collini et al. 2006; Fujii et al. 2006; Noda et al. 2008). However, the cellular and molecular mechanisms involved in CIPS are still largely unknown. Although Ca2+ channel blockers can reduce pain in some patients, effective treatment of CIPS remains a therapeutic challenge. In this study, we first examined the effect of chronic administration of FK506 on mechanical hypersensitivity in rats. We found that in all animals tested, systemic injection of FK506 for 7 days caused long-lasting tactile allodynia and mechanical hyperalgesia, which subsided 2 weeks after discontinuing FK506. It is not yet clear why calcineurin inhibitors cause pain particularly in both legs and feet in humans. Because tonic calcineurin activity is readily augmented when neuronal activity and intracellular Ca2+ levels are increased (Misonou et al. 2004; Wu et al. 2005), it is possible that the constitutive activity of calcineurin may be high in primary sensory neurons innervating weight-bearing lower extremities in humans. We observed that in the majority of lamina II neurons in the lumbar spinal cord, the glutamate release (reflected in the baseline frequency of sEPSCs and mEPSCs) was profoundly increased in rats treated with FK506. This increased tonic glutamatergic input could explain the spontaneous pain and pain hypersensitivity in the legs and feet of patients with CIPS. Our in vivo study indicates the critical role of calcineurin as an intrinsic regulator of nociceptive transmission at the spinal level. Consistent with this notion, it has been shown that intrathecal injection of calcineurin attenuates pain behaviours in a nerve constriction injury model (Miletic et al. 2013).
The first sensory synapse between primary afferent terminals and spinal dorsal horn neurons is critically involved in the transmission and modulation of sensory information during painful conditions (Koltzenburg et al. 1994; Zhou et al. 2012). To identify the substrates or molecular targets involved in pain hypersensitivity induced by calcineurin inhibitors, we used whole-cell recordings of glutamatergic EPSCs in spinal cord slices, which provide a sensitive and accurate measurement of spinal synaptic transmission. Another advantage of spinal slice recordings is that the experiment can be done in the absence of anaesthetics. We considered that spinal NMDARs may be involved in CIPS, because previous studies have shown that calcineurin can tonically reduce the activity of synaptic NMDARs by inhibiting the duration of NMDAR channel opening in the brain (Lieberman & Mody, 1994; Tong et al. 1995). Persistent stimulation of NMDARs plays a major role in spinal synaptic plasticity and development of neuropathic pain (Leem et al. 1996; Chaplan et al. 1997; Zhou et al. 2012). We found in this study that treatment with FK506 profoundly increased the amplitude of monosynaptic NMDAR-EPSCs, but not of AMPAR-EPSCs, of spinal dorsal horn neurons. The preferential sensitivity of NMDARs to calcineurin inhibition suggests a selective means of regulating increased excitatory synaptic transmission in the spinal cord by tonically active calcineurin. Also, the amplitude of NMDAR, but not of AMPAR, currents elicited by direct puff application of NMDA or AMPA to lamina II neurons was much larger in FK506-treated rats than in vehicle-treated rats. These findings suggest that inhibition of calcineurin potentiates the activity of postsynaptic NMDARs present in second-order sensory neurons in the spinal cord.
We also found that FK506 treatment caused a large increase in the baseline frequency, but not the amplitude, of sEPSCs and mEPSCs in the majority of lamina II neurons examined. Furthermore, blocking NMDARs with AP5 significantly reduced the high frequency of sEPSCs and mEPSCs in these neurons caused by FK506 treatment. To specifically determine the contribution of presynaptic NMDARs to increased glutamate release onto lamina II neurons, we recorded mEPSCs in neurons by blocking postsynaptic NMDAR channels with intracellular dialysis of MK-801. We found that AP5 also significantly reduced the frequency of mEPSCs in these neurons recorded with MK-801-containing pipette solution in FK506-treated rats. These results indicate that calcineurin inhibition can also augment presynaptic NMDARs in the spinal cord. The decay time of GluN2B-containing NMDARs is much slower than that of GluN2A-containing NMDARs (Vicini et al. 1998). Because the decay time of NMDAR-EPSCs did not differ significantly between vehicle-treated and FK506-treated rats, the increased spinal NMDAR activity by calcineurin inhibitors is less likely to be caused by the switch of GluN2 subunits. In support of this hypothesis, we found that blocking GluN2A or GluN2B subunits similarly reduced the amplitude of evoked NMDAR-EPSCs and the frequency of mEPSCs in lamina II neurons of FK506-treated rats, suggesting that both GluN2A and GluN2B subunits mediate the increased activity of presynaptic and postsynaptic NMDARs in the spinal cord by the calcineurin inhibitor. The remaining NMDAR-EPSCs after blocking GluN2A and GluN2B subunits may be mediated by other NMDAR subunits (i.e. GluN2C, GluN2D and GluN3) or tri-heteromeric GluN1/2A/2B subunits (Rauner & Köhr, 2011). Thus, calcineurin inhibitors can result in pain hypersensitivity by removing tonic calcineurin inhibition of presynaptic and postsynaptic NMDARs, thereby potentiating glutamate-mediated nociceptive input to dorsal horn neurons in the spinal cord.
