Abstract
Generating a diverse T-cell memory population through vaccination is a promising strategy to overcome pathogen epitope variability and tolerance to tumor antigens. The effector and memory pool becomes broad in TCR diversity by recruiting high and low affinity T-cells. We wanted to determine which factors dictate whether a memory T-cell pool has a broad vs. focused repertoire. We find that inflammation increases the magnitude of low and high affinity T-cell responses equally well, arguing against a synergistic effect of TCR and inflammatory signals on T cell expansion. We dissect the differential effects of TCR signal strength and inflammation and demonstrate that they control effector T-cell survival in a bim-dependent manner. Importantly, bim-dependent cell death is overcome with a high antigen dose in the context of an inflammatory environment. Our data define the framework for the generation of a broad T-cell memory pool to inform future vaccine design.
Introduction
During the course of an infection, antigen-specific CD8 T cells become activated, acquire effector function and a fraction of these cells will then differentiate into long-lived memory CD8 T cells (1–3). The generation of large numbers of high affinity T cells during this process is generally considered a desirable and advantageous outcome as it selects for the best responders. Recent studies have strongly suggested a significant biological role for low affinity T cells in creating a successful vaccination strategy. CD8 T cells activated by low affinity ligands are recruited into the primary CD8 T cell response as well as the ensuing CD8 memory T cell pool (4). T cell mediated protection against pathogens that are capable of a high mutation rate may benefit from a repertoire of antigen-specific T cells that is very broad. A broad repertoire includes T cells which react only weakly to the initial priming antigen (low affinity T cells), but may recognize subsequently mutated epitopes with high affinity. Recent evidence from an HIV vaccine trial shows that the mere presence of memory CD8 T cells is not enough to provide protection against HIV infection (5) and this lack of protection could be attributed to insufficient potency or breadth of epitope recognition (6). Importantly, previous animal studies indeed underline a crucial role for TCR repertoire diversity to prevent viral escape mutants (7, 8). This protective benefit of a broad T cell repertoire is presumably true for all highly variable pathogens whether they are chronic infections such as described in the aforementioned studies or acute infections like influenza (9). Moreover, anti-tumor responses may depend on recruiting lower affinity T cells since higher affinity T cells are often eliminated by tolerance mechanisms. The main focus in the cancer immunotherapy field is on generating high numbers of functional CTLs to eliminate tumor burden.
In the context of vaccine design it is a major challenge to trigger potent T cell responses and the parameters that allow for induction of T cell responses with a broad repertoire are still poorly understood. Historically, vaccines using live attenuated viruses such as the smallpox, polio (Sabin) and yellow fever vaccine have been highly successful and shown to prime long-lived, potent T cell responses (10–12). More recently, an SIV vaccine based on a recombinant rhesus cytomegalovirus (RhCMV) provided protection against highly pathogenic SIV (13). Two major differences between live and inactivated vaccines are the amount of inflammation and antigen dose elicited by each vaccine, with live vectors generating more potent inflammatory responses and presumably providing more antigen. It has been established that TCR signal strength, antigen availability and inflammation are the driving forces of the primary and memory T cell response (1–3, 14), but it is still unclear how these signals interact to shape the breadth of the T cell response and affect high and low affinity T cell responses (2, 14). Previous studies suggest that cytokine and TCR signals can synergize to mediate memory CD4 T cell survival (15). Whether comparable synergistic events take place in CD8 T cells is still unknown, but such an integration of different signals could enhance the selective outgrowth of the highest affinity T cells and regulate affinity maturation of the memory T cell pool (16). Gaining a more thorough understanding of the mechanisms involved in efficient priming and survival of low affinity T cells is thus of broad general interest in the context of infectious disease as well as cancer therapy.
The primary goal of this study is to address how inflammation, TCR signal strength and antigen availability interact to shape the breadth and function of the effector and memory T cell response. We used an experimental system, which gave us tight control over factors that can vary between different vaccine formulations or types of infection. This system allowed us demonstrate that (1) concurrent inflammation enhances effector T cell responses to the same extent regardless of the strength of the TCR stimulus, (2) inflammation and TCR signal strength control distinct and overlapping phenotypic and functional characteristics of the effector cell population, (3) bim-mediated cell death is responsible for the loss of low affinity effector cells under conditions of limited inflammation and antigen availability, but (4) this can be overcome with increased antigen availability and inflammation.
Material and Methods
Mice
C57BL/6 (B6) and B6 congenic (CD45.1+ and Thy1.1+) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in SPF conditions at the University of Washington and the Fred Hutchinson Cancer Research Center (Seattle, WA). OT-I TCR transgenic mice congenic for CD45.1 or Thy1.1 and bim−/− OT-I mice were bred and maintained in the same facilities.
Adoptive transfer and cell sorting: naïve CD44low OT-I T cells were isolated from lymph nodes by separation over a magnetic column (Miltenyi) as previously described (17). A total number of 1×104 naïve OT-I T cells was transferred i.v. per recipient. This number was chosen based on a 10% take of transferred cells in a new host following adoptive transfer (17, 18) and a precursor frequency of antigen-specific, endogenous T cells that can range from less than 102 to over 103 (19, 20).
Dendritic cell isolation
Dendritic cells (DC) were expanded in B6 mice with a Flt3L secreting cell line (B16-Flt3L). 10–14 days after injection of the B16-Flt3L cells CD11c+ cells were purified, LPS (1μg/ml) and peptide (1μg/ml) pulsed in vitro for 1 hour (37°C) prior transfer as previously described (17). A total number of 1×106 DCs was transferred i.v. per recipient.
Infections
Listeria monocytogenes was grown as previously described (17). For primary infections, mice were injected i.v. with 2×103 cfu LM. In some experiments memory cells were boosted with a recombinant VSV strain expressing the SIINFEKL epitope (21) or a recombinant vaccinia strain expressing the SIINFEKL epitope (22) and were sacrificed 5 to 7 days later (2×106 pfu, i.v., in each case).
Flow Cytometry
Recipient mice were sacrificed at the time points indicated. For intracellular staining, cells were prepared with the Cytofix/Cytoperm kit in the presence of brefeldin A (BD) and incubated with or without 100nM SIINFEKL peptide for 4–5 hours in RP10. Cells were analyzed using a FACSCanto (BD) and FlowJo (TreeStar) software.
Results
Inflammation enhances generation of CD8 effector cells
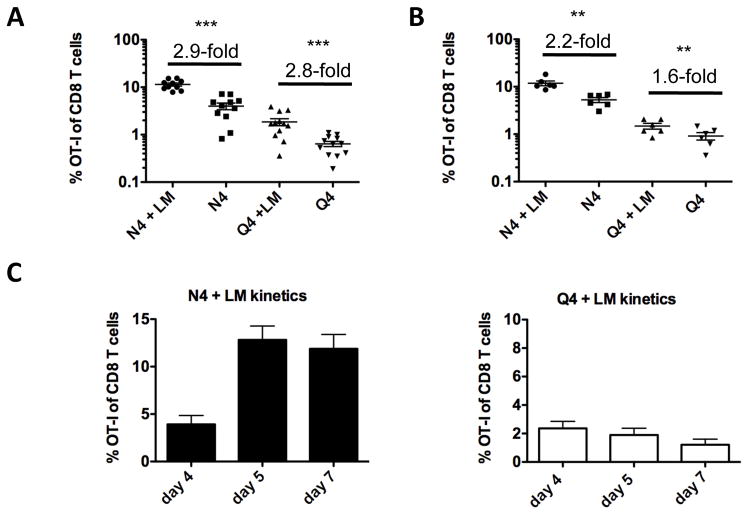
We first addressed whether cytokine signals synergize with the strength of a TCR signal. For this purpose we relied on the OT-I system to provide defined TCR specificity. OT-I T cells are activated by the high affinity SIINFEKL (N4) peptide of chicken ovalbumin (OVA) presented by H2-Kb (23). A large number of altered peptide ligands (APLs) with equal ability to bind to H-2Kb but gradually reduced TCR affinity have been described which nevertheless all still activate OT-I T cells (4). We decided to use the low affinity APL SIIQFEKL (Q4) for this study, because it elicits an OT-I T cell response that can still be easily measured and it is well above the threshold of thymic negative selection (24, 25). In order to keep antigen dose and availability constant, we used a system where antigen is provided by ex vivo isolated (from B16-Flt3L injected animals), LPS activated, peptide pulsed DCs and inflammation is provided by a concurrent infection with wild-type Listeria monocytogenes (LM) that does not provide antigen for the OT-I T cells (17, 26). A small number of naïve OT-I T cells was transferred into recipient mice together with high (N4) or low (Q4) affinity antigen pulsed DCs. In the presence of an accompanying LM infection N4 pulsed DCs generated an approx. 2 to 3-fold bigger OT-I effector population compared to the peptide pulsed DC only group in the blood (Fig. 1A) and spleen (Fig. 1B) on day 5. Interestingly, a similar enhancement by bystander Listeria infection was observed for Q4 stimulated OT-I T cells (Fig. 1A, B). We chose day 5 as a time-point to examine OT-I T cell phenotype and function after the expansion stage was completed in both (N4 and Q4 primed) conditions (Fig. 1C) to allow for a meaningful comparison. Together, these data suggest that inflammation enhanced low and high affinity T cell responses equally and independently of ligand potency. It is important to note that CD4 T cell help is provided even in the absence of a bystander LM infection since DCs are exposed to antigen (fetal calf serum, enzymes, etc.) during the isolation process which results in presentation of antigenic MHC class II restricted epitopes (27, 28).