Clinical studies have shown that NMDAR antagonists are effective for treating certain chronic pain conditions (Sinis et al. 2007; Schwartzman et al. 2009; Sigtermans et al. 2009). In this study, we showed that blocking NMDARs at the spinal cord level by intrathecal injection of low doses of AP5 profoundly reversed mechanical hypersensitivity induced by FK506 treatment. This finding provides further evidence about the causal relationship between augmented NMDAR activity in the spinal cord and pain hypersensitivity induced by calcineurin inhibitors. In addition, we observed that systemic injection of small doses of memantine, a clinically available and orally active NMDAR antagonist, effectively reduced tactile allodynia and mechanical hyperalgesia in FK506-treated rats. Our findings have important translational implications because it is conceivable that memantine is potentially effective for treating patients with CIPS.
Although the balance between phosphorylated and dephosphorylated NMDARs critically defines the synaptic NMDAR activity, we are uncertain as to how calcineurin inhibitors augment NMDAR activity in the spinal cord. It is possible that calcineurin inhibitors may directly increase the phosphorylation of NMDAR subunits (Krupp et al. 2002) or indirectly increase the phosphorylation of NMDAR-interacting proteins such as calmodulin (Zhang et al. 1998; Rycroft & Gibb, 2004). In this study, we measured the NMDAR activity in spinal cords removed 3–5 days after the last injection of FK506. In addition to increased glutamatergic input from nociceptive primary afferent nerves, calcineurin inhibition-initiated increases in spinal NMDAR activity may cause sustained changes in other synaptic plasticity such as diminished synaptic inhibition because of NMDAR-mediated proteolytic cleavage of K+–Cl− cotransporter-2 (Zhou et al. 2012). These synaptic and signalling events may explain why calcineurin inhibitors produce long-lasting changes in nociceptive transmission and pain hypersensitivity in CIPS. It should also be recognized that calcineurin may control the phosphorylation of other proteins to influence nociceptive transmission. For example, calcineurin can regulate Kv2.1 channel phosphorylation (Park et al. 2006), and increased phosphorylation of Kv2.1 by calcineurin inhibitors may increase the excitability of nociceptive neurons. Furthermore, calcineurin inhibitors may increase the activity of voltage-activated Ca2+ channels to increase the neurotransmitter release from nociceptive primary sensory neurons (Wu et al. 2005). Thus, further studies are required to define the contribution of other possible targets/substrates of calcineurin to altered nociceptive transmission and CIPS.
In summary, our present study provides new information about the important physiological function of calcineurin in the regulation of pain transmission and synaptic NMDAR plasticity at the spinal level. Calcineurin inhibitors can lead to loss of the normal ‘gating’ function of calcineurin at the spinal cord level and result in unrestrained nociceptive input by potentiating presynaptic and postsynaptic NMDARs. This information advances our understanding of the molecular mechanisms underlying CIPS and helps the design of effective strategies for treating CIPS.
Acknowledgments
None declared.
Glossary
- aCSF
artificial cerebrospinal fluid
- AMPAR
AMPA receptor
- AP5
d-2-amino-5-phospho-nopentanoate
- CIPS
calcineurin inhibitor-induced pain syndrome
- EPSCs
excitatory postsynaptic currents
- i.p.
intraperitoneally
- mEPSCs
miniature excitatory postsynaptic currents
- NMDAR
NMDA receptor
- sEPSCs
spontaneous excitatory postsynaptic currents
Additional information
Competing interests
None declared.