Fig. 1. Impact of inflammation and TCR signal strength on the size, function and phenotype of the primary response.
C57BL/6 mice were injected intravenously (i.v.) with 1×106 N4 or Q4 peptide pulsed DCs and 1×104 naïve OT-I T cells (Thy1.1) with or without an accompanying wild-type Listeria monocytogenes (LM) infection. The frequency of OT-I T cells of total CD8 T cells (A) in the blood and (B) in the spleen was determined 5 days later and fold increase of N4 and Q4 primed OT-I T cells (+/− LM) was calculated. Data shown are representative for at least 5 independently performed experiments with a minimum of 3 mice per group. Horizontal bars represent the mean. (C) The frequency of OT-I T cells in the blood is shown on days 4, 5 and 7 after priming with N4 peptide pulsed DCs + LM and Q4 peptide pulsed DCs + LM. Data shown are pooled from 2 independently performed experiments with a minimum of 3 mice per group. Statistical significance was determined by student t-test. p-values >0.05 were considered not significant (ns), 0.01 to 0.05 significant (*), 0.001 to 0.01 very significant (**) and <0.001 extremely significant (***).
Integrating inflammation and TCR signals to shape T cell function
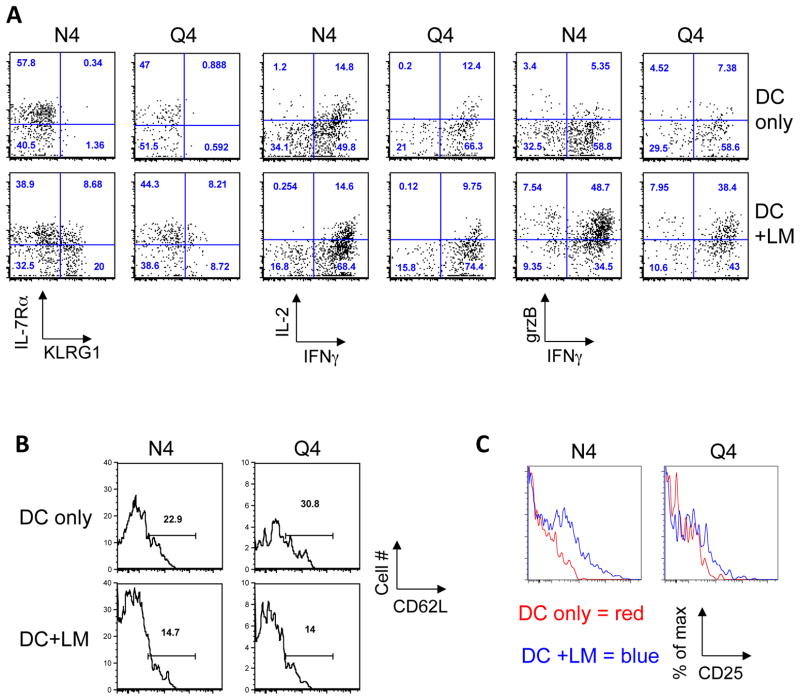
We previously demonstrated that priming high affinity CD8 T cells in the context of minimal inflammation induces robust T cell expansion and IFN-γ production but the cells lack granzyme expression and remain KLRG1 low (26). We wanted to determine which aspects of T cell phenotype and function are regulated primarily by inflammatory stimuli versus TCR signal strength. We found that production of IFNγ and granzyme B was enhanced by inflammation, while the affinity of the TCR ligand played only a limited role (Fig. 2A). In contrast, KLRG1 expression levels were dependent on both, inflammation and TCR signal strength (Fig. 2A). While inflammation was necessary to induce KLRG1 expression, a greater percentage of cells expressed KLRG1 when stimulated with a high affinity ligand compared to an intermediate ligand (N4 vs. Q4, about 2x difference). OT-I T cells that were expanded in the context of systemic inflammation expressed less CD62L on day 5 post priming regardless of the nature of the ligand (Fig. 2B). Inflammation had very little impact on the ability of effector cells to produce IL-2, while priming with high affinity ligand generated cells that produced more IL-2 (Fig. 2A). In contrast, expression of IL-2Rα (CD25) was directly correlated to both the extent of inflammation present and the strength of the TCR stimulus on day 5, when both N4 and Q4 primed OT-I T cells had finished expanding following peptide-pulsed DC immunization (Fig 1C). Together these data show that TCR signal strength and inflammatory conditions exclusively control certain functional aspects of the T cell response, while other features are clearly the result of integrating both signals.
Fig. 2. Impact of inflammation and TCR signal strength on function and phenotype of the primary response.
C57BL/6 mice were injected intravenously (i.v.) with 1×106 N4 or Q4 peptide pulsed DCs and 1×104 naïve OT-I T cells (Thy1.1) with or without an accompanying LM infection. (A) OT-I T cell surface phenotype (IL-7Rα, KLRG-1), function (IL-2, IFNγ and granzyme B expression), (B) CD62L expression and (C) CD25 expression was determined 5 days post priming. Data are representative of at least 5 independently performed experiments with a minimum of 3 mice per group.
Bim-mediated cell death dictates the size of the effector pool
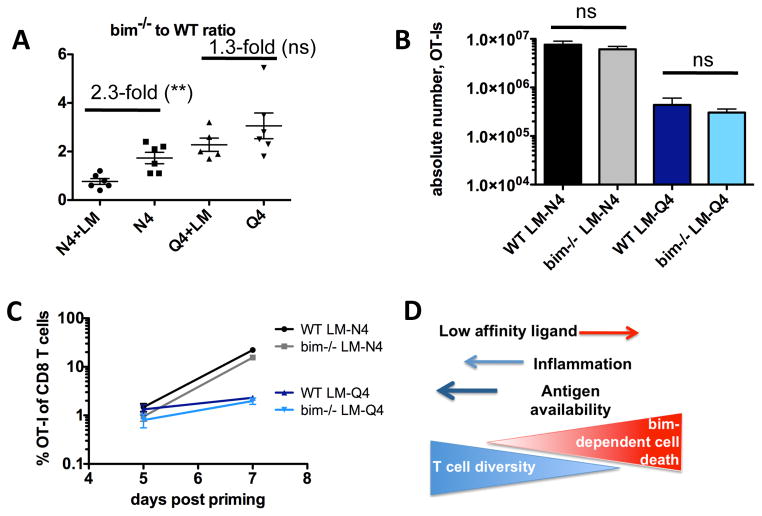
We wanted to directly address the respective roles of TCR signal strength and inflammatory signals in regulating effector cell apoptosis and determining the magnitude of the T cell response. Using the same experimental approach as described in Fig. 1, we primed a 1:1 mix of WT and bim−/− OT-I cells with high or low affinity ligand pulsed DCs (+/− LM) and analyzed the magnitude of the OT-I response 5 days later (Fig. 3A). We found that bim-mediated T cell death does not play a role when high affinity effector cells are primed in the context of an inflammatory environment (N4+LM) while in the absence of abundant inflammation high affinity T cells are lost in a bim-dependent manner (N4) (Fig. 3A). The ratio of bim−/− to WT OT-I T cells is skewed towards the bim−/− cells when primed with Q4-pulsed DC alone and in contrast to N4 immunization, in this case inflammation was not sufficient to prevent bim-mediated cell death for low affinity primed T cells (Q4+LM compared to Q4 only). These data establish a central role for bim in regulating the breadth of the T cell repertoire and provide a mechanism of how TCR signal strength and inflammation integrate to determine T cell fate. When peptide pulsed DCs are used for immunization, only a limited quantity of antigen is provided. This differs from a situation in which a pathogen expresses antigen, which is available at higher quantities for a longer time-period and with potentially wider tissue-distribution of the antigen. We therefore next tested the effect of increasing antigen dose on bim-mediated cell death using the previously described recombinant strains LM-N4 and LM-Q4 to prime a 1:1 mix of WT and bim−/− OT-I T cells (Fig. 3B). Strikingly in contrast to immunization with peptide-pulsed DC we found that bim-mediated cell death did not occur following immunization with recombinant LM strains even after low affinity (LM-Q4) priming (Fig. 3B). To exclude the possibility that the different expansion and contraction kinetics of high and low affinity responders may skew interpretation of the results, we tracked WT and bim−/− OT-I T cells on day 5 and 7 post priming with LM-N4 and LM-Q4 confirming that bim-dependent cell death of effector cells during the expansion phase does not play a role in conditions of high dose antigen and systemic inflammation (Fig 3C). Together these data show that bim-mediated cell death affects the size of the effector pool through TCR signal strength- and inflammation-mediated signals, but is overcome when low affinity cells are primed in the context of a replicating pathogen (Fig. 3D).