Author contributions
All experiments were carried out at the University of Texas MD Anderson Cancer Center (Houston, TX, USA). S.-R.C., Y.-M.H. and H.C. contributed to the collection, and analysis and interpretation of data. S.-R.C. and H.-L.P. contributed to the conception and design of the experiments, interpretation of data, and drafting and revising the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by grants from the National Institutes of Health (NS073935 and DE022015) and by the N. G. and Helen T. Hawkins endowment (to H.-L.P.).
References
- Bao J, Li JJ, Perl ER. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci. 1998;18:8740–8750. doi: 10.1523/JNEUROSCI.18-21-08740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, Chen SR, Pan HL. Upregulation of nuclear factor of activated T-cells by nerve injury contributes to development of neuropathic pain. J Pharmacol Exp Ther. 2013;345:161–168. doi: 10.1124/jpet.112.202192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Chen SR, Eisenach JC, McCaslin PP, Pan HL. Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology. 2000;92:500–506. doi: 10.1097/00000542-200002000-00033. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal μ opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol. 2006;95:3086–3096. doi: 10.1152/jn.01343.2005. [DOI] [PubMed] [Google Scholar]
- Chen SR, Samoriski G, Pan HL. Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology. 2009;57:121–126. doi: 10.1016/j.neuropharm.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collini A, De Bartolomeis C, Barni R, Ruggieri G, Bernini M, Carmellini M. Calcineurin-inhibitor induced pain syndrome after organ transplantation. Kidney Int. 2006;70:1367–1370. doi: 10.1038/sj.ki.5001833. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Cummings JL. Effective pharmacologic management of Alzheimer’s disease. Am J Med. 2007;120:388–397. doi: 10.1016/j.amjmed.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Fujii N, Ikeda K, Koyama M, Aoyama K, Masunari T, Kondo E, Matsuzaki T, Mizobuchi S, Hiraki A, Teshima T, Shinagawa K, Ishimaru F, Tanimoto M. Calcineurin inhibitor-induced irreversible neuropathic pain after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2006;83:459–461. doi: 10.1532/IJH97.05154. [DOI] [PubMed] [Google Scholar]
- Grandtnerova B, Spisiakova D, Lepej J, Markova I. Reflex sympathetic dystrophy of the lower limbs after kidney transplantation. Transpl Int. 1998;11(Suppl. 1):S331–333. doi: 10.1007/s001470050491. [DOI] [PubMed] [Google Scholar]
- Grotz WH, Breitenfeldt MK, Braune SW, Allmann KH, Krause TM, Rump JA, Schollmeyer PJ. Calcineurin-inhibitor induced pain syndrome (CIPS): a severe disabling complication after organ transplantation. Transpl Int. 2001;14:16–23. doi: 10.1007/s001470000285. [DOI] [PubMed] [Google Scholar]
- Kakihana K, Ohashi K, Murata Y, Tsubokura M, Kobayashi T, Yamashita T, Sakamaki H, Akiyama H. Clinical features of calcineurin inhibitor-induced pain syndrome after allo-SCT. Bone Marrow Transplant. 2012;47:593–595. doi: 10.1038/bmt.2011.120. [DOI] [PubMed] [Google Scholar]
- Kida A, Ohashi K, Tanaka C, Kamata N, Akiyama H, Sakamaki H. Calcineurin-inhibitor pain syndrome following haematopoietic stem cell transplantation. Br J Haematol. 2004;126:288. doi: 10.1111/j.1365-2141.2004.05066.x. [DOI] [PubMed] [Google Scholar]
- Kimura R, Matsuki N. Protein kinase CK2 modulates synaptic plasticity by modification of synaptic NMDA receptors in the hippocampus. J Physiol. 2008;586:3195–3206. doi: 10.1113/jphysiol.2008.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Torebjörk HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117:579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology. 2002;42:593–602. doi: 10.1016/s0028-3908(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Leem JW, Choi EJ, Park ES, Paik KS. N-Methyl-d-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett. 1996;211:37–40. doi: 10.1016/0304-3940(96)12714-2. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. Role of presynaptic muscarinic and GABAB receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lukyanetz EA. Evidence for colocalization of calcineurin and calcium channels in dorsal root ganglion neurons. Neuroscience. 