Fig. 3. TCR signal strength and inflammation dictate the magnitude of the primary response in a bim-dependent manner.
A mix of 5×103 WT (Thy1.1+) and 5×103 bim−/− CD45.2 OT-I T cells was transferred into CD45.1+ congenic B6 hosts. (A) Mice were injected intravenously (i.v.) with 1×106 N4 or Q4 peptide pulsed DCs with or without an accompanying LM infection. Spleens were harvested 5 days post priming and the ratio of bim−/− to WT OT-I was determined. (B) Mice were primed with LM-N4 or LM-Q4 and the number of WT and bim−/− OT-I cells in the spleen was determined on day 7 after infection. (C) Mice were primed with LM-N4 or LM-Q4. The number of WT and bim−/−OT-I cells in the blood was determined on days 5 and 7 after infection. Data shown are representative for 2–3 independently performed experiments with a minimum of 3 mice per group. Horizontal bars represent the mean. (D) Overview of the mechanisms dictating the composition of the T cell response. Low affinity stimulated T cells are more susceptible to bim-mediated cell death, but this can be partially overcome by increasing inflammation. Increasing antigen availability in the context of inflammation can fully overcome the bim-mediated cell death and preserve low affinity T cell responses. Statistical significance was determined by student t-test. p-values >0.05 were considered not significant (ns), 0.01 to 0.05 significant (*), 0.001 to 0.01 very significant (**) and <0.001 extremely significant (***).
Generating functional memory
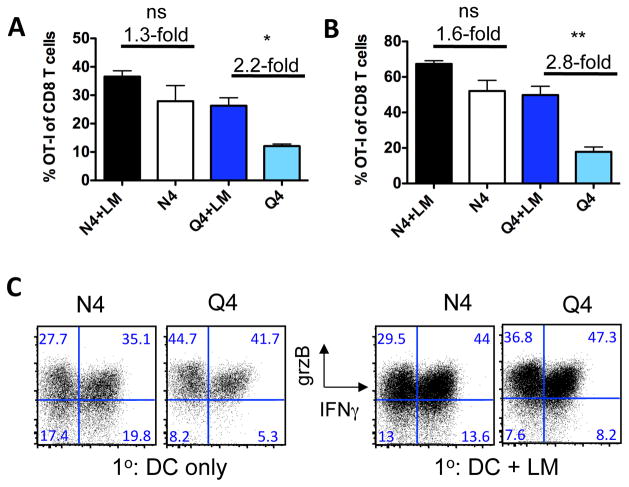
We previously demonstrated that high affinity CD8 T cells primed in the context of minimal inflammation give rise to effector cells that lack granzyme expression, but which contract normally and give rise to fully functional memory T cells (26). Since inflammation and TCR signal strength both affected aspects of the CD8 effector phenotype (Fig. 2A), we next tested the ability of high and low affinity memory cells primed with our without an accompanying LM infection to respond to a recall response. Specifically, we wanted to address whether low affinity memory T cells generated in the presence of limited inflammation are actually functional. OT-I T cells were primed with N4- or Q4-pulsed DCs +/− LM and rested for at least 30 days. Mice were challenged with a recombinant vaccinia (VAC) or vesicular stomatitis virus (VSV) expressing OVA. This heterologous rechallenge strategy allowed us to directly address the recall ability of the memory OT-I T cells without engaging endogenous, Listeria-specific memory cells. This is important since pre-existing immunity to a vaccine vector can affect the ensuing memory response to the targeted antigens (29). Moreover, this secondary response delivers the same quality of stimulus to T cells in all experimental groups, thus specifically addressing possible differences induced during the initial T cell priming. Mice were euthanized 5 days after the rechallenge and OT-I T cell frequency, function and phenotype were determined. We found that both high and low affinity primed OT-I T cells mounted a robust recall response regardless of the inflammatory environment during priming (Fig. 4A). This suggests that a low affinity TCR signal mediated by a limited amount of antigen during the primary response is still sufficient to generate functional memory T cells. Following VAC-OVA secondary immunization, progeny of the memory cells were also found in non-lymphoid tissue such as the lung (Fig. 4B) indicating that memory trafficking to non-lymphoid compartments is intact and independent of the initial TCR signal strength encountered during priming. Most importantly, this secondary challenge elicited a comparable functional response from all memory T cells regardless of the priming conditions as measured by IFN-γ and granzyme B production (Fig. 4C).