1997;78:625–628. doi: 10.1016/s0306-4522(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Miletic G, Lippitt JA, Sullivan KM, Miletic V. Loss of calcineurin in the spinal dorsal horn contributes to neuropathic pain, and intrathecal administration of the phosphatase provides prolonged analgesia. Pain. 2013;154:2024–2033. doi: 10.1016/j.pain.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Muñoz-Gomez J, Collado A, Gratacós J, Campistol JM, Lomeña F, Llena J, Andreu J. Reflex sympathetic dystrophy syndrome of the lower limbs in renal transplant patients treated with cyclosporin A. Arthritis Rheum. 1991;34:625–630. doi: 10.1002/art.1780340515. [DOI] [PubMed] [Google Scholar]
- Murase N, Starzl TE, Demetris AJ, Valdivia L, Tanabe M, Cramer D, Makowka L. Hamster-to-rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993;55:701–707. doi: 10.1097/00007890-199304000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Kodama K, Yasuda T, Takahashi S. Calcineurin-inhibitor-induced pain syndrome after bone marrow transplantation. J Anesth. 2008;22:61–63. doi: 10.1007/s00540-007-0574-2. [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23:2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004;91:2413–2421. doi: 10.1152/jn.01242.2003. [DOI] [PubMed] [Google Scholar]
- Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- Puig IMJM, Martinez-Miralles E, Perich X, Lloveras J, Mir M, Inigo V, Barbosa F, Orfila A, Masramon J. Reflex sympathetic dystrophy syndrome of the lower limbs in a renal transplant patient treated with tacrolimus. Transplantation. 2000;70:210–211. [PubMed] [Google Scholar]
- Rauner C, Köhr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-d-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286:7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft BK, Gibb AJ. Inhibitory interactions of calcineurin (phosphatase 2B) and calmodulin on rat hippocampal NMDA receptors. Neuropharmacology. 2004;47:505–514. doi: 10.1016/j.neuropharm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581:241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–311. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Sinis N, Birbaumer N, Gustin S, Schwarz A, Bredanger S, Becker ST, Unertl K, Schaller HE, Haerle M. Memantine treatment of complex regional pain syndrome: a preliminary report of six cases. Clin J Pain. 2007;23:237–243. doi: 10.1097/AJP.0b013e31802f67a7. [DOI] [PubMed] [Google Scholar]
- Strack S, Wadzinski BE, Ebner FF. Localization of the calcium/calmodulin-dependent protein phosphatase, calcineurin, in the hindbrain and spinal cord of the rat. J Comp Neurol. 1996;375:66–76. doi: 10.1002/(SICI)1096-9861(19961104)375:1<66::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Takama Y, Miyagawa S, Yamamoto A, Firdawes S, Ueno T, Ihara Y, Kondo A, Matsunami K, Otsuka H, Fukuzawa M. Effects of a calcineurin inhibitor, FK506, and a CCR5/CXCR3 antagonist, TAK-779, in a rat small intestinal transplantation model. Transpl Immunol. 2011;25:49–55. doi: 10.1016/j.trim.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Tze WJ, Tai J, Cheung SS. Prolongation of pig islet xenograft survival in rats immunosuppressed with FK506. Diabetes Res Clin Pract. 1997;37:149–156. doi: 10.1016/s0168-8227(97)00068-5. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem. 2005;280:18142–18151. doi: 10.1074/jbc.M501229200. [DOI] [PubMed] [Google Scholar]
- Ybarra J, Crespo M, Torregrosa JV, Fuster D, Campistol JM, Oppenheimer F. Reflex sympathetic dystrophy syndrome in renal transplanted patients under immunosuppression with tacrolimus. Transplant Proc. 2003;35:2937–2939. doi: 10.1016/j.transproceed.2003.10.067. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- Zhao YL, Chen SR, Chen H, Pan HL. Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-d-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem. 2012;287:25073–25085. doi: 10.1074/jbc.M112.378737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK, Pan HL. N-methyl-d-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem. 2012;287:33853–33864. doi: 10.1074/jbc.M112.395830. [DOI] [PMC free article] [PubMed] [Google Scholar]