Fig. 4. Functional memory is generated even from low affinity T cells primed in an environment with limited inflammation.
(A) More than 30 days after the primary response (as described in Fig. 1), mice of all 4 experimental groups were immunized with recombinant vaccinia virus expressing Ova (VAC-OVA) and T cell responses in (A) the spleen and (B) lung were determined 5 days later. (C) Memory cell function was assessed in an in vitro restimulation assay by measuring IFNγ and granzyme B production. Data shown are representative for 2 independently performed experiments with a minimum of 3 mice per group. Similar data was obtained from 2 experiments using recombinant vesicular stomatitis virus (VSV-OVA). Statistical significance was determined by student t-test. p-values >0.05 were considered not significant (ns), 0.01 to 0.05 significant (*), 0.001 to 0.01 very significant (**) and <0.001 extremely significant (***).
Discussion
In the context of vaccine design, generating a broad memory T cell repertoire including high and low affinity responders is desirable in order to provide a diverse repertoire of responding memory T cells upon subsequent pathogen encounter. This is thought to be particularly beneficial for highly variable pathogens such as HIV or influenza. While the benefits of generating a broad memory T cell repertoire have been established, the parameters needed to achieve such a response remain poorly defined. We addressed how inflammatory signals, TCR signal strength (in this context defined as the functional avidity of a T cell for a given epitope) and antigen dose interact to shape the breadth and function of an effector and memory T cell response.
We show that inflammation provided by a bystander LM infection enhanced low and high affinity T cell responses equally and independently of ligand potency when keeping the antigen dose consistent (Fig. 1). TCR signal strength and inflammatory conditions exclusively control certain functional aspects of the T cell response, while other features are clearly the result of integrating both signals (Fig. 2). It is important to keep in mind that certain functional properties might not change between two ligands of different affinity if an affinity ceiling is reached (30). However, given the phenotypic and functional differences that we observed between N4 and Q4 primed OT-I T cells, our data argue against an affinity threshold model that equally affects all aspects of T cell function. This notion is particularly interesting when considering that at least certain features of the primary CD8 T cell response are programmed: brief exposure to high affinity antigen is sufficient to initiate long lasting T cell proliferation and induce effector function, however prolonged exposure to antigen further enhances the magnitude of the response (17, 31–33).
Our study also defines the requirements for preserving low affinity responders in a CD8 T cell response. Low TCR signal strength and limited inflammation lead to impaired expansion partially due to bim-dependent apoptosis of low affinity responders (Fig. 3C). However, a high antigen dose is sufficient to overcome bim-dependent cell death as shown by the use of recombinant LM strains compared to OT-I cells primed by peptide pulsed DCs in the presence of an LM infection. We cannot formally exclude the possibility that there is a concurrent effect on cell division mediated by bim enhancing the difference in WT and bim−/− T cell accumulation specifically in situations of low affinity stimulation. WT and bim−/− OT-I T cells have comparable TCR surface expression levels, thus excluding differential sensitivity of these cells to TCR stimulation (34). An increase in epitope density has been previously shown to regulate CD8 viability via the Nur77 family member Nor-1 (35). Nor-1 and bim have been proposed to converge at the mitochondrial level to regulate cell fate in thymic selection (36), but whether similar mechanisms are in place in effector cells to regulate apoptosis remains to be determined. The difference in cell accumulation in the LM-N4 and LM-Q4 infected animals could be due to differences in the number of cell divisions as suggested by BrdU incorporation experiments (4) without a significant role for bim-mediated apoptosis. Although we cannot measure the fold difference in antigen dose at different time points following immunization with peptide pulsed DCs versus recombinant LM, it is important to consider that we are comparing limited versus replicating antigen scenarios. The number of infected cells in the spleen increases dramatically in the first days following LM infection (37) and antigen-presentation persists for more than 10 days (38) supporting our notion that there is a higher antigen availability in the recombinant LM immunization scenario.
Following a secondary challenge OT-I T cells from all four experimental groups expanded very robustly, but fold-expansion across the different groups has to be carefully interpreted due to the different memory precursory frequencies in each group. Importantly, we show that functional memory cells can be generated in even suboptimal conditions with a limited antigen dose, low affinity TCR signal and limited inflammation (Fig. 4). Together, our data provide mechanistic insight into the success of vaccines that are based on using live, replicating vectors and suggest that this approach is likely the most successful approach to generate a broad CD8 memory pool.
Acknowledgments
This work was supported by NIH grants R00 AI079159 and DP2 DE023321 (to M.P.), the Howard Hughes Medical Institute (to M.J.B.), ProDoc grant (PDFMP3_137128) and project grant (310030_130512) from the Swiss National Science Foundation (to DZ).
References
- 1.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Daniels MA, Teixeiro E. The persistence of T cell memory. Cell Mol Life Sci. 2010;67:2863–2878. doi: 10.1007/s00018-010-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenport MP, Price DA, McMichael AJ. The T cell repertoire in infection and vaccination: implications for control of persistent viruses. Curr Opin Immunol. 2007;19:294–300. doi: 10.1016/j.coi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Price DA, West SM, Betts MR, Ruff LE, Brenchley JM, Ambrozak DR, Edghill-Smith Y, Kuroda MJ, Bogdan D, Kunstman K, Letvin NL, Franchini G, Wolinsky SM, Koup RA, Douek DC. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity. 2004;21:793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 9.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten Brinke A, Tak PP, Eldering E, Nolte MA, van Lier RA. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed R, Akondy RS. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol. 2011;89:340–345. doi: 10.1038/icb.2010.155. [DOI] [PubMed] [Google Scholar]
- 12.Wahid R, Cannon MJ, Chow M. Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J Virol. 2005;79:5988–5995. doi: 10.1128/JVI.79.10.5988-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cell Mol Life Sci. 2012;69:1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caserta S, Zamoyska R. Memories are made of this: synergy of T cell receptor and cytokine signals in CD4(+) central memory cell survival. Trends Immunol. 2007;28:245–248. doi: 10.1016/j.it.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 17.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 19.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrancois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci U S A. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prlic M, Gibbs J, Jameson SC. Characteristics of NK cell migration early after vaccinia infection. J Immunol. 2005;175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 23.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 24.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 25.Enouz S, Carrie L, Merkler D, Bevan MJ, Zehn D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J Exp Med. 2012;209:1769–1779. doi: 10.1084/jem.20120905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prlic M, Sacks JA, Bevan MJ. Dissociating markers of senescence and protective ability in memory T cells. PloS one. 2012;7:e32576. doi: 10.1371/journal.pone.0032576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingstone AM, Kuhn M. Dendritic cells need T cell help to prime cytotoxic T cell responses to strong antigens. Eur J Immunol. 1999;29:2826–2834. doi: 10.1002/(SICI)1521-4141(199909)29:09<2826::AID-IMMU2826>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, Casimiro DR, Duerr A, Robertson MN, Buchbinder SP, Huang Y, Spies GA, De Rosa SC, McElrath MJ. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122:359–367. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179:2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 31.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 33.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suen AY, Baldwin TA. Proapoptotic protein Bim is differentially required during thymic clonal deletion to ubiquitous versus tissue-restricted antigens. Proc Natl Acad Sci U S A. 2012;109:893–898. doi: 10.1073/pnas.1114834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leignadier J, Labrecque N. Epitope density influences CD8+ memory T cell differentiation. PloS one. 2010;5:e13740. doi: 10.1371/journal.pone.0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, Jung S, Hochrein H, Russmann H, Brocker T, Busch DH. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